Abstract
The goals of this cross-sectional study were to explore correlates of walking speed in a large wide age-ranged population and to identify factors affecting lower walking speed at older ages. Participants were 3,872 community-dwelling adults in the first follow-up of the SardiNIA study who completed a 4-m walking test. Sex-specific correlates of walking speed included marital status, height, waist circumference, pulse wave velocity, comorbidity, subjective health, strength, and personality. Effect modifiers of the age–walking speed association included extraversion (<55 years, p = .019) and education (<55 years, p = .021; ≥55 years, p = .012) in women, and openness (<55 years, p = .005), waist circumference (<55 years, p = .010), and subjective health (<55 years, p = .014) in men. The strong impact of personality suggests that certain personality traits may be associated with behaviors that affect physical performance and condition the reduced mobility mostly at younger ages. If these patterns are confirmed in longitudinal studies, personality may be an important target for prevention.
Keywords: Cross-sectional design, Personality, Walking speed
AGE-RELATED decline in physical function represents a major public health concern as it infringes on the ability to live independently. Difficulty in walking is a critical stage in this process (Ferrucci et al., 2000). Poor walking performance increases with aging even in healthy older adults (Himann, Cunningham, Rechnitzer, & Paterson, 1988) and indicates depletion of functional reserve and failure of compensatory mechanisms (Chou & Draganich, 1997) often in the context of frailty (Fried, 1992). Mobility is thus a critical aspect of functioning in old age (Ferrucci et al., 2000) that promotes physical, social (Simonsick, Kasper, & Phillips, 1998), and psychological well-being (Penninx et al., 1998). Physical function and mobility in particular represent in older persons global measures of health status that are more valid and predictive of negative health outcomes compared with more traditional measures such as disease nosology and biomarkers. In fact, poor lower extremity performance is a strong and robust risk factor for incident disability, hospitalization, institutionalization, and mortality independent of potential confounders (Gill, Williams, & Tinetti, 1995; Guralnik et al., 1994; Newman et al., 2006).
Interestingly, the prognostic value of walking speed is best captured by simple tests performed at self-selected pace. A preferred walking speed of less than 1 m/s, for example, already identifies older individuals at risk for health-related outcomes (Cesari et al., 2005). Walking speed is superior to other measures (i.e., self-report, clinical evaluation) in terms of characterizing older individuals with varying degrees of lower extremity function (Guralnik et al., 1994) and is now measured as part of clinical assessment of older persons as a tool for screening persons most likely to benefit from interventions of disability prevention.
However, the mechanism by which walking speed is such a powerful measure of health status is not understood. Chronic medical conditions; impairments such as muscle weakness, poor vision, and poor cognition; and behavioral and physiological factors have been independently associated with slower gait (Bootsma-van der Wiel et al., 2002; Brach et al., 2003; Volpato et al., 2008). Despite the numerous studies examining correlates of gait speed, some important gaps remain in understanding patterns of decline over the life span as well as factors likely to influence these patterns. For example, it may be hypothesized that certain personality characteristics are associated with specific motor behaviors and attitudes known to affect performance early in life but also influence performance maintenance over the life span. Such behaviors include physical activity, smoking, abusive alcohol consumption, and unhealthy diet (Bogg & Roberts, 2004; Booth-Kewley & Vickers, 1994) and have been linked to personality via different mechanisms including biological (e.g., dopaminergic and serotonergic neurotransmission), social (e.g., sensation seeking, sociability, and conventionality), affective (e.g., control of negative affect), and genetic (Donovan, Jessor, & Costa, 1991; Eysenck, Grossarth-Maticek, & Everitt, 1991).
In addition to health behaviors, psychophysiological/emotional reactions could constitute a secondary mechanism potentially explaining the association between personality and walking speed (Friedman, 2000). Increased levels of chronic inflammation with aging have been reported and linked to poor physical performance (Cesari et al., 2004) and functional decline possibly through an effect on muscle atrophy and/or a pathophysiological role in certain chronic conditions (Ferrucci et al., 1999); they are also linked to certain personality traits (e.g., hostility; Marsland, Prather, Petersen, Cohen, & Manuck, 2008).
Studies that reported on the relationship between personality and disability suggest that individual differences in personality traits (e.g., anxiety, neuroticism, mastery, extraversion, and conscientiousness) play an instrumental role in self-reported and performance-based functional status (Friedman, 2000; Krueger, Wilson, Shah, Tang, & Bennett, 2006). Yet, although psychological factors had been related to mobility, they were limited to positive affect (Lord & Menz, 2002; Ostir, Markides, Black, & Goodwin, 2000), although a new taxonomy of personality has gained wide acceptance (McCrae & Costa, 1987).
In this study, we explored correlates of walking speed and identified factors that modify the patterns of walking speed with higher age in a large wide age-ranged sample of male and female participants in the SardiNIA study. Moreover, the study investigates a multitude of correlates of walking speed, including personality, which, through different health-related behaviors, could affect gait changes during the aging process.
METHODS
Study Participants
The SardiNIA project is an ongoing observational study initiated in 2001 in the Ogliastra province of Sardinia to explore the genetic contribution to different age-related phenotypes, with a focus on cardiovascular risk factors and personality traits. Study design and rationale, presented elsewhere (Pilia et al., 2006), were approved by the National Institute on Aging and relevant institutional review boards, and written informed consents were obtained from the participants. Briefly, residents of four towns in the Ogliastra province aged older than 14 and their family were invited to participate in the study. Approximately 62% of the entire population in the targeted age range joined the study, yielding a baseline sample of 6,191 participants, of which 3,891 (63%) participated 3 years later in a follow-up evaluation, which included for the first time a test of walking speed.
Measures
Length divided by time to complete a 4-m course at usual pace was used to compute walking speed (m/s), our outcome measure. Instructions including demonstrations on the test were provided prior to testing and assistive devices allowed when necessary. The participants, who wore comfortable clothing and shoes, were positioned behind a marked starting line and instructed to start walking when prompted. The finish line was not marked to prevent slowing down at the end of the course. The time was recorded using a chronometer. The 4-m walking test had been previously reported to be a highly reliable (Ferrucci et al., 1996; Rolland et al., 2004) and an accurate and practical instrument for screening large populations for mobility limitations (Rolland et al., 2004). Based on an extensive literature review, the following variables potentially associated with walking speed were selected. Self-reported sociodemographic characteristics included age, sex, education, and marital status. Anthropometrical variables included waist circumference (girth) and height. Personality was measured using the revised NEO Personality Inventory, which has high internal consistency and test–retest reliability both in the English version (Costa & McCrae, 1992) and in the Italian translation (Terracciano, 2003). Domain and facet scores for neuroticism, extraversion, openness, agreeableness, and conscientiousness were used in this study as continuous variables. Behavioral risk factors included self-reported moderate activity (min/week), walking (min/week), and smoking. Risk factors for cardiovascular disease included blood pressure measured supinely three times (average of last two measurements used), lipid levels (total cholesterol, high-density lipoproteins cholesterol, and triglycerides) from fasting blood samples, and pulse wave velocity (PWV), a marker of arterial stiffness measured via time delay of the pulse propagation between carotid and femoral sites. Disease-related variables included self-reported health (1 = poor/average; 2 = good/very good; and 3 = excellent health), depression, anxiety, other major chronic conditions (self-reported angina, myocardial infarction, stroke, arthritis, asthma, cancer, eye disease, and diabetes [measured by a combination of fasting glucose ≥126 mg/dl, diagnosis, or use of antidiabetic drugs]), as well as self-reported number of medications. Handgrip strength was measured by asking the participants to squeeze a dynamometer (Jamar hydraulic hand dynamometer; Sammons Preston Corp., Bolingbrook, IL) with maximum strength three times with each hand; the average of the two hands was used in the analysis. The Mini-Mental State Examination (MMSE) score (Folstein, Folstein, & McHugh, 1975), a measure of cognitive status, was used as a continuous variable. A log transformation was used to obtain more normally distributed triglyceride, PWV, and physical activity values.
Data Analysis
Statistical analyses were stratified by sex due to sex differences in gait speed. Initial inspection showed a nonlinear age–walking speed relationship. Therefore, using spline regression analysis, bivariate and multivariate models were run to identify factors that correlated with walking speed without and with adjustment for other factors. To explore the correlates of walking speed, factors significantly associated with walking speed in bivariate analysis were added to an initial multivariate model, which was then reduced to a parsimonious model by manually selecting out insignificant terms (Model 1). Due to the large number of correlates being tested at the bivariate level, a Bonferroni correction was applied, leading to a p level of ≤.002 being used to indicate significance at this level. To identify effect modifiers of the age-related patterns of walking speed, all significant correlates in bivariate analyses were tested for their interaction with age, controlling for Model 1 covariates. Two factors were identified as significant (p < .05) modifiers in women and three in men, and analyses concerning them are presented separately (Models 2–4). Associations between walking speed and age are presented as slopes per decade of age. Statistical analyses were carried out using SAS version 9.1 (SAS Institute, Cary, NC) software package.
RESULTS
Sample Characteristics
The analytical sample consisted of 3,872 (59% women) participants representing 62.5% of the baseline sample with valid data on the 4-m walking test. Participants were on average 48 years old (age range: 18–98 years), with men slightly but significantly older than women. Collectively, the prevalence of social (except being married), behavioral (except smoking), and biological risk factors increased with age and differed by sex (Table 1). Compared with women, men were taller and thicker, had stronger muscles, rated their health higher, and walked more for exercise. However, they were also more likely to be uneducated, to smoke, and to have higher blood pressure and triglyceride levels. Women tended to report higher levels of neuroticism, openness, and agreeableness. In terms of usual walking speed, women walked on average with a speed of 1.05 m/s (SD = 0.24, median = 1.05, interquartile range = 0.90–1.21), whereas men had a speed of 1.14 m/s (SD = 0.23, median = 1.13, interquartile range = 0.99–1.29).
Table 1.
Sample Characteristics According to Sex and Age
| Women |
Men |
Test statistic, χ2 |
||||||||
| Variable | <35 years (n = 595) | 35–54 years (n = 932) | 55–74 years (n = 645) | 75+ years (n = 112) | <35 years (n = 401) | 35–54 years (n = 600) | 55–74 years (n = 489) | 75+ years (n = 98) | Sex differences | Age differences |
| n (%) | ||||||||||
| Low education (less than high school) | 180 (30) | 604 (65) | 563 (87) | 104 (93) | 195 (49) | 442 (74) | 437 (89) | 94 (96) | 41*** | 565*** |
| Married | 146 (25) | 720 (77) | 454 (70) | 40 (3) | 53 (13) | 456 (76) | 449 (92) | 90 (92) | 13*** | 497*** |
| Smoker | 147 (25) | 155 (17) | 29 (5) | 0 (0) | 162 (40) | 169 (28) | 51 (10) | 4 (1) | 70*** | 234*** |
| Comorbidity | 13 (2) | 65 (7) | 219 (34) | 71 (63) | 6 (2) | 41 (7) | 149 (31) | 53 (54) | 3 | 502*** |
| Subjective health | 69*** | 495*** | ||||||||
| Poor/average | 56 (9) | 249 (27) | 279 (43) | 65 (58) | 21 (5) | 106 (18) | 144 (29) | 49 (50) | ||
| Good/very good | 428 (72) | 590 (63) | 346 (54) | 44 (39) | 262 (65) | 407 (68) | 304 (62) | 47 (48) | ||
| Excellent | 111 (19) | 93 (10) | 20 (3) | 3 (3) | 118 (29) | 86 (14) | 41 (8) | 2 (2) | ||
| M (SD) | ||||||||||
| Waist circumference (cm) | 73 (8) | 80 (10) | 87 (11) | 89 (12) | 84 (9) | 93 (9) | 98 (10) | 98 (10) | 1,229*** | 1,155*** |
| Height (cm) | 159 (6) | 156 (6) | 152 (6) | 147 (6) | 171 (7) | 168 (7) | 163 (6) | 159 (6) | 3,319*** | 1,106*** |
| Neuroticism | 60 (10) | 59 (9) | 60 (9) | 61 (9) | 52 (8) | 53 (8) | 54 (7) | 54 (7) | 505*** | 7** |
| Extraversion | 51 (10) | 47 (9) | 45 (7) | 44 (6) | 52 (8) | 48 (8) | 46 (7) | 46 (7) | 9** | 265*** |
| Openness | 50 (9) | 48 (9) | 43 (8) | 40 (8) | 47 (9) | 46 (8) | 43 (8) | 39 (8) | 30*** | 336*** |
| Agreeableness | 48 (9) | 52 (8) | 54 (9) | 57 (9) | 42 (9) | 45 (8) | 49 (8) | 53 (9) | 380*** | 391*** |
| Conscientiousness | 49 (11) | 52 (9) | 53 (8) | 57 (7) | 48 (10) | 52 (9) | 53 (8) | 55 (8) | 1 | 180*** |
| Systolic BP (mmHg) | 112 (10) | 120 (15) | 134 (16) | 143 (19) | 122 (11) | 128 (14) | 138 (16) | 140 (18) | 203*** | 1,215*** |
| Diastolic BP (mmHg) | 69 (9) | 78 (10) | 84 (10) | 84 (10) | 73 (9) | 83 (11) | 85 (11) | 80 (11) | 83*** | 754*** |
| Cholesterol (mg/dl) | 190 (33) | 209 (36) | 224 (36) | 220 (38) | 186 (39) | 215 (36) | 219 (38) | 201 (37) | 2 | 343*** |
| Triglycerides (mg/dl) | 68 (40) | 74 (42) | 92 (52) | 105 (65) | 93 (89) | 124 (117) | 112 (72) | 96 (59) | 182*** | 45*** |
| HDL (mg/dl) | 67 (13) | 68 (14) | 68 (13) | 68 (14) | 55 (10) | 57 (12) | 61 (13) | 62 (19) | 526*** | 24*** |
| PWV (m/s) | 6.2 (0.2) | 6.4 (0.2) | 6.7 (0.2) | 6.9 (0.2) | 6.3 (0.2) | 6.4 (0.2) | 6.7 (0.2) | 6.9 (0.3) | 19*** | 2,472*** |
| Moderate activity (min/week) | 1,171 (975) | 1,691 (1,046) | 1,595 (1,029) | 1,381 (604) | 1,472 (1,153) | 1,723 (1,216) | 1,511 (1,074) | 1,171 (1,119) | 1 | 5* |
| Walking (min/week) | 330 (661) | 500 (860) | 508 (868) | 330 (423) | 499 (748) | 559 (857) | 610 (961) | 443 (730) | 10** | 5* |
| Handgrip strength (kg) | 25 (5) | 25 (6) | 21 (6) | 17 (5) | 48 (9) | 46 (9) | 36 (8) | 26 (7) | 5,788*** | 859*** |
| Walking speed (m/s) | 1.2 (0.2) | 1.1 (0.2) | 0.9 (0.2) | 0.7 (0.2) | 1.2 (0.2) | 1.2 (0.2) | 1.1 (0.2) | 0.9 (0.2) | 203*** | 861*** |
Notes: BP = blood pressure; HDL = high-density lipoprotein; PWV = pulse wave velocity. Sex and age differences were determined by means of regression analysis. Sex differences were controlled for age and age differences for sex. Values presented in the table represent number (%) for categorical variables and mean (SD) for continuous variables.
*p < .05; **p < .01; ***p < .001.
Correlates of Walking Speed
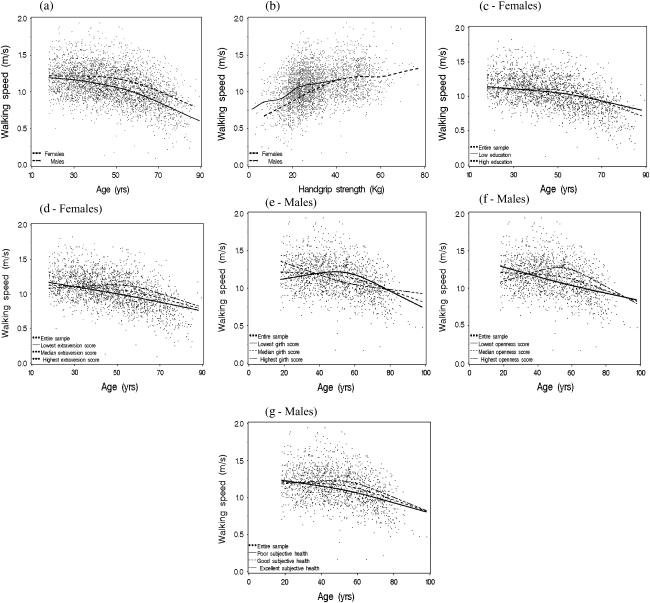
As expected given the wide age range, the association between age and walking speed was not linear. In both sexes, walking speed did not differ across the younger age-groups, but reduced speed was evident for older age-groups beginning around 50–55 years (Figure 1a). To determine a more exact inflection point at which the age–walking speed association departs from linearity, we conducted a sensitivity analysis testing every age from 35 to 80 years as such inflector. This was done by comparing models with inflection points set at different ages in terms of their goodness of fit based on the deviance criterion. The results of the sensitivity analysis indicated age 55 as the best inflection point for both sexes. Therefore, all subsequent analyses, bivariate and multivariate, were conducted with spline regression models with one inflection point set at the age of 55. Generally, women walked slower than men at every age, with more marked differences after age 55, and older women walked slower than younger women.
Figure 1.
Patterns of walking speed from ages 18 to 100 according to gender (a); education (c), and extraversion (d) among women; and waist circumference (e), openness (f), and subjective health (g) among men. (b) The association between walking speed and muscle strength according to gender.
Bivariate analysis.—
Among those younger than 55 years, age was negatively associated with walking speed, and women walked slower than men. After the age of 55, the negative association between age and walking speed was even more pronounced, but patterns in men and women were not substantially different (Tables 2 and 3). Besides age, being married (men only), waist circumference, agreeableness, conscientiousness (women only), blood pressure, cholesterol (women only), triglycerides (women only), PWV, depression (women only), comorbidity, and number of medications were also negatively associated with walking speed. Positive correlates of walking speed included education, height, extraversion, openness, subjective health, handgrip strength, and MMSE score in both sexes. Unexpectedly, among women, smoking was positively and physical activity negatively associated with walking speed.
Table 2.
Independent Correlates of Walking Speed and Effect Modifiers of the Age–Walking Speed Association Among Women
| Multivariate analysis, β (p value) |
||||
| Correlates | Bivariate analysis, β (p value) | Model 1 | Model 2 | Model 3 |
| Age pre-55 | −.045 (<.001) | −.016 (.017) | −.081 (.002) | −.025 (.001) |
| Age post-55 | −.128 (<.001) | −.063 (<.001) | .017 (.792) | −.044 (.002) |
| Education (high vs. low) | .134 (<.001) | — | — | −.086 (.038) |
| Married (vs. unmarried) | −.023 (.027) | — | — | — |
| Height (cm) | .013 (<.001) | .004 (<.001) | .004 (<.001) | .004 (<.001) |
| Waist circumference (cm) | −.006 (<.001) | −.001 (.045) | −.001 (.054) | −.001 (.070) |
| Neuroticism | −.002 (.006) | — | — | — |
| Extraversion | .005 (<.001) | .001 (.039) | −.004 (.036) | .001 (.036) |
| Openness | .007 (<.0001) | .002 (<.001) | .002 (<.001) | .002 (.001) |
| Agreeableness | −.004 (<.001) | — | — | — |
| Conscientiousness | −.002 (<.001) | — | — | — |
| Systolic blood pressure (mmHg) | −.004 (<.001) | — | — | — |
| Diastolic blood pressure (mmHg) | −.005 (<.001) | — | — | — |
| Cholesterol (mg/dl) | −.001 (<.001) | — | — | — |
| HDL (mg/dl) | .001 (.005) | — | — | — |
| Triglycerides (log) | −.076 (<.001) | — | — | — |
| PWV (log) | −.350 (<.001) | −.056 (.027) | −.058 (.021) | −.051 (.044) |
| Moderate activity (log) | −.018 (.002) | — | — | — |
| Walking activity (log) | −.011 (.019) | — | — | — |
| Smoker (vs. nonsmoker) | .079 (<.001) | — | — | — |
| Subjective health | .117 (<.001) | .040 (<.001) | .040 (<.001) | .041 (<.001) |
| Depression | −.080 (<.001) | — | — | — |
| Anxiety | −.050 (.028) | — | — | — |
| At least 2 other major conditions (yes vs. no) | −.227 (<.001) | −.043 (.007) | −.044 (.006) | −.043 (.007) |
| Number of meds | −.191 (<.001) | — | — | — |
| Handgrip strength | .025 (<.001) | .002 (.041) | .001 (.079) | .002 (.049) |
| Handgrip strength (quadratic term) | −.0003 (.002) | — | — | — |
| MMSE score | .015 (<.001) | — | — | — |
| Extraversion × Age Pre-55 | — | — | .001 (.010) | — |
| Extraversion × Age Post-55 | — | — | −.002 (.258) | — |
| Education × Age Pre-55 | — | — | — | .026 (.014) |
| Education × Age Post-55 | — | — | — | −.072 (.006) |
| Deviance | — | 72.32 | 72.01 | 71.97 |
Notes: Age pre-55 and age post-55 indicate the effect of age on walking speed among those younger than 55 years and those aged 55 years and older, respectively. Only variables significant at p ≤ .002 were considered significant and entered in the multivariate analysis. Model 1: obtained by reducing the full model of correlates of walking speed significant at p ≤ .002 in the bivariate analysis. Model 2: Model 1 + interaction terms between extraversion and age. Model 3: Model 1 + interaction terms between education and age. Note that only effect modifiers that contributed significantly to the model fit are reflected in this table. Due to the spline regression analysis design, the actual estimates for the age–walking speed association among those aged 55 years and older require summation of the slopes before and after the inflection (presented in Table 4). MMSE = Mini-Mental State Examination; HDL = high-density lipoprotein; PWV = pulse wave velocity.
Table 3.
Independent Correlates of Walking Speed and Effect Modifiers of the Age–Walking Speed Association Among Men
| Multivariate analysis |
|||||
| Correlates | Bivariate analysis, β (p value) | Model 1, β (p value) | Model 2, β (p value) | Model 3, β (p value) | Model 4, β (p value) |
| Age pre-55 | −.013 (.029) | −.008 (.365) | −.096 (.003) | .139 (.017) | −.037 (.011) |
| Age post-55 | −.121 (<.001) | −.074 (<.001) | .064 (.357) | −.332 (.023) | −.031 (.243) |
| Education (high vs. low) | .084 (<.001) | — | — | — | — |
| Married (vs. unmarried) | −.067 (<.001) | .035 (.043) | .036 (.038) | .032 (.062) | .035 (.041) |
| Height | .009 (<.001) | .004 (<.001) | .003 (.001) | .004 (<.001) | .004 (<.001) |
| Waist circumference (cm) | −.003 (<.001) | — | — | .006 (.017) | — |
| Neuroticism | −.003 (.006) | — | — | — | — |
| Extraversion | .004 (<.001) | — | — | — | — |
| Openness | .004 (<.001) | — | −.007 (.017) | — | — |
| Agreeableness | −.005 (<.001) | −.001 (.048) | −.001 (.059) | −.002 (.033) | −.001 (.040) |
| Conscientiousness | −.0001 (.907) | — | — | — | — |
| Systolic blood pressure (mmHg) | −.003 (<.001) | — | — | — | — |
| Diastolic blood pressure (mmHg) | −.001 (.014) | — | — | — | — |
| Cholesterol (mg/dl) | −.0003 (.063) | — | — | — | — |
| HDL (mg/dl) | −.001 (.011) | — | — | — | — |
| Triglycerides (log) | −.004 (.658) | — | — | — | — |
| PWV (log) | −.236 (<.001) | — | — | — | — |
| Moderate activity (log) | .003 (.724) | — | — | — | — |
| Walking activity (log) | −.004 (.401) | — | — | — | — |
| Smoker (vs. nonsmoker) | .040 (.002) | — | — | — | — |
| Subjective health | .093 (<.001) | .037 (.001) | .035 (.001) | .036 (.001) | −.072 (.095) |
| Depression | −.095 (.019) | — | — | — | — |
| Anxiety | −.056 (.519) | — | — | — | — |
| At least 2 other chronic conditions (yes vs. no) | −.160 (<.001) | — | — | — | — |
| Number of meds | −.155 (<.001) | — | — | — | — |
| Handgrip strength | .027 (<.001) | .010 (.001) | .010 (.002) | .011 (.001) | .010 (.001) |
| Handgrip strength (quadratic term) | −.0002 (<.001) | −.0001 (.005) | −.0001 (.006) | −.0001 (.003) | −.0001 (.004) |
| MMSE score | .014 (<.001) | — | — | — | — |
| Openness × Age Pre-55 | — | — | .002 (.005) | — | — |
| Openness × Age Post-55 | — | — | −.003 (.059) | — | — |
| Waist × Age Pre-55 | — | — | — | −.002 (.010) | — |
| Waist × Age Post-55 | — | — | — | .003 (.065) | — |
| Subjective Health × Age Pre-55 | .026 (.014) | ||||
| Subjective health × Age Post-55 | −.040 (.096) | ||||
| Deviance | — | 58.83 | 58.37 | 58.49 | 58.53 |
Notes: Age pre-55 and age post-55 indicate the effect of age on walking speed among those younger than 55 years and those aged 55 years and older, respectively. Only variables significant at p ≤ .002 were considered significant and entered in the multivariate analysis. Model 1: obtained by reducing the full model of correlates of walking speed significant at p ≤ .002 in the bivariate analysis. Model 2: Model 1 + interaction terms between openness and age. Model 3: Model 1 + interaction terms between waist circumference and age. Model 4: Model 1 + interaction terms between subjective health and age. Note that only effect modifiers that contributed significantly to the model fit are reflected in this table. Due to the spline regression analysis design, the actual estimates for the age–walking speed association among those aged 55 years and older require summation of the slopes before and after the inflection (presented in Table 4). MMSE = Mini-Mental State Examination; HDL = high-density lipoprotein; PWV = pulse wave velocity.
Multivariate analysis.—
Results of the multivariate regression analysis are also presented in Tables 2 and 3. Model 1 represents the most parsimonious set of correlates of walking speed, controlling for the effect of other significant factors. Age was negatively associated with walking speed only among women younger than 55 years (p = .017) but in both sex groups among those aged 55 years or older (p < .001). It is worth noting that after adjusting for other covariates, the size of these coefficients was reduced in half (bivariate vs. model 1). Taller stature and better subjective health were independently associated with faster walking speed in both sex groups after adjusting for other covariates. Also, higher extraversion, higher openness, lower PWV, absence of comorbidity, and higher handgrip strength remained significantly associated with faster walking speed in women. Married, less agreeable, and stronger men were more likely to walk faster. Of note, handgrip strength was nonlinearly associated with walking speed in men (Table 3) but not in women, as illustrated by the insignificant quadratic terms (Model 1, Table 2) and in Figure 1b.
Effect Modifiers
Extraversion and education in women and openness, waist circumference, and subjective health in men significantly modified the age–walking speed relationship after adjustment for covariates in Model 1. The significant interaction terms with age are presented in Tables 2 and 3. The individual effect of these modifiers can be observed in Models 2–4 separately for each factor and in a graphical form in Figure 1c and d for women and in Figure 1e–g for men. Because extraversion, openness, and waist circumference were analyzed as continuous variables, their interaction with age is summarized graphically as estimates for the lowest, intermediate (median), and highest scores.
In women with the highest extraversion score, estimated walking speed was the same for those aged 18–54 years (p = .675) compared with women with intermediate (p < .001) and lowest extraversion (p < .001) scores, who walked more slowly across older age-groups; differences between them were statistically significant (Table 4). However, in women aged 55 years and older, there were no significant differences among the three groups. The highly educated group did not differ in walking speed among those younger than 55 years, but walking speed decreased across increasingly older groups (p < .001). In contrast, women with low education walked slower with higher age both before the inflection point (p < .001) and after (p < .001), although the latter association was weaker than in the high-education group. Differences between low- and high-education groups were statistically significant both among women younger than 55 years and among those 55 years and older (Table 4).
Table 4.
Actual Estimates of the Association Between Walking Speed and Age According to Different Categories (i.e., education) or Scores (i.e., extraversion, girth, and openness) of Effect Modifiers
| Women |
Men |
|||||||
| <55 years |
≥55 years |
<55 years |
≥55 years |
|||||
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | |
| Individual estimates | ||||||||
| Low education | −.025 | −0.040 to −0.010 | −.069 | −0.089 to −0.048 | — | — | — | — |
| High education | −.002 | −0.016 to 0.019 | −.115 | −0.149 to −0.080 | — | — | — | — |
| Lowest extraversion | −.056 | −0.089 to −0.023 | −.069 | −0.125 to −0.012 | — | — | — | — |
| Median extraversion | −.018 | −0.030 to −0.005 | −.076 | −0.096 to −0.055 | — | — | — | — |
| Highest extraversion | .022 | −0.009 to 0.053 | −.083 | −0.155 to −0.011 | — | — | — | |
| Lowest girth | — | — | — | — | .035 | −0.003 to 0.073 | −.116 | −0.188 to −0.044 |
| Median girth | — | — | — | — | −.012 | −0.031 to 0.007 | −.081 | −0.104 to −0.059 |
| Highest girth | — | — | — | — | −.080 | −0.139 to −0.022 | −.031 | −0.116 to 0.055 |
| Lowest openness | — | — | — | — | −.062 | −0.103 to −0.022 | −.051 | −0.106 to 0.004 |
| Median openness | — | — | — | — | −.012 | −0.029 to 0.005 | −.079 | −0.101 to −0.057 |
| Highest openness | — | — | — | — | .065 | 0.010 to 0.120 | −.122 | −0.215 to −0.028 |
| Poor subjective health | — | — | — | — | −.037 | −0.065 to −0.008 | −.068 | −0.099 to −0.036 |
| Good subjective health | — | — | — | — | −.011 | −0.028 to 0.007 | −.082 | −0.105 to −0.059 |
| Excellent subjective health | — | — | — | — | .015 | −0.011 to 0.041 | −.096 | −0.142 to −0.050 |
| Differences between groups of moderators | ||||||||
| High vs. low education | .026 | 0.005 to 0.047 | −.046 | −0.083 to −0.009 | — | — | — | — |
| Highest vs. lowest extraversion | .078 | 0.019 to 0.138 | −.015 | −0.137 to 0.107 | — | — | — | — |
| Highest vs. median extraversion | .040 | 0.009 to 0.070 | −.007 | −0.069 to 0.055 | — | — | — | — |
| Median vs. lowest extraversion | .038 | 0.009 to 0.068 | −.007 | −0.067 to 0.053 | — | — | — | — |
| Highest vs. lowest girth | — | — | — | — | −.115 | −0.203 to −0.027 | .085 | −0.066 to 0.236 |
| Highest vs. median girth | — | — | — | — | −.068 | −0.120 to −0.016 | .051 | −0.039 to 0.140 |
| Median vs. lowest girth | — | — | — | — | −.047 | −0.083 to −0.011 | .035 | −0.027 to 0.097 |
| Highest vs. lowest openness | — | — | — | — | .127 | 0.038 to 0.216 | −.071 | −0.212 to 0.071 |
| Highest vs. median openness | — | — | — | — | .076 | 0.023 to 0.130 | −.042 | −0.128 to 0.043 |
| Median vs. lowest openness | — | — | — | — | .051 | 0.015 to 0.086 | −.028 | −0.085 to 0.028 |
| Excellent vs. poor health | — | — | — | — | .052 | 0.011 to 0.093 | .028 | −0.093 to 0.036 |
| Excellent vs. good health | — | — | — | — | .026 | 0.005 to 0.047 | −.014 | −0.046 to 0.018 |
| Good vs. poor health | — | — | — | — | .026 | 0.005 to 0.047 | −.014 | −0.046 to 0.018 |
Notes: This table presents estimates of the association between age and walking speed for women and men aged 18–54 years and 55 years and older according to their level of education and subjective health, and for different scores of extraversion, girth, and openness. It also compares estimates for different groups and scores. For example, the individual estimate for low-educated women was −0.035 m/s per decade for those younger than 55 years (obtained from Table 2, Model 2) and was significantly different from 0 as indicated by the 95% CI (−0.048 to −0.021), which can be interpreted as a significant association between age and walking speed in this group. Additionally, the estimate of 0.027 m/s per decade along with its 95% CI indicate that before age 55, the estimates for the high-education (−0.008 m/s) and the low-education (−0.035 m/s) groups were significantly different among women in that age range. CI = confidence interval.
In similar analyses, the association of age with walking speed differed by openness score among men. Among those younger than 55 years, lower openness score was associated with slower speed (p = .003); differences were statistically significant (Table 4). However, although these associations seem to flip in those aged 55 years and older, differences between openness groups were not statistically significant. Moreover, among those younger than 55 years, individuals with the highest girth walked significantly slower with higher age, compared with those with smaller girth who were more stable, whereas a reverse in direction was noted among those aged 55 years and older. Differences among these three categories of girth were significant only among individuals younger than 55 years (Table 4). Also, poor subjective health was associated with slower speed in those younger than 55 years, whereas among those aged 55 years and older, the group rating their subjective health as excellent seemed to experience the slowest speed. Similarly, differences between groups were significant only among those younger than 55 years and not among those aged 55 years and older.
However, it should be noted that for the most part, the magnitude of the effect of these modifiers was relatively low. Nonetheless, the significance of the interaction terms along with smaller deviances for the models that include the interaction terms as compared with the reduced model (Model 1) is a reasonable indication that these factors synergistically interact with age to affect walking speed beyond their individual effects.
DISCUSSION
In this cohort of Sardinians aged 18 years and older, we found that correlates of walking speed previously reported for older individuals exert their effects at younger ages as well. Beyond the traditional correlates of walking speed, we found a clear predictive association between personality characteristics and walking speed, a relationship little explored previously. Our study is also one of the few to explore effect modifiers of the age–walking speed association in a wide-aged population. For example, Callisaya, Blizzard, Schmidt, McGinley, and Srikanth (2008) reported that in older individuals, the patterns of walking speed with higher age differs by sex, being linear in men but curvilinear in women. We found that women and men differ in terms of patterns of walking speed with higher ages not only in late life but also at younger ages. Our findings also suggest that education influences walking speed in both young and old adulthood, whereas other factors such as waist circumference, subjective health, and personality appear to be important only early in life.
Most studies of walking speed conducted in older samples have described a linear pattern of slower walking speed with increasing age (Escalante, Lichtenstein, & Hazuda, 2001), patterns that differ by sex (Callisaya et al., 2008). In line with other studies encompassing a broader age range (Bohannon, 1997), we found a curvilinear association between walking speed and age, the pattern of slower walking speed with higher age being more pronounced in old age. Age 55 as the inflection is in line with some reports (Bohannon, Andrews, & Thomas, 1996) but about 10 years sooner than in another (Himann et al., 1988).
Several correlates of walking speed were identified in this study. Taller stature probably because of longer strides (Escalante et al., 2001) and better subjective health, which might reflect fewer underlying medical conditions (Lord & Menz, 2002), were positive correlates in both sex groups. Additionally, men who were married or had higher handgrip strength, used in our study as a proxy for muscle strength (Rantanen, Era, & Heikkinen, 1994), and women who had smaller waist circumference, had no other major comorbidities, or had lower PWV (log) walked faster. The association with marital status might reflect the propensity of married men to engage in health-promoting behaviors such as good nutritional health (Locher et al., 2005) and higher leisure-time physical activity (Hughes, McDowell, & Brody, 2008), with a positive effect on their walking speed. The observed relationship between handgrip strength and walking speed, which echoes other reports on walking performance (Bootsma-van der Wiel et al., 2002), was curvilinear, suggesting that after a certain threshold, additional increases in hand strength are not associated with further increases in walking speed (Buchner, Larson, Wagner, Koepsell, & de Lateur, 1996). However, we could not demonstrate a significant departure from linearity in the relationship between strength and walking speed in women (Figure 1b), explained by the fact that very few women in our study had very high handgrip strength.
The association between comorbidity and walking speed, consistent with other reports (Cesari et al., 2006), can be explained via impairment of different types such as poor muscle strength or impaired circulation or cardiovascular function (Verbrugge & Jette, 1994). Increased arterial stiffness might be related to slower walking speed via microvascular and conduit artery dysfunction (Malik, Kondragunta, & Kullo, 2008) and other consequences of increased arterial stiffness such as increased systolic blood pressure and reduced diastolic blood pressure (Amoh-Tonto, Malik, Kondragunta, Ali & Kullo, 2009), which may have a negative effect on exercise capacity. However, it could also be that lower walking speed might indicate poor health status leading to decreased physical activity and, subsequently, arterial stiffness. Higher PWV had been previously associated with reduced walking distance among patients with suspected peripheral artery disease (Amoh-Tonto et al., 2009).
Our findings of an independent effect of personality traits on walking speed are particularly interesting. Despite evidence that personality is associated with certain chronic diseases (e.g., cardiovascular disease, depression) and with cause-specific and all-cause mortality, the association of personality and physical function has not been fully investigated (Kendler, Gatz, Gardner, & Pedersen, 2006; Shekelle, Gale, Ostfeld, & Paul, 1983; Shipley, Weiss, Der, Taylor, & Deary, 2007). In this study, walking speed was positively correlated with extraversion and openness in women and agreeableness in men. Thus, openness, a personality trait that predisposes to intellectual curiosity and availability to new experiences, may protect against physical decline and possibly through the same causal pathway against mortality (Iwasa et al., 2008), at least among women. More open individuals are probably inclined to try new types of exercises, which might promote continued engagement in physical activities, with its beneficial effect on health and physical function as reflected by walking speed. Moreover, the positive association between extraversion and walking speed might be explained by a tendency toward support-seeking behavior (Amirkhan, Risinger, & Swickert, 1995) and lower risk of depression (Kendler et al.) and disability (Krueger et al., 2006).
The finding of a negative effect of agreeableness among men was surprising. Most reports on agreeableness and health are negative, although straight forwardness (a facet of agreeableness) was found modestly associated with reduced total mortality (Weiss & Costa, 2005). Agreeable individuals tend to be trustful, altruistic, compliant, and tender minded, and to have a lower risk for cardiovascular disease (Costa, Stone, McCrae, Dembroski, & Williams, 1987). In the context of walking speed, we found agreeableness to indicate slowness and antagonism (low agreeableness) fastness, probably a reflection of the former's general mildness and compliance and the latter's rigidity and opposition.
Our findings also add to the literature by identifying some of the factors that influence the age–walking speed relationship. Identification of these moderators is very important as it could lead to therapies targeted at reducing the age-related decline in physical function and overall health. Our study highlights several potential moderators of this age-related decline in physical function as being social, behavioral, and psychological in nature. Although future longitudinal studies will be needed to confirm these associations, our findings suggest that extraverted women and men who are open, think they are in good health, and have lower waist circumferences might experience a slower decline in walking speed as they grow older. However, these effects appear to operate primarily in early life. It is possible that these factors are important correlates of behavior in healthy persons and contribute to build their functional reserve. However, later on, their effect may diminish and they cannot substantially counteract the effect of aging. The only exception was education, which significantly influenced the age–walking speed association both among women younger than 55 and among those aged 55 years and older.
Our results should be considered with caution in light of study limitations. First of all, the risk of making Type I errors was increased due to the multiple comparisons involved. We partially overcame this problem by selecting a significance p level of .002, which took into account the total number of correlates being tested at the bivariate level. However, in the moderation analyses, because there was no prior indication from the literature as to what factors might moderate the age–walking speed association (except for sex), we wanted to test all the significant correlates as potential moderators. We felt that by applying a corrective methodology (i.e., Bonferroni correction), the likelihood of making Type II errors would have been excessively increased. As other authors suggested, taking into account the infancy of this field of research, avoiding missing a true effect is important (Goodwin & Friedman, 2006). Second, we did not have information to account for secular trends in factors likely to affect walking speed such as changes in lifestyle, occupation, health care, and access to care. Third, the cross-sectional design does not allow inferences on causality and even temporality in regard to walking speed and its correlates. Slower walking speed per decade was based on mean differences between individuals at different ages and does not reflect differences within the same individual over time. Fourth, we might have been limited in our ability to capture the importance of physical activity on walking speed by the way walking and moderate activity were measured in the SardiNIA study. Participants were asked whether they walked for at least 10 min during the past week, and the moderate activity was limited to activities done at work during the previous week. Finally, although our study results suggest that personality might be part of the causal pathway by which walking speed is such a good marker of health status, further work is necessary to fully explain the specific mechanisms that link walking speed, personality, and health status/adverse health outcomes. In spite of these limitations, our study has the advantage of a large cohort of individuals aged 18 years and older and also of comprehensive assessment of the different factors hypothesized to influence walking speed.
In conclusion, our findings suggest that slower walking speed with higher age is not limited to late life but can also be seen at much younger ages and that the magnitude of this association may be affected by different factors in different periods of the life span. The results of a strong association between personality and walking speed and its effect on the age-related patterns support its proposed moderating effect on the disablement process (Verbrugge & Jette, 1994) particularly early in life. Our findings also have significance from a clinical perspective. They suggest that personality has an impact on physical function as indicated by walking speed even before usual declines become evident. Women with low extraversion and men with low openness should be made aware of their increased risk early on in life, such that steps can be taken to prevent or alleviate their risk of decline. Although we did not find an effect of personality on the association between age and walking speed in late life, individuals with high scores continued to walk faster, probably a residual of their earlier advantage. Thus, if measures are taken early in their adulthood to prevent future risk, these at-risk individuals might be able to achieve higher levels of walking speed in later life. However, whether health behaviors represent the link between personality and physical performance, and disability and other negative health outcomes remain to be determined. Moreover, longitudinal studies should provide a more informative analysis of the effects of personality domains and their facets on function loss.
FUNDING
This research and the SardiNIA team were supported (in part) by the Intramural Research Program of the National Institutes of Health, National Institute on Aging (contract N01-AG-1-2109). P.T.C. receives royalties from the Revised NEO Personality Inventory.
CONFLICT OF INTEREST
The sponsor had no role in either of the following: design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation and review of the manuscript.
Acknowledgments
All authors approved the submitted manuscript and have participated sufficiently in the research report to take public responsibility (E.M.S., S.S.N., D.S., and A.T. for part of the content and the rest of the coauthors for the whole content). M.I.T., L.F., P.T.C., A.T., and D.S. were involved in conception and design of the study; P.T.C., A.T., A.S., M.O., B.D., M.M., M.U., and S.S.N. in acquisition of data; and M.I.T., L.F., P.T.C., A.T., E.M.S., and S.S.N. in analysis and interpretation of data. M.I.T. drafted the manuscript; all the other authors critically revised the manuscript for important intellectual content. M.I.T., L.F., P.T.C., and A.T. were involved in statistical analysis; D.S. and M.U. obtained funding; L.F., E.M.S., and S.S.N. provided administrative, technical, or material support; and L.F. provided supervision of the research report.
References
- Amirkhan JH, Risinger RT, Swickert RJ. Extraversion: A “hidden” personality factor in coping? Journal of Personality. 1995;63:189–212. doi: 10.1111/j.1467-6494.1995.tb00807.x. [DOI] [PubMed] [Google Scholar]
- Amoh-Tonto CA, Malik AR, Kondragunta V, Ali Z, Kullo IJ. Brachial-ankle pulse wave velocity is associated with walking distance in patients referred for peripheral arterial disease evaluation. Atherosclerosis. 2009;206:173–178. doi: 10.1016/j.atherosclerosis.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogg T, Roberts BW. Conscientiousness and health-related behaviors: A meta-analysis of the leading behavioral contributors to mortality. Psychological Bulletin. 2004;130:887–919. doi: 10.1037/0033-2909.130.6.887. [DOI] [PubMed] [Google Scholar]
- Bohannon RW. Comfortable and maximum walking speed of adults aged 20-79 years: Reference values and determinants. Age and Ageing. 1997;26:15–19. doi: 10.1093/ageing/26.1.15. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, Andrews AW, Thomas MW. Walking speed: Reference values and correlates for older adults. Journal of Orthopaedic and Sports Physical Therapy. 1996;24:86–90. doi: 10.2519/jospt.1996.24.2.86. [DOI] [PubMed] [Google Scholar]
- Booth-Kewley S, Vickers RR., Jr. Associations between major domains of personality and health behavior. Journal of Personality. 1994;62:281–298. doi: 10.1111/j.1467-6494.1994.tb00298.x. [DOI] [PubMed] [Google Scholar]
- Bootsma-van der Wiel A, Gussekloo J, De Craen AJ, Van Exel E, Bloem BR, Westendorp RG. Common chronic diseases and general impairments as determinants of walking disability in the oldest-old population. Journal of the American Geriatrics Society. 2002;50:1405–1410. doi: 10.1046/j.1532-5415.2002.50363.x. [DOI] [PubMed] [Google Scholar]
- Brach JS, FitzGerald S, Newman AB, Kelsey S, Kuller L, VanSwearingen JM, Kriska AM. Physical activity and functional status in community-dwelling older women: a 14-year prospective study. Archives of Internal Medicine. 2003;163:2565–2571. doi: 10.1001/archinte.163.21.2565. [DOI] [PubMed] [Google Scholar]
- Buchner DM, Larson EB, Wagner EH, Koepsell TD, de Lateur BJ. Evidence for a non-linear relationship between leg strength and gait speed. Age and Ageing. 1996;25:386–391. doi: 10.1093/ageing/25.5.386. [DOI] [PubMed] [Google Scholar]
- Callisaya ML, Blizzard L, Schmidt MD, McGinley JL, Srikanth VK. Sex modifies the relationship between age and gait: A population-based study of older adults. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2008;63:165–170. doi: 10.1093/gerona/63.2.165. [DOI] [PubMed] [Google Scholar]
- Cesari M, Kritchevsky SB, Penninx BW, Nicklas BJ, Simonsick EM, Newman AB, Tylavsky FA, Brach JS, Satterfield S, Bauer DC, et al. Prognostic value of usual gait speed in well-functioning older people–results from the Health, Aging and Body Composition Study. Journal of the American Geriatrics Society. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- Cesari M, Onder G, Russo A, Zamboni V, Barillaro C, Ferrucci L, Pahor M, Bernabei R, Landi F. Comorbidity and physical function: results from the aging and longevity study in the Sirente geographic area (ilSIRENTE study) Gerontology. 2006;52:24–32. doi: 10.1159/000089822. [DOI] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Pahor M, Lauretani F, Corsi AM, Rhys Williams G, Guralnik JM, Ferrucci L. Inflammatory markers and physical performance in older persons: the InCHIANTI study. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2004;59:242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- Chou LS, Draganich LF. Stepping over an obstacle increases the motions and moments of the joints of the trailing limb in young adults. Journal of Biomechanics. 1997;30:331–337. doi: 10.1016/s0021-9290(96)00161-3. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FF) Professional Manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Costa PT, Stone SV, McCrae RR, Dembroski TM, Williams RB., Jr. Hostility, agreeableness-antagonism, and coronary heart disease. Holistic Medicine. 1987;2:161–167. [Google Scholar]
- Donovan JE, Jessor R, Costa FM. Adolescent health behavior and conventionality-unconventionality: An extension of problem-behavior theory. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association. 1991;10:52–61. [PubMed] [Google Scholar]
- Escalante A, Lichtenstein MJ, Hazuda HP. Walking velocity in aged persons: Its association with lower extremity joint range of motion. Arthritis and Rheumatism. 2001;45:287–294. doi: 10.1002/1529-0131(200106)45:3<287::AID-ART262>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ, Grossarth-Maticek R, Everitt B. Personality, stress, smoking, and genetic predisposition as synergistic risk factors for cancer and coronary heart disease. Integrative Physiological and Behavioral Science: The Official Journal of the Pavlovian Society. 1991;26:309–322. doi: 10.1007/BF02691067. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, Guralnik JM. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. Journal of the American Geriatrics Society. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Guralnik JM, Salive ME, Fried LP, Bandeen-Roche K, Brock DB, Simonsick EM, Corti MC, Zeger SL. Effect of age and severity of disability on short-term variation in walking speed: the Women's Health and Aging Study. Journal of Clinical Epidemiology. 1996;49:1089–1096. doi: 10.1016/0895-4356(96)00231-4. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ. Serum IL-6 level and the development of disability in older persons. Journal of the American Geriatrics Society. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fried LP. Conference on the physiologic basis of frailty. April 28, 1992, Baltimore, Maryland. Introduction. Aging. 1992;4:251–252. [PubMed] [Google Scholar]
- Friedman HS. Long-term relations of personality and health: Dynamisms, mechanisms, tropisms. Journal of Personality. 2000;68:1089–1107. doi: 10.1111/1467-6494.00127. [DOI] [PubMed] [Google Scholar]
- Gill TM, Williams CS, Tinetti ME. Assessing risk for the onset of functional dependence among older adults: The role of physical performance. Journal of the American Geriatrics Society. 1995;43:603–609. doi: 10.1111/j.1532-5415.1995.tb07192.x. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Friedman HS. Health status and the five-factor personality traits in a nationally representative sample. Journal of Health Psychology. 2006;11:643–654. doi: 10.1177/1359105306066610. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journal of Gerontology. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- Himann JE, Cunningham DA, Rechnitzer PA, Paterson DH. Age-related changes in speed of walking. Medicine and Science in Sports and Exercise. 1988;20:161–166. doi: 10.1249/00005768-198820020-00010. [DOI] [PubMed] [Google Scholar]
- Hughes JP, McDowell MA, Brody DJ. Leisure-time physical activity among US adults 60 or more years of age: Results from NHANES 1999–2004. Journal of Physical Activity and Health. 2008;5:347–358. doi: 10.1123/jpah.5.3.347. [DOI] [PubMed] [Google Scholar]
- Iwasa H, Masui Y, Gondo Y, Inagaki H, Kawaai C, Suzuki T. Personality and all-cause mortality among older adults dwelling in a Japanese community: A five-year population-based prospective cohort study. American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry. 2008 doi: 10.1097/JGP.0b013e3181662ac9. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. Personality and major depression: A Swedish longitudinal, population-based twin study. Archives of General Psychiatry. 2006;63:1113–1120. doi: 10.1001/archpsyc.63.10.1113. [DOI] [PubMed] [Google Scholar]
- Krueger KR, Wilson RS, Shah RC, Tang Y, Bennett DA. Personality and incident disability in older persons. Age and Ageing. 2006;35:428–433. doi: 10.1093/ageing/afl028. [DOI] [PubMed] [Google Scholar]
- Locher JL, Ritchie CS, Roth DL, Baker PS, Bodner EV, Allman RM. Social isolation, support, and capital and nutritional risk in an older sample: Ethnic and gender differences. Social Science & Medicine. 2005;60:747–761. doi: 10.1016/j.socscimed.2004.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord SR, Menz HB. Physiologic, psychologic, and health predictors of 6-minute walk performance in older people. Archives of Physical Medicine and Rehabilitation. 2002;83:907–911. doi: 10.1053/apmr.2002.33227. [DOI] [PubMed] [Google Scholar]
- Malik AR, Kondragunta V, Kullo IJ. Forearm vascular reactivity and arterial stiffness in asymptomatic adults from the community. Hypertension. 2008;51:1512–1518. doi: 10.1161/HYPERTENSIONAHA.107.106088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Prather AA, Petersen KL, Cohen S, Manuck SB. Antagonistic characteristics are positively associated with inflammatory markers independently of trait negative emotionality. Brain, Behavior, and Immunity. 2008;22:753–761. doi: 10.1016/j.bbi.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae RR, Costa PT., Jr. Validation of the five-factor model of personality across instruments and observers. Journal of Personality and Social Psychology. 1987;52:81–90. doi: 10.1037//0022-3514.52.1.81. [DOI] [PubMed] [Google Scholar]
- Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA: the Journal of the American Medical Association. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- Ostir GV, Markides KS, Black SA, Goodwin JS. Emotional well-being predicts subsequent functional independence and survival. Journal of the American Geriatrics Society. 2000;48:473–478. doi: 10.1111/j.1532-5415.2000.tb04991.x. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Guralnik JM, Simonsick EM, Kasper JD, Ferrucci L, Fried LP. Emotional vitality among disabled older women: The Women's Health and Aging Study. Journal of the American Geriatrics Society. 1998;46:807–815. doi: 10.1111/j.1532-5415.1998.tb02712.x. [DOI] [PubMed] [Google Scholar]
- Pilia G, Chen WM, Scuteri A, Orru M, Albai G, Dei M, Lai S, Usala G, Lai M, Loi P, et al. Heritability of cardiovascular and personality traits in 6,148 Sardinians. PLoS Genetics. 2006;2:e132. doi: 10.1371/journal.pgen.0020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantanen T, Era P, Heikkinen E. Maximal isometric strength and mobility among 75-year-old men and women. Age and Ageing. 1994;23:132–137. doi: 10.1093/ageing/23.2.132. [DOI] [PubMed] [Google Scholar]
- Rolland YM, Cesari M, Miller ME, Penninx BW, Atkinson HH, Pahor M. Reliability of the 400-m usual-pace walk test as an assessment of mobility limitation in older adults. Journal of the American Geriatrics Society. 2004;52:972–976. doi: 10.1111/j.1532-5415.2004.52267.x. [DOI] [PubMed] [Google Scholar]
- Shekelle RB, Gale M, Ostfeld AM, Paul O. Hostility, risk of coronary heart disease, and mortality. Psychosomatic Medicine. 1983;45:109–114. doi: 10.1097/00006842-198305000-00003. [DOI] [PubMed] [Google Scholar]
- Shipley BA, Weiss A, Der G, Taylor MD, Deary IJ. Neuroticism, extraversion, and mortality in the UK Health and Lifestyle Survey: A 21-year prospective cohort study. Psychosomatic Medicine. 2007;69:923–931. doi: 10.1097/PSY.0b013e31815abf83. [DOI] [PubMed] [Google Scholar]
- Simonsick EM, Kasper JD, Phillips CL. Physical disability and social interaction: Factors associated with low social contact and home confinement in disabled older women (the Women's Health and Aging Study) Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 1998;53:S209–S217. doi: 10.1093/geronb/53b.4.s209. [DOI] [PubMed] [Google Scholar]
- Terracciano A. The Italian version of the NEO PI-R: Conceptual and empirical support for the use of targeted rotation. Personality and Individual Differences. 2003;35:1859–1872. doi: 10.1016/S0191-8869(03)00035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugge LM, Jette AM. The disablement process. Social Science & Medicine. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Volpato S, Ble A, Metter EJ, Lauretani F, Bandinelli S, Zuliani G, Fellin R, Ferrucci L, Guralnik JM. High-density lipoprotein cholesterol and objective measures of lower extremity performance in older nondisabled persons: the InChianti study. Journal of the American Geriatrics Society. 2008;56:621–629. doi: 10.1111/j.1532-5415.2007.01608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A, Costa PT., Jr. Domain and facet personality predictors of all-cause mortality among Medicare patients aged 65 to 100. Psychosomatic Medicine. 2005;67:724–733. doi: 10.1097/01.psy.0000181272.58103.18. [DOI] [PubMed] [Google Scholar]