Abstract
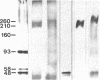
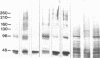
The postsynaptic glycine receptor of rat spinal cord is a glycosylated membrane protein that, after affinity purification, contains membrane-spanning subunits of Mr 48,000 and 58,000 and an associated peripheral polypeptide of Mr 93,000. Here, the quaternary structure of the transmembrane core of the receptor was investigated by chemically crosslinking its subunits. Upon treatment with crosslinking reagents of different side-chain specificities and lengths, a consistent set of adducts up to Mr 260,000 was detected after separation by NaDodSO4/PAGE. The observed pattern of adducts was similar irrespective of whether purified receptor protein or synaptosomal membranes were crosslinked. Compositional analysis revealed that the crosslinked adducts contained the Mr 48,000 and 58,000 subunits in varying ratios but not the peripheral Mr 93,000 polypeptide. Thus adducts of intermediate molecular weight represent dimers, trimers, and tetramers of the transmembrane subunits, whereas the major adduct of Mr 260,000 corresponds to a pentameric assembly of subunits forming the ion channel of the glycine receptor. This subunit arrangement is similar to that reported for the nicotinic acetylcholine receptor of fish electric organ and skeletal muscle. Hence, we suggest that the different ligand-gated ion channels of excitable membranes share a similar quaternary structure.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschuler R. A., Betz H., Parakkal M. H., Reeks K. A., Wenthold R. J. Identification of glycinergic synapses in the cochlear nucleus through immunocytochemical localization of the postsynaptic receptor. Brain Res. 1986 Mar 26;369(1-2):316–320. doi: 10.1016/0006-8993(86)90542-1. [DOI] [PubMed] [Google Scholar]
- Becker C. M., Hermans-Borgmeyer I., Schmitt B., Betz H. The glycine receptor deficiency of the mutant mouse spastic: evidence for normal glycine receptor structure and localization. J Neurosci. 1986 May;6(5):1358–1364. doi: 10.1523/JNEUROSCI.06-05-01358.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J., Evans K., Goldman D., Martin G., Treco D., Heinemann S., Patrick J. Isolation of a cDNA clone coding for a possible neural nicotinic acetylcholine receptor alpha-subunit. 1986 Jan 30-Feb 5Nature. 319(6052):368–374. doi: 10.1038/319368a0. [DOI] [PubMed] [Google Scholar]
- Brisson A., Unwin P. N. Quaternary structure of the acetylcholine receptor. Nature. 1985 Jun 6;315(6019):474–477. doi: 10.1038/315474a0. [DOI] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. The specific ionic conductances and the ionic movements across the motoneuronal membrane that produce the inhibitory post-synaptic potential. J Physiol. 1955 Nov 28;130(2):326–374. doi: 10.1113/jphysiol.1955.sp005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S. The size and detergent binding of membrane proteins. J Biol Chem. 1975 Jul 25;250(14):5459–5469. [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Frye L. D., Edidin M. The rapid intermixing of cell surface antigens after formation of mouse-human heterokaryons. J Cell Sci. 1970 Sep;7(2):319–335. doi: 10.1242/jcs.7.2.319. [DOI] [PubMed] [Google Scholar]
- Furth A. J., Bolton H., Potter J., Priddle J. D. Separating detergent from proteins. Methods Enzymol. 1984;104:318–328. doi: 10.1016/s0076-6879(84)04098-2. [DOI] [PubMed] [Google Scholar]
- GRAY E. G., WHITTAKER V. P. The isolation of nerve endings from brain: an electron-microscopic study of cell fragments derived by homogenization and centrifugation. J Anat. 1962 Jan;96:79–88. [PMC free article] [PubMed] [Google Scholar]
- Gaffney B. J. Chemical and biochemical crosslinking of membrane components. Biochim Biophys Acta. 1985 Dec 9;822(3-4):289–317. doi: 10.1016/0304-4157(85)90012-7. [DOI] [PubMed] [Google Scholar]
- Giraudat J., Dennis M., Heidmann T., Haumont P. Y., Lederer F., Changeux J. P. Structure of the high-affinity binding site for noncompetitive blockers of the acetylcholine receptor: [3H]chlorpromazine labels homologous residues in the beta and delta chains. Biochemistry. 1987 May 5;26(9):2410–2418. doi: 10.1021/bi00383a003. [DOI] [PubMed] [Google Scholar]
- Graham D., Pfeiffer F., Betz H. Photoaffinity-labelling of the glycine receptor of rat spinal cord. Eur J Biochem. 1983 Apr 5;131(3):519–525. doi: 10.1111/j.1432-1033.1983.tb07292.x. [DOI] [PubMed] [Google Scholar]
- Graham D., Pfeiffer F., Simler R., Betz H. Purification and characterization of the glycine receptor of pig spinal cord. Biochemistry. 1985 Feb 12;24(4):990–994. doi: 10.1021/bi00325a027. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Gundelfinger E., Schmitt B., Betz H., Darlison M. G., Barnard E. A., Schofield P. R., Seeburg P. H. Glycine vs GABA receptors. Nature. 1987 Nov 5;330(6143):25–26. doi: 10.1038/330025b0. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Rienitz A., Schmitt B., Methfessel C., Zensen M., Beyreuther K., Gundelfinger E. D., Betz H. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature. 1987 Jul 16;328(6127):215–220. doi: 10.1038/328215a0. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Properties of human brain glycine receptors expressed in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1984 Apr 24;221(1223):235–244. doi: 10.1098/rspb.1984.0032. [DOI] [PubMed] [Google Scholar]
- Hajdu J., Bartha F., Friedrich P. Crosslinking with bifunctional reagents as a means for studying the symmetry of oligomeric proteins. Eur J Biochem. 1976 Sep 15;68(2):373–383. doi: 10.1111/j.1432-1033.1976.tb10824.x. [DOI] [PubMed] [Google Scholar]
- Hermans-Borgmeyer I., Zopf D., Ryseck R. P., Hovemann B., Betz H., Gundelfinger E. D. Primary structure of a developmentally regulated nicotinic acetylcholine receptor protein from Drosophila. EMBO J. 1986 Jul;5(7):1503–1508. doi: 10.1002/j.1460-2075.1986.tb04389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucho F., Bandini G., Suárez-Isla B. A. The acetylcholine receptor as part of a protein complex in receptor-enriched membrane fragments from Torpedo californica electric tissue. Eur J Biochem. 1978 Feb;83(2):335–340. doi: 10.1111/j.1432-1033.1978.tb12099.x. [DOI] [PubMed] [Google Scholar]
- Hucho F., Oberthür W., Lottspeich F. The ion channel of the nicotinic acetylcholine receptor is formed by the homologous helices M II of the receptor subunits. FEBS Lett. 1986 Sep 1;205(1):137–142. doi: 10.1016/0014-5793(86)80881-x. [DOI] [PubMed] [Google Scholar]
- Imoto K., Methfessel C., Sakmann B., Mishina M., Mori Y., Konno T., Fukuda K., Kurasaki M., Bujo H., Fujita Y. Location of a delta-subunit region determining ion transport through the acetylcholine receptor channel. Nature. 1986 Dec 18;324(6098):670–674. doi: 10.1038/324670a0. [DOI] [PubMed] [Google Scholar]
- Kiehm D. J., Ji T. H. Photochemical cross-linking of cell membranes. A test for natural and random collisional cross-links by millisecond cross-linking. J Biol Chem. 1977 Dec 10;252(23):8524–8531. [PubMed] [Google Scholar]
- Kubalek E., Ralston S., Lindstrom J., Unwin N. Location of subunits within the acetylcholine receptor by electron image analysis of tubular crystals from Torpedo marmorata. J Cell Biol. 1987 Jul;105(1):9–18. doi: 10.1083/jcb.105.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mamalaki C., Stephenson F. A., Barnard E. A. The GABAA/benzodiazepine receptor is a heterotetramer of homologous alpha and beta subunits. EMBO J. 1987 Mar;6(3):561–565. doi: 10.1002/j.1460-2075.1987.tb04791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Furutani Y., Hirose T., Takashima H., Inayama S., Miyata T. Structural homology of Torpedo californica acetylcholine receptor subunits. Nature. 1983 Apr 7;302(5908):528–532. doi: 10.1038/302528a0. [DOI] [PubMed] [Google Scholar]
- Peters K., Richards F. M. Chemical cross-linking: reagents and problems in studies of membrane structure. Annu Rev Biochem. 1977;46:523–551. doi: 10.1146/annurev.bi.46.070177.002515. [DOI] [PubMed] [Google Scholar]
- Pfeiffer F., Graham D., Betz H. Purification by affinity chromatography of the glycine receptor of rat spinal cord. J Biol Chem. 1982 Aug 25;257(16):9389–9393. [PubMed] [Google Scholar]
- Pfeiffer F., Simler R., Grenningloh G., Betz H. Monoclonal antibodies and peptide mapping reveal structural similarities between the subunits of the glycine receptor of rat spinal cord. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7224–7227. doi: 10.1073/pnas.81.22.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rienitz A., Becker C. M., Betz H., Schmitt B. The chloride channel blocking agent, t-butyl bicyclophosphorothionate, binds to the gamma-aminobutyric acid-benzodiazepine, but not to the glycine receptor in rodents. Neurosci Lett. 1987 Apr 23;76(1):91–95. doi: 10.1016/0304-3940(87)90198-4. [DOI] [PubMed] [Google Scholar]
- Schmitt B., Knaus P., Becker C. M., Betz H. The Mr 93,000 polypeptide of the postsynaptic glycine receptor complex is a peripheral membrane protein. Biochemistry. 1987 Feb 10;26(3):805–811. doi: 10.1021/bi00377a022. [DOI] [PubMed] [Google Scholar]
- Schofield P. R., Darlison M. G., Fujita N., Burt D. R., Stephenson F. A., Rodriguez H., Rhee L. M., Ramachandran J., Reale V., Glencorse T. A. Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor super-family. Nature. 1987 Jul 16;328(6127):221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Bennett J. P., Jr Neurotransmitter receptors in the brain: biochemical identification. Annu Rev Physiol. 1976;38:153–175. doi: 10.1146/annurev.ph.38.030176.001101. [DOI] [PubMed] [Google Scholar]
- Triller A., Cluzeaud F., Pfeiffer F., Betz H., Korn H. Distribution of glycine receptors at central synapses: an immunoelectron microscopy study. J Cell Biol. 1985 Aug;101(2):683–688. doi: 10.1083/jcb.101.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting P., Esch F., Shimasaki S., Lindstrom J. Neuronal nicotinic acetylcholine receptor beta-subunit is coded for by the cDNA clone alpha 4. FEBS Lett. 1987 Jul 27;219(2):459–463. doi: 10.1016/0014-5793(87)80272-7. [DOI] [PubMed] [Google Scholar]
- Whiting P., Lindstrom J. Purification and characterization of a nicotinic acetylcholine receptor from rat brain. Proc Natl Acad Sci U S A. 1987 Jan;84(2):595–599. doi: 10.1073/pnas.84.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A. B., Snyder S. H. Strychnine binding associated with glycine receptors of the central nervous system. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2832–2836. doi: 10.1073/pnas.70.10.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A. B., Snyder S. H. The glycine synaptic receptor: evidence that strychnine binding is associated with the ionic conductance mechanism. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4002–4005. doi: 10.1073/pnas.71.10.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]