Abstract
OBJECTIVES
To evaluate the ability of MEG to detect medial temporal spikes in patients with known medial temporal lobe epilepsy (MTLE) and to use magnetic source imaging (MSI) with equivalent current dipoles to examine localization and orientation of spikes and their relation to surgical outcome.
METHODS
We prospectively obtained MSI on a total of 25 patients previously diagnosed with intractable MTLE. MEG was recorded with a 275 channel whole-head system with simultaneous 21-channel scalp EEG during inpatient admission one day prior to surgical resection. The patients’ surgical outcomes were classified based on one-year follow-up after surgery.
RESULTS
Nineteen of the 22 patients (86.4%) had interictal spikes during the EEG and MEG recordings. Thirteen of 19 patients (68.4%) demonstrated unilateral temporal dipoles ipsilateral to the site of surgery. Among these patients, five (38.5%) patients had horizontal dipoles, one (7.7%) patient had vertical dipoles, and seven (53.8%) patients had both horizontal and vertical dipoles. Sixty percent of patients with nonlocalizing ictal scalp EEG had well-localized spikes on MSI ipsilateral to the side of surgery and 66.7% of patients with nonlocalizing MRI had well-localized spikes on MSI ipsilateral to the side of surgery. Concordance between MSI localization and the side of lobectomy was not associated with a likelihood of an excellent postsurgical outcome.
CONCLUSIONS
MSI can detect medial temporal spikes. It may provide important localizing information in patients with MTLE, especially when MRI and/or ictal scalp EEG are not localizing.
SIGNIFICANCE
This study demonstrates that MSI has a good ability to detect interictal spikes from mesial temporal structures.
Keywords: MEG, EEG, Predictors, Epilepsy surgery
INTRODUCTION
Although magnetic source imaging (MSI), the combination of magnetoencephalography (MEG) recordings with source localization and MRI overlay, appears to be very sensitive and useful for localization of the epileptogenic zone in neocortical epilepsy, its ability to detect deep sources such as the medial temporal lobe and the interhemispheric surface of the frontal lobe remains in question (Rampp and Stefan, 2007). Several studies suggest that MSI can distinguish between neocortical and medial temporal lobe sources (Baumgartner et al., 2000; Stephen et al., 2005). In addition, combined MEG and EEG dipole modeling has been used to classify spikes arising from subcompartments of the temporal lobe and may thus be helpful in differentiating subtypes of mesial temporal lobe epilepsy (MTLE) (Pataraia et al., 2005). However, a recent report using a whole-head magnetoencephalography (MEG) system to study patients with MTLE found that the yield of MEG to record medial temporal spikes was very low and equivalent current dipole (ECD) modeling showed only partial correlation with electrocorticography (Leijten et al., 2003). Another study demonstrated that MEG was unable to detect spikes arising from the medial temporal lobe in patients who had medial temporal lobe spikes identified by intracranial electrodes (Shigeto et al., 2002). The goals of our study were to evaluate the ability of MEG to detect medial temporal spikes in patients with known MTLE and to localize and describe corresponding magnetic source images. We also explored whether MSI concordance with side of surgery could predict surgical outcome.
METHODS
We prospectively obtained MEG recordings on a total of 25 patients previously diagnosed with intractable MTLE who were being admitted for anterior temporal lobectomy. The mean age of these patients was 42.8 years (range, 24–68 years). Informed consent for the study was obtained from all subjects. MEG studies were performed under a protocol approved by the UCSF Committee on Human Research.
Prior to the MEG, all patients had already undergone a standard clinical presurgical evaluation including ictal scalp EEG monitoring, high resolution MRI, neuropsychological testing and Wada testing. For ictal scalp EEG analysis, we used primarily longitudinal bipolar and referential montages. The ictal EEG onset was localized to the temporal lobe when the amplitude ratio of the temporal versus the parasagittal chain was higher than 2:1 in bipolar montages and was higher than 2:1 for the two sides in referential montages (Steinhoff et al., 1995). In some cases, functional neuroimaging (such as a PET scan) was performed in order to determine the surgical plan, but none of the patients had had MEG prior to admission for surgery and so MSI was not used to direct the surgical plan.
Selection of patients for resective temporal lobe epilepsy surgery was based on standard criteria, which included medical intractability, identification of epileptogenic zone in the temporal lobe and a relatively low risk of new deficits (Ojemann and Valiante, 2005). Identification of epileptogenic zone was based on convergence of findings from clinical semiology, electrophysiology, neuroimaging and neuropsychology (Rosenow and Luders, 2001). In general, patients with unilateral anterior temporal epileptic discharges on interictal scalp EEG with ictal scalp EEG onset in the same region and imaging evidence of ipsilateral mesial temporal sclerosis were offered surgery. Patients who had non-localizing ictal EEG onset with or without imaging changes might have invasive EEG monitoring with subdural strips and depth electrodes.
All patients underwent surgical resection by a single neurosurgeon who was experienced in surgery for epilepsy and who was unaware of the MSI results. Surgeries were anterior medial temporal lobectomies (AMTL) tailored by intraoperative electrocorticography (ECoG) and direct cortical electrical stimulation mapping of language cortex (in the case of dominant hemisphere lobectomies). Typically, the resection included a maximum of 5.5 cm of the anterior lateral non-dominant temporal lobe or 4.5 cm of the dominant temporal lobe as measured from the temporal tip. The superior temporal gyrus was usually spared in the dominant hemisphere. The medial resections included the amygdala and the hippocampus to 2–3.5 cm.
MEG data acquisition & analysis
Simultaneous EEG and MEG recordings were performed inside a magnetically shielded room during inpatient admission one day prior to surgical resection, at a time when patients’ antiepileptic medications were being reduced to maximize the utility of ECoG. MEG was recorded with a 275 channel whole-head axial gradiometer system (VSM MedTech, Port Coquitlam, British Columbia). Data were recorded from each patient in a passband of 0–75 Hz (300 Hz sample rate) using a CTF 275-channel whole cortex MEG helmet. Twenty-one channel scalp EEG was recorded simultaneously using a modified international 10–20 system that included subtemporal electrodes (American Clinical Neurophysiology Society, 2006). Thirty to forty minutes of spontaneous data were obtained in 10 to 15 minute intervals with the patient asleep and awake. The position of the head in the MEG dewar relative to the MEG sensors was determined via indicator coils before and after each interval to ensure adequate sampling of the entire magnetic field. The data were bandpass filtered offline at 1–70 Hz.
Spikes were visually identified by a certified EEG technologist (MM) and were confirmed by a board-certified clinical neurophysiologist and epileptologist (HEK). EEG spikes were identified based on the criteria defined by the International Federation of Clinical Neurophysiology (IFCN) for EEG epileptiform discharges (Deuschl and Eisen, 1999). MEG spikes were chosen for analyses based on duration (< 80 ms), morphology, field map, and lack of associated artifact (e.g., ECG, EMG, EOG). The onset of each spike, defined as the rising deflection of the first sharp negativity from the baseline, was marked and equivalent current dipoles (ECD) were fit using commercial software provided by CTF Systems (VSM MedTech, Port Coquitlam, British Columbia). Only sources with a goodness of fit higher than 90% were accepted. Co-registration of dipoles to MRI scans was performed using fiducials (nasion and preauricular points) to produce magnetic source images of dipoles superimposed on anatomic images. The authors then inspected these MSI results and classified the spike dipoles according to their location and orientation. The dipoles were considered to have vertical orientation if they lay between 0 and 30 degrees from the vertical axis. If the dipoles lay between 60 and 90 degrees from the vertical axis, they were considered to have horizontal orientation (Pataraia et al., 2005).
Clinical data collection
Ictal scalp EEG was recorded using a 21-channel system with the electrodes arranged according to a modified international 10–20 system that included subtemporal electrodes (American Clinical Neurophysiology Society, 2006). In addition to noninvasive studies, four patients underwent intracranial EEG recordings with depth electrodes and subdural strips. Results of ictal scalp and/or intracranial EEG and MRI were determined by retrospective review of their reports. These results are shown along with MSI localizations in Table 1.
Table 1.
Demographic and clinical information of 22 patients with MTLE
| Case | Age | Ictal EEG | MRI | MSI | Intraoperative ECoG spikes | Surgery | Outcome | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LOC | NO | OR | Hipp | STG | MTG | ||||||
| 1 | 35 | RT | Rt HA | RAT | 19 | H, V | Rare | Freq | Freq | RT | Ia |
| 2 | 26 | LT | Lt HA | LAT | 6 | V>H | Freq | NS | NS | LT | IIc |
| 3 | 56 | LT | Lt HA | LAT | 16 | H | NA | NA | NA | LT | Ia |
| 4 | 41 | LT* | NL | LAT | 32 | V>H | Freq | Rare | Occ | LT | IId |
| 5 | 45 | LT | Lt HA | RAT | 9 | V≫>H | Freq | Occ | NS | LT | Ia |
| 6 | 36 | LT | Lt HA | LAT | 52 | V≫>H | Freq | Freq | Freq | LT | Ia |
| 7 | 33 | LT | NL | NS | 0 | NS | Freq | NS | Occ | LT | Ia |
| 8 | 38 | LT | Lt HA | LAT RAT |
7 6 |
V V |
Freq | Rare | NS | LT | Ia |
| 9 | 62 | RT | Bi HA | RAT | 19 | V≫>H | Freq | NS | NS | RT | Ia |
| 10 | 52 | RT | Rt HA | LAT RAT |
35 2 |
V>H V |
Occ | Occ | NS | RT | IV |
| 11 | 34 | LT* | Lt HA | NS | 0 | NS | Freq | Freq | Freq | LT | Ia |
| 12 | 46 | RT | NL | LAT RAT |
2 1 |
H=V H |
Rare | NS | Occ | RT | Ib |
| 13 | 68 | LT | Lt HA | LAT | 16 | H | Occ | NS | NS | LT | Ia |
| 14 | 53 | RT | Rt HA | NS | 0 | NS | NS | NS | NS | RT | Ia |
| 15 | 56 | LT | Lt HA | LAT RAT |
20 27 |
V≫>H V≫>H |
Freq | Occ | Occ | LT | Ia |
| 16 | 33 | LT | Lt HA | LAT | 8 | H≫>V | NA | NA | NA | LT | IIc |
| 17 | 35 | NL | Lt HA | LAT | 16 | H | Freq | Freq | Occ | LT | IIa |
| 18 | 35 | RT | NL | RAT | 3 | V | Freq | None | None | RT | Ia |
| 19 | 40 | RT | Rt HA | RAT | 4 | H | NS | Freq | None | RT | Ia |
| 20 | 31 | LT* | Lt HA | RAT | 6 | V | Occ | NS | NS | LT | Ia |
| 21 | 47 | RT | Rt HA | RAT | 28 | H | Occ | NS | NS | RT | Ia |
| 22 | 40 | LT* | Rt HA | LAT | 23 | H,V | Freq | NS | NS | LT | Id |
Invasive monitoring; LOC, Location of dipoles; NO, Number of dipoles; OR, Orientation of dipoles.
HA, Hippocampal atrophy; Bi HA, Bilateral hippocampal atrophy; LT, Left temporal; RT, Right temporal.
LAT, Left anterior temporal; RAT, Right anterior temporal; BAT, Bilateral anterior temporal; H, Horizontal; V, Vertical.
NA, Not available; NL, Normal; NS, No spike.
Hipp, Hippocampus; STG, Superior temporal gyrus; MTG, Middle temporal gyrus; Freq, Frequent; Occ, Occasional.
Surgical outcome
We classified patients’ surgical outcomes based on one year post-surgery follow-up using a modified Engel classification (Engel et al., 1993). Patients with Engel Class I were considered as having an excellent outcome.
Statistical analysis
Data were analyzed using Stata statistical software version 10.1 (Stata Corp, College Station, Texas). Because the decision to perform surgery in our patients was based largely on the result of ictal scalp or intracranial EEG and MRI, only the positive predictive value of MSI was calculated. The positive predictive value of MSI was the ratio of patients with excellent outcome who had well-localized spikes on MSI ipsilateral to the side of surgery to all patients with well-localized spikes on MSI ipsilateral to the side of surgery regardless of the outcome. To demonstrate the relationship between MSI localization and surgical outcome, the patients were divided into two groups: those in whom the MSI localization was concordant with the side of surgery and those in whom it was not. Fisher’s exact test was used for analysis of whether the proportion of those with excellent outcome differed between the concordance and the non-concordance groups. Logistic regression analysis was used to determine if concordance of ictal scalp EEG with the side of lobectomy, concordance of MRI with the side of lobectomy, concordance of MSI with the side of lobectomy and concordance of a combination of these tests with the side of lobectomy could independently predict excellent outcome after surgery. Significance level was set at P<0.05.
RESULTS
Twenty-five patients underwent simultaneous EEG and MEG recording. Due to significant artifact from metallic dental objects on MEG, which resulted in unreliable spike detection and localization, three out of the 25 patients were excluded from subsequent data analyses. Of the remaining 22 patients, 14 underwent left temporal lobectomy and eight underwent right temporal lobectomy. Table 1 shows the demographic and clinical information for the remaining 22 patients. Ten of the 14 left temporal lobectomy patients had a surgical outcome of Engel class I, while the remaining four had a surgical outcome of Engel class II. Only one out of the nine right temporal lobectomy patients had a poor outcome of Engel class IV.
MEG data
Nineteen of the 22 patients (86.4%) had clearly identifiable interictal spikes in MEG and EEG recordings, and the MEG spikes could be accurately modeled by a single equivalent current dipole. All spike dipoles were localized to the temporal lobe. No extratemporal dipoles were detected. Thirteen of 19 patients (68.4%) demonstrated unilateral temporal dipoles ipsilateral to the site of surgery (e.g. Figure 1, Case 22 in Table 1), four patients (21.0%) had independent bilateral temporal dipoles (e.g. Figure 2, Case 12 in Table 1) and only two patients (10.5%) had unilateral temporal dipoles in the contralateral temporal lobe (Case 5 and 20 in Table 1).
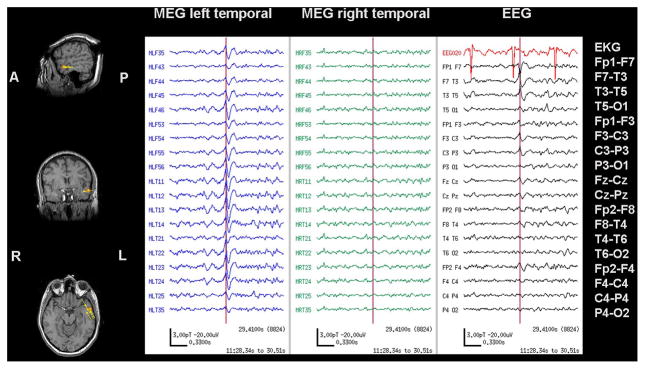
Figure 1.
MSI, MEG with simultaneous scalp EEG of Case 22 showing interictal unilateral temporal spikes. Single dipole source locations are shown as yellow triangles with vector tails proportional to dipole strength, superimposed on the anatomical MRI. MEG and EEG show a representative left temporal spike marked with a vertical cursor. The corresponding dipole is outlined in orange on the MSI.
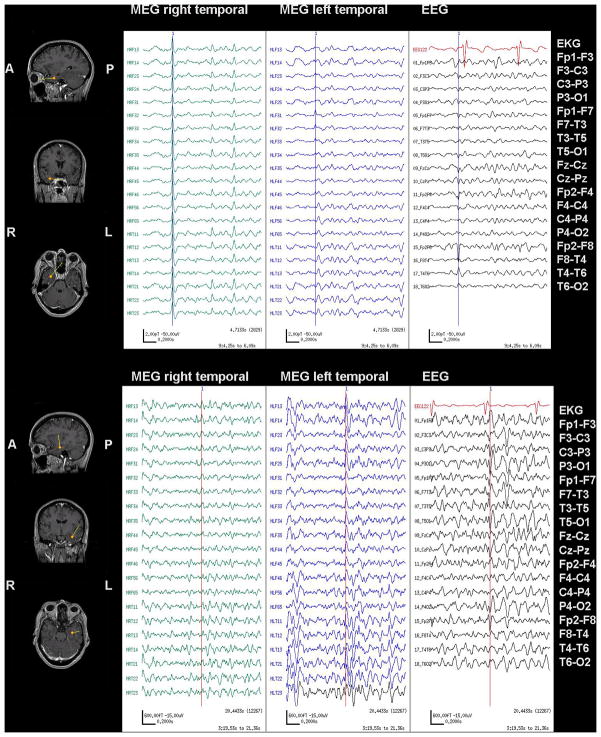
Figure 2.
MSI, MEG with simultaneous scalp EEG recorded from Case 12 in Table 1, showing interictal bilateral independent temporal spikes. Dipoles are shown as yellow triangles with vector tails proportional to dipole strength.
Top Panel: MEG and EEG show a right temporal spike (marked with a vertical cursor). MSI demonstrates horizontal dipoles modeled in the right anterior temporal lobe.
Bottom Panel: MEG and EEG show a left temporal spike (marked with a vertical cursor). MSI shows vertical dipoles modeled in the left anterior temporal lobe.
Among patients that had concordance between MSI localization and the side of lobectomy, two types of dipole orientations were observed. Five patients (38.5%) had horizontal dipoles, one (7.7%) patient had vertical dipoles, and seven (53.8%) patients had both horizontal and vertical dipoles.
Interestingly, patients with unilateral temporal dipoles localized to the contralateral temporal lobe (Case 5 & 20 in Table 1) and three out of four patients with bilateral temporal dipoles (Case 8, 10 and 15 in Table 1) demonstrated mainly vertical dipole orientation.
Comparison of MSI localization with ictal scalp EEG and MRI
Ictal EEG was non-localizing in one out of 22 cases. Sixteen patients (72.7%) had unilateral hippocampal atrophy (HA) on the side of lobectomy. One patient had unilateral HA contralateral to the side of lobectomy. One patient had bilateral HA. Ictal scalp EEG agreed with the eventual side of lobectomy in 17 patients (77.3%). In all but one patient, ictal scalp and/or intracranial EEG identified an epileptogenic focus ipsilateral to the lobectomy.
Interictal MSI lobar localization was in agreement with that of the ictal scalp EEG in 10 of 22 patients (45.4%), with that of the ictal scalp and intracranial EEG in 12 of 22 patients (54.5%), and with that of the abnormal MRI in nine of 16 patients (56.2%). In patients with well-localized MEG spikes, ictal EEG was non-localizing in one case (Case 17 in Table 1) and the discordance was only found in two cases (Case 5 & 20 in Table 1). Of five patients with non-localizing ictal scalp EEG, three patients (60%) had well-localized spikes on MSI ipsilateral to the side of surgery and four of six patients (66.7%) with nonlocalizing MRI had well-localized spikes on MSI ipsilateral to the side of surgery.
Surgical outcome
Seventeen of 22 (77.3%) patients had an excellent outcome. Among the patients with an excellent outcome, nine (69.2%) patients had well-localized spikes on MSI ipsilateral to the side of surgery.
Four patients with Engel class II outcome showed unilateral temporal dipoles ipsilateral to the site of surgery. Two (50%) of the patients with Engel class II outcome had non-localized ictal scalp EEG and one patient had non-localized ictal scalp EEG and MRI (Case 4 in Table 1). One patient with Engel class IV (Case 10 in Table 1) demonstrated bitemporal spikes on MSI with well-localized ictal scalp EEG and MRI. The concordance between interictal MSI localization and the side of lobectomy was not associated with a likelihood of an excellent outcome (P>0.05).
Table 2 summarizes the association of well-localized and concordant MSI or non-localizing and discordant MSI with potentially surgically remediable epilepsy. The positive predictive value of MSI for patients with excellent outcome was 0.69 (95% CI, 0.39–0.91).
Table 2.
Comparison of postsurgical outcome according to the concordance and the discordance or non-localization of MSI with the site of surgery.
| Magnetic source imaging (MSI) | ||
|---|---|---|
| Concordance (n = 13) | Discordance or non-localization (n = 9) | |
| Engel class I | 9 (69.2%) | 8 (88.9%) |
| Engel class II | 4 (30.8%) | None |
| Engel class III | None | None |
| Engel class IV | None | 1 (11.1%) |
DISCUSSION
In our study, MEG was able to detect mesial temporal spikes in over 85% of our patients with known MTLE. This finding is within the range of earlier studies with patient groups comprised of those with extratemporal as well as temporal lobe epilepsy (Wheless et al., 1999; Ishibashi et al., 2002; Iwasaki et al., 2002; Pataraia et al., 2005). Our detection rate is higher than that reported of 32% by Leijten et al. (2003); this difference may be due to the different sensor position with respect to the head and underlying cortex and/or the reduction of antiepileptic medications in our presurgical patient group (Quesney & Ortiz, 2004). In addition, the difference of sensor types, densities and configurations should always be taken into consideration (Santiuste et al., 2008). A recent study comparing two different types of MEG pick up coils, the magnetometer and the planar gradiometer, suggested that the magnetometer is superior in recording spikes from the mesial temporal lobe and the planar gradiometer is superior in detecting spikes from the temporal neocortices (Enatsu et al., 2008). In theory, the loss of signal magnitude as a function of dipole depth is fastest for planar gradiometers (Vrba et al., 2006). The planar gradiometer therefore seems likely to perform worse than the axial gradiometer for deep sources. Our study, which employed the axial gradiometer system, demonstrated similar results as reported in a recent study that used the magnetometer system (Santiuste et al., 2008).
The majority of MEG spikes observed in our patients were oriented in the horizontal or in mixed horizontal and vertical directions. Interestingly, most of the MEG dipoles in patients with non-concordant MSI localization (Case 5, 8, 10, 15 and 20 in Table 1) were oriented vertically. Horizontal dipoles have been shown to originate from temporal tip and lateral temporal cortical sources, while vertical dipoles are associated with basomedial temporal sources and probably represent propagation rather than direct involvement of the mesial temporal structures (Pataraia et al., 2005; Ebersole and Hawes-Ebersole, 2007). The intraoperative ECoG recordings in patients with non-localizing MSI demonstrated frequent spontaneous discharges from the hippocampus but only occasional or no spikes from the temporal neocortices. On the other hand, intraoperative ECoG recordings in the patients with well-localized MEG spikes (Table 1) demonstrated spontaneous discharges from lateral or subtemporal neocortices as well as hippocampus. The widespread involvement extending beyond mesial temporal structures recorded by intraoperative ECoG has been well documented (Alarcon et al., 1997; Schwartz et al., 1997; Fernandez Torre et al., 1999; Pataraia et al., 2005). Our findings and the findings from intraoperative ECoG studies suggest that epileptic spikes are likely the product of propagation and recruitment of neuronal activity from complex interaction along specific neuronal pathways. This concept has been confirmed by several functional neuroimaging studies using PET and SPECT that described an extensive involvement of the entire temporal lobe or farther in patients with MTLE (Sakamoto et al., 2003; Kaiboriboon et al., 2005).
MRI and ictal scalp EEG are routinely used for presurgical evaluation in patients with refractory MTLE. A previous study in a group of consecutive presurgical patients with extratemporal and temporal lobe epilepsy suggested that MEG localization was superior to 1990-era MRI and ictal scalp EEG (Wheless et al., 1999). In our patient group with pathology-proven mesial temporal lobe epilepsy, we did not find that MSI was more sensitive than ictal scalp EEG or high-resolution epilepsy protocol MRI. This may be due to subsequent improvement in neuroimaging or to the likelihood of interictal MEG to record some contralateral temporal spikes. It has been shown that patients with MTLE commonly have independent spikes from bilateral temporal lobes (Krendl et al., 2008). Ictal EEG recording, therefore, has been used as the most important piece of information in making surgical decision in this group of patients. In general, MEG studies are conducted on an outpatient basis and focus on interictal epileptiform activity. Only limited attempts have been made to record itcal MEG in patients with MTLE (Sutherling et al., 1987; Stefan et al., 1992; Tilz et al., 2002; Assaf et al., 2003). These studies demonstrated that ictal MEG findings were in close agreement with the invasive and noninvasive preoperative studies. Recent advances in detection and correction of head movement by continuous registration of head positioning have resulted in improved localization accuracy of MEG (Uutela et al., 2001; Wilson, 2004; Wilson et al., 2007). These techniques allow longer recording sessions and may increase our ability to record seizures. Further studies are required to assess the utility of ictal MEG in identification of the epileptogenic zone and its role in predicting outcome after epilepsy surgery.
Compared to scalp EEG, MEG has been considered to be less sensitive in recording spikes from the medial temporal lobe because the decay of magnetic fields is more pronounced than that of electrical fields with increased distance from the generator (Rampp and Stefan, 2007). Moreover, MEG selectively measures spikes produced by the neurons lining the sulci because only tangential components of a current source can be detected (Barkley and Baumgartner, 2003). Nevertheless, MEG signals are not distorted by the dura, skull and scalp and are independent of a reference (Barkley and Baumgartner, 2003). Several studies using simultaneous MEG and EEG recordings have shown that some spikes are seen only in the EEG, some are seen only in the MEG and some are seen in both EEG and MEG (Wheless et al., 1999; Lin et al., 2003; Ramantani et al., 2006; Kirsch et al., 2007). MEG and scalp EEG, therefore, provide complementary information when used together in presurgical evaluation for epilepsy surgery.
Approximately two-thirds of the patients with normal or nonlocalizing MRI and about half of the patients with nonlocalizing ictal scalp EEG had well-localized MSI that was concordant with the side of lobectomy. In this subgroup of patients where one of the other pieces of clinical information was nonlocalizing, MSI would have provided additional helpful information. From a clinical decision standpoint, patients with normal or nonlocalizing MRI were the most difficult, because surgical planning relied mainly on functional neuroimaging studies and/or intracranial EEG recordings. In fact, two of these patients (Patients 4 and 22 in Table 1) eventually underwent intracranial EEG recordings. Both patients had well-localized MSI and invasive monitoring would have potentially been avoided had MSI been used to confirm the significance of functional neuroimaging studies.
Different predictive factors that are associated with favorable outcome of anterior temporal lobectomy have been reported by several studies. A recent meta-analysis of the overall outcome of epilepsy surgery including both temporal and extratemporal epilepsy demonstrated that history of febrile seizures, mesial temporal sclerosis, tumor, abnormal MRI as well as extensive surgical resection were strong predictors of excellent surgical outcome (Tonini et al., 2004). Although concordance of EEG and MRI appeared to be a strong predictor of good outcome, the conclusion cannot be made with certainty due to the heterogeneity of the studies and potential confounding effects. We found that the concordance of MSI localization with the side of surgery as well as the concordance of ictal scalp EEG, MRI or a combination of these tests with the site of surgery was unable to predict excellent surgical outcome. It is possible that this is a false negative finding due to the small number of patients in our study. Patients with bilateral spikes may have poorer outcome as a group, though they may also have more frequent spikes, which is an independent predictor of surgical failure (Krendl et al., 2008). In this patient group, those with frequent bilateral spikes, MSI is more likely to be falsely lateralizing and at the same time outcome is less certain; this confound biases against the predictive value of MSI. In addition, other known and unknown prognostic factors that were not included in our analysis could have an effect or interaction with the test results.
In conclusion, our study demonstrates that MSI can detect mesial temporal spikes. It may provide important localizing information in patients with MTLE, especially when MRI and/or ictal scalp EEG are not localizing. Further prospective investigations of MSI in a large number of patients with MTLE will be useful to determine the predictive value of MSI concordance with seizure outcome after surgery. As multiple lines of evidence converge to suggest that TLE is not an homogeneous process, we must learn in which subgroups MSI can best be deployed as a presurgical planning tool.
Acknowledgments
Dr. Kitti Kaiboriboon was supported by a grant from the National Epifellows Foundation and the Epilepsy Foundation. Portions of this work were funded by NIH DC004855 to S.S.N. and by K23 NS047100 to H.E.K.
Footnotes
Disclosure Of Conflicts Of Interest: None of the authors has any conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alarcon G, Garcia Seoane JJ, Binnie CD, Martin Miguel MC, Juler J, Polkey CE, et al. Origin and propagation of interictal discharges in the acute electrocorticogram. Implications for pathophysiology and surgical treatment of temporal lobe epilepsy. Brain. 1997;120:2259–2282. doi: 10.1093/brain/120.12.2259. [DOI] [PubMed] [Google Scholar]
- American Clinical Neurophysiology Society. Guideline 5: Guidelines for standard electrode position nomenclature. J Clin Neurophysiol. 2006;23:107–110. doi: 10.1097/00004691-200604000-00006. [DOI] [PubMed] [Google Scholar]
- Assaf BA, Karkar KM, Laxer KD, Garcia PA, Austin EJ, Barbaro NM, Aminoff MJ. Ictal magnetoencephalography in temporal and extratemporal lobe epilepsy. Epilepsia. 2003;44:1320–1327. doi: 10.1046/j.1528-1157.2003.14303.x. [DOI] [PubMed] [Google Scholar]
- Barkley GL, Baumgartner C. MEG and EEG in epilepsy. J Clin Neurophysiol. 2003;20:163–178. doi: 10.1097/00004691-200305000-00002. [DOI] [PubMed] [Google Scholar]
- Baumgartner C, Pataraia E, Lindinger G, Deecke L. Neuromagnetic recordings in temporal lobe epilepsy. J Clin Neurophysiol. 2000;17:177–189. doi: 10.1097/00004691-200003000-00007. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Eisen A. Recommendations for the practice of clinical neurophysiology: guidelines of the International Federation of Clinical Neurophysiology. Amsterdam; New York: Elsevier; 1999. [PubMed] [Google Scholar]
- Ebersole JS, Hawes-Ebersole S. Clinical application of dipole models in the localization of epileptiform activity. J Clin Neurophysiol. 2007;24:120–129. doi: 10.1097/WNP.0b013e31803ece13. [DOI] [PubMed] [Google Scholar]
- Enatsu R, Mikuni N, Usui K, Matsubayashi J, Taki J, Begum T, Matsumoto R, Ikeda A, Nagamine T, Fukuyama H, Hashimoto N. Usefulness of MEG magnetometer for spike detection in patients with mesial temporal epileptic focus. Neuroimage. 2008;41:1206–1219. doi: 10.1016/j.neuroimage.2008.03.038. [DOI] [PubMed] [Google Scholar]
- Engel JJ, Van Ness P, Rasmussen T, Ojemann L. Outcome with respect to epileptic seizures. In: Engel JJ, editor. Surgical treatment of the epilepsies. 2. New York: Raven Press; 1993. pp. 609–621. [Google Scholar]
- Fernandez Torre JL, Alarcon G, Binnie CD, Seoane JJ, Juler J, Guy CN, et al. Generation of scalp discharges in temporal lobe epilepsy as suggested by intraoperative electrocorticographic recordings. J Neurol Neurosurg Psychiatry. 1999;67:51–58. doi: 10.1136/jnnp.67.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi H, Simos PG, Castillo EM, Maggio WW, Wheless JW, Kim HL, et al. Detection and significance of focal, interictal, slow-wave activity visualized by magnetoencephalography for localization of a primary epileptogenic region. J Neurosurg. 2002;96:724–730. doi: 10.3171/jns.2002.96.4.0724. [DOI] [PubMed] [Google Scholar]
- Iwasaki M, Nakasato N, Shamoto H, Nagamatsu K, Kanno A, Hatanaka K, et al. Surgical implications of neuromagnetic spike localization in temporal lobe epilepsy. Epilepsia. 2002;43:415–424. doi: 10.1046/j.1528-1157.2002.30801.x. [DOI] [PubMed] [Google Scholar]
- Kaiboriboon K, Bertrand ME, Osman MM, Hogan RE. Quantitative analysis of cerebral blood flow patterns in mesial temporal lobe epilepsy using composite SISCOM. J Nucl Med. 2005;46:38–43. [PubMed] [Google Scholar]
- Kirsch HE, Mantle M, Nagarajan SS. Concordance between routine interictal magnetoencephalography and simultaneous scalp electroencephalography in a sample of patients with epilepsy. J Clin Neurophysiol. 2007;24:215–231. doi: 10.1097/WNP.0b013e3180556095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krendl R, Lurger S, Baumgartner C. Absolute spike frequency predicts surgical outcome in TLE with unilateral hippocampal atrophy. Neurology. 2008;71:413–418. doi: 10.1212/01.wnl.0000310775.87331.90. [DOI] [PubMed] [Google Scholar]
- Leijten FS, Huiskamp GJ, Hilgersom I, Van Huffelen AC. High-resolution source imaging in mesiotemporal lobe epilepsy: a comparison between MEG and simultaneous EEG. J Clin Neurophysiol. 2003;20:227–238. doi: 10.1097/00004691-200307000-00001. [DOI] [PubMed] [Google Scholar]
- Lin YY, Shih YH, Hsieh JC, Yu HY, Yiu CH, Wong TT, Yeh TC, Kwan SY, Ho LT, Yen DJ, Wu ZA, Chang MS. Magnetoencephalographic yield of interictal spikes in temporal lobe epilepsy: comparison with scalp EEG recordings. Neuroimage. 2003;19:1115–1126. doi: 10.1016/s1053-8119(03)00181-2. [DOI] [PubMed] [Google Scholar]
- Ojemann GA, Valiante T. Epilepsy surgery: principles and controversies. In: Miller JW, Silbergeld DL, editors. Neurological disease and therapy. Vol. 76. New York: Taylor & Francis; 2005. pp. 403–413. [Google Scholar]
- Pataraia E, Lindinger G, Deecke L, Mayer D, Baumgartner C. Combined MEG/EEG analysis of the interictal spike complex in mesial temporal lobe epilepsy. Neuroimage. 2005;24:607–614. doi: 10.1016/j.neuroimage.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Quesney LF, Ortiz T. MEG in the presurgical investigation of temporal lobe epilepsy. J Clin Neurophysiol. 2004;21:132. doi: 10.1097/00004691-200403000-00009. [DOI] [PubMed] [Google Scholar]
- Ramantani G, Boor R, Paetau R, Ille N, Feneberg R, Rupp A, Boppel T, Scherg M, Rating D, Bast T. MEG versus EEG: influence of background activity on interictal spike detection. J Clin Neurophysiol. 2006;23:498–508. doi: 10.1097/01.wnp.0000240873.69759.cc. [DOI] [PubMed] [Google Scholar]
- Rampp S, Stefan H. Magnetoencephalography in presurgical epilepsy diagnosis. Expert Rev Med Devices. 2007;4:335–347. doi: 10.1586/17434440.4.3.335. [DOI] [PubMed] [Google Scholar]
- Rosenow F, Luders H. Presurgical evaluation of epilepsy. Brain. 2001;124:1683–1700. doi: 10.1093/brain/124.9.1683. [DOI] [PubMed] [Google Scholar]
- Sakamoto S, Tsuyuguchi N, Takami T, Morino M, Goto T, Hattori H, et al. Interictal patterns of cerebral glucose metabolism, perfusion, and magnetic field in mesial temporal lobe epilepsy. Epilepsia. 2003;44:1196–1206. doi: 10.1046/j.1528-1157.2003.08603.x. [DOI] [PubMed] [Google Scholar]
- Santiuste M, Nowak R, Russi A, Tarancon T, Oliver B, Ayats E, Scheler G, Graetz G. Simultaneous magnetoencephalography and intracranial EEG registration: technical and clinical aspects. J Clin Neurophysiol. 2008;25:331–339. doi: 10.1097/WNP.0b013e31818e7913. [DOI] [PubMed] [Google Scholar]
- Schwartz TH, Bazil CW, Walczak TS, Chan S, Pedley TA, Goodman RR. The predictive value of intraoperative electrocorticography in resections for limbic epilepsy associated with mesial temporal sclerosis. Neurosurgery. 1997;40:302–309. doi: 10.1097/00006123-199702000-00014. [DOI] [PubMed] [Google Scholar]
- Shigeto H, Morioka T, Hisada K, Nishio S, Ishibashi H, Kira D, et al. Feasibility and limitations of magnetoencephalographic detection of epileptic discharges: simultaneous recording of magnetic fields and electrocorticography. Neurol Res. 2002;24:531–536. doi: 10.1179/016164102101200492. [DOI] [PubMed] [Google Scholar]
- Stefan H, Schneider S, Feistel H, Pawlik G, Schuler P, Abraham-Fuchs K, Schlegel T, Neubauer U, Huk WJ. Ictal and interictal activity in partial epilepsy recorded with multichannel magnetoelectroencephalography: correlation of electroencephalography/electrocorticography, magnetic resonance imaging, single photon emission computed tomography, and positron emission tomography findings. Epilepsia. 1992;33:874–887. doi: 10.1111/j.1528-1157.1992.tb02195.x. [DOI] [PubMed] [Google Scholar]
- Steinhoff BJ, So NK, Lim S, Luders HO. Ictal scalp EEG in temporal lobe epilepsy with unitemporal versus bitemporal interictal epileptiform discharges. Neurology. 1995;45:889–896. doi: 10.1212/wnl.45.5.889. [DOI] [PubMed] [Google Scholar]
- Stephen JM, Ranken DM, Aine CJ, Weisend MP, Shih JJ. Differentiability of simulated MEG hippocampal, medial temporal and neocortical temporal epileptic spike activity. J Clin Neurophysiol. 2005;22:388–401. [PubMed] [Google Scholar]
- Sutherling WW, Crandall PH, Engel JJ, Darcey TM, Cahan LD, Barth DS. The magnetic field of complex partial seizures agrees with intracranial localizations. Ann Neurol. 1987;21:548–558. doi: 10.1002/ana.410210605. [DOI] [PubMed] [Google Scholar]
- Tilz C, Hummel C, Kettenmann B, Stefan H. Ictal onset localization of epileptic seizures by magnetoencephalography. Acta Neurol Scand. 2002;106:190–195. doi: 10.1034/j.1600-0404.2002.02047.x. [DOI] [PubMed] [Google Scholar]
- Tonini C, Beghi E, Berg AT, Bogliun G, Giordano L, Newton RW, et al. Predictors of epilepsy surgery outcome: a meta-analysis. Epilepsy Res. 2004;62:75–87. doi: 10.1016/j.eplepsyres.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Uutela K, Taulu S, Hamalainen M. Detecting and correcting for head movements in neuromagnetic measurements. Neuroimage. 2001;14:1424–1431. doi: 10.1006/nimg.2001.0915. [DOI] [PubMed] [Google Scholar]
- Vrba J, Nenonen J, Trahms L. Biomagnetism. In: Clarke J, Braginski AI, editors. The SQUID Handbook, Volume 2: Applications of SQUIDs and SQUID Systems. Wiley-VCH; 2006. pp. 269–390. [Google Scholar]
- Wheless JW, Willmore LJ, Breier JI, Kataki M, Smith JR, King DW, et al. A comparison of magnetoencephalography, MRI, and V-EEG in patients evaluated for epilepsy surgery. Epilepsia. 1999;40:931–941. doi: 10.1111/j.1528-1157.1999.tb00800.x. [DOI] [PubMed] [Google Scholar]
- Wilson HS. Continuous head-localization and data correction in a whole-cortex MEG sensor. Neurol Clin Neurophysiol . 2004;2004:56. [PubMed] [Google Scholar]
- Wilson H, Moiseev A, Podin S, Quraan M. Continuous head localization and data correction in MEG. International Congress Series. 2007;1300:623–626. [Google Scholar]