Abstract
Stress and drug-associated cues can trigger craving and relapse in abstinent drug-dependent individuals. Although the role of these two critical factors in relapse has been extensively studied, the interaction between stress and drug-associated cues in relapse has been less well characterized. Using an animal model of relapse, we assessed the effects of the pharmacological stressor, yohimbine (1.25 or 2.5 mg/kg), on reinstatement of extinguished heroin-seeking in rats either in the presence or absence of heroin-associated cues. Yohimbine, in the absence of heroin-associated cues, and cues by themselves reliably reinstated heroin-seeking over extinction levels. Notably, animals showed significantly potentiated responding when yohimbine preceded cue-induced reinstatement (3 to 4x higher over cues or yohimbine alone). These results demonstrate that exposure to heroin-paired cues during yohimbine-induced stress greatly potentiates heroin-seeking, and support the simultaneous targeting of both stress and cue activation during relapse intervention.
Keywords: cue, heroin, reinstatement, relapse, self-administration, stress, yohimbine
1. Introduction
Opioid dependence is a chronic relapsing disease associated with significant physical and mental health problems, including infectious diseases (e.g., hepatitis, human immunodeficiency virus, tuberculosis), high mortality rates (e.g., 10 per 1,000 untreated persons), depression and anxiety, legal problems, and unemployment [1,2]. The use of opioids has increased substantially since the 1990s and continues to rise [3,4]. While many patients can complete detoxification and abstain from opioid use for short periods of time, relapse is extremely common. One study involving 242 opiate-dependent patients found that 34% relapsed within 3 days, 45% within 7 days, and 57% within 30 days [5]. A better understanding of factors that provoke opiate relapse is critical for the development of effective prevention and treatment interventions.
One particularly salient feature that occurs during abstinence from drug use is the ability of drug-associated environmental cues (e.g., drug paraphernalia) to elicit drug craving and subsequent relapse [6,7,8]. Conditioned drug cues, therefore, play a critical role in ongoing drug-seeking behavior and relapse, even after significant periods of abstinence. In addition to drug cues, most major theories of addiction postulate that stress, both acute and chronic, plays an important role in relapse [9,10,11]. Research using rodent models of addiction and relapse demonstrates that exposure to certain types of stressors (e.g., footshock, food deprivation, and social stress) increase drug-seeking and drug-taking behaviors [12,13,14,15,16]. Furthermore, studies have demonstrated that exposure to pharmacological stressors, particularly the norepinephrine (NE) α2 receptor antagonist yohimbine, increases stress and anxiety and provokes craving in drug-dependent humans [17], as well as drug-seeking in animals with a history of chronic drug self-administration [18,19,20,21].
Although drug-paired cues and stress have been independently studied for their ability to prompt drug-seeking behavior in preclinical and clinical studies, little attention has been given to the interaction of cues and stress in relapse. Only a few animal studies [18,20,22,23], and no clinical studies to our knowledge, have explicitly evaluated the interactive effects of drug cues and stress on relapse. This question is of importance since the combination of drug-related cues and stress is likely involved in most human relapse situations. Liu and Weiss [22] reported that footshock stress and conditioned drug cues (i.e., a light previously paired with ethanol) interacted to augment the resumption of ethanol-seeking following extinction. Rats exposed to both footshock stress and ethanol cues, as compared to rats exposed to either stress or cues, demonstrated approximately twice as many lever presses and the response rate was sustained for a longer period of time. More recently, we examined the interactive effects of yohimbine-induced stress and drug-associated cues on the reinstatement of cocaine-seeking in rats [18]. When cocaine cues were preceded by yohimbine pretreatment, a significant potentiation (i.e., responding at 3–5 times greater over cues or yohimbine alone) of cocaine-seeking was observed.
In order to further assess the nature of stress-cue interactions in relapse to opiate-seeking, the current study utilized a model of heroin self-administration, extinction, and reinstatement [24,25] to examine the separate and interactive effects of previously heroin-paired stimuli and yohimbine-induced stress. Based on previous findings with cocaine [18], we hypothesized that yohimbine-induced stress would significantly potentiate reinstatement of heroin-seeking in the presence of discrete conditioned cues.
2. Materials and methods
2.1. Subjects
Male, Sprague Dawley rats (final n=22) weighing 270–366 g at the beginning of the study (Charles River, Wilmington, MA, USA) served as subjects. Rats were housed individually in a temperature- and humidity-controlled vivarium on a reverse 12h-12h light-dark cycle (lights on at 0700). Subjects were handled and allowed to acclimate to their surroundings for a minimum of 7 days prior to catheter implantation. Water and standard rat chow were available ad libitum in home cages, except during initial lever press training (2–3 days). During this period, food was restricted to 20–25 g per day and was provided immediately following experimental sessions. Animals were housed and all procedures were conducted in an AAALAC accredited facility, and housing and care of the rats were carried out in accordance with the guidelines of the Institute of Laboratory Animal Resources on Life Sciences, National Research Council.
2.2. Apparatus
All procedures were carried out in standard operant chambers (30×20×20 cm), which were enclosed in sound-attenuating cubicles and connected to a computerized data collection program (MED-PC, Med Associates, Inc., St. Albans, VT, USA). Two retractable levers, two white stimulus lights (one above each lever), and a tone generator (ENV-223HAM, Med Associates), were located on one panel of the chamber. A house light located on the opposite panel provided illumination throughout each test session.
2.3. Surgery
Approximately one week after arrival, jugular catheters were surgically implanted in each rat. Subjects were anesthetized using a mixture of ketamine hydrochloride and xylazine (66 and 1.33 mg/kg, respectively, IP) followed by equithesin (0.5 ml/kg with a solution of 9.72 mg/ml pentobarbital sodium, 42.5 mg/ml chloral hydrate, and 21.3 mg/ml magnesium sulfate heptahydrate dissolved in a 44% propylene glycol, 10% ethanol solution, IP). Surgical procedures were conducted using aseptic techniques. Catheters were constructed using previously described methods [24] and consisted of external guide cannulae with screw-type connectors (Plastics One Inc., Roanoke, VA, USA), Silastic tubing (10 cm; i.d. = 0.64 mm; o.d. = 1.19 mm; Dow Corning, Midland, MI, USA), prolite polypropylene monofilament mesh (2 cm diameter, Atrium Medical Corporation, Hudson, NH, USA), and cranioplastic cement. The end of the catheter was inserted into the right jugular vein and secured to surrounding tissue with sutures. The catheter ran subcutaneously and exited on the rat’s back, posterior to the shoulder blades. On the 4 days following surgery, catheters were flushed once per day with 0.1 ml each of an antibiotic solution of cefazolin (10.0 mg/ml; Schein Pharmaceuticals, Florham Park, NJ, USA) dissolved in heparinized saline (70 U/ml; Elkins-Sinn, Cherry Hill, NJ, USA) and heparinized saline in order to maintain patency. During the remainder of the study, subjects received 0.1 ml of heparinized saline (10 U/ml) immediately prior to self-administration sessions and the cefazolin and 70 U/ml heparinized saline regimen immediately after each session. On the day immediately preceding initiation of self-administration and occasionally thereafter, rats received a 0.1 ml infusion of methohexital sodium (10.0 mg/ml IV; Eli Lilly and Co., Indianapolis, IN, USA). Intravenous administration of this short-acting barbiturate produces a rapid loss of muscle tone, which is indicative of catheter patency.
2.4. Heroin self-administration
Five to seven days after surgery, rats began self-administration of heroin (diacetylmorphine HCl, provided by the National Institute on Drug Abuse, Research Triangle Park, NC). Using previously established methods [24,26], rats self-administered heroin on a fixed ratio 1 (FR1) schedule of reinforcement at a dose of 25 µg/50 µl/infusion. At the start of each 3-h session, the catheter was connected to a liquid swivel (Instech, Plymouth Meeting, PA) via polyethylene 20 tubing encased in steel spring leashes (Plastics One, Inc.). The swivels were suspended above the operant conditioning chamber and were connected to infusion pumps (model PHM-100, Med-Associates). Active lever responses resulted in a 2 s activation of the infusion pump and a 5 s presentation of a conditioned stimulus complex (CS), which consisted of a cue light above the active lever and a tone (78 dB, 4.5 kHz). Following each infusion, responding on the active lever had no consequences during a 20 s time-out period. Inactive lever presses also had no consequences, but were recorded. Self-administration sessions were conducted 6 days per week and continued until a minimum of 10 infusions had been earned on each of 12 days.
2.5. Extinction and reinstatement testing
Following the self-administration period, subjects began daily extinction sessions during which responding on the active lever had no programmed consequences (i.e., no heroin infusions or CS presentations). Extinction sessions were conducted for a minimum of 10 days or until extinction criteria were met (i.e., the number of responses made during 3-h sessions may not have exceeded 25% of the average number of responses made during the last 3 days of heroin self-administration on two consecutive days).
Reinstatement testing was conducted using a within subjects experimental design at the end of the extinction period. Thirty minutes prior to each session, subjects received an injection of either saline or yohimbine (1.25 or 2.5 mg/kg, i.p.). Sessions were further designated as either extinction or cue tests. Conditions during the former were identical to those during the extinction period described above. Responding during cue tests resulted in a 5-s presentation of the CS that previously accompanied heroin infusions. A 20-s timeout followed CS termination, during which time responding was recorded, but had no programmed consequences. Each subject was tested under all combinations of dose and session type, yielding six reinstatement tests per rat. Order of testing was randomized with the following constraints: (1) each yohimbine dose was administered exactly once within the first three tests and (2) the schedule for each animal could contain no more than two consecutive cue or extinction tests. Reinstatement tests were separated by at least two days of extinction sessions or until extinction criteria were met.
2.6. Data analysis
Data were analyzed using repeated measures analysis of variance (RM-ANOVA) and by paired comparisons for determination of changes in responding over extinction baseline. Post hoc comparisons were conducted using the Tukey HSD and an overall α of 0.05.
3. Results
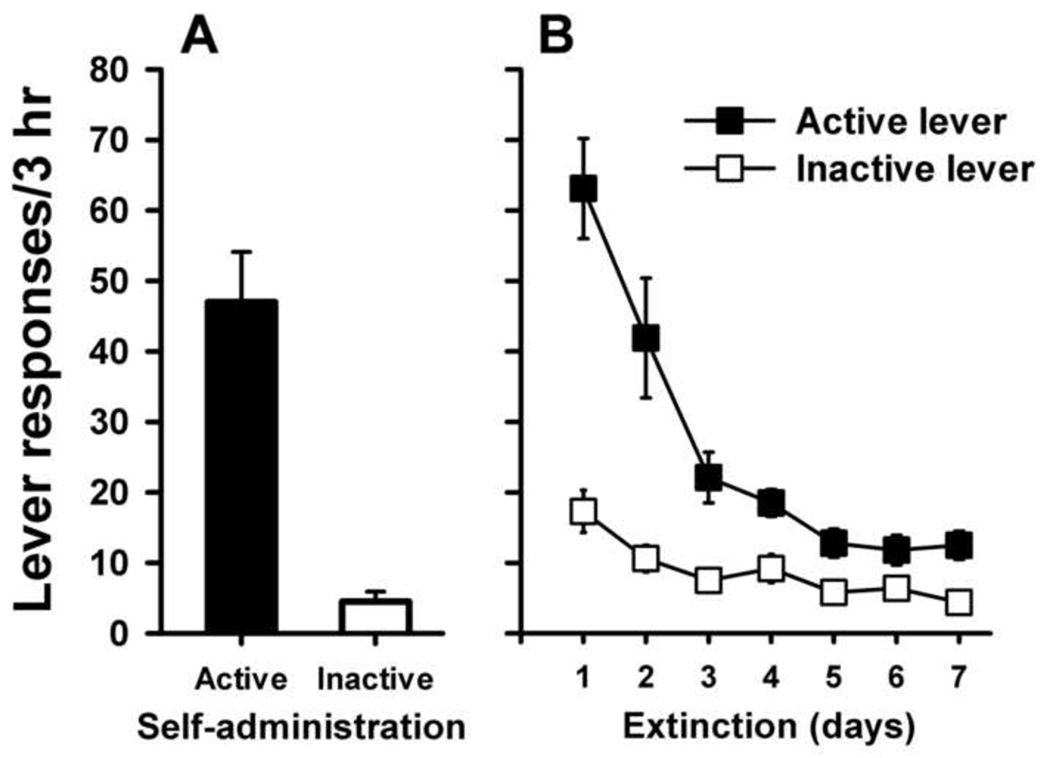
Figure 1 shows the number of responses on the active and inactive levers averaged over the last three days of heroin self-administration (Panel A). Subjects exhibited stable and preferential responding on the active (heroin-paired) lever. Heroin intake over the last three days showed an average of 1.22 ± 0.08 mg/kg/session. Responding on the active lever decreased rapidly following termination of the heroin reinforcer (Panel B). RM-ANOVA showed a significant decrease over time for active lever responding (F(6,21) = 22.18, p < 0.001). Inactive lever responding showed a typical pattern of an initial increase during extinction that likely reflects increased drug-seeking on the alternative lever in the absence of the reinforcer. Responding on this lever also decreased significantly over time across extinction trials (F(6,21) = 9.96, p < 0.001).
Fig. 1.
Average active and inactive lever responses (mean ± SEM) per session over the last 3 days of heroin self-administration (Panel A) and during the first 7 days of extinction (Panel B).
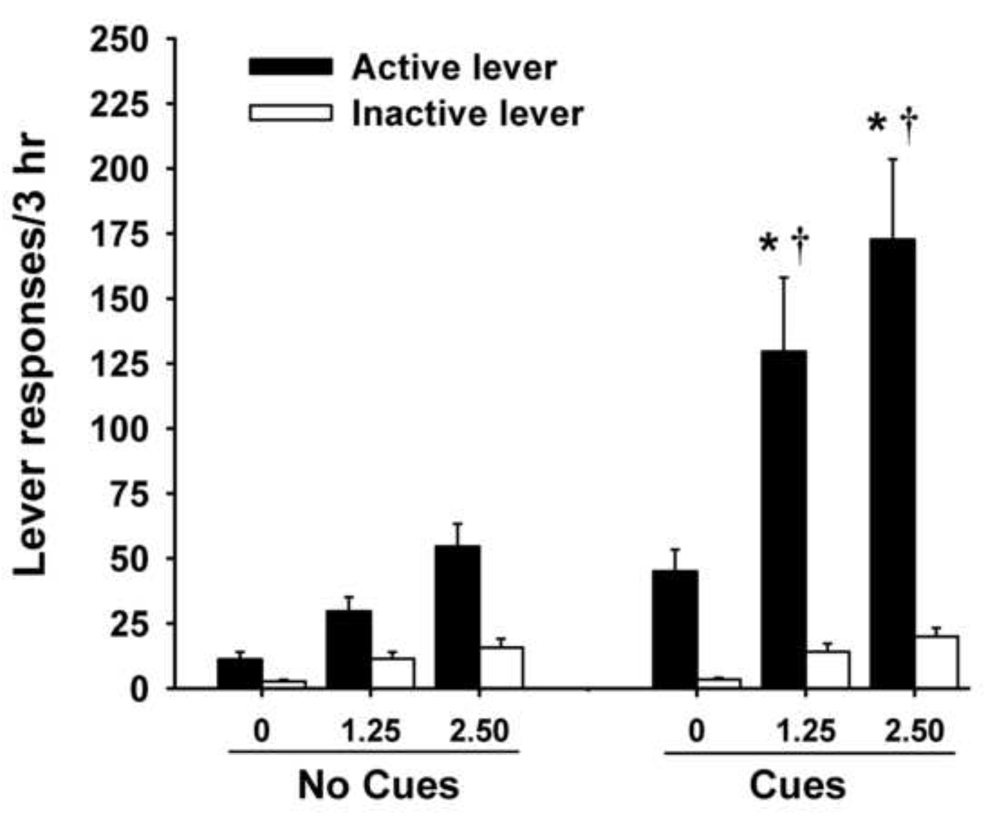
Figure 2 illustrates responding during the reinstatement test trials. Both yohimbine-stress alone and previously heroin-paired cues alone produced significant reinstatement of heroin-seeking over extinction baseline responding (ps < 0.05). RM-ANOVA for active lever presses revealed main effects for session type (F(1,21) = 27.19, p < 0.001) and dose (F(1,21) = 13.23, p < 0.001), and a significant session × dose interaction (F(2,42) = 4.57, p < 0.05). Post hoc tests indicated that yohimbine-stress induced responding during cue tests was significantly elevated over yohimbine alone for both the 1.25 and 2.5 mg/kg doses (ps < 0.05). While there was a dose dependent trend, responding did not significantly differ between the two different yohimbine doses in either condition (no cues and cues present). Furthermore, cue-induced reinstatement of heroin-seeking during yohimbine-induced stress showed significant potentiation (3 – 4x) over cues alone (ps < 0.05). Analysis of inactive lever responding during reinstatement trials showed only a main effect of dose (F(1,21) = 22.4, p < 0.001), but not for session type (F(1,21) = 2.09, p = 0.16), or for the session × dose interaction (F(2,42) = 0.40, p = 0.68).
Fig. 2.
Lever responding during reinstatement tests (mean ± SEM) on active and inactive levers. Test sessions occurred after yohimbine pretreatment (0, 1.25, or 2.50 mg/kg) in the absence (No Cues) or presence (Cues) of previously paired heroin cues. Significant differences from the No Cues condition (*p < 0.05) and Cues alone (†p < 0.05) are indicated.
4. Discussion
The current study examined the separate and interactive effects of heroin-associated cues and yohimbine-induced stress on the reinstatement of extinguished heroin-seeking. The findings demonstrate that heroin-associated cues or yohimbine-induced stress alone significantly augmented reinstatement of heroin-seeking over extinction. Furthermore, when heroin-associated cues were preceded by yohimbine, resumption to heroin-seeking was potentiated 3 to 4 times over cues alone. Thus, while both cues and stress were independent relapse-promoting factors, their combined presentation generated substantial enhancement of reinstated heroin-seeking.
The relapse-promoting properties of conditioned environmental cues and stress have been extensively studied separately; however, only five studies to our knowledge have previously investigated the combined ability of stress and cues to induce reinstatement of drug-seeking in animal models of relapse. Similar to the current findings, previous investigations using other drugs of abuse found that stress augments the effects of conditioned drug cues on drug-seeking behavior. In a study of the interactive effects of alcohol conditioned cues and footshock-induced stress in rats, Liu and Weiss [22] demonstrated that the combination of stress and cues produced levels of reinstatement that were twice as high as cues or stress alone, with sustained responding to combined cues and stress lasting longer than either modality alone. Lee and colleagues [20] showed in squirrel monkeys that response rates for cocaine-taking were higher when drug-associated cues and yohimbine were combined. In another study in monkeys with a history of cocaine self-administration, spiradoline, a kappa agonist that produces stress, potentiated responding to a cocaine-paired stimulus [27]. Shelton et al. [23] found that while footshock stress alone in rats failed to reinstate responding, the combination of footshock and conditioned cues resulted in significant reinstatement of cocaine-seeking. Finally, Feltenstein and See [18] showed that conditioned cocaine cues combined with yohimbine in rats produced reinstatement responding that was 10 to 13 times higher than extinction levels and 3 to 5 times higher than cues or yohimbine alone. Taken together, these prior studies and our current findings provide evidence of a consistent additive effect of stress and drug-associated cues across a variety of abused substances (i.e., alcohol, cocaine, heroin) and across different types of stressors (i.e., pharmacologic and footshock).
In the current study, we used the α2–noradrenergic receptor antagonist yohimbine to induce stress for several reasons. The use of a pharmacological stressor has some obvious limitations, particularly in that it does not fully encompass the impact of a typical external stressor, such as social stress. However, from an experimental perspective, pharmacologically-induced stress offers several advantages. First, yohimbine has been shown in previous animal models to reliably induce stress in subjects with a history of chronic self-administration of several different abused drugs [18,19,20,21]. In comparison to footshock, which has generally been employed in animal models of stress-induced relapse [10,28], yohimbine also provides the advantages of a prolonged half-life (7–8 hr) that ensures sustained overlap between stress activation and cue exposure. In addition, yohimbine’s effects on drug-seeking are not limited by several factors that constrain the use of footshock, including the inconsistent nature of footshock on reinstatement [23], the variant level of shock needed across subjects [29,30], and the need for specific procedural approaches for footshock administration (e.g., footshock must be administered within the drug-taking context) [10]. Finally, yohimbine can be reliably employed in parallel studies of stress activation of drug desire in humans and drug-seeking in animal models, thus facilitating translational investigation better than other forms of laboratory-induced stress. Of particular note in this regard is the reported ability of yohimbine stress to enhance craving in opioid-dependent human subjects [17], a finding that corresponds well with our results of yohimbine-potentiated reinstatement of drug-seeking in heroin-experienced rats. While yohimbine is a useful pharmacological tool across species, it should be noted that the neuropharmacology of yohimbine is complex, as seen by a recent study that reported a lack of blockade of yohimbine’s effects by corticotropin-releasing factor (CRF) receptor antagonism or the α2–noradrenergic receptor agonist, clonidine [31].
To date, the majority of studies investigating the neural circuitry of stress-induced and cue-induced reinstatement have examined the mechanisms of these two forms of reinstatement separately. Few studies have explored possible substrates of stress potentiation of drug-paired cues. Liu and Weiss [22] examined whether stress- and cue-induced reinstatement recruit overlapping neural mechanisms in rats exposed to footshock and conditioned alcohol cues. Their results showed that the interactive effects of stress and cues were dependent on the concurrent activation of both CRF and opioid receptors. More recently, Duncan et al. [32] examined brain activation in response to cues (i.e., personalized scripts about cocaine use) and stress (i.e., anticipation of electrical shock) among ten cocaine-dependent males. The findings revealed that stress might provoke relapse to drugs by activating brain areas associated with reward processing, attentional bias, and memory recall of conditioned drug cues [32]. A role for the bed nucleus of the stria terminalis (BNST) in footshock stress-induced reinstatement has been found, in that both blockade of CRF [33] or beta norepinephrine [34] receptors attenuated footshock stress-induced reinstatement of cocaine-seeking in rats. The BNST has been previously implicated in mediating affective information [35,36,37] and shows multiple neuroadaptations after chronic drug use [38]. Thus, it is a likely structure that may serve as a critical neural node for processing stress potentiation of drug paired cue information. Since distinct, yet overlapping neural pathways mediate cue- and stress-induced reinstatement of drug-seeking [39,40], future studies will need to carefully discern the unique circuits that mediate the interaction of cues and stress in relapse.
In conclusion, the current findings demonstrate that exposure to heroin-associated cues during a stressful state can greatly potentiate heroin-seeking behavior. These findings have important clinical implications for relapse prevention and treatment efforts, as relapse to drug use in humans is precipitated by a combination of factors that often involve stress and conditioned drug cues. The current findings, coupled with previous research, support the simultaneous targeting of both stress and cue activation during relapse intervention. Further support is provided by research demonstrating that the co-administration of CRF and opiate receptor antagonists is necessary to reverse stress- and cue-induced reinstatement [22], as well as recent neuroimaging studies detecting differential brain activation to cocaine cues in the presence or absence of stress [32]. Future research should target the dual prevention of stress- and cue-induced reinstatement, and the stress-cue paradigm employed in this study may provide a useful model for testing putative pharmacotherapies in both animal models and opiate-dependent individuals.
Acknowledgements
This research was supported by National Institute on Drug Abuse (NIDA) grants DA015369 and DA021690 (RES), K23 DA021228 (SEB), and NIH grant C06 RR015455.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brooner RK, King VL, Kidorf M, Schmidt CW, Jr., Bigelow GE. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71–80. doi: 10.1001/archpsyc.1997.01830130077015. [DOI] [PubMed] [Google Scholar]
- 2.Frances RJ, Miller SI. Clinical Textbook of Addictive Disorders. New York: Guilford Press; 1998. [Google Scholar]
- 3.Blanco C, Alderson D, Ogburn E, Grant BF, Nunes EV, Hatzenbuehler ML, Hasin DS. Changes in the prevalence of non-medical prescription drug use and drug use disorders in the United States: 1991–1992 and 2001–2002. Drug Alcohol Depend. 2007;90:252–260. doi: 10.1016/j.drugalcdep.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81:103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Gossop M, Stewart D, Browne N, Marsden J. Factors associated with abstinence, lapse or relapse to heroin use after residential treatment: protective effect of coping responses. Addiction. 2002;97:1259–1267. doi: 10.1046/j.1360-0443.2002.00227.x. [DOI] [PubMed] [Google Scholar]
- 6.Childress AR, McLellan AT, Ehrman R, O'Brien CP. Classically conditioned responses in opioid and cocaine dependence: a role in relapse? NIDA Res Monogr. 1988;84:25–43. [PubMed] [Google Scholar]
- 7.Drummond DC, Cooper T, Glautier SP. Conditioned learning in alcohol dependence: implications for cue exposure treatment. Br J Addict. 1990;85:725–743. doi: 10.1111/j.1360-0443.1990.tb01685.x. [DOI] [PubMed] [Google Scholar]
- 8.O'Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- 9.Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- 11.Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- 12.Covington HE, 3rd, Miczek KA. Vocalizations during withdrawal from opiates and cocaine: possible expressions of affective distress. Eur J Pharmacol. 2003;467:1–13. doi: 10.1016/s0014-2999(03)01558-9. [DOI] [PubMed] [Google Scholar]
- 13.Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology. 1996;128:408–412. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- 14.Goeders NE, Clampitt DM. Potential role for the hypothalamo-pituitary-adrenal axis in the conditioned reinforcer-induced reinstatement of extinguished cocaine seeking in rats. Psychopharmacology. 2002;161:222–232. doi: 10.1007/s00213-002-1007-4. [DOI] [PubMed] [Google Scholar]
- 15.Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology. 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- 16.Shalev U, Highfield D, Yap J, Shaham Y. Stress and relapse to drug seeking in rats: studies on the generality of the effect. Psychopharmacology. 2000;150:337–346. doi: 10.1007/s002130000441. [DOI] [PubMed] [Google Scholar]
- 17.Stine SM, Southwick SM, Petrakis IL, Kosten TR, Charney DS, Krystal JH. Yohimbine-induced withdrawal and anxiety symptoms in opioid-dependent patients. Biol Psychiatry. 2002;51:642–651. doi: 10.1016/s0006-3223(01)01292-6. [DOI] [PubMed] [Google Scholar]
- 18.Feltenstein MW, See RE. Potentiation of cue-induced reinstatement of cocaine-seeking in rats by the anxiogenic drug yohimbine. Behav Brain Res. 2006;174:1–8. doi: 10.1016/j.bbr.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 19.Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology. 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- 20.Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- 21.Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shelton KL, Beardsley PM. Interaction of extinguished cocaine-conditioned stimuli and footshock on reinstatement in rats. Int J Comp Psychol. 2005;18:154–166. [Google Scholar]
- 24.Fuchs RA, See RE. Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology. 2002;160:425–433. doi: 10.1007/s00213-001-0997-7. [DOI] [PubMed] [Google Scholar]
- 25.Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–588. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24:10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valdez GR, Platt DM, Rowlett JK, Ruedi-Bettschen D, Spealman RD. Kappa agonist-induced reinstatement of cocaine seeking in squirrel monkeys: a role for opioid and stress-related mechanisms. J Pharmacol Exp Ther. 2007;323:525–533. doi: 10.1124/jpet.107.125484. [DOI] [PubMed] [Google Scholar]
- 28.Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev. 2003;27:457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 29.Buffalari DM, See RE. Footshock stress potentiates cue-induced cocaine-seeking in an animal model of relapse. Physiol Behav. 2009;98:614–617. doi: 10.1016/j.physbeh.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown ZJ, Tribe E, D'Souza NA, Erbx ErbS. Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology. 2009;203:121–130. doi: 10.1007/s00213-008-1376-4. [DOI] [PubMed] [Google Scholar]
- 32.Duncan E, Boshoven W, Harenski K, Fiallos A, Tracy H, Jovanovic T, Hu X, Drexler K, Kilts C. An fMRI study of the interaction of stress and cocaine cues on cocaine craving in cocaine-dependent men. Am J Addict. 2007;16:174–182. doi: 10.1080/10550490701375285. [DOI] [PubMed] [Google Scholar]
- 33.Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cullinan WE, Herman JP, Watson SJ. Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol. 1993;332:1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- 36.Forray MI, Gysling K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in the regulation of the hypothalamic-pituitary-adrenal axis. Brain Res Brain Res Rev. 2004;47:145–160. doi: 10.1016/j.brainresrev.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 37.Herman JP, Mueller NK, Figueiredo H. Role of GABA and glutamate circuitry in hypothalamo-pituitary-adrenocortical stress integration. Ann N Y Acad Sci. 2004;1018:35–45. doi: 10.1196/annals.1296.004. [DOI] [PubMed] [Google Scholar]
- 38.Le Moal M, Koob GF. Drug addiction: pathways to the disease and pathophysiological perspectives. Eur Neuropsychopharmacol. 2007;17:377–393. doi: 10.1016/j.euroneuro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology. 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- 40.See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]