Abstract
Background
Eosinophils are likely key cells involved in the pathogenesis of asthma and allergic diseases; however, the mechanisms that regulate eosinophil dynamics and functions in mucosal tissues are incompletely understood. IL-33, which is produced by mucosal cells, is a new member of the IL-1 cytokine family. Mice injected with IL-33 display profound mucosal eosinophilia with associated pathologic changes. Although mast cells and Th2 cells express the IL-33 receptor, ST2, the roles of IL-33 and ST2 in eosinophil biology are unknown.
Objectives
We investigated the effects of IL-33 on human eosinophils in vitro.
Methods
Eosinophils and neutrophils were isolated from blood of normal individuals and mildly atopic patients. Real-time RT-PCR and flow cytometry were used to detect ST2. Granulocyte responses to IL-33 were monitored by superoxide anion production and by degranulation; IL-5, IL-1β, and TNF-α served as controls. Eosinophil survival and cytokine production were assessed by flow cytometry and ELISA, respectively.
Results
ST2 mRNA and protein were detected on eosinophils. IL-33 induced eosinophil superoxide anion production and degranulation as potently as IL-5. IL-33 also increased eosinophil survival and induced production of IL-8. Anti-ST2 inhibited eosinophil responses to IL-33. Neutrophils did not express ST2 nor did they respond to IL-33.
Conclusion
IL-33 and its receptor, ST2, may play important roles in eosinophil-mediated inflammation; they may provide new therapeutic targets for controlling mucosal eosinophilic inflammation.
Clinical Implications
Exploration of IL-33/ST2 may lead to novel therapeutics for eosinophilic inflammation.
Keywords: Eosinophils, IL-33, Activation, Inflammation, ST2
INTRODUCTION
The eosinophil is a proinflammatory granulocyte implicated in various human diseases, such as bronchial asthma, atopic dermatitis, and in immunity to parasites 1. While eosinophils exist in the peripheral circulation, they primarily reside in tissues where epithelial surfaces are exposed to the external environment 2. Current evidence suggests that the pathophysiology of these conditions is closely related to pro-inflammatory mediators, such as eosinophil major basic protein and reactive oxygen species, released when eosinophils are exposed to appropriate stimuli in mucosal tissues 1. Several factors activate and promote the survival and/or effector functions of human eosinophils, such as IL-5, GM-CSF, IL-3, immunoglobulins and complement fragments 1, 3. Of these, IL-5 is probably the best-studied; it is a potent stimulant for eosinophil survival and effector functions, including superoxide production, degranulation, phagocytosis, and leukotriene generation. IL-5 may be “the most critical cytokine in the pathophysiology of eosinophil-associated inflammation” 1, 4, 5. However, treatment of asthma patients with anti-IL-5 monoclonal antibody (mAb) only partially reduces eosinophilia in bronchial tissue, suggesting contributions by other mucosal factors that enhance eosinophil survival and function 6, 7.
IL-33 was identified by bioinformatic analysis of the human genome, and it is now designated as a novel member of the IL-1 cytokine family, IL-1F11/IL-33 8, 9. There are four other members of the IL-1 cytokine family, including IL-1α, IL-1β, IL-1R and IL-18 10. In general, IL-1 family members are widely expressed in hematopoietic cells and are important for inflammatory responses and host defenses 10. However, the expression pattern of human IL-33 appears restricted to epithelial cells from bronchus and small airways, fibroblasts, and smooth muscle cells 8, suggesting its involvement in regulating mucosal organ function. Receptors for the IL-1 cytokine family all possess a conserved protein sequence in their cytoplasmic domain called the Toll-IL-1 receptor domain. The orphan IL-1 receptor, ST2 (also called DER4, Fit-1, or T1), serves as the binding subunit for IL-33 8. ST2 is expressed on murine mast cells and Th2 cells, but not on Th1 cells, and is essential for Th2 cell effector function 11–13. Indeed, mice injected with IL-33 display profound pathological changes in mucosal tissues, including eosinophilia in the lung, esophagus, and intestine and increased serum levels of IgA and IgE 8. Furthermore, administration of a mAb against ST2, administration of recombinant ST2 fusion protein or lack of the ST2 gene attenuates eosinophilic airway inflammation after transfer of Th2 cells 12 or Schistosoma mansoni antigen challenge 14. Thus, these studies suggest a role for IL-33 and its receptor ST2 in allergic inflammation.
Despite expression of IL-33 by mucosal cells, its role in human diseases is largely unknown. Furthermore, little information has been available regarding the role of IL-33 in the activation of human cells that are implicated in allergic or Th2-type inflammation. Importantly, recent studies showed that IL-33 promotes survival and cytokine production by human mast cells 15, 16. Herein, we found that IL-33 potently activates human eosinophils and induces production and release of several inflammatory mediators through ST2. Interestingly, ST2 was not expressed by neutrophils, and IL-33 did not induce superoxide release from neutrophils. Thus, IL-33 and its receptor ST2 may play pivotal roles in mucosal eosinophilic inflammation and may provide novel and specific therapeutic targets for treatments directed against allergic inflammation in humans.
METHODS
Reagents
Recombinant human IL-33, IL-1β/IL-1F2, IL-17E/IL-25, GM-CSF and TNF-α/TNFSF1A were from R&D Systems (Minneapolis, MN). Recombinant human IL-5 was a generous gift from the Schering-Plough Research Institute (Kenilworth, NJ). Anti-human IL-1 R4/ST2 polyclonal goat IgG, biotinylated anti-human IL-1 R4/ST2 polyclonal goat IgG, normal goat IgG and biotinylated normal goat IgG were from R&D Systems. FITC-conjugated anti-CD9 Ab was from BD Bioscience (San Jose, CA). Anti-CD16-conjugated immunomagnetic beads and anti-FITC immunomagnetic beads were from Miltenyi Biotec (Auburn, CA). Cytochrome c was from Sigma (St. Louis, MO). Percoll was from GE Healthcare Biosciences AB (Uppsala, Sweden). HybriCare culture media was from ATCC (Manassas, VA) and α-Calf Serum (α-CS) was from HyClone (Logan, UT). α-CS was heat inactivated at 56° C for 30 minutes before use.
Eosinophil isolation
Eosinophils were isolated from the blood of 17 normal and mildly atopic volunteers using negative immunomagnetic bead selection as previously described with only one slight modification 17. Granulocytes were incubated with an equal volume of anti-CD16-conjugated magnetic beads on ice for 30 minutes rather than 60 minutes. This protocol consistently yielded >95% eosinophil purity. The study was approved by the Institutional Review Board at Mayo Clinic in Rochester, MN; all volunteers provided informed consent.
Neutrophil isolation
Neutrophils were isolated from the blood of the same donors used for eosinophil isolation. The eosinophil isolation protocol referenced above was followed with 10 µL of the granulocyte pellet being re-suspended in buffer and incubated with FITC-conjugated anti-CD9 Ab and anti-FITC magnetic beads. Neutrophils were isolated by negative immunomagnetic selection similarly to eosinophils 17. This yielded 98±2% neutrophil purity (mean±SEM) and allowed us to do parallel experiments on neutrophils and eosinophils on the same day from the same donor.
ST2 detection by real-time PCR
Total RNA was purified from eosinophils and neutrophils with TRIzol and Invitrogen PureLink Micro-to-Midi columns (Invitrogen, Carlsbad, CA). RNA samples were treated with DNase I (Invitrogen) and cDNA was reverse-transcribed using Bio-Rad iScript. Human IL1RL1 (ST2) mRNA transcripts were quantified by real-time PCR with Taqman Gene Expression Array primer-probe sets (Applied Biosystems, Foster City, CA) and iCycler with iQ5 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA). Transcription was normalized to the 18S rRNA transcription in each sample. Amplified PCR products were observed by gel electrophoresis using 2% agarose.
ST2 detection by flow cytometry
Purified eosinophils and neutrophils were resuspended in HybriCare media with 2 mM L-glutamine and 10% α-CS with or without GM-CSF (10 ng/mL) and incubated at 37 °C and 5% CO2 for 24 hours in 24 well tissue culture (TC) plates. Supernatants were removed, and the cells were resuspended in flow cytometry buffer (PBS with 1% bovine serum albumin and 0.01% NaN3). Cells were then incubated with biotinylated anti-human ST2 Ab (10 µg/mL) or biotinylated normal goat IgG as control Ab for 60 minutes on ice. After washing with buffer, cells were incubated with 2.5 µL of streptavidin conjugated to R-phycoerythrin for 20 minutes on ice and then fixed with 1% paraformaldehyde; cells were then analyzed with flow cytometry (FACScan; BD Biosciences, San Jose, CA).
Superoxide anion production
Eosinophil and neutrophil superoxide production was measured by cytochrome c reduction as previously described with slight modifications 18. Cells were resuspended in reaction buffer with cytochrome c (HBSS with 20 mM Hepes, 0.01% gelatin and 2.4 mg/mL cytochrome c). Flat-bottom 96-well TC plates were incubated with 50 µL of reaction buffer before adding 100 µL of cell suspension and 50 µL of the indicated concentration of cytokine. To analyze superoxide production, the kinetics of absorbance at 550 nm was measured at 37 °C with a microplate reader (SpectraMax Plus; Molecular Devices, Sunnyvale, CA). All assays were conducted in duplicate. For assays performed with anti-ST2 neutralizing Ab, 50 µL of Ab at the desired concentration was added with 100 µL of eosinophil suspension to each well and incubated at room temperature for 30 minutes. After incubation, 50 µL of cytokine was added. Ab remained present for the entire experiment.
Eosinophil degranulation assay
Supernatants from superoxide production experiments were collected at 180 minutes and stored at −20 °C. Eosinophil-derived neurotoxin (EDN) released into the supernatant by degranulation was then quantified with ELISA. Briefly, 96-well flat-bottom plates (Immulon® 4HXB; Thermo Electron Corporation, Milford, MA) were coated with mouse anti-human EDN mAb (5 µg/mL in PBS, clone 167-6C5), followed by blocking with 1% bovine serum albumin in assay buffer (0.05% Tween in PBS). Dilutions of the EDN standard or test sample were added to the wells and incubated for 2 hours. The plates were then washed and incubated with horseradish peroxidase-labeled anti-human EDN detection mAb (1:15,000 dilution in PBS, clone 167-2G4). After washing, 3,3’,5,5’-tetramethylbenzidine substrate (Pierce Protein Research Products; Thermo Fischer Scientific, Rockford, IL) was added. Absorbance at 450 nm was measured immediately after stopping the reaction with 50 µL of 2N H2SO4 with a microplate reader (SpectraMax Plus; Molecular Devices, Sunnyvale, CA). The lowest point of the standard curve was 0.09 ng/mL.
Eosinophil adhesion assay
Eosinophil adhesion was quantitated by measuring the EDN content of adherent cells as described previously 18. Briefly, after 90-minute incubation with cytokines, the supernatants were removed and the plates were gently rinsed with warm PBS. Cell morphology was examined and recorded by inverted phase microscopy. Adherent eosinophils were then lysed with 0.5% Nonidet P-40 in 0.01 M HCl, and the EDN content in the lysates was measured by radioimmunoassay. Percent adhesion was calculated as a ratio of EDN content in adherent eosinophils to total EDN in replicate samples.
Eosinophil survival assay
Eosinophils were resuspended in HybriCare medium with 2 mM L-glutamine and 10% α-CS. Cells were incubated in 96-well flat bottom TC plates with serial dilutions of IL-5, IL-33 or IL-25 at 37 °C and 5% CO2 for 96 hours. After culture, cell viability was determined using flow cytometry (FACScan) to identify dead cells that stained with propidium iodide (PI). Cell viability was calculated as the number of cells not stained with PI divided by total number of cells × 100%.
Cytokine production
Eosinophils (2×106 cells/mL) were resuspended in HybriCare medium with 2 mM L-glutamine and 10% α-CS. Cells were incubated with medium alone, IL-33 (100 ng/mL), IL-5 (100 ng/mL) or IL-33 plus IL-5 (each 100 ng/mL) in 24 well TC plates for 24 hours at 37 °C and 5% CO2. After culture, supernatants were collected and assayed for cytokine/chemokine production using a cytokine/chemokine array according to the manufacturer’s instructions (RayBio® Human Cytokine Antibody Array 5, RayBiotech, NorCross, GA). The levels of IL-8 were quantified using ELISA kits as per the manufacturer’s instructions (R&D Systems).
Statistical analysis
Results were expressed as means ± SEM. Data from granulocytes from atopic volunteers were pooled with those from normal individuals. Statistical analysis was performed using One-way Analysis of Variance (Tukey-Kramer Multiple Comparisons Test) or paired t test. P values <0.05 were considered statistically significant.
RESULTS
Human eosinophils, but not neutrophils, express ST2
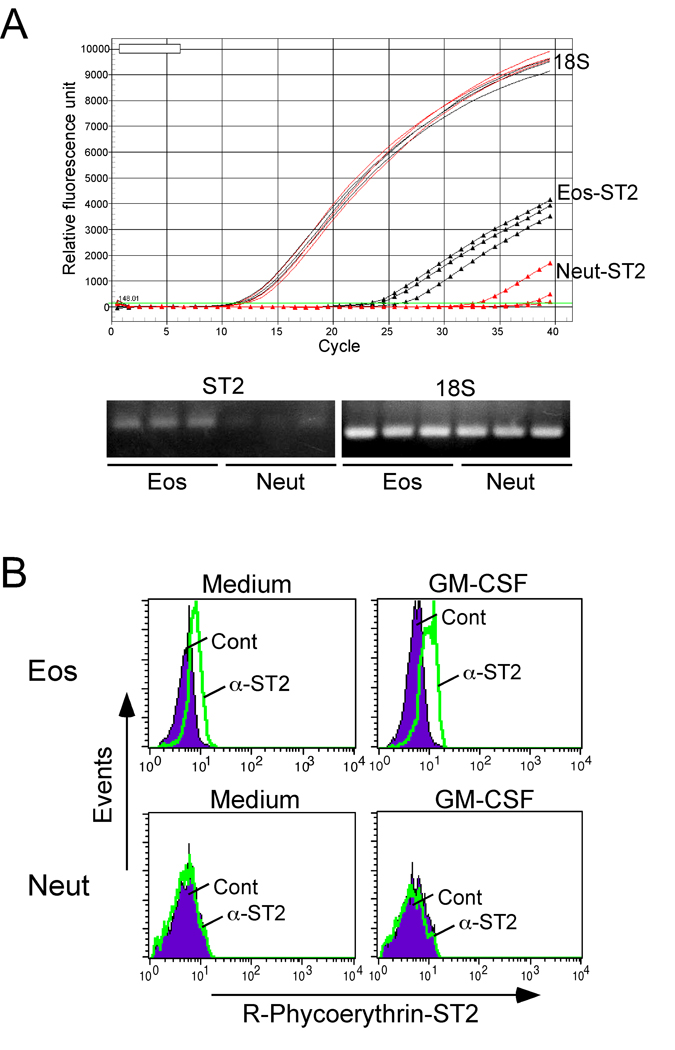
Expression of the receptor for IL-33, ST2, has been shown on murine and human mast cells and Th2 cells 11, 12, 15, 16. However, no information exists on granulocyte expression of ST2. During our gene microarray analysis of human eosinophils, ST2 mRNA was constitutively expressed by freshly isolated eosinophils 3(data not shown). Real-time PCR analysis showed that ST2 mRNA was reproducibly expressed by eosinophils freshly isolated from 3 different individuals (Figure 1A). In contrast, no or minimal ST2 mRNA was detectable in neutrophils from these same individuals. By flow cytometry, ST2 protein was undetectable on freshly isolated eosinophils (data not shown). However, after eosinophils were cultured in medium for 24 hours, the cells clearly showed surface expression of ST2; incubation with GM-CSF (10 ng/mL) tended to increase ST2 expression (Figure 1B). Surface expression of ST2 protein was undetectable in fresh or cultured neutrophils.
Figure 1.
Detection of ST2 in eosinophils and neutrophils. (A) Total RNA was purified from eosinophils and neutrophils, and ST2 mRNA transcripts were quantified by real-time PCR. Amplified PCR products were observed by gel electrophoresis. (B) After incubation for 24 hours in culture media or with 10 ng/ml GM-CSF, the cells were stained with isotype control Ab (Cont) or anti-ST2 Ab (α-ST2) and analyzed by FACS. Filled histogram shows Cont; green line shows α-ST2. One representative experiment of three is shown.
IL-33 potently induces effector functions in human eosinophils
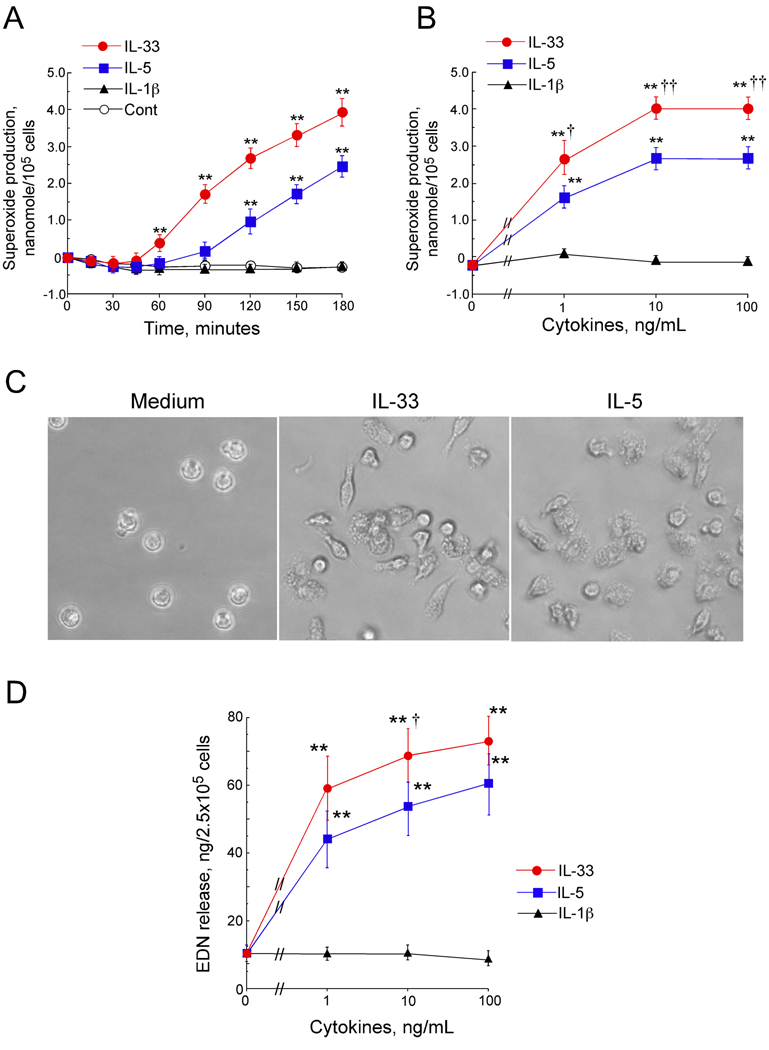
To examine the biological significance of ST2 expression on human eosinophils, we examined the ability of IL-33 to induce eosinophil effector functions, including superoxide production and degranulation. IL-5, a potent activator of human eosinophils1, 5, and another IL-1 family cytokine, IL-1β 10, were used as controls. IL-33 (100 ng/mL) induced eosinophil superoxide production in a time-dependent manner, starting after 60 minutes of incubation (Figure 2A). IL-5 (100 ng/mL) also induced superoxide production, although it took about 120 minutes to detect a significant response. At 180 minutes, eosinophils stimulated with IL-33 (100 ng/mL) produced about 40 percent more superoxide compared to IL-5 (100 ng/mL), suggesting that IL-33 activates eosinophils at least as potently as IL-5 if not more so at higher IL-33 concentrations. For example, the concentration-response experiments show that IL-33 (1 ng/mL) induced superoxide in comparable amounts to that induced by IL-5 (10 ng/mL) (Figure 2B). Furthermore, at 10 and 100 ng/mL, IL-33 induced significantly more superoxide compared to the same concentrations of IL-5 (4.0±0.2 vs. 2.7±0.3 nanomole/105 cells at 10 ng/mL of IL-33 and IL-5, respectively, p<0.01, n=6). In contrast, up to 100 ng/mL of IL-1β did not induce detectable superoxide production by eosinophils.
Figure 2.
Eosinophil functions in response to IL-33. (A) Eosinophils were incubated with medium alone (Cont), IL-33, IL-5, or IL-1β (100 ng/mL). Kinetics of superoxide production were analyzed. (B) Eosinophils were incubated with IL-33, IL-5 or IL-1β; superoxide production was measured at 180 minutes. (C) Eosinophils were incubated with medium, IL-33 (100 ng/mL) or IL-5 (100 ng/mL) for 180 minutes. Photomicrograph shows the morphology of eosinophils. Original magnification x400. (D) Eosinophils were incubated with IL-33, IL-5 or IL-1β for 180 minutes. EDN in the supernatants was quantified by ELISA. Results show means ± SEM, n=6. **, p < 0.01 compared with medium, † and ††, p<0.05 and p < 0.01 compared with IL-5.
Morphological analysis using inverted microscopy showed that eosinophils incubated with IL-33 appear elongated and adhere tightly to the plastic surface of TC plate wells (Figure 2C), similarly to eosinophils incubated with IL-5. Indeed, compared to medium alone (0.7±0.4%), IL-33 and IL-5 induced 36±17 and 38±15% eosinophil adhesion, respectively (mean±SEM, n=3). Furthermore, IL-33 induced eosinophil degranulation in a concentration-dependent manner as examined by EDN release into the cell-free supernatants (Figure 2D). Again, the effects of IL-33 on eosinophil degranulation appeared at least as potent as IL-5, and IL-1β did not stimulate EDN release. Thus, IL-33, unlike another IL-1 family cytokine, IL-1β, potently activates and induces adhesion, superoxide production and degranulation of human eosinophils.
IL-33 stimulates eosinophils through ST2
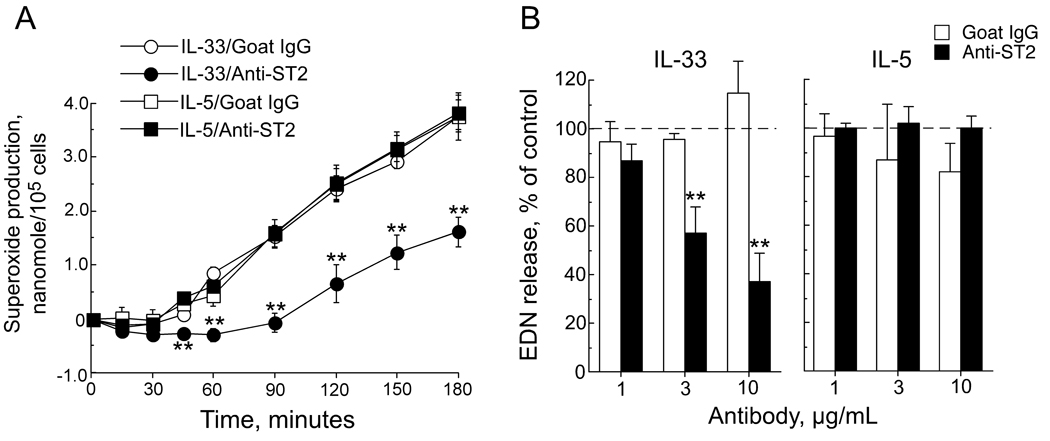
Having shown both the expression of ST2 on eosinophils and the activation of eosinophils by IL-33, we next examined whether IL-33 exerts its effects on eosinophils through ST2. This experiment was important because ST2 was not detected by flow cytometry on freshly isolated eosinophils (see above). We repeated the superoxide production and EDN release assays in the presence of blocking Ab for human ST2. Anti-ST2 (3 µg/mL), but not control goat IgG, significantly inhibited superoxide production induced by 10 ng/mL of IL-33 (p<0.01, n=3) (Figure 3A). In contrast, anti-ST2 did not affect superoxide production induced by 100 ng/mL of IL-5, demonstrating Ab specificity. Furthermore, anti-ST2, but not goat IgG, significantly inhibited IL-33-mediated EDN release in a concentration-dependent manner with about 70% inhibition with anti-ST2 (10 µg/mL) (p<0.01, n=3); EDN release induced by IL-5 was not affected by anti-ST2 (Figure 3B).
Figure 3.
Role of ST2 in eosinophil response to IL-33. (A) Eosinophils were incubated at room temperature with anti-ST2 Ab (3 µg/mL) or control goat IgG for 30 minutes. Next, cells were exposed to IL-33 (10 ng/mL) or IL-5 (100 ng/mL) with subsequent measurement of superoxide production. (B) Supernatants from (A) using three different antibody concentrations were assayed at 180 minutes for EDN using ELISA. Results show means ± SEMs, n=3. **, p< 0.01 compared with control antibody.
IL-33 does not activate neutrophils
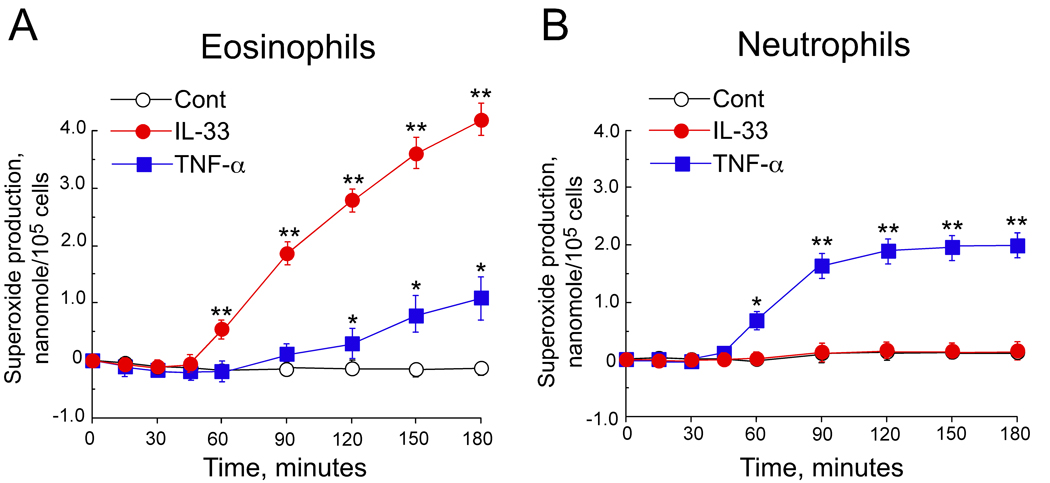
Eosinophils and neutrophils are derived from common myeloid cell precursors, share some common receptors and are involved in airway inflammation 1. Thus, we examined the cellular specificity of the IL-33 responses in these cell types by concurrently comparing eosinophils and neutrophils isolated from the same donors. As before, eosinophils were strongly activated by IL-33 to produce superoxide (Figure 4A), but neutrophils from the same donors showed no increase in superoxide production when stimulated with IL-33 (n=3, Figure 4B). Moreover, TNF-α induced modest superoxide production in neutrophils, but minimal superoxide production in eosinophils (Figures 4A and 4B). Thus, IL-33 and TNF-α seem to differentially activate eosinophils and neutrophils, respectively.
Figure 4.
Eosinophil and neutrophil superoxide production after stimulation with IL-33 or TNF-α. Eosinophils (A) or neutrophils (B) were incubated with medium alone (Cont), IL-33 (100 ng/mL) or TNF-α (100 ng/mL). The kinetics of superoxide production was measured by reduction of cytochrome c. Results are means ± SEM, n=3. * and **, p<0.05 and p< 0.01 compared with medium alone.
IL-33 induces cytokine production and prolongs eosinophil survival
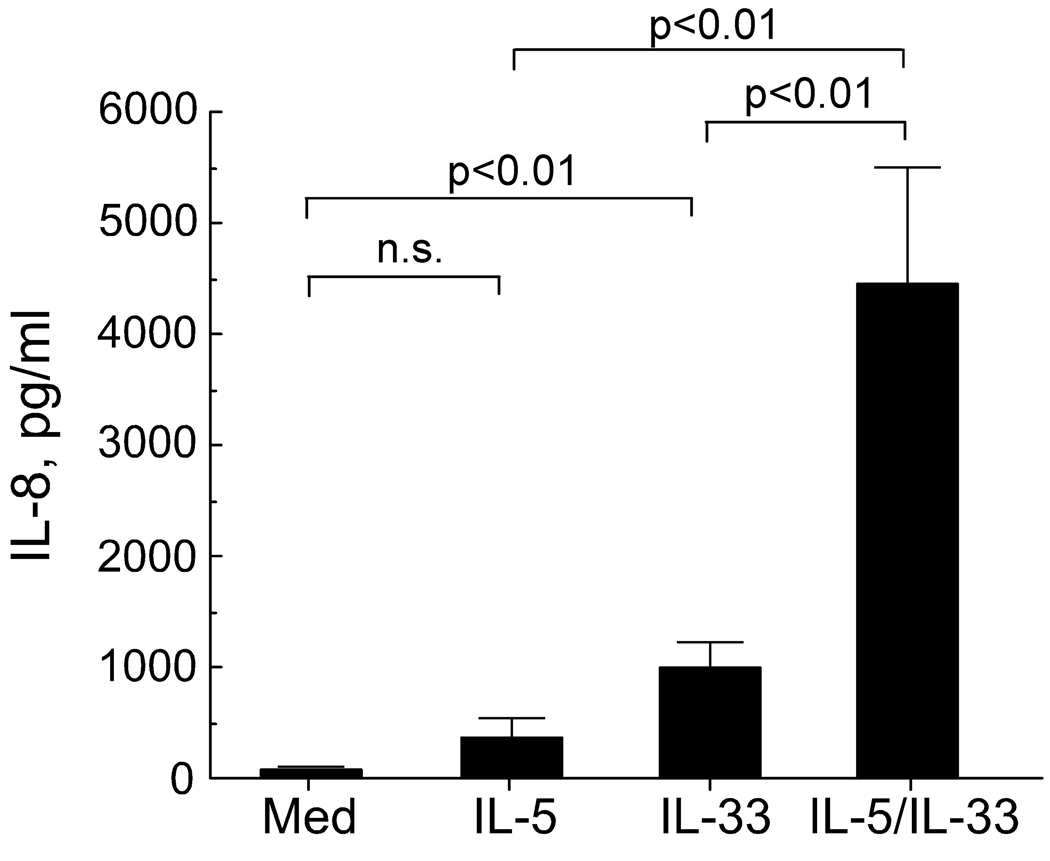
We next examined the long-term effects of IL-33 on eosinophils, namely cytokine production and survival. When exposed to appropriate stimuli, such as soluble immune complexes, eosinophils produce cytokines and chemokines 3, 19. Thus, we used a protein array assay to screen cytokines produced by eosinophils cultured for 24 hours with IL-33, IL-5, or IL-33 plus IL-5. In the supernatants from eosinophils incubated with IL-5 plus IL-33, we observed increases in IL-8 and MCP-1 (data not shown). When eosinophils were stimulated with IL-33 (100 ng/mL), an IL-8 ELISA showed significant IL-8 production (Figure 5); IL-5 alone did not induce IL-8 production. Importantly, cells stimulated with both IL-33 and IL-5 showed strikingly increased IL-8 production about 4-fold > IL-33 alone.
Figure 5.
Eosinophil IL-8 production induced by IL-5 and IL-33. Eosinophils (2×106 cells/mL) were incubated for 24 hours with medium, IL-5 (100 ng/mL), IL-33 (100 ng/mL) or IL-5 and IL-33 (each at 100 ng/mL). Cell-free supernatants were collected and assayed for IL-8 using ELISA. Results are means ± SEM, n=5.
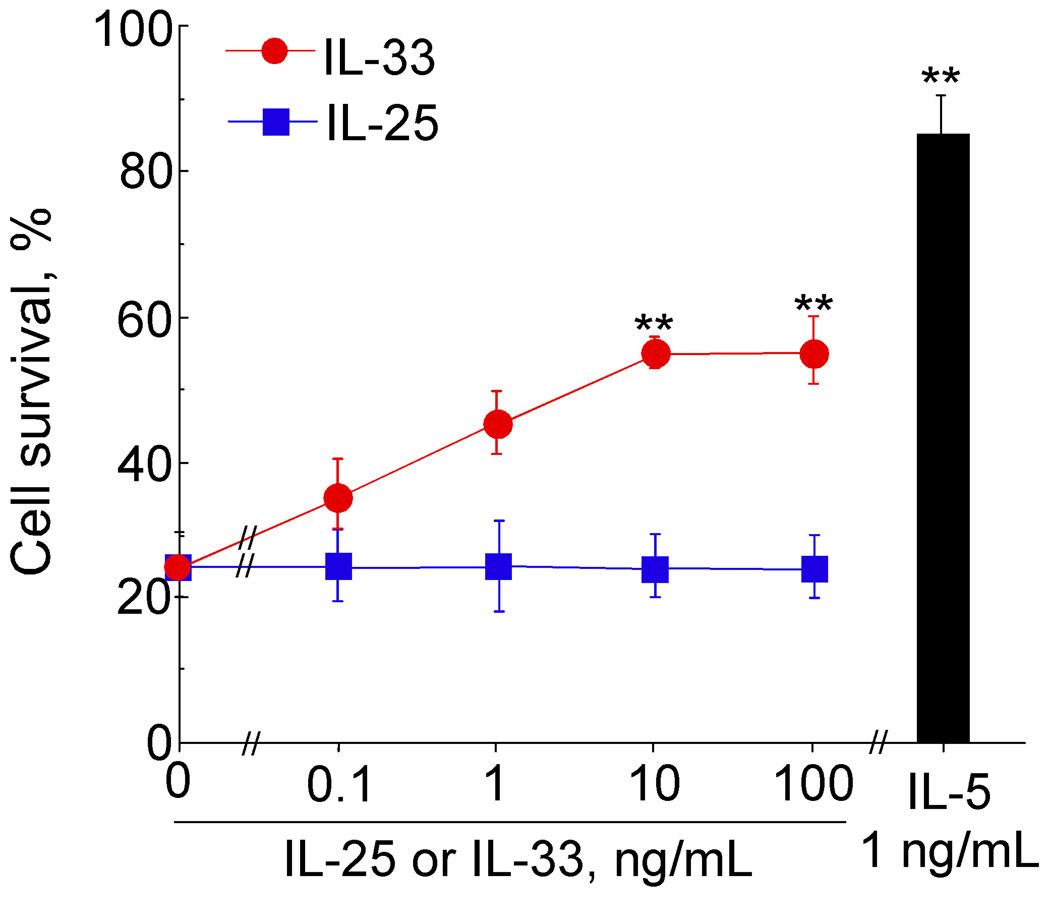
Eosinophils undergo apoptosis in vitro without exogenous growth factors. Cytokines, such as IL-5, potently inhibit apoptosis and prolong eosinophil survival 1, 5. After 96 hours of incubation with IL-5 or IL-33, eosinophil survival was significantly enhanced; however, survival with IL-33 was less robust than with IL-5 (Figure 6). For example, IL-33 at 10 and 100 ng/mL increased survival from the baseline (medium alone) of 26±5% to 53±2 % and 53±4% at 96 hours, respectively (mean ± SEM, n=3). IL-5 at 1 ng/mL increased survival to 84±6%, but a novel IL-17 family cytokine, IL-25, did not enhance eosinophil survival.
Figure 6.
Survival of eosinophils cultured with IL-33, IL-25 or IL-5. Isolated eosinophils were cultured with serial dilutions of IL-33 or IL-25 or 1 ng/mL IL-5 for 96 hours. Cell viability was determined using propidium iodide (PI) staining and FACS. Results show percent of viable cells ± SEM, n=3. **, p< 0.01 compared with no cytokines.
DISCUSSION
Our key observation is the potent direct effect of a novel IL-1 family cytokine, IL-33 8, on human eosinophils. IL-33 appeared stronger than or at least as strong as IL-5 in activating eosinophil functions (Figure 2 and Figure 5), in particular production and release of pro-inflammatory mediators, such as superoxide anion, a granule protein and IL-8. In addition, IL-33 prolonged eosinophil survival, although less robustly than IL-5 (Figure 6). Unlike the ubiquitous nature of other IL-1 family cytokines, IL-33 expression seems restricted to epithelial cells from the bronchus and small airways, to fibroblasts, and to smooth muscle cells 8. Because eosinophil functions were not affected by another IL-1 family cytokine, IL-1β, human eosinophils likely differ in their responses to individual members of this cytokine family. In contrast to eosinophils, neutrophil production of superoxide was not affected by IL-33. Furthermore, IL-33 promotes survival and cytokine production by other cells involved in eosinophilic inflammation, including mast cells and Th2 cells 12, 15, 16. Therefore, the biology of IL-33 may be unique among the IL-1 family cytokines with potentially profound implications for pathologic eosinophilic inflammation in mucosal tissues.
In our study we used in vitro models, and extrapolation from these findings to in vivo systems should be made cautiously. Nevertheless, it is tempting to speculate about potential implications of these findings. After IL-5 was demonstrated to have potent survival, chemokinetic and activating effects on eosinophils, it emerged as a potential target for asthma treatment 1, 4, 5. However, clinical trials with an anti-IL-5 mAb showed that this approach did not improve late phase bronchial hyperreactivity in asthma despite almost eliminating blood and sputum eosinophilia 6. This observation has partially been explained by an only 55% decrease in eosinophil levels in bronchial mucosa and by unchanged levels of eosinophil major basic protein in BAL fluid despite anti-IL-5 treatment 7. Furthermore, human eosinophils entering the airway lumen apparently lose their surface receptors for IL-5 20. Therefore, eosinophil dynamics may be regulated by different molecules depending where the cells are located (e.g. blood vs. tissues). Furthermore, novel tissue-derived factor(s), such as IL-33, may play critical roles in eosinophilic inflammation of the airway mucosa. Thus, further studies are necessary to dissect the role(s) and regulation of IL-33 in both normal human subjects and those with atopic diseases by using biological specimens from patients and animal models. Areas of future investigation include identifying natural triggers, which induce the expression, processing, and release of IL-33 in mucosal tissues, elucidating the molecular mechanisms, which may modulate eosinophil responsiveness to IL-33, and analyzing ST2 expression by eosinophils from different body compartments (e.g. mucosal tissues) and among patients with different eosinophil-related disorders.
Because of their profound biological functions and the severe pathophysiological consequences of dysregulated expression, all IL-1 family members are tightly regulated, either at the ligand or receptor level or both 10. The IL-33 receptor, ST2, was barely detectable on the surface of freshly isolated eosinophils by flow cytometry, while it was clearly detected on eosinophils cultured for 24 hours. This observation may reflect lack of sensitivity of flow cytometry to a small number of receptor molecules or the blocking of anti-ST2 binding sites by endogenous soluble ligands or cellular accessory molecules. Nonetheless, the effect of IL-33 on freshly isolated eosinophils appears to be mediated through ST2 (Figure 3). ST2 represents a lineage marker for mouse Th2 cells 21 and mast cell precursors 22 and is involved in mast cell and Th2 cell responses to IL-33 15, 23, 24. Thus, manipulation of the IL-33/ST2 pathway by anti-ST2 or by recombinant ST2 fusion protein could be new therapeutic approaches for allergic inflammation where several cell types, including mast cells, Th2 cells and eosinophils, likely play important and interactive roles. Indeed, this novel concept has been partially tested in mouse models of Th2 immune responses. Mice deficient in ST2 have demonstrated severe impairment of Th2 cytokine production in a Schistosoma mansoni granuloma model 14, and in mice sensitized to ovalbumin, recombinant ST2 fusion protein decreases splenocyte production of IL-4, IL-5 and IL-13 24. Administration of either mAb against ST2 or recombinant ST2 fusion protein also attenuates eosinophilic inflammation of mouse airways following transfer of Th2 cells 12. Furthermore, a single-nucleotide polymorphism in the promoter region of the t1/st2 gene may influence susceptibility to atopic dermatitis 25.
In conclusion, our study demonstrates that IL-33 and its receptor, ST2, play important roles in the pro-inflammatory effector functions and survival of human eosinophils. Thus, in addition to classical Th2 cytokines, such as IL-4, IL-5 and IL-13, non-lymphoid cell-derived cytokines, such as IL-33, may also be involved in the development or exacerbation of asthma and allergic diseases through the induction of Th2 cell responses, activation of mast cells and eosinophils or any combination of these factors. Manipulation of these new classes of cytokines may provide a novel therapeutic strategy to control human diseases.
ACKNOWLEDGEMENTS
The authors wish to thank Cheryl R. Adolphson for editorial assistance and LuRaye S. Eischens for secretarial help.
Funding:
NIH grant R01AI34486 and Mayo Foundation
ABBREVIATIONS
- CS
calf serum
- EDN
eosinophil-derived neurotoxin
- mAb
monoclonal antibody
- PI
propidium iodide
- TC
tissue culture
REFERENCES
- 1.Kita H, Adolphson CR, Gleich GJ. Biology of eosinophils. In: Adkinson NF, Yunginger JW, Busse WW, Bochner BS, Holgate ST, Simons FER, editors. Middleton’s Allergy Principles and Practice. 6th ed. Philadelphia, PA: Mosby-Year Book, Inc; 2003. pp. 305–332. [Google Scholar]
- 2.Matthews AN, Friend DS, Zimmermann N, Sarafi MN, Luster AD, Pearlman E, et al. Eotaxin is required for the baseline level of tissue eosinophils. Proc Natl Acad Sci U S A. 1998;95:6273–6278. doi: 10.1073/pnas.95.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartemes KR, Cooper KM, Drain KL, Kita H. Secretory IgA induces antigen-independent eosinophil survival and cytokine production without inducing effector functions. J Allergy Clin Immunol. 2005;116:827–835. doi: 10.1016/j.jaci.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Kita H, Weiler DA, Abu-Ghazaleh R, Sanderson CJ, Gleich GJ. Release of granule proteins from eosinophils cultured with IL-5. J Immunol. 1992;149:629–635. [PubMed] [Google Scholar]
- 5.Lopez AF, Sanderson CJ, Gamble JR, Campbell HD, Young IG, Vadas MA. Recombinant human interleukin 5 is a selective activator of human eosinophil function. J Exp Med. 1988;167:219–224. doi: 10.1084/jem.167.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leckie MJ, ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356:2144–2148. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 7.Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil's role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003;167:199–204. doi: 10.1164/rccm.200208-789OC. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 9.Sims JE, Nicklin MJ, Bazan JF, Barton JL, Busfield SJ, Ford JE, et al. A new nomenclature for IL-1-family genes. Trends Immunol. 2001;22:536–537. doi: 10.1016/s1471-4906(01)02040-3. [DOI] [PubMed] [Google Scholar]
- 10.Dinarello CA. Interleukin-1. Cytokine Growth Factor Rev. 1997;8:253–265. doi: 10.1016/s1359-6101(97)00023-3. [DOI] [PubMed] [Google Scholar]
- 11.Moritz DR, Rodewald HR, Gheyselinck J, Klemenz R. The IL-1 receptor-related T1 antigen is expressed on immature and mature mast cells and on fetal blood mast cell progenitors. J Immunol. 1998;161:4866–4874. [PubMed] [Google Scholar]
- 12.Lohning M, Stroehmann A, Coyle AJ, Grogan JL, Lin S, Gutierrez-Ramos JC, et al. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc Natl Acad Sci U S A. 1998;95:6930–6935. doi: 10.1073/pnas.95.12.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coyle AJ, Lloyd C, Tian J, Nguyen T, Erikkson C, Wang L, et al. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J Exp Med. 1999;190:895–902. doi: 10.1084/jem.190.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J Exp Med. 2000;191:1069–1076. doi: 10.1084/jem.191.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol. 2007;179:2051–2054. doi: 10.4049/jimmunol.179.4.2051. [DOI] [PubMed] [Google Scholar]
- 16.Iikura M, Suto H, Kajiwara N, Oboki K, Ohno T, Okayama Y, et al. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab Invest. 2007;87:971–978. doi: 10.1038/labinvest.3700663. [DOI] [PubMed] [Google Scholar]
- 17.Bartemes KR, McKinney S, Gleich GJ, Kita H. Endogenous platelet-activating factor is critically involved in effector functions of eosinophils stimulated with IL-5 or IgG. J Immunol. 1999;162:2982–2989. [PubMed] [Google Scholar]
- 18.Horie S, Kita H. CD11b/CD18 (Mac-1) is required for degranulation of human eosinophils induced by human recombinant granulocyte-macrophage colony-stimulating factor and platelet-activating factor. J Immunol. 1994;152:5457–5467. [PubMed] [Google Scholar]
- 19.Lacy P, Moqbel R. Eosinophil cytokines. Chem Immunol. 2000;76:134–155. doi: 10.1159/000058782. [DOI] [PubMed] [Google Scholar]
- 20.Liu LY, Sedgwick JB, Bates ME, Vrtis RF, Gern JE, Kita H, et al. Decreased expression of membrane IL-5 receptor alpha on human eosinophils: I. Loss of membrane IL-5 receptor alpha on airway eosinophils and increased soluble IL-5 receptor alpha in the airway after allergen challenge. J Immunol. 2002;169:6452–6458. doi: 10.4049/jimmunol.169.11.6452. [DOI] [PubMed] [Google Scholar]
- 21.Xu D, Chan WL, Leung BP, Huang F, Wheeler R, Piedrafita D, et al. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J Exp Med. 1998;187:787–794. doi: 10.1084/jem.187.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen CC, Grimbaldeston MA, Tsai M, Weissman IL, Galli SJ. Identification of mast cell progenitors in adult mice. Proc Natl Acad Sci U S A. 2005;102:11408–11413. doi: 10.1073/pnas.0504197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komai-Koma M, Xu D, Li Y, McKenzie AN, McInnes IB, Liew FY. IL-33 is a chemoattractant for human Th2 cells. Eur J Immunol. 2007;37:2779–2786. doi: 10.1002/eji.200737547. [DOI] [PubMed] [Google Scholar]
- 24.Hayakawa H, Hayakawa M, Kume A, Tominaga S. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J Biol Chem. 2007;282:26369–26380. doi: 10.1074/jbc.M704916200. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu M, Matsuda A, Yanagisawa K, Hirota T, Akahoshi M, Inomata N, et al. Functional SNPs in the distal promoter of the ST2 gene are associated with atopic dermatitis. Hum Mol Genet. 2005;14:2919–2927. doi: 10.1093/hmg/ddi323. [DOI] [PubMed] [Google Scholar]