Abstract
Primary graft dysfunction (PGD) after lung transplantation causes significant morbidity and mortality. We aimed to determine the role of cytokines and chemokines in PGD. This is a multicenter case–control study of PGD in humans. A Luminex analysis was performed to determine plasma levels of 25 chemokines and cytokines before and at 6, 24, 48 and 72 h following allograft reperfusion in 25 cases (grade 3 PGD) and 25 controls (grade 0 PGD). Biomarker profiles were evaluated using a multivariable logistic regression and generalized estimating equations. PGD cases had higher levels of monocyte chemotactic protein-1 (MCP-1)/chemokine CC motif ligand 2 (CCL2) and interferon (IFN)-inducible protein (IP-10)/chemokine CXC motif ligand 10 (CXCL10) (both p < 0.05), suggesting recruitment of monocytes and effector T cells in PGD. In addition, PGD cases had lower levels of interleukin (IL-13) (p = 0.05) and higher levels of IL-2R (p = 0.05). Proinflammatory cytokines, including tumor necrosis factor (TNF)-α, and IFN-γ decreased to very low levels after transplant in both PGD cases and controls, exhibiting no differences between the two groups. These findings were independent of clinical variables including diagnosis in multivariable analyses, but may be affected by cardiopulmonary bypass. Profound injury in clinical PGD is distinguished by the upregulation of selected chemokine pathways, which may useful for the prediction or early detection of PGD if confirmed in future studies.
Keywords: Chemokines, cytokines, lung transplantation, primary graft dysfunction, reperfusion injury
Introduction
Primary graft dysfunction (PGD) is a form of ischemia/reperfusion (I/R) acute lung injury that complicates an estimated 10–25% of lung transplantations and is the leading cause of early posttransplantation morbidity and mortality (1–9). Seen pathologically, there is a predominance of the diffuse alveolar damage lung injury pattern, and the pathophysiology is felt to be most likely due to I/R injury (10–13). Recent studies have implicated a patho-physiological role for cytokines and chemokines in PGD or I/R injury, often focusing on small groups of markers in animal models, lung tissue or bronchoalveolar lavage (BAL) (5,14,15). Likewise, there is a growing interest in anti-cytokine and -chemokine therapy in PGD prevention and therapy (12).
Our goal was to determine the time course of changes in the systemic expression of selected cytokines and chemokines after transplantation in human subjects with PGD, compared with controls without PGD. To achieve this, we measured the following categories of mediators: (a) proinflammatory cytokines involved in the activation of lymphocytes and neutrophils, including interleukin (IL)-1β, IL-2, IL-2 receptor (IL-2R), IL-5, IL-7, IL-12 and tumor necrosis factor (TNF)-α; (b) anti-inflammatory and pleiotropic cytokines IL-2 receptor antagonist (IL-1Ra), IL-4, IL-6, IL-10 and IL-13; (c) chemokines involved in the recruitment of neutrophils, IL-8 and (d) chemokines involved in the recruitment of monocytes and lymphocytes, inter-feron (IFN)-inducible protein (IP-10), monocyte chemotactic protein-1 (MCP-1), monokine induced by IFN-γ (MIG), macrophage inflammatory protein (MIP-1α) and MIP-1β. The mediators were measured in plasma samples collected before lung transplantation and at 6, 24, 48 and 72 h following allograft reperfusion.
Methods
Study population
The Lung Transplant Outcomes Group (LTOG) is an ongoing prospective cohort study of patients undergoing first lung transplantation at nine centers in the United States, designed to study the risks and pathogenesis of PGD (see Appendix for institutions and investigators). Subjects in this cohort study underwent postoperative immunosuppression, according to local protocols, that included induction with IL-2Ra, followed by maintenance with a calcineurin inhibitor, azathioprine or mycophenolate mofetil, and steroids.
We performed a case–control study nested within the LTOG cohort. We chose the nested case–control study approach using our most severe cases of PGD (grade 3, at 72 h after transplantation [T72]) and our ‘cleanest’ non-PGD controls (grade 0 at all time points) to best uncover the differences in biomarkers between the ends of the spectrum of PGD, as well as for efficiency due to the high cost of these multiple assays (16). Because of the high cost of the assay platform, we sought to maximize the efficiency by choosing a nested sample of 25 cases and 25 controls. Specifically, 25 cases were selected from the first 128 lung transplant recipients enrolled between April 2002 and November 2005 (17). PGD cases met the criteria for International Society of Heart and Lung Transplantation (ISHLT) grade 3 PGD defined at 72 h after transplantation, as defined in prior studies (1,2,6,10). The cases had (a) diffuse alveolar infiltrates involving the lung allograft(s) (in the case of single lung transplant, infiltrates spare the native lung), (b) PaO2/FiO2 less than 200 mmHg and (c) no other secondary cause of graft dysfunction identified. In previous studies, PGD defined by these criteria was associated with poor outcomes after lung transplantation including an increased risk of death (1,2,6).
From the same time period, 25 controls were chosen, characterized by ISHLT grade 0 PGD defined at 72 h after lung transplantation. Matching of controls was not performed on clinical variables to avoid errors due to overmatching (16).
Data collection and management
Informed consent for this study was obtained prior to organ transplantation. Blood samples were obtained in citrated Vacutainers (Becton Dickinson, Franklin Lakes, NJ) from the recipients immediately before transplantation and at 6, 24, 48 and 72 h after reperfusion of the lung allograft(s). The samples were centrifuged within 30 min of collection and stored at –80°C. Clinical variables were categorized and defined using methods published previously (3,17). Some patients from this study have been included in other studies of biomarkers of coagulation and endothelial injury in PGD (17,18).
Multiplex analysis of plasma chemokines and cytokines
The levels of 25 cytokines and chemokines in 25 μL of plasma were assayed simultaneously, in duplicate, using a human cytokine 25-plex antibody bead kit (BioSource, Camarillo, CA) and a Luminex-100 array assay reader (Luminex Corp., Austin, TX). The analytes were IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13, IL-15, IL-17, eotaxin, IFN)-γ-inducible protein (IP)-10, MIG, MCP-1, MIP-1α, MIP-1β, Regulated upon Activation, Normal T-cell Expressed (RANTES), also known as CCL5, TNF-α,IFN-α,IFN-γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-1Ra and IL-2R.
Statistical analysis
The continuous variables were compared between the cases and controls using t-tests and rank-sum tests. Generalized estimating equations (GEE), a statistical tool to test the relationships of longitudinal variables between groups, were used to assess the differences in biomarker profiles over time between PGD cases and non-PGD controls. To assess the potential confounding effects of imbalances in clinical variables between the groups, a multivariable logistic regression was used for biomarker levels at the 24-h time point. For each mediator analyzed in multivariable analyses, the cutoffs were chosen based on the median levels. The confounders were included one at a time in adjusted models if p < 0.20 in bivariable analyses. Because of the potential confounding effect of diagnosis category, in addition to adjusting, we performed analyses excluding individuals with a given diagnosis (such as idiopathic pulmonary fibrosis [IPF] or idiopathic pulmonary artery hypertension [IPAH]). With α = 0.05, the study had an 80% power to detect differences of 0.8 standard deviations (SD) for mediators at each time point. Statistical comparisons were performed using STATA version 9.1 (STATA Corp., College Station, TX) and SAS version 9.1 (SAS Institute, Cary, NC). This research protocol was approved by the institutional review board at each participating center.
Results
The characteristics of the study population are shown in Table 1. Subjects in the PGD group had higher preoperative pulmonary artery systolic pressures and more frequent use of cardiopulmonary bypass during transplantation and were more likely to have a diagnosis of diffuse parenchymal lung disease or idiopathic pulmonary arterial hypertension. Ages, ischemic times and the use of medications were similar between groups.
Table 1.
Donor and recipient characteristics by PGD status, with continuous variables expressed as means with 95% confidence intervals, and dichotomous variables as column percentages
| Donor variable | No PGD (n = 25) | PGD (n = 25) | p-Value |
|---|---|---|---|
| Mean donor age in years (95% CI) | 30 (24–36) | 33 (27–39) | 0.44 |
| Female gender | 28% | 46% | 0.20 |
| Race/ethnicity | 0.53 | ||
| Caucasian | 70% | 65% | |
| African American | 17% | 9% | |
| Hispanic | 13% | 22% | |
| Other | 0% | 4% | |
| Head trauma as cause of death | 65% | 53% | 0.46 |
| Recipient and surgical variable | |||
| Age, years | 53 (46–60) | 42 (32–52) | 0.07 |
| Female gender | 40% | 36% | 0.77 |
| Race/ethnicity | 0.27 | ||
| Caucasian | 92% | 72% | |
| African American | 8% | 20% | |
| Other | 0% | 8% | |
| Recipient diagnosis | 0.004 | ||
| Chronic obstructive pulmonary disease | 68% | 20% | |
| Diffuse parenchymal lung disease | 16% | 52% | |
| Cystic fibrosis (CF) | 16% | 12% | |
| Pulmonary arterial hypertension | 0% | 12% | |
| Congenital heart disease | 0% | 4% | |
| Other | 0% | 0% | |
| Receipt of preprocedure steroids | 44% | 40% | 0.77 |
| Receipt of induction therapy | 100% | 100% | – |
| Procedure type | 0.24 | ||
| Single | 48% | 28% | |
| Bilateral | 52% | 72% | |
| Use of cardiopulmonary bypass | 16% | 68% | <0.001 |
| Pulmonary artery systolic pressure | 39 (33–44) | 68 (53–83) | <0.001 |
| Ischemic time in minutes | |||
| First lung | 232 (211–253) | 241 (224–259) | 0.50 |
| Second lung | 345 (318–373) | 344 (281–406) | 0.96 |
Chemokines
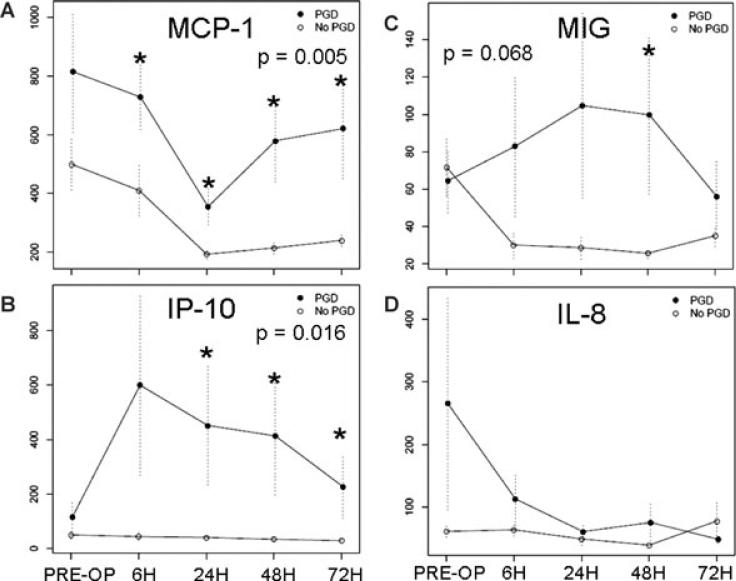
The largest differences between PGD and non-PGD were observed for two chemokines, MCP-1 and IP-10. As seen in Figure 1A, MCP-1 levels appeared higher preoperatively, and remained higher in PGD subjects throughout the period of observation (p = 0.005). There were no associations between MCP-1 levels and preoperative diagnosis or elevated pulmonary arterial pressures. A multivariable explanatory logistic regression model for the relationship of higher MCP-1 at 24 h with PGD revealed no confounding by diagnosis category, age, sex or other demographic variables (Table 3). Notably, when IPAH or diffuse parenchymal lung disease (DPLD) were excluded from the analyses, higher MCP-1 levels remained associated with PGD. However, this relationship was slightly attenuated by both the degree of elevated right heart pressures and the use of cardiopulmonary bypass.
Figure 1. Mean plasma levels (pg/mL) of chemokines in PGD cases and controls.
(A) MCP-1. (B) IP-10. (C) MIG. (D) IL-8. Standard errors are shown as error bars, and the GEE p-value is displayed. Asterisks signify significant differences at time points between PGD case and control plasma levels (p < 0.05).
Table 3.
Multivariable analysis of association of MCP-1 and IP-10 levels at 24 h with PGD
| MCP-1 >200 pg/mL |
IP-10 >50 pg/mL |
|||
|---|---|---|---|---|
| Variable | Odds ratio (95% CI) | p-Value | Odds ratio (95% CI) | p-Value |
| Unadjusted base model | 5.3 (1.4–19.5) | 0.013 | 14.7 (3.0–73.0) | 0.001 |
| Adjusted for | ||||
| Donor gender | 4.9 (1.3–18.9) | 0.023 | 12.8 (2.5–65.3) | 0.002 |
| Recipient age | 4.7 (1.2–18.0) | 0.024 | 14.7 (2.8–76.2) | 0.001 |
| Procedure type | 4.6 (1.2–17.7) | 0.025 | 18.9 (3.2–111.5) | 0.001 |
| Cardiopulmonary bypass | 3.5 (0.8–15.8) | 0.109 | 8.3 (1.5–46.8) | 0.017 |
| Total Ischemic time | 4.4 (1.1–17.3) | 0.035 | 15.7 (2.9–84.5) | 0.001 |
| Pulmonary artery systolic pressure at transplant | 4.3 (0.82–22.8) | 0.084 | 9.8 (1.6–58.7) | 0.012 |
| Recipient diagnosis | 8.2 (1.4–49.7) | 0.022 | 9.2 (1.7–50.3) | 0.011 |
| Excluding subjects with DPLD | 5.5 (1.3–22.7) | 0.019 | 14.7 (2.7–78.9) | 0.002 |
| Excluding subjects with CF | 8.4 (1.4–49.9) | 0.020 | 10.5 (1.5–73.4) | 0.018 |
| Excluding subjects with IPAH | 4.0 (1.1–15.5) | 0.041 | 12.5 (2.5–63.0) | 0.002 |
The reported odds ratios are for the relationship between change in each biomarker and PGD risk, adjusted individually for each confounder variable listed in the table. Odds ratios for MCP-1 and IP-10 are reported for cutoff values of 200 pg/mL and 50 pg/mL, respectively.
The two related chemokines IP-10 (p = 0.02) and MIG (p = 0.07) were similar preoperatively, then diverged postoperatively between PGD cases and non-PGD controls, peaking at 24 h postoperatively in PGD cases, then dropping to control levels at later time points (Figure 1B and C). Although the MIG profile did not achieve overall statistical significance, early postoperative time points diverged. In the multivariable analyses, IP-10 levels at 24 h remained associated with PGD, independent of the potential confounding effects of clinical variables (Table 3), including the use of cardiopulmonary bypass, right heart pressures and diagnostic categories. Furthermore, when patients from an unbalanced diagnostic group (such as DPLD) were excluded from the analyses, the results remained significant.
Chemokines that did not have statistically significant differences at any time points included IL-8, eotaxin, MIP-1α, MIP-1β and RANTES. Preoperative IL-8 plasma levels were widely variable in PGD cases and consistently low in controls, with plasma levels becoming low in both groups postoperatively (Figure 1D). However, we found no association of preoperative IL-8 levels with diagnosis category, hemodynamic variables or recipient demographic variables.
Cytokines and their receptors
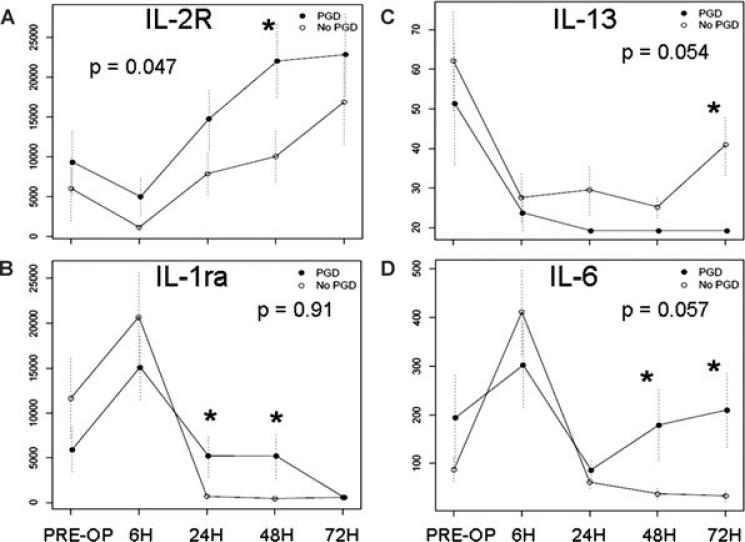
Similar to the chemokines, cytokines and/or their receptors had varying changes in plasma levels over time (Figures 2 and 3). Cytokine levels that differed between the cases and controls included IL-2R (p = 0.05), with PGD case subject levels consistently greater than control subject levels (Figure 2A). Plasma levels diverged at later time points between PGD cases and controls for IL-6 (p < 0.05 at 48 and 72 h) and IL-13 (p < 0.05 at 72 h) (Figure 2C and D), although the overall profile p-values did not reach significance for these mediators.
Figure 2. Mean plasma levels (pg/mL) of cytokines in PGD cases and controls.
(A) IL-2R. (B) IL-1ra. (C) IL-13. (D) IL-6. Standard errors are shown as error bars, and the GEE p-value is displayed. Asterisks signify significant differences at time points between PGD case and control plasma levels (p < 0.05).
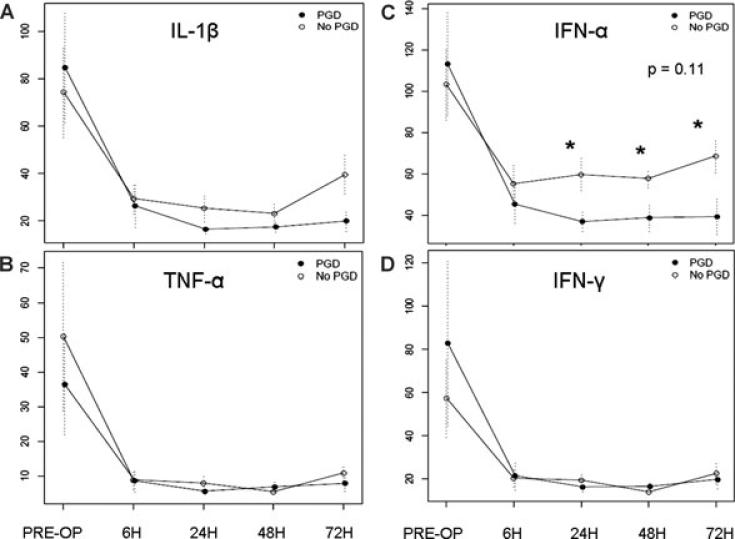
Figure 3. Mean plasma levels (pg/mL) of inflamma-tory cytokines and interferons in PGD cases and controls.
(A) IL-1β. (B) TNF-α. (C) INF-α. (D) IFN-γ. Standard errors are shown as error bars, and the GEE p-value is displayed. Asterisks signify significant differences at time points between PGD case and control plasma levels (p < 0.05).
A number of cytokines showed marked reductions in postoperative plasma levels, and did not differ between PGD cases and controls, including IL-2, IL-4, IL-5, IL-7, IL-10, IL-12, IL-15 and IL-17. Notably, as illustrated in Figure 3A and B, TNF-α and IL-1β levels likewise fell precipitously in the postoperative period in both cases and controls.
Interferons
The IFNs exhibited patterns similar to inflammatory cytokines (Figure 3C and D). IFN-α plasma levels initially dropped postoperatively in both groups, but at 24 h postoperatively and beyond, PGD cases had lower levels than controls (p < 0.05 at 24, 48, and 72 h).
Discussion
We evaluated the relationship of plasma cytokines and chemokines with PGD after lung transplantation. Building on previous laboratory investigations, our study is the first to simultaneously measure a comprehensive panel of immune-modulating mediators in the plasma of human lung transplant recipients at numerous perioperative time points. Several interesting observations arise from this investigation, particularly regarding the large differences in plasma MCP-1 and IP-10 levels between PGD cases and controls as well as the lack of difference in proinflammatory cytokine response in plasma. Furthermore, we showed that associations of MCP-1 and IP-10 with PGD were independent of preoperative clinical factors, and that higher MCP-1 levels were related to the use of cardiopulmonary bypass.
Importantly, we demonstrated that the associations of MCP-1 and IP-10 with PGD were independent of the differences in preoperative clinical variables, including diagnosis categories. Confounding effects of clinical variables can be addressed using several methods in human studies, including matching, adjustment in multivariable regression models and exclusion of categories that are imbalanced. We did not performing upfront matching, since this strategy may lead to errors due to overmatching (16). Instead, we demonstrated that chemokine associations were robust when confounding clinical variables were included in multivariable logistic regression models as well as when we excluded subjects with diagnoses that appeared imbalanced between the groups (such as DPLD or IPAH). However, we were unable to definitively determine whether MCP-1 was simply higher concurrent with cardiopulmonary bypass use due to pulmonary arterial hypertension, or whether MCP-1, elevated right heart pressures and cardiopulmonary bypass use are part of a causal pathway in PGD pathogenesis. Future laboratory and clinical studies may address this issue.
MCP-1 and IP-10 are implicated in myocardial I/R injury (19), and clinical (20,21) and experimental (22,23) data show that IP-10 plays a key role in early injury after cardiac and kidney transplantation. Elevated postoperative levels of each of the observed chemokines may simply be an epiphenomenon of lung injury, or else be part of the causal pathway in PGD pathogenesis. Potential explanations for elevated levels of IP-10, MCP-1 and MIG include: (a) higher IFN production in response to injury, (b) increased sensitivity to IFN production in PGD cases and/or (c) overproduction of these mediators despite normal tissue IFN levels and sensitivity. Regardless, the findings suggest that PGD risk reduction may potentially be achieved via modulation of the chemokine CC motif receptor 2 (CCR2) and chemokine CXC motif receptor 3 (CXCR3) receptors. Additional studies in animal models may help further unravel these associations and identify key points for a rational therapy. Furthermore, our findings suggest that these mediators may be useful in the future for the prediction or early recognition of PGD. However, future studies of clinical utility of these biomarkers will require validation and will need to include the entire spectrum of PGD.
One of the striking findings of our investigation was a marked reduction in both case and control plasma levels of multiple proinflammatory mediators, including (but not limited to) TNF-α, IL-1β and IL-2. There are three potential explanations for this global trend in our study. First, an early proinflammatory response may not have been captured by study blood drawing time points, particularly prior to 6 h postoperatively. In human lung transplantation, lung tissue levels of TNF-α, IFN-γ and IL-8 elevate during the ischemic time, and subsequently decrease within 2 h of reperfusion (5); after 24 h posttransplantation, there are no noticeable elevations in plasma levels of TNF-α (24). Second, reduction in plasma proinflammatory mediators may be due to a postoperative suppression of NF-κB-mediated transcription, as seen in a canine lung allograft model, despite temporary elevations in these cytokines in the BAL (14). Lastly, these mediators may have bound to soluble receptors in the plasma, reducing measured plasma levels.
Our study has several limitations. First, although our study had adequate power to detect moderate differences between mediators, the sample size may be too low to detect subtle differences, or to detect differences where there is a large variance. Therefore, negative results should be interpreted with caution. Second, the case and control groups had differences in clinical variables, including diagnoses and pulmonary hypertension. However, the multivariable analyses performed revealed little effects of diagnosis category on the relationship of mediators with PGD. Third, since we assessed multiple biomarkers, it is possible that some of our differences are false-positive results. However, since each of our mediators was assessed at multiple time points, statistical significance can be interpreted in the setting of consistency with other time points within the same individual, strengthening the confidence that the results are not just ‘random noise’. Fourth, we did not have access to validly collected donor samples; therefore, future studies will need to focus on donors. Fifth, we chose to analyze the extremes of the PGD spectrum grade 0 and grade 3, given the high cost of the assay platform. Future studies will need to focus on other PGD grades. Sixth, many of our mediators had levels at or below the detection limits of the assays (Table 2). Therefore, our negative results should be interpreted with caution. Finally, BAL samples were not available, and correlations between plasma and lung compartments cannot be made. Our findings therefore suggest the importance of these data for directing future scientific inquiry, both clinical and basic, including future studies of the clinical utility of these biomarkers in predicting PGD.
Table 2.
Detection limits and percentages of total assays with values at lower limits of detection
| Mediator | Lower limit of detection (pg/mL) | Percentage of assays with levels at lower limit of detection |
|---|---|---|
| Eotaxin | 5 | 2 |
| GM-CSF | 15 | 59 |
| IFN-α | 15 | 22 |
| IFN-γ | 5 | 82 |
| IL-1β | 15 | 67 |
| IL-1Ra | 30 | 1 |
| IL-2 | 6 | 93 |
| IL-2R | 30 | 0 |
| IL-4 | 5 | 91 |
| IL-5 | 3 | 89 |
| IL-6 | 3 | 21 |
| IL-7 | 10 | 65 |
| IL-8 | 3 | 81 |
| IL-10 | 5 | 80 |
| IL-12 | 15 | 46 |
| IL-13 | 10 | 83 |
| IL-15 | 10 | 72 |
| IL-17 | 10 | 76 |
| IP-10 | 5 | 2 |
| MCP-1 | 10 | 0 |
| MIG | 4 | 8 |
| MIP-1α | 10 | 60 |
| MIP-1β | 10 | 12 |
| RANTES | 15 | 3 |
| TNF-α | 10 | 39 |
In conclusion, this study provides useful data on early plasma levels of multiple cytokines and chemokines from a well-characterized cohort undergoing lung transplantation. In subjects developing PGD, there was an increase in plasma levels of MCP-1 and IP-10, suggesting an IFN-induced activation of macrophages, endothelium and epithelium in these subjects and attraction of monocytes (via CCR2) and effector T cells (via CXCR3). These findings suggest promising targets for further investigation as mediators of lung injury as well as potential clinical biomarkers for the prediction or early recognition of PGD.
Acknowledgments
The authors report the following funding sources and addresses:
S.A.H.1,2,3,4 Hospital of the University of Pennsylvania, 839 West Gates Building, 3400 Spruce Street, Philadelphia, PA, 19104; L.W.5,6,7 916B Abramson Research Center, Children's Hospital of Philadelphia, 3615 Civic Center Boulevard, Philadelphia, PA 19104; C.V.S.2,3,4,8 Hospital of the University of Pennsylvania, 839 West Gates Building, 3400 Spruce Street, Philadelphia, PA, 19104; V.N.A.4,5 Hospital of the University of Pennsylvania, 832 West Gates Building, 3400 Spruce Street, Philadelphia, PA, 19104; A.P .5 Hospital of the University of Pennsylvania, 2nd Floor Dulles Building, 3400 Spruce Street, Philadelphia, PA, 19104; K.O.5 Hospital of the University of Pennsylvania, 2nd Floor Dulles Building, 3400 Spruce Street, Philadelphia, PA, 19104; A.S.5 Hospital of the University of Pennsylvania, 2nd Floor Dulles Building, 3400 Spruce Street, Philadelphia, PA, 19104; K.W.2,3 University of Alabama at Birmingham, 1808 7th Avenue South, BDB 300, Birmingham, AL 35294–0006; V.N.L.2,3,9 University of Michigan, 6301 Medical Sciences Research Building III, Ann Arbor, MI 48109–5642; A.M.2,3 Vanderbilt University Medical Center, 913 Oxford House, Nashville, TN 37232; L.B.W.2,3,10 Vanderbilt University School of Medicine, T1218 MCN, 116 21st Avenue South, Nashville, TN 37232– 2650; J.O.2,3 Johns Hopkins University Hospital, 1830 East Monument Street (5th floor), Baltimore, Maryland 21205; A.W.2,3 Stanford University, 300 Pasteur Drive #S102, Stanford, CA 94305–5110; E.D.2,3 Hospital of the University of Pennsylvania, 839 West Gates Building, 3400 Spruce Street, Philadelphia, PA, 19104; S.B.2,3 629 Blockley Hall, 423 Guardian Drive, Philadelphia, PA 19104–6021; S.M.K.2,3,5,11 622 West 168th Street, PH 8E, Room 101, New York, NY 10032; W.W.H.5,6,7 916B Abramson Research Center, Children's Hospital of Philadelphia, 3615 Civic Center Boulevard, Philadelphia, PA 19104; J.D.C.2,3,4,5,12 University of Pennsylvania School of Medicine, 423 Guardian Drive, 719 Blockley Hall, Philadelphia, PA 19104; 1HL00756822; 2HL087115; 3HL081619; 4The Craig and Elaine Dobbin Pulmonary Research Fund; 5AI063589; 6AI54720; 7AI68061; 8HL07891; 9HL077719; 10HL081332; 11HL67771; 12HL04243.
Abbreviations
- CCR2
chemokine CC motif receptor 2
- CXCR3
chemokine CXC motif receptor 3
- CXCL
chemokine CXC motif ligand
- CCL
chemokine CC motif ligand
- IP-10 (CXCL10)
interferon-inducible protein
- I/R
ischemia/reperfusion
- ISHLT
International Society of Heart and Lung Transplantation
- LTOG
Lung Transplant Outcomes Group
- MIG (CXCL9)
monokine induced by gamma-interferon
- MCP-1 (CCL2)
monocyte chemotactic protein-1
- PGD
primary graft dysfunction
Appendix Participants in the Lung Transplant Outcomes Group by Site
University of Alabama, Birmingham Keith Wille, MD (PI) Joao de Andrade, MD Tonja Meadows, RN
Columbia University Steven Kawut, MD, MS (PI) Selim Arcasoy, MD Joshua Sonett, MD Jessie Wilt, MD David Lederer, MD Frank D'Ovidio, MD Catherine Forster, BA Michael Koeckert, BA Debbie Rybak, BA
Johns Hopkins University Jonathan Orens, MD (PI) Ashish Shah, MD University of Michigan Vibha Lama, MD, MS (PI) Fernando Martinez, MD, MS Emily Galopin, BS
University of Pennsylvania (Coordinating site) Jason D. Christie, MD, MS (PI) Alberto Pocchetino, MD Ejigayehu Demissie, MSN Robert M. Kotloff, MD Vivek N. Ayha, MD Jeffrey Sager, MD, MS Denis Hadjiliadis, MD, MHS Lillian Geunther, BS Richard Aplenc, MD
Stanford University Ann Weinacker, MD (PI) Ramona Doyle, MD Susan Spencer Jacobs, MSN Val Scott, MSN
Vanderbilt University Aaron Milstone, MD (PI) Lorraine Ware, MD E. Wesley Ely, MD, MPH Stacy Kelley-Blackburn, RN
Footnotes
Conflict of Interest Statement
There are no conflicts of interest to disclose.
References
- 1.Christie JD, Bavaria JE, Palevsky HI, et al. Primary graft failure following lung transplantation. Chest. 1998;114:51–60. doi: 10.1378/chest.114.1.51. [DOI] [PubMed] [Google Scholar]
- 2.King RC, Binns OA, Rodriguez F, et al. Reperfusion injury significantly impacts clinical outcome after pulmonary transplantation. Ann Thorac Surg. 2000;69:1681–1685. doi: 10.1016/s0003-4975(00)01425-9. [DOI] [PubMed] [Google Scholar]
- 3.Christie JD, Kotloff RM, Pochettino A, et al. Clinical risk factors for primary graft failure following lung transplantation. Chest. 2003;124:1232–1241. doi: 10.1378/chest.124.4.1232. [DOI] [PubMed] [Google Scholar]
- 4.Arcasoy SM, Kotloff RM. Lung transplantation. N Engl J Med. 1999;340:1081–1091. doi: 10.1056/NEJM199904083401406. [DOI] [PubMed] [Google Scholar]
- 5.De Perrot M, Sekine Y, Fischer S, et al. Interleukin-8 release during early reperfusion predicts graft function in human lung transplantation. Am J Respir Crit Care Med. 2002;165:211–215. doi: 10.1164/ajrccm.165.2.2011151. [DOI] [PubMed] [Google Scholar]
- 6.Christie JD, Sager JS, Kimmel SE, et al. Impact of primary graft failure on outcomes following lung transplantation. Chest. 2005;127:161–165. doi: 10.1378/chest.127.1.161. [DOI] [PubMed] [Google Scholar]
- 7.Arcasoy SM, Fisher A, Hachem RR, Scavuzzo M, Ware LB. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part V: Predictors and outcomes. J Heart Lung Transplant. 2005;24:1483–1488. doi: 10.1016/j.healun.2004.11.314. [DOI] [PubMed] [Google Scholar]
- 8.Christie JD, Kotloff RM, Ahya VN, et al. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med. 2005;171:1312–1316. doi: 10.1164/rccm.200409-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christie JD, Van Raemdonck D, de Perrot M, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part I: Introduction and methods. J Heart Lung Transplant. 2005;24:1451–1453. doi: 10.1016/j.healun.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: Definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24:1454–1459. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 11.de Perrot M, Bonser RS, Dark J, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part III: Donor-related risk factors and markers. J Heart Lung Transplant. 2005;24:1460–1467. doi: 10.1016/j.healun.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 12.de Perrot M, Liu M, Waddell TK, Keshavjee S, Ischemiareperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167:490–511. doi: 10.1164/rccm.200207-670SO. [DOI] [PubMed] [Google Scholar]
- 13.Barr ML, Kawut SM, Whelan TP, et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part IV: Recipient-related risk factors and markers. J Heart Lung Transplant. 2005;24:1468–1482. doi: 10.1016/j.healun.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 14.Serrick C, Adoumie R, Giaid A, Shennib H. The early release of interleukin-2, tumor necrosis factor-alpha and interferon-gamma after ischemia reperfusion injury in the lung allograft. Transplantation. 1994;58:1158–1162. [PubMed] [Google Scholar]
- 15.Naidu BV, Farivar AS, Woolley SM, Grainger D, Verrier ED, Mulligan MS. Novel broad-spectrum chemokine inhibitor protects against lung ischemia-reperfusion injury. J Heart Lung Transplant. 2004;23:128–134. doi: 10.1016/s1053-2498(03)00102-5. [DOI] [PubMed] [Google Scholar]
- 16.Wacholder S. Design issues in case control studies. Stat Methods Med Res. 1995;4:293–309. doi: 10.1177/096228029500400403. [DOI] [PubMed] [Google Scholar]
- 17.Christie JD, Robinson N, Ware LB, et al. Association of protein C and type 1 plasminogen activator inhibitor with primary graft dysfunction. Am J Respir Crit Care Med. 2007;175:69–74. doi: 10.1164/rccm.200606-827OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Covarrubias M, Ware LB, Kawut SM, et al. Plasma intercellular adhesion molecule-1 and von Willebrand factor in primary graft dysfunction after lung transplantation. Am J Transplant. 2007;7:2573–2578. doi: 10.1111/j.1600-6143.2007.01981.x. [DOI] [PubMed] [Google Scholar]
- 19.Frangogiannis NG. The role of the chemokines in myocardial ischemia and reperfusion. Curr Vasc Pharmacol. 2004;2:163–174. doi: 10.2174/1570161043476375. [DOI] [PubMed] [Google Scholar]
- 20.Tatapudi RR, Muthukumar T, Dadhania D, et al. Noninvasive detection of renal allograft inflammation by measurements of mRNA for IP-10 and CXCR3 in urine. Kidney Int. 2004;65:2390–2397. doi: 10.1111/j.1523-1755.2004.00663.x. [DOI] [PubMed] [Google Scholar]
- 21.Melter M, Exeni A, Reinders ME, et al. Expression of the chemokine receptor CXCR3 and its ligand IP-10 during human cardiac allograft rejection. Circulation. 2001;104:2558–2564. doi: 10.1161/hc4601.098010. [DOI] [PubMed] [Google Scholar]
- 22.Hancock WW, Gao W, Csizmadia V, Faia KL, Shemmeri N, Luster AD. Donor-derived IP-10 initiates development of acute allograft rejection. J Exp Med. 2001;193:975–980. doi: 10.1084/jem.193.8.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hancock WW, Lu B, Gao W, et al. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J Exp Med. 2000;192:1515–1520. doi: 10.1084/jem.192.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathur A, Baz M, Staples ED, et al. Cytokine profile after lung transplantation: Correlation with allograft injury. Ann Thorac Surg. 2006;81:1844–1849. doi: 10.1016/j.athoracsur.2005.11.053. discussion 1849–1850. [DOI] [PubMed] [Google Scholar]