Summary
Tumor stroma, consisting of the extracellular matrix and multiple cell types such as immune cells, fibroblasts and vascular cells, contributes to the malignancy of solid tumors by a variety of mechanisms. Intravital imaging by different microscopy techniques, especially by confocal and multi-photon microscopy, has proven to be a powerful method for analyzing the cell-cell and cell-matrix interactions in the dynamic tumor microenvironments. Intravital imaging has fostered the acquisition of data on parameters such as motility of different cell types in distinct tumor regions or manipulated with defined challenges, kinetics of tumor cell killing by T cells or macrophage-assisted tumor cell extravasation, functionality of the vasculature, protease activity and metabolic state. Achieving the direct observation of intact tumors offered by intavital imaging provides unique insights into tumor biology that will continue to deepen our understanding of the processes leading to malignancy and of the ways they can be targeted.
Introduction
It is now widely recognized that the stromal microenvironment of mutated tumor cells is a major determinant of malignancy in epithelial cancers (reviewed in [1]). Composed of a variety of extracellular matrix (ECM) components and several different cell types, the stroma of solid tumors varies according to tumor type, location and stage of disease progression. The stromal cells include carcinoma-associated fibroblasts (CAFs) and the blood and lymphatic vascular cells and other tissue type-specific mesenchymal cells [1], as well as infiltrating immune and inflammatory cells. Infiltrates contain adaptive immune cells, but are dominated by innate immune cells such as macrophages, mast cells, granulocytes, dendritic cells and natural killer (NK) cells that can account for a significant part of the tumor volume. During cancer progression, dynamic interactions between tumor cells, stromal cells and the surrounding ECM are required for the tumor cells to exploit the functionality of stromal cells and generate a microenvironment favorable to malignancy.
In recent years, research has elucidated the distinct roles that stromal cells can play in tumors. The link between inflammation and cancer has long been recognized, and there are striking parallels between tumor stroma and wound healing tissue [2]. Infiltrates of innate immune cells such as macrophages and mast cells correlate with poor prognosis in cancer patients and, along with other myeloid cells such as neutrophils, these cells promote experimental cancers by producing growth factors, cytokines and proteases that stimulate angiogenesis and tissue remodeling, by generating free radicals that induce DNA damage and in some cases by suppressing antitumor immunity [3]. On the other hand, natural killer (NK) cell and lymphocyte infiltrates can correlate with favorable prognosis, and these cells have antitumor activity in experimental systems [3]. However, adaptive immune cells such as regulatory T cells, B lymphocytes and CD4+ helper T cells have also been shown to have tumor-promoting functions, illustrating the importance of the context in which these cells function in determining their properties [3,4].
CAFs also have many cancer promoting functions, through their extensive ECM synthesis and remodeling capacity, as well as through their secretion of growth factors and chemokines that can be directly mitogenic to tumor cells or stimulate angiogenesis or immune cells [5,6]. Contact with CAFs can induce otherwise nontumorigenic cells to form tumors, whereas ‘normal’ fibroblasts may suppress tumor formation; but the mechanisms by which CAFs regulate tumor formation and progression are still incompletely understood [5]. The concerted research effort on the mechanisms of tumor angiogenesis has led to the development of anti-angiogenic cancer therapeutics, three of which are currently approved for clinical use in a range of cancers. However, it has become evident that tumors do evade anti-angiogenic therapy [7]. The potential for targeting lymphatic vessels to stop metastasis [8], as well as the mechanisms that tumors use to evade anti-angiogenic therapy [7], are currently under intense investigation.
The dynamic nature of cell-cell and cell-matrix interactions in the tumor stroma has led to the need for direct observation of the events in tumor tissue to develop a better understanding of the processes involved and to observe the effects of experimental manipulation. With the development of intravital imaging techniques with subcellular resolution, it has become possible to analyze cell behavior in tumor tissues in situ. Intravital microscopy of experimental animals has been used to analyze cell movement and interactions since the transillumination brightfield observations of blood flow and leukocyte rolling in the 19th century, and techniques have evolved through epifluorescence to confocal and multi-photon microscopy (MPM) and second harmonic generation (SHG) microscopy of various in vivo preparations with different visualization methods (reviewed in [9–11]). Table 1 lists the major challenges that must be addressed to obtain meaningful and/or quantifiable information by live imaging of the tumor microenvironment, and summarizes some of the approaches that can be taken to meet these challenges. The references given are some examples of studies relevant to cancer biology where these techniques were used, and the list is by no means exhaustive. In this review, we highlight the recent advances in our understanding of tumor-stroma interactions that have resulted from the application of intravital microscopy to animal models of cancer.
Table 1.
Challenges and solutions for intravital microscopy
| Challenge | Possible solutions | Refs. |
|---|---|---|
| Visualization of tumor components |
|
[42–44] [19,30,45,46] [47,48] [19,31,45,49] [22,25,26,33,50] [19,35,47,51] [17,19,51] [46,52,53] [19,31,36,45,46] |
| Minimal out-of-focus signal detection |
|
[19,54] [14,51] |
| Minimally invasive tissue preparation |
|
[35,43,44] [19,45,54] [27–30] |
| Long-term anesthesia |
|
[19] |
| Deep tissue imaging |
|
[14,36,51] [37] |
| Minimal photobleaching and phototoxicity |
|
[19] [14,36,51] |
| Minimal motion artefacts |
|
[19] |
| Ability to image several regions in the same mouse |
|
[19] |
Dynamics of inflammatory cells in tumor infiltrates
Multi-photon microscopy has been the intravital imaging method of choice for immunologists analyzing dense tissues such as lymph nodes and tumors (reviewed in [9,12,13]). MPM uses short pulses of near-infrared light for excitation, and the combined energy of two or more photons is required to excite the fluorophore (i.e., two photons at twice the normal excitation wavelength). Unlike one-photon confocal microscopy, one excitation wavelength is typically used for multiple fluorophores. Near-infrared light also has the ability to produce SHG signals from non-centrosymmetric organic structures such as fibrillar collagen. MPM has intrinsic confocality, since the probability of near-simultaneous excitation by multiple photons is extremely low anywhere other than at the focal point. This property eliminates the need for a pinhole, and minimizes out-of-focus photobleaching and phototoxicity. Near-infrared light also has superior tissue penetration and less scattering compared to shorter wavelengths. For an excellent overview of the principles of multi-photon microscopy and its application in tumor biology, with special emphasis on techniques such as fluorescence lifetime imaging microscopy (FLIM), spectral-lifetime imaging microscopy (SLIM) and Förster (or fluorescence) resonance energy transfer (FRET) that have been used to study cellular and molecular interactions as well as the metabolic status of tumors see Provenzano et al. [14]. Table 2 presents a comparison between the different microscopy methods used for intravital imaging of tumors.
Table 2.
Comparison between different microscopy modalities for intravital imaging
| Modality | Depth* | Advantages | Limitations |
|---|---|---|---|
| Wide-field epi- fluorescence | 20 μm |
|
|
| Confocal (point- scanning) | 50–100 μm |
|
|
| Spinning disk confocal | 50–100 μm |
|
|
| Near-infrared multi-photon | 400–1000 μm |
|
|
| Infrared multi-photon | Increased from near infra-red |
|
|
| Optical frequency domain imaging | > 1 mm |
|
|
Cytotoxic T lymphocytes (CTLs) can infiltrate into tumors, recognize tumor antigens and kill tumor cells. Boissonnas and coworkers used intravital MPM of subcutaneous tumors to analyze the dynamics of adoptively transferred, T cells expressing green fluorescent protein (GFP), and found profound differences in CTL motility and infiltration depending on the expression of their cognate antigen by the tumor cells [15]. In the same experimental model, the actual tumor cell killing by T cells was observed by an elegant strategy, where tumor cells express a cyan fluorescent protein (CFP)-yellow fluorescent protein (YFP) construct with a caspase-cleavable linker, resulting in disruption of FRET between CFP and YFP in apoptotic tumor cells that can be quantified by MPM [16]. MPM has also been used to show that macrophages, visualized by transgenic expression of GFP under specific promoters or by their ability to take up fluorescent dextran leaking from blood vessels, are directly involved in the intravasation of tumor cells in mammary tumors. Moreover, this process depends on a paracrine signaling loop between macrophages and tumor cells involving the epidermal growth factor (EGF) and colony-stimulating factor (CSF)-1 [17].
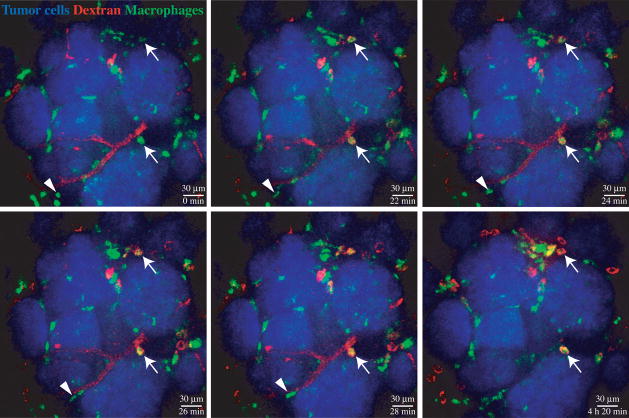
Despite the advantages of MPM listed above, single photon confocal microscopy techniques are popular for intravital imaging as they are considerably less expensive, user-friendly and allow more convenient use of a wide range of different fluorophores (Table 2). Spinning (Nipkow) disk confocal microscopy is particularly useful for long-term continuous imaging of the tumor microenvironment. The advantages of this system include very rapid image acquisition, which combined with sensitive detection by an intensified charge-coupled device (ICCD) allows continuous imaging with minimal photobleaching [18,19]. The imaging can be done in a lit room, which aids in the observation of the mouse necessary for long-term anesthesia, and the rapid acquisition helps to minimize motion artifacts resulting from respiratory movement of the animal [18,19]. Taking advantage of labeling, it is possible to visualize tumor cells, macrophages, fibroblasts or regulatory T cells through transgenic fluorescent protein reporters, blood vessels and macrophages by intravenous injection of fluorescent dextrans and Gr1+ myeloid cells by injecting a labeled anti-Gr1 antibody [18]. In the mouse mammary tumor virus (MMTV) promoter-polyoma middle T antigen transgenic breast carcinoma model, analysis of the dynamics of at least four different cell types in parallel over periods exceeding 24 hours has indicated distinct cellular behaviors in different areas of the tumor and tumor microenvironments [18]. Figure 1 presents a series of images of MMTV-PyMT tumor nodules with surrounding blood vessels and macrophages, obtained with a spinning disk confocal microscope in a time-lapse experiment. Irrespective of method, the data acquired by intravital imaging of immune cells in tumors have demonstrated that in vitro systems or analysis ex vivo samples cannot give us a complete picture of the mechanisms by which these cells exert their complex and sometimes paradoxical effects in the tumor stroma.
Figure 1.
A series of stills from a time lapse movie of over 4 hours of an early MMTV-PyMT tumor taken with a spinning confocal microscope. Tumor cells are visualized by the high transgenic expression of CFP under the β-actin promoter (blue). The c-fms promoter directs GFP expression to myeloid cell, mostly macrophages (green). Rhodamine-dextran (red) injected into the tail vein immediately before imaging accumulates first in the blood vessels, slowly leaks and is taken up by stromal macrophages, and eventually disappears from the blood vessels over time. Macrophages that have ingested dextran (arrows) remain sessile, whereas some other myeloid cells, especially on the tumor margins, are seen to migrate (arrowheads). The panels are maximum intensity projections of three z planes at 4 m intervals. The images have been modified to improve brightness and contrast.
Functional probes for protease activity
ECM remodeling proteases, such as matrix metalloproteinases (MMPs), serine proteinases and cysteine cathepsins, contribute to cancer progression by multiple mechanisms, including the facilitation of angiogenesis and tumor cell invasion by ECM remodeling and regulation of the bioavailability and activity of growth factors, cytokines and chemokines [20]. Autoquenched protease substrates that fluoresce upon cleavage were first used to image tumor protease activity in vivo in 1999 [21], and since then various autoquenched or FRET-based probes, including a near-infrared FRET probe for MMP-7 [22], have been developed for detection of MMP activity (reviewed in [23]). The group of Roger Tsien has developed fluorescently labeled activatable cell-penetrating peptides (ACCPs) that translocate into cells upon cleavage by MMP-2 or MMP-9, allowing accumulation in tumor cells in the vicinity of protease activity and intravital visualization [24,25]. In agreement with previous data for MMP-2/9 activity, the highest uptake of the peptide in MMTV-PyMT tumors is observed in the tumor-stroma interface. Importantly, small (100 μm) metastatic foci in the lungs are also visualized [26]. These tools can be used to localize regions of protease activity during tumor progression and to assess the efficiency of protease inhibitor treatments. They could be modified for other extracellular proteases as well. ACPPs can also be used for clinical applications, such as magnetic resonance imaging, by conjugating them to contrast agents; they could be used to carry therapeutic payloads into tumors with high protease activity [24,25]. In another recent example of the utility of protease probes for cancer research, the autoquenched near-infrared cathepsin B probe ProSense 680, used in an intravital imaging study of a mouse model of hereditary polyposis, has shown that cathepsin activity is localized to CD11b+Gr1+ myeloid cells, and is important for their tumor-promoting activity [26].
Windows to vasculature and metastasis
All of the studies introduced thus far were done in rodents where minimal surgery, namely the generation of a skin flap, is used to expose the tumor. However, following tumor development over a longer period of time with repeated imaging sessions raises additional challenges. Implanted imaging windows allow access to the tumor without the need for repeated surgery. The dorsal skin fold chamber [27] and the cranial window [28] have been used for a long time, especially for examining tumor angiogenesis, permeability and diffusion (reviewed in [11]), and imaging windows over the inguinal mammary gland have been developed more recently [29,30]. Kedrin and coworkers recently used the mammary imaging window to follow tumor cells expressing the photoswitchable protein Dendra2, which allows subpopulations of tumor cells in different microenvironments to be switched from green to red fluorescent when exposed to blue light [30]. This study showed that tumor cells, which are close to blood vessels, are more motile that those further away from vasculature, and noted that the perivascular microenvironments are rich in macrophages and ECM [30]. Migration of tumor cells along blood and lymph vessels and collagen fibers was also observed in a slightly modified dorsal skin fold chamber model [31].
Window chamber models have also been used in combination with imaging to evaluate the response of tumors to anti-vascular therapy. The results show that the therapy leads to normalization of the disorganized tumor vasculature, resulting in better penetration of large molecules such as therapeutics into the tumor [11,32]. In the cranial window model, a functional diamonofluorescein probe that reacts with nitric oxide (NO) was used to monitor NO gradients after silencing of tumor cell NO synthase [33]. Normalization of tumor vasculature was observed when the NO gradient is restricted to perivascular area [33]. Furthermore, platelet-derived growth factor (PDGF)-C was identified as a potential factor in the resistance to current anti-angiogenic therapy in a cranial window model of glioblastoma [34].
A new model of lymphatic metastasis, where a small tumor grown in the tip of the mouse ear can be directly imaged by MPM, is an example of a completely non-invasive method for intravital tumor imaging [35]. Similarly to the dorsal window, however, the tumor size is very limited and the location is non-orthotopic for most tumors.
Conclusions and Perspectives
Application of intravital microscopy is still a recent event in the effort to understand tumor biology. However, it has become clear that observation of the dynamics of tumor cells and stromal cells in context is essential for complete understanding of tumor development, metastasis and responses to therapy. The combination of intravital imaging in small animal models with ex vivo, in vitro and clinical analyses of the players involved in cancer and their interactions will enable the development of more powerful strategies for treatment of human cancer, possibly by combinatorial approaches that target both tumor cells and the stroma.
Technological advances in instrumentation and molecular tools for intravital imaging have been rapid during the last couple of decades, and this development shows no signs of slowing down. MPM is moving from near infrared to infrared excitation, allowing the use of red fluorophores and fluorescent proteins [36]. Infrared MPM achieved by using an optical parametric oscillator to convert a shorter wavelength pump beams into two tuneable longer wavelength beams was demonstrated to cause less photobleaching and photodamage than near infrared MPM, while being capable of markedly improved tissue penetration and better SHG [36]. Additionally, a recently developed infrared fluorescent protein engineered from a bacterial phytochrome will allow better deep tissue imaging of reporters also by single photon excitation [37]. Miniaturised epifluorescence and 2-photon fiber optic microscopes that can be used to image even freely moving animals, and confocal endomicroscopy in humans are bringing the advantages of intravital microscopy to the clinic, and offer less invasive imaging solutions also for small animal models [38–41]. A second-generation optical coherence tomography technology termed optical frequency domain imaging can generate truly impressive time lapse images with measurable parameters of blood- and lymphatic vasculature, or viable versus necrotic tumor tissue, over much wider regions than feasible with confocal microscopy [42]. As technology improves, we will obtain a more and more accurate view of the actual real-time events in tumors that combine and collaborate to result in malignancy. The promise that this information can be translated to enable earlier detection, better treatment selection, and eventually better therapy for human cancer is just beginning to be realized.
Acknowledgments
Supported by grants from the National Cancer Institute (R01 CA057621 and R01 CA057621-17S1), Amgen, Inc., and fellowships from the Academy of Finland (M.L.) and the Emil Aaltonen Foundation (M.L.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 2.Schafer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9:628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 3.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 4.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 6.Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth--bystanders turning into key players. Curr Opin Genet Dev. 2009;19:67–73. doi: 10.1016/j.gde.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stacker SA, Achen MG. From anti-angiogenesis to anti-lymphangiogenesis: emerging trends in cancer therapy. Lymphat Res Biol. 2008;6:165–172. doi: 10.1089/lrb.2008.1015. [DOI] [PubMed] [Google Scholar]
- 9.Mempel TR, Scimone ML, Mora JR, von Andrian UH. In vivo imaging of leukocyte trafficking in blood vessels and tissues. Curr Opin Immunol. 2004;16:406–417. doi: 10.1016/j.coi.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 10*.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–589. doi: 10.1038/nature06917. A good review of the broad scope of imaging modalities available for cancer research from the lab to the clinic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukumura D, Jain RK. Imaging angiogenesis and the microenvironment. Apmis. 2008;116:695–715. doi: 10.1111/j.1600-0463.2008.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pittet MJ. Behavior of immune players in the tumor microenvironment. Curr Opin Oncol. 2009;21:53–59. doi: 10.1097/CCO.0b013e32831bc38a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mempel TR, Bauer CA. Intravital imaging of CD8+ T cell function in cancer. Clin Exp Metastasis. 2009;26:311–327. doi: 10.1007/s10585-008-9196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Provenzano PP, Eliceiri KW, Keely PJ. Multiphoton microscopy and fluorescence lifetime imaging microscopy (FLIM) to monitor metastasis and the tumor microenvironment. Clin Exp Metastasis. 2009;26:357–370. doi: 10.1007/s10585-008-9204-0. [DOI] [PubMed] [Google Scholar]
- 15.Boissonnas A, Fetler L, Zeelenberg IS, Hugues S, Amigorena S. In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J Exp Med. 2007;204:345–356. doi: 10.1084/jem.20061890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breart B, Lemaitre F, Celli S, Bousso P. Two-photon imaging of intratumoral CD8+ T cell cytotoxic activity during adoptive T cell therapy in mice. J Clin Invest. 2008;118:1390–1397. doi: 10.1172/JCI34388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 18**.Egeblad M, Ewald AJ, Askautrud HA, Truitt ML, Welm BE, Bainbridge E, Peeters G, Krummel MF, Werb Z. Visualizing stromal cell dynamics in different tumor microenvironments by spinning disk confocal microscopy. Dis Model Mech. 2008;1:155–167. doi: 10.1242/dmm.000596. This study establishes spinning disk confocal microscopy combined with an advanced anesthesia protocol, a transgenic tumor model and a combination of transgenic markers with injectable agents as powerful tools for simultaneously examining the behavior of multiple players in different tumor microenvironments over extended periods of time. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ewald AJ, Werb Z, Egeblad M. Dynamic, long-term, in vivo imaging of tumor-stroma interactions in mouse models of breast cancer using spinning disk confocal microscopy. In: Goldman RD, Swedlow JR, Spector DL, editors. Live Cell Imaging, A Laboratory Manual. 2. Chapter 23. Cold Spring Harbor Laboratory Press; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Affara NI, Andreu P, Coussens LM. Delineating protease functions during cancer development. Methods Mol Biol. 2009;539:1–32. doi: 10.1007/978-1-60327-003-8_1. [DOI] [PubMed] [Google Scholar]
- 21.Weissleder R, Tung CH, Mahmood U, Bogdanov A., Jr In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol. 1999;17:375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 22.Scherer RL, VanSaun MN, McIntyre JO, Matrisian LM. Optical imaging of matrix metalloproteinase-7 activity in vivo using a proteolytic nanobeacon. Mol Imaging. 2008;7:118–131. [PMC free article] [PubMed] [Google Scholar]
- 23.Scherer RL, McIntyre JO, Matrisian LM. Imaging matrix metalloproteinases in cancer. Cancer Metastasis Rev. 2008;27:679–690. doi: 10.1007/s10555-008-9152-9. [DOI] [PubMed] [Google Scholar]
- 24.Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc Natl Acad Sci U S A. 2004;101:17867–17872. doi: 10.1073/pnas.0408191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25**.Olson ES, Aguilera TA, Jiang T, Ellies LG, Nguyen QT, Wong EH, Gross LA, Tsien RY. In vivo characterization of activatable cell penetrating pepides for targeting protease activity in cancer. Integrative Biology. 2009;1:382–393. doi: 10.1039/b904890a. In this work the authors have developed improved activatable cell penetrating peptides as in vivo tools for visualizing matrix metalloproteinase activity in tumors, with uses for research into stromal biology and treatment responses as well as potential to be modified for clinical use. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gounaris E, Tung CH, Restaino C, Maehr R, Kohler R, Joyce JA, Ploegh HL, Barrett TA, Weissleder R, Khazaie K. Live imaging of cysteine-cathepsin activity reveals dynamics of focal inflammation, angiogenesis, and polyp growth. PLoS One. 2008;3:e2916. doi: 10.1371/journal.pone.0002916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehr HA, Leunig M, Menger MD, Nolte D, Messmer K. Dorsal skinfold chamber technique for intravital microscopy in nude mice. Am J Pathol. 1993;143:1055–1062. [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan F, Salehi HA, Boucher Y, Vasthare US, Tuma RF, Jain RK. Vascular permeability and microcirculation of gliomas and mammary carcinomas transplanted in rat and mouse cranial windows. Cancer Res. 1994;54:4564–4568. [PubMed] [Google Scholar]
- 29.Shan S, Sorg B, Dewhirst MW. A novel rodent mammary window of orthotopic breast cancer for intravital microscopy. Microvasc Res. 2003;65:109–117. doi: 10.1016/s0026-2862(02)00017-1. [DOI] [PubMed] [Google Scholar]
- 30*.Kedrin D, Gligorijevic B, Wyckoff J, Verkhusha VV, Condeelis J, Segall JE, van Rheenen J. Intravital imaging of metastatic behavior through a mammary imaging window. Nat Methods. 2008;5:1019–1021. doi: 10.1038/nmeth.1269. This study introduces a photoswitchable protein as an innovative tool for following cell populations in tumors over time, utilizing an implanted imaging window for an orthotopic tumor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexander S, Koehl GE, Hirschberg M, Geissler EK, Friedl P. Dynamic imaging of cancer growth and invasion: a modified skin-fold chamber model. Histochem Cell Biol. 2008;130:1147–1154. doi: 10.1007/s00418-008-0529-1. [DOI] [PubMed] [Google Scholar]
- 32.Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 33*.Kashiwagi S, Tsukada K, Xu L, Miyazaki J, Kozin SV, Tyrrell JA, Sessa WC, Gerweck LE, Jain RK, Fukumura D. Perivascular nitric oxide gradients normalize tumor vasculature. Nat Med. 2008;14:255–257. doi: 10.1038/nm1730. In this study, intravital imaging of a functional probe for nitric oxide is used to establish the importance of nitric oxide gradients for vascular stability in tumors. [DOI] [PubMed] [Google Scholar]
- 34.di Tomaso E, London N, Fuja D, Logie J, Tyrrell JA, Kamoun W, Munn LL, Jain RK. PDGF-C induces maturation of blood vessels in a model of glioblastoma and attenuates the response to anti-VEGF treatment. PLoS One. 2009;4:e5123. doi: 10.1371/journal.pone.0005123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoshida T, Isaka N, Hagendoorn J, di Tomaso E, Chen YL, Pytowski B, Fukumura D, Padera TP, Jain RK. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res. 2006;66:8065–8075. doi: 10.1158/0008-5472.CAN-06-1392. [DOI] [PubMed] [Google Scholar]
- 36*.Andresen V, Alexander S, Heupel WM, Hirschberg M, Hoffman RM, Friedl P. Infrared multiphoton microscopy: subcellular-resolved deep tissue imaging. Curr Opin Biotechnol. 2009;20:54–62. doi: 10.1016/j.copbio.2009.02.008. This paper presents the setup for infrared multiphoton microscopy, which allows a wider use of red fluorophores than conventional near-infrared multiphoton microscopy, and also has improved tissue penetration and second harmonic generation properties. [DOI] [PubMed] [Google Scholar]
- 37**.Shu X, Royant A, Lin MZ, Aguilera TA, Lev-Ram V, Steinbach PA, Tsien RY. Mammalian expression of infrared fluorescent proteins engineered from a bacterial phytochrome. Science. 2009;324:804–807. doi: 10.1126/science.1168683. This report presents a novel monomeric infrared fluorescent protein engineered from a bacterial phytochrome that can be used for improved deep tissue or whole-body imaging of genetic reporters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goetz M, Kiesslich R. Advanced imaging of the gastrointestinal tract: research vs. clinical tools? Curr Opin Gastroenterol. 2009;25:412–421. doi: 10.1097/MOG.0b013e32832d62c1. [DOI] [PubMed] [Google Scholar]
- 39.Flusberg BA, Cocker ED, Piyawattanametha W, Jung JC, Cheung EL, Schnitzer MJ. Fiber-optic fluorescence imaging. Nat Methods. 2005;2:941–950. doi: 10.1038/nmeth820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flusberg BA, Nimmerjahn A, Cocker ED, Mukamel EA, Barretto RP, Ko TH, Burns LD, Jung JC, Schnitzer MJ. High-speed, miniaturized fluorescence microscopy in freely moving mice. Nat Methods. 2008;5:935–938. doi: 10.1038/nmeth.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thiberville L, Salaun M, Lachkar S, Dominique S, Moreno-Swirc S, Vever-Bizet C, Bourg-Heckly G. Confocal fluorescence endomicroscopy of the human airways. Proc Am Thorac Soc. 2009;6:444–449. doi: 10.1513/pats.200902-009AW. [DOI] [PubMed] [Google Scholar]
- 42**.Vakoc BJ, Lanning RM, Tyrrell JA, Padera TP, Bartlett LA, Stylianopoulos T, Munn LL, Tearney GJ, Fukumura D, Jain RK, et al. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat Med. 2009;15:1219–1223. doi: 10.1038/nm.1971. This work introduces an impressive new method for intravital imaging tumor vasculature and viability, based on endogenous contrast only and thus eliminating the need for contrast agents, which allows rapid imaging of very large areas with good resolution. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mempel TR, Moser C, Hutter J, Kuebler WM, Krombach F. Visualization of leukocyte transendothelial and interstitial migration using reflected light oblique transillumination in intravital video microscopy. J Vasc Res. 2003;40:435–441. doi: 10.1159/000073902. [DOI] [PubMed] [Google Scholar]
- 44.Yanagi K, Ohshima N. Angiogenic vascular growth in the rat peritoneal disseminated tumor model. Microvasc Res. 1996;51:15–28. doi: 10.1006/mvre.1996.0003. [DOI] [PubMed] [Google Scholar]
- 45.McElroy M, Bouvet M, Hoffman RM. Chapter 2. Color-coded fluorescent mouse models of cancer cell interactions with blood vessels and lymphatics. Methods Enzymol. 2008;445:27–52. doi: 10.1016/S0076-6879(08)03002-4. [DOI] [PubMed] [Google Scholar]
- 46.Sahai E, Wyckoff J, Philippar U, Segall JE, Gertler F, Condeelis J. Simultaneous imaging of GFP, CFP and collagen in tumors in vivo using multiphoton microscopy. BMC Biotechnol. 2005;5:14. doi: 10.1186/1472-6750-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stroh M, Zimmer JP, Duda DG, Levchenko TS, Cohen KS, Brown EB, Scadden DT, Torchilin VP, Bawendi MG, Fukumura D, et al. Quantum dots spectrally distinguish multiple species within the tumor milieu in vivo. Nat Med. 2005;11:678–682. doi: 10.1038/nm1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swirski FK, Berger CR, Figueiredo JL, Mempel TR, von Andrian UH, Pittet MJ, Weissleder R. A near-infrared cell tracker reagent for multiscopic in vivo imaging and quantification of leukocyte immune responses. PLoS One. 2007;2:e1075. doi: 10.1371/journal.pone.0001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berk DA, Yuan F, Leunig M, Jain RK. Direct in vivo measurement of targeted binding in a human tumor xenograft. Proc Natl Acad Sci U S A. 1997;94:1785–1790. doi: 10.1073/pnas.94.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- 51.Brown EB, Campbell RB, Tsuzuki Y, Xu L, Carmeliet P, Fukumura D, Jain RK. In vivo measurement of gene expression, angiogenesis and physiological function in tumors using multiphoton laser scanning microscopy. Nat Med. 2001;7:864–868. doi: 10.1038/89997. [DOI] [PubMed] [Google Scholar]
- 52.Campagnola PJ, Millard AC, Terasaki M, Hoppe PE, Malone CJ, Mohler WA. Three-dimensional high-resolution second-harmonic generation imaging of endogenous structural proteins in biological tissues. Biophys J. 2002;82:493–508. doi: 10.1016/S0006-3495(02)75414-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown E, McKee T, diTomaso E, Pluen A, Seed B, Boucher Y, Jain RK. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat Med. 2003;9:796–800. doi: 10.1038/nm879. [DOI] [PubMed] [Google Scholar]
- 54.Farina KL, Wyckoff JB, Rivera J, Lee H, Segall JE, Condeelis JS, Jones JG. Cell motility of tumor cells visualized in living intact primary tumors using green fluorescent protein. Cancer Res. 1998;58:2528–2532. [PubMed] [Google Scholar]
- 55.Wang E, Babbey CM, Dunn KW. Performance comparison between the high-speed Yokogawa spinning disc confocal system and single-point scanning confocal systems. J Microsc. 2005;218:148–159. doi: 10.1111/j.1365-2818.2005.01473.x. [DOI] [PubMed] [Google Scholar]
- 56.Graf R, Rietdorf J, Zimmermann T. Live cell spinning disk microscopy. Adv Biochem Eng Biotechnol. 2005;95:57–75. doi: 10.1007/b102210. [DOI] [PubMed] [Google Scholar]