Abstract
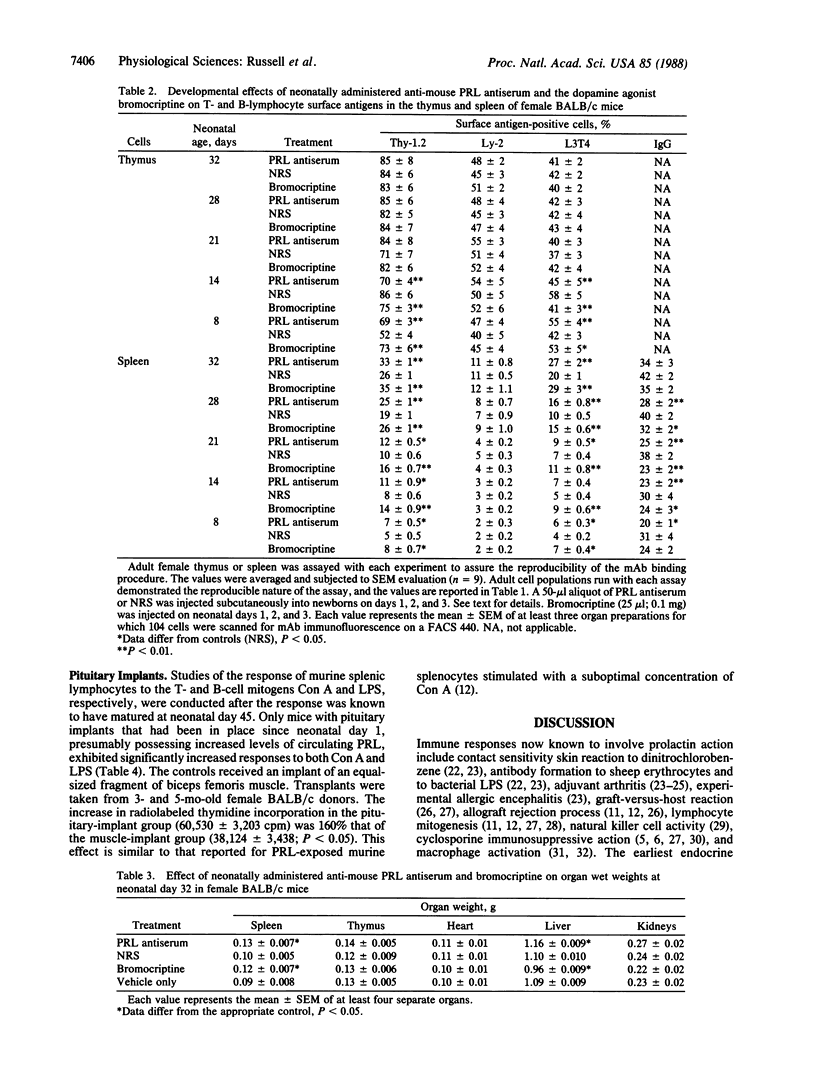
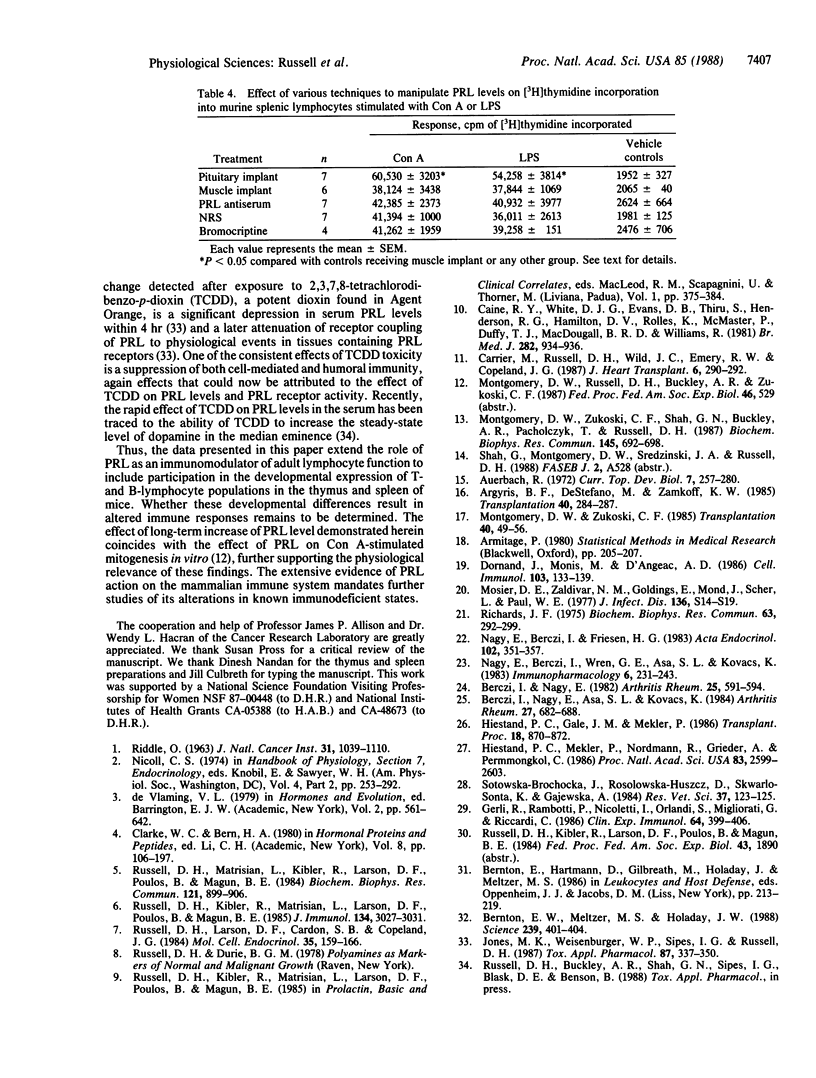
We have evaluated the effect of neonatal administration of mouse prolactin (PRL) antiserum on the developmental expression of T- and B-lymphocytes in the thymus and spleen of female BALB/c mice. Newborn female mice were injected subcutaneously with a 50-microliters aliquot of PRL antiserum or normal rabbit serum on days 1, 2, and 3. On neonatal day 5, the PRL antiserum-treated group had a significantly (P less than 0.05) increased population of cells in the thymus and the spleen that were positive for Thy-1.2 and for L3T4. Increases in Thy-1.2- and L3T4-positive cells in the thymus were detectable also on days 8 and 14 in mice that received the PRL antiserum and in mice injected with bromocriptine, a dopamine agonist that inhibits PRL release from the anterior pituitary. On neonatal days 21, 28, and 32, there were no significant differences in the percentage of cells positive for Thy-1.2, Ly-2 (formerly Lyt-2), or L3T4 antigens in the thymus. However, there were significant increases in the percentage of Thy-1.2- and L3T4-positive spleen cells in the bromocriptine-treated group at all times monitored and in the PRL antiserum-treated group except on day 14. In addition, the percentage of splenocytes that were positive for IgG was significantly increased in the PRL antiserum-treatment group on days 8-28, although not on neonatal day 32. Of tissues known to contain PRL receptors, neonatal administration of PRL antiserum or bromocriptine resulted in a significant alteration in the wet weight of spleen and liver, with no significant effect in thymus, heart, and kidney. Pituitary implants also resulted in a significant increase in both concanavalin A- and lipopolysaccharide-stimulated thymidine incorporation into murine splenic lymphocytes prepared from 45-day-old female mice. These data extend the role of PRL as an immunomodulator of adult lymphocyte function to a role in the developmental expression of T- and B-lymphocyte populations in the thymus and spleen of mice.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argyris B. F., DeStefano M., Zamkoff K. W. Interleukin-2 production in the neonatal mouse. Transplantation. 1985 Sep;40(3):284–287. doi: 10.1097/00007890-198509000-00013. [DOI] [PubMed] [Google Scholar]
- Auerbach R. Studies on the development of immunity: the response to sheep red blood cells. Curr Top Dev Biol. 1972;7:257–280. doi: 10.1016/s0070-2153(08)60074-5. [DOI] [PubMed] [Google Scholar]
- Berczi I., Nagy E. A possible role of prolactin in adjuvant arthritis. Arthritis Rheum. 1982 May;25(5):591–594. doi: 10.1002/art.1780250517. [DOI] [PubMed] [Google Scholar]
- Berczi I., Nagy E., Asa S. L., Kovacs K. The influence of pituitary hormones on adjuvant arthritis. Arthritis Rheum. 1984 Jun;27(6):682–688. doi: 10.1002/art.1780270612. [DOI] [PubMed] [Google Scholar]
- Bernton E. W., Meltzer M. S., Holaday J. W. Suppression of macrophage activation and T-lymphocyte function in hypoprolactinemic mice. Science. 1988 Jan 22;239(4838):401–404. doi: 10.1126/science.3122324. [DOI] [PubMed] [Google Scholar]
- Calne R. Y., White D. J., Evans D. B., Thiru S., Henderson R. G., Hamilton D. V., Rolles K., McMaster P., Duffy T. J., MacDougall B. R. Cyclosporin A in cadaveric organ transplantation. Br Med J (Clin Res Ed) 1981 Mar 21;282(6268):934–936. doi: 10.1136/bmj.282.6268.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier M., Russell D. H., Wild J. C., Emery R. W., Copeland J. G. Prolactin as a marker of rejection in human heart transplantation. J Heart Transplant. 1987 Sep-Oct;6(5):290–292. [PubMed] [Google Scholar]
- Dornand J., Monis M., Dupuy d'Angeac A. 5'-nucleotidase activity of murine T-cell subpopulations separated according to their Lyt2 and L3T4 phenotypes. Cell Immunol. 1986 Nov;103(1):133–139. doi: 10.1016/0008-8749(86)90074-2. [DOI] [PubMed] [Google Scholar]
- Gerli R., Rambotti P., Nicoletti I., Orlandi S., Migliorati G., Riccardi C. Reduced number of natural killer cells in patients with pathological hyperprolactinemia. Clin Exp Immunol. 1986 May;64(2):399–406. [PMC free article] [PubMed] [Google Scholar]
- Hiestand P. C., Mekler P., Nordmann R., Grieder A., Permmongkol C. Prolactin as a modulator of lymphocyte responsiveness provides a possible mechanism of action for cyclosporine. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2599–2603. doi: 10.1073/pnas.83.8.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. K., Weisenburger W. P., Sipes I. G., Russell D. H. Circadian alterations in prolactin, corticosterone, and thyroid hormone levels and down-regulation of prolactin receptor activity by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 1987 Feb;87(2):337–350. doi: 10.1016/0041-008x(87)90295-x. [DOI] [PubMed] [Google Scholar]
- Montgomery D. W., Zukoski C. F. Didemnin B: a new immunosuppressive cyclic peptide with potent activity in vitro and in vivo. Transplantation. 1985 Jul;40(1):49–56. [PubMed] [Google Scholar]
- Montgomery D. W., Zukoski C. F., Shah G. N., Buckley A. R., Pacholczyk T., Russell D. H. Concanavalin A-stimulated murine splenocytes produce a factor with prolactin-like bioactivity and immunoreactivity. Biochem Biophys Res Commun. 1987 Jun 15;145(2):692–698. doi: 10.1016/0006-291x(87)91020-5. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Zaldivar N. M., Goldings E., Mond J., Scher I., Paul W. E. Formation of antibody in the newborn mouse: study of T-cell-independent antibody response. J Infect Dis. 1977 Aug;136 (Suppl):S14–S19. doi: 10.1093/infdis/136.supplement.s14. [DOI] [PubMed] [Google Scholar]
- Nagy E., Berczi I., Friesen H. G. Regulation of immunity in rats by lactogenic and growth hormones. Acta Endocrinol (Copenh) 1983 Mar;102(3):351–357. doi: 10.1530/acta.0.1020351. [DOI] [PubMed] [Google Scholar]
- Nagy E., Berczi I., Wren G. E., Asa S. L., Kovacs K. Immunomodulation by bromocriptine. Immunopharmacology. 1983 Oct;6(3):231–243. doi: 10.1016/0162-3109(83)90023-1. [DOI] [PubMed] [Google Scholar]
- RIDDLE O. PROLACTIN IN VERTEBRATE FUNCTION AND ORGANIZATION. J Natl Cancer Inst. 1963 Nov;31:1039–1110. [PubMed] [Google Scholar]
- Richards J. F. Ornithine decarboxylase activity in tissues of prolactin-treated rats. Biochem Biophys Res Commun. 1975 Mar 3;63(1):292–299. doi: 10.1016/s0006-291x(75)80042-8. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Kibler R., Matrisian L., Larson D. F., Poulos B., Magun B. E. Prolactin receptors on human T and B lymphocytes: antagonism of prolactin binding by cyclosporine. J Immunol. 1985 May;134(5):3027–3031. [PubMed] [Google Scholar]
- Russell D. H., Larson D. F., Cardon S. B., Copeland J. G. Cyclosporine inhibits prolactin induction of ornithine decarboxylase in rat tissues. Mol Cell Endocrinol. 1984 May;35(2-3):159–166. doi: 10.1016/0303-7207(84)90012-1. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Matrisian L., Kibler R., Larson D. F., Poulos B., Magun B. E. Prolactin receptors on human lymphocytes and their modulation by cyclosporine. Biochem Biophys Res Commun. 1984 Jun 29;121(3):899–906. doi: 10.1016/0006-291x(84)90762-9. [DOI] [PubMed] [Google Scholar]
- Sotowska-Brochocka J., Rosołowska-Huszcz D., Skwarło-Sońta K., Gajewska A. Effect of exogenous prolactin on immunity in chickens. Res Vet Sci. 1984 Jul;37(1):123–125. [PubMed] [Google Scholar]



