Abstract
This study was designed to assess the in vitro gene expression efficiency and therapeutic effectiveness of polymer mediated transfection of primary myoblasts. Autologous primary myoblasts transplantation may improve the function of infarcted myocardium via myogenesis. In addition, primary myoblasts can carry exogenous angiogenic genes that encode angiogenic factors to promote therapeutic angiogenesis. Viral vectors have limited clinical application due to the induction of inflammatory reactions, tumorigenic mutations and genome integration. To overcome these problems, two new biodegradable poly(disulfide—amine)s, poly(cystaminebisacryamide-diaminohexane) [poly(CBA-DAH)] and poly(cystaminebisacryantide-diaminohexane-arginine) [poly(CBA-DAH-R)], were synthesized as polymer carriers for gene delivery. In this study, primary myoblasts were isolated and purified from rat skeletal muscles. Based on an optimized polymer mediated transfection procedure using a luciferase assay and confocal microscopy, these two poly(disulfide amine)s induced up to 16-fold higher luciferase expression and much higher green fluorescence protein expression than branched poy(ethylenimine) (bPEI, 25kDa) in primary myoblasts. By flow cytometry, poly(CBA-DAH) and poly(CBA-DAH-R) promote rates of cellular uptake of florescence-labeled polymer/pDNA complexes of 97% and 99%, respectively, which are rats higher than that of bPEI 25kDa (87%). Both poly(disulfide amine)s were much less cytotoxic than bPEI 25kDa. The in vitro time course and co-culture experiments verified that polymer engineered primary myoblasts have the ability to stimulate endothelial proliferation. These data confirmed that poly(disulfide amine)s are the safe and feasible polymeric gene carriers to transfect VEGF165 into primary myoblasts. Polymer engineered primary myoblasts have potential for therapeutic application in the treatment of ischemic heart diseases.
Keywords: skeletal primary myoblasts, poly(disulfide amine)s, VEGF165, ischemic heart disease
1. Introduction
Over the past several years, cellular transplantation has received growing attention as a potential treatment for patients with ischemic heart diseases (IHD). Among the potential candidate cells, skeletal primary myoblasts have been widely investigated due to their ability to regenerate functionally contractile myocardium[1–4]. Primary myoblasts are quiescent mono-nucleated progenitor cells along the basal membrane of skeletal muscular fibers. Following injury, they rapidly mobilize, proliferate and fuse to neighboring myotubes and myofibers to regenerate damaged tissues[5–8]. From a clinical perspective, these cells have several attractive features including: (i) autologous origin; (ii) easy of access and culture, proliferative potential, and the ability to amplify and regenerate under appropriate culture conditions; (iii) commitment to a well-differentiated myogenic lineage, thereby eliminating the risk of tumoreginicity; (iv) high resistance to ischemia, which allows them to engraft and survive under conditions of myocardial ischemia (v) the potential to protect agaist subsequent injury to the myocardium[3, 5, 9]. As a result, reduced infarct size and improved cardiac function have been observed after primary myoblasts transplantation in both animal and clinical studies of myocardial infarction[3, 9–13].
Therapeutic angiogenesis is the use of exogenous angiogenic genes to stimulate the neovasculaturization in ischemic tissue[14, 15]. More than twenty growth factors have been identified with angiogenic activities, among which vascular endothelial growth factor (VEGF) is the major regulator of blood vessel formation, both during development and in the adulthood. VEGF has been widely studied both in animal and human studies. VEGF165 is the predominant isoform, with strong angiogenic potency[16, 17]. Primary myoblasts have been investigated as a cellular reservoir to produce biologically active angiogenic factor VEGF165 in a localized and sustained manner restore damaged vasculature. Therefore, skeletal primary myoblasts mediated angiogenic gene therapy is emerging as a new treatment for ischemic heart disease to both functionally regenerate contractile myocardium and restore blood supply[18–20].
A novel approach to cardiac repair is to combine cellular transplanation and angiogenic gene therapy. The gene carriers that can transduce angiogenic genes into primary myoblasts, however, have not been optimized. While viral vectors, including adenovirus, adeno-associated virus, and retrevirus, have high gene transfection efficiency, they have limited clinical application due to their inherent potential for immunognicity, tumorigenicity, induction of an inflammatory response, and integration into the host genome[1, 21, 22]. Furthermore, long-term over-expression of VEGF via viral vectors has been observed to lead to hemangioma formation instead of functional vessels in animal models[23–26]. These findings exclude viruses as clinically viable vectors to transduce angiogenic genes into primary myoblasts. It is therefore necessary to develop a safer and more efficient non-viral gene carrier that can circumvent the limitations of viral vectors.
Polymer gene carriers have several advantages compared to viral vectors including safety, stability, large plasmid DNA loading capacity, and capacity for ease of large-scale production[27, 28]. Polymer carriers can transducer angiogenic factors into primary myoblasts for therapeutic benefits. By condensing plasmid DNA into nanoparticles through electrostatic interactions, polymeric carriers can protect DNA from nuclease degradation, facilitate endosome-lysosome escape and induce efficient cellular uptake and gene expression[28, 29]. The current available cationic polymers, such as poly(ethylenimine) (PEI), poly(L-lysine) (PLL) and poly(amidoamine)s dendrimers, have significant toxicity concerns, mostly due to their poor biocompatibility and non-degradability[28]. The newer generation of hydrolyzable polymers, such as poly(β-amino ester)s[30, 31], poly(amino ester)s[32, 33], and poly(amido amine)s[29], are less cytotoxic but only modestly increase transfection efficiency. In addition, skeletal primary myoblasts are generally difficult to transfect with many cationic polymers, imposing additional challenges for polymeric gene transfection.
We have designed two new biodegradable poly(disulfide amine)s, poly(cystaminebisacryamide-diaminohexane) [poly(CBA-DAH)] and poly(cystaminebisacryamide-diaminohexane-arginine) [poly(CBA-DAH-R)], as polymer gene carriers and demonstrated high transfection efficiency in 293T, Hela, NIH3T3 and C2C12 cell lines in our prior work[34, 35]. Therefore, we hypothesized that these poly(disulfide amine)s would be effective polymer gene carriers to induce efficient gene expression, with low cytotoxicity, in skeletal primary myoblasts. After transfection with the angiogenic gene VEGF165, these polymer engineered primary myoblasts would have ability to induce angiogenic proliferation.
The primary myoblasts were isolated following rat skeletal muscle biopsy, and identified by desmin immunostaining. The reporter gene transfection efficiency was evaluated by luciferase assay and green fluorescent protein (GFP) expression study. The cellular uptake of polymer/DNA complexes (polyplexes) was studied by flow cytometry. The viability of primary myoblasts undergoing polymeric transfection was determined by MTT assay. An ELISA assay was used to evaluate functional gene VEGF165 expression from primary myoblasts after polymer transfection. The capability of inducing angiogenic proliferation was determined by in vitro time course and co-culture endothelial proliferation assay.
2. Materials and methods
2.1. Materials
Two poly(disulfide amine)s were synthesized as previously described[34, 35]. Branched polyethylenimine (bPEI, 25kDa), 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), bovine serum albumin (BSA) and 0.01% type I calf skin collagen with 0.1 N acetic acid were purchased from Sigma-Aldrich (St. Louis, MO). Ham’s F-10 Nutrient Medium, penicillin-streptomycin, fetal bovine serum (FBS), trypsin-like enzyme (TrypLE Express), and Dubelcco’s phosphate buffered saline (PBS) were purchased from Invitrogen-Gibco (Carlsbad, CA). Endothelial Cell Growth Medium-2 Bullet kit (EGM-2 Bullet kit) was purchased from Lonza Inc. (Allendale, NJ). Collagenase D and Dispase II were purchased from Roche Molecular Biochemicals (Indianapolis, IN). Human basic fibroblast growth factor (bFGF) was purchased from Promega (Madison, WI). Mouse-anti-human desmin IgG1/κ was purchased from Lab Vision (Fremont, CA). Streptavidin-horseradish peroxidase (SAv-HRP) conjugated biotinylated anti-mouse IgG and diaminobenzidine (DAB) substrate solution were purchased from BD Pharmingen (San Diego, CA). The plasmids, pCMV-Luc, pCMV-GFP, and pCMV-VEGF165, containing a firefly luciferase reporter gene, green fluorescent protein gene and vascular endothelial growth factor gene 165, respectively, were amplified in E. coli DH5α and purified by standard Maxiprep kit (Invitrogen, Carlsbad, CA), separately. The luciferase assay system with reporter lysis buffer was purchased from Promega (Madison, WI). BCA™ protein assay kit was purchased from Thermo Scientific (Rockford, IL). YOYO-1 iodide (1 mM solution in DMSO) was purchased from Molecular Probes (Eugene, OR). All materials were used without further purification.
2.2. Isolation and culture of rat skeletal primary myoblasts
All experiments were approved by the University of Utah Institutional Animal Care and Use Committee and followed the guidelines provided by the National Institutes of Health in Guide for the Care and Use of Laboratory Animals. The modified process of isolating and culturing rat primary skeletal myoblasts was summarized as below[8, 36]. Briefly, skeletal muscle was harvested from a young rat hindlimb, rinsed with 70% ethanol and sterile PBS, minced and digested in solution of 1.5 U/mL of collagenase D, 2.4 U/mL of dispase II and 2.5 mM CaCl2. The minced tissue was incubated at 37°C for approximately 45 min with occasional mixing till it reduced to a fine slurry. The dissociated cells were strained through 80 μm nylon mesh and centrifuged at 1500 rpm for 5 min. Then cells in the pellet were plated into a 25 mm2 collagen-coated culture flask (prepared by 0.01% type I calf skin collagen with 0.1 N acetic acid) and re-suspended in growth medium consisting of Ham’s F-10 supplemented with 20% FBS, 2.5 ng/mL human bFGF, penicillin (200 U/mL), and streptomycin (200 μg/mL). Ham’s F-10 gives a growth advantage to myoblasts over fibroblasts. Primary myoblasts were separated from contaminating cells such as fibroblasts by taking advantages of their differential adherence to the flask over several passages (selective growth and passaging conditions). Cells are incubated at 37°C in 5% CO2 and medium is changed every other day. After a couple of weeks of selective growth, cells can be passaged by trypsinization. Cells can be frozen in liquid nitrogen tank using standard cell culture protocols, e.g., in 90% serum and 10% dimethyl sulfoxide (DMSO).
2.3. Characterization of primary myoblasts
Primary myoblasts were plated on Lab-Tek™ Chamber Slides (Nunc, Naperville, IL) at about 60–70% confluence. After 24 h incubation, cells were immunostained by the primary antibody (mouse-anti-human desmin IgG1/κ) and the secondary antibody (SAv-HRP conjugated biotinylated anti-mouse IgG). Cells were fixed by 2% paraformaldehyde in PBS for 30 min, followed by rinsing with PBS three times and then with block buffer (3% BSA with 0.5% Triton X-100 in PBS) for 1 h at room temperature. The slides were incubated with 1:100 primary anti-desmin IgG at 4°C overnight. The next day, cells were rinsed 3 times with PBS and then the slides were incubated with 1:100 biotinylated anti-mouse IgG secondary antibody for 1 h at room temperature. After rinsing again, SAv-HRP was applied and the slides were incubated for 30 min at room temperature. DAB substrate solution was prepared and applied to the slides for 5 min or until the desired color intensity was reached. The cells were dehydrated using 95% alcohol and xylene. The slides were covered with Fluoromount™ Mount Gel (Sigma-Aldrich, St. Louis, MO). The proportion of desmin-positive cells was counted and expressed as a percentage of the total cell number.
2.4. In vitro luciferase reporter gene transfection
Poly(CBA-DAH) and poly(CBA-DAH-R) mediated in vitro gene transfection of primary myoblasts was evaluated by using a luciferase reporter gene (pCMV-Luc, 1.0 μg/mL) and measured at polyplex w/w ratios of 1, 5, 10, and 20 in the absence of serum. bPEI (25kDa)/DNA complexes at a w/w ratio of 0.6:1 were used as a positive control. Cells were seeded 24 h prior to transfection in collagen-coated 24-well plates at an initial density of 4.5 × 104 cells/well. Plasmid DNA was complexed with poly(CBA-DAH), poly(CBA-DAH-R) and bPEI at predetermined w/w ratios in 20 mM HEPES buffer (pH 7.4) and incubated for 30 min at room temperature before use. At the time of transfection, the medium in each well was replaced with 500 μL of fresh serum-free medium. Polyplexes were added into each well and incubated with the cells for 4 h at 37°C. Then the serum-free medium was replaced with 500 μL of fresh complete medium. After an additional 44 h incubation, cells were washed with pre-warmed PBS, treated with 100 μL cell lysis buffer and subjected to a freezing-thawing cycle. Cellular debris was removed by centrifugation at 16,000 rpm for 2 min. The luciferase activity in cell lysate (25 μL) was measured using a luciferase assay kit (100 μL luciferase assay buffer) on a luminometer (Dynex Technologies Inc., Chantilly, VA). The relative luminescent unit (RLU) of luciferase expression was normalized against protein concentration in the cell extracts, measured by a BCA protein assay kit. All transfection assays were carried out in triplicate.
2.5. Confocal study
Primary myoblasts (1 × 105 cells/well) were seeded on 8-well Lab-Tek™ Chamber Slides (NUNC, Rochester, NY) and transfected with poly (disulfide amine)s/pCMV-GFP complexes at w/w ratio of 1, 5, 10 and 20 (1.0 μg/mL DNA) using the same process described above. After 48 h of culturing, the medium was removed by aspiration and the cells were washed with cold PBS and fixed with 0.5 mL/well of 2% paraformaldehyde in PBS for 10 min at room temperature and ice-cold acetone for 10 min. The fixation agent was aspirated and cells were washed twice with cold PBS. The slides were covered using Fluoromount™ Mount Gel. Primary myoblasts expressing GFP protein were detected using an Olympus IX81 microscope (FV1000-XY confocal, Minneapolis, MN, for GFP, excitation: 488 nm, emission: 505 nm long-pass filter). Untreated cells and bPEI 25kDa/pCMV-GFP (w/w 0.6:1) transfected cells were used as negative and positive controls, respectively.
2.6. Cellular uptake assay
Primary myoblasts (2 × 105 cells/well) were seeded in collagen-coated 12-well plates for 24 h. YOYO-1 iodide tagged pCMV-Luc (1 molecule of the dye per 20 base pairs of the nucleotide) was prepared in the dark for 30 min. Polyplexes were prepared by mixing poly(CBA-DAH) and poly(CBA-DAH-R) with YOYO-1 labeled plasmid DNA at w/w ratios of 1, 5, 10 and 20, incubating at room temperature for 30 min before transfection. bPEI (25kDa)/DNA (w/w 0.6:1) complex was used as the positive control. At the time of transfection, the medium in each well was replaced with fresh serum-free medium. The polyplexes were incubated with cells at 37°C for 4 h. The medium was removed by aspiration and the cells were washed with PBS, harvested by trypsin-like enzyme (TrypLE Express) and neutralized with serum containing medium. After centrifugation at 1500 rpm for 2 min, cells were re-suspended in 0.4 mL ice-cold PBS. Samples were kept in ice and analyzed by the flow cytometer (FACS Caliber, BD Biosciences, San Jose, CA) at a minimum of 1 × 104 cells using FL1-height channel for YOYO-1 dye. Untreated cells were used as a negative control for calibration. All the assays were performed in duplicate. Data were analyzed using Windows Multiple Document Interface Software version 2.9 (WinMDI, Microsoft, Redmond, WA).
2.7. In vitro cytotoxicity
In vitro cytotoxicity of poly(CBA-DAH) and poly(CBA-DAH-R) in primary myoblasts was evaluated by MTT assay. Plasmid DNA was complexed with the poly(CBA-DAH), poly(CBA-DAH-R) or bPEI at w/w ratios of 1, 5, 10, and 20 in 20 mM HEPES buffer (pH 7.4) and incubated for 30 min at room temperature before use. Each of the polyplexes (1.0 μg/mL DNA) were incubated with primary myoblasts at 37°C for 4 h. After exchanging to and incubating with serum containing medium for an additional 20 h, MTT solution (50 μL, 2 mg/mL in PBS) was added into each well and incubated for 2 h. The medium was then removed and 300 μL DMSO was added to dissolve the formazan crystal formed by viable cells. The absorption was measured at 570 nm using a microplate reader (Model 680, Bio-Rad Lab, Hercules, CA). Untreated cells were used as control. Plain DMSO was used as a blank. All cytotoxicity experiments were performed in triplicate. The relative cell viability was calculated according to the equation ([Abs]sample−[Abs]blank)/([Abs]control−[Abs]blank)×100%.
2.8. ELISA for VEGF165 expression
The VEGF165 therapeutic protein secreted from primary myoblast transfected with poly(CBA-DAH) and poly(CBA-DAH-R) was measured using a Quantikine Human VEGF Immunoassay ELISA kit (R&D, Minneapolis, MN). The primary myoblasts were seeded in collagen-coated 12-well plates at an initial cell density of 1 × 105 cells/well. Cells were transfected by poly(CBA-DAH)/pCMV-VEGF165 and poly(CBA-DAH-R)/pCMV-VEGF165 complexes at a w/w ratio of 20:1 (1.0 μg/mL DNA) in the same manner as described above. Non-transfected myoblasts were used as control. At the same time, cell culture medium from each well was collected from day 0 (pre-transfection) to day 7 (post-transfection) and kept frozen at −70°C until use. ELISA assay was performed according to the instructions from the supplier. Briefly, 200 μL of cell medium samples and VEGF standards were coated onto each designated well and incubated at room temperature for 2 h. After washed 3 times with wash buffer, 200 μL of conjugate solution was added. The plate was incubated at room temperature for another 2 h, washed three times and incubated with 200 μL of substrate solution at room temperature for 20 min, and then 20 μL of stop solution was added into each well. The final quantification of VEGF165 protein in culture medium secreted from transfected primary myoblasts was detected under 450 nm minus 570 nm using ELISA plate reader (Model 680, Bio-Rad Lab, Hercules, CA). All experiments were performed in triplicate.
2.9. In vitro endothelial cell proliferation assay
The in vitro time-course and co-culture endothelial cell proliferation assays using human umbilical vein endothelial cells (HUVEC) and polymer transfected primary myoblasts were performed. HUVEC were purchased from American Type Culture Collection (ATCC, Manassas, VA) and cultured in EGM-2 medium. For the time-course cell proliferation assay experiment, HUVEC were seeded in 12-well plates at an initial density of 1 × 104 cells/well. At the same time, primary myoblasts were seeded in collagen-coated 12-well plates at a density of 3.5 × 104 cells/well and then transfected with poly(disulfide amine)s/pCMV-VEGF165 complexes (w/w 20:1, 1.0 μg/mL DNA) in the same manner as described above. From day 0 (pre-transfection) to day 5 (post-transfection), 500 μL of culture medium from polymer transfected myoblasts was collected and transferred to HUVEC every day. HUVEC proliferation was measured by MTT assay at day 0, 1, 3, and 5 incubated with medium from primary myoblasts. HUVEC treated with medium from non-transfected myoblasts were used as the control. HUVEC proliferation at day 0 was normalized at 100%. At the same time point of MTT assay, cell culture medium from HUVEC incubation was collected and stored at −70°C for VEGF concentration measurement.
2.10. Co-culture study
Cell culture insert (PET track-etched membrane, 0.4 μm pore size for 12-well plates, Becton Dickinson Labware, Franklin Lakes, NJ) was used for co-culture endothelial cell proliferation assay. In this experiment, HUVEC was plated in 12-well plates at an initial density of 2 × 104 cells/well. Primary myoblasts were plated in cell culture inserts at a density of 5 × 104 cells/well. After 24 h incubation, primary myoblasts were transfected by poly(disulfide amine)s/pCMV-VEGF165 (w/w 20:1, 1.0 μg/mL DNA) in the same manner as described above. At the same time, EGM-2 medium was exchanged to complete Ham’s F-10 medium for HUVEC cells. After 4 h transfection, the cell culture inserts plated with transfected primary myoblasts were put into 12-well plates with HUVEC cells. By using the cell culture inserts system, monolayers of HUVEC and transfected primary myoblasts could be co-cultured. With additional 4 days incubation, HUVEC proliferation was measured by MTT assay. HUVEC co-cultured with non-transfected primary myoblasts were used as the control, and whose proliferation was normalized to 100%. Just before MTT assay, the cell culture medium from co-culture system was collected and stored at −70°C for VEGF measurement. All experiments were performed in triplicate. Statistical calculations were carried by using GraphPad Prism 4.0 for Windows. Results were expressed as mean values ± standard deviations. One-way ANOVA followed by Tukey/Bonferroni post-hoc correction for multiple comparison test with significance at a 95% confidence interval was used to analyze in vitro HUVEC proliferation and VEGF concentration data.
3. Results
3.1. Skeletal primary myoblasts: isolation, culture and identification
By using the modified methods of selective growth and conditional passaging, primary myoblasts can be isolated and purified following rat skeletal muscle biopsy. After 2 weeks continuous culturing, the primary myoblast is the dominant cell type present. By 3 weeks, nearly all the cells are stellate-shaped primary myoblasts (Figure 1a). Desmin is a muscle-specific structural protein and one of the earliest proteins expressed in myogenic cells. Desmin immunostaining confirmed the primacy of primary myoblasts. Under light microscopy, the cytoplasm of primary myoblasts is clearly stained brown. There was no staining in the control group, which was treated with the secondary antibody but not the primary antibody (Figure 1b and 1c). The proportion of desmin-positive primary myoblasts was almost 100% after 3 weeks of culture. The cells were then stored and cultured for the next experiments without further purification.
Figure 1.
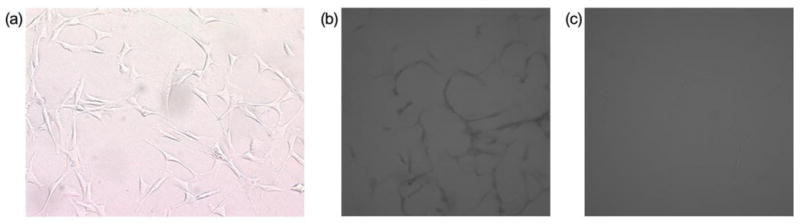
Primary myoblasts were isolated from rat skeletal muscle. (a) With 3 weeks culturing, stellate-shaped primary myoblasts were detected under light microscopy (× 200). (b) All cells demonstrated desmin expression by immunostaining (light-brown staining by DAB substrate). (c) Control, cells had no staining treated only by SAv-HRP conjugated anti-mouse IgG but not mouse-anti-human desmin (light microscopy × 200).
3.2. In vitro reporter genes transfection and cytotoxicity
In vitro transfection was performed by using poly(CBA-DAH) and poly(CBA-DAH-R) to transfect reporter gene pCMV-Luc into primary myoblasts at w/w ratio of 1:1 to 20:1 in the absence of serum. bPEI (25kDa)/pCMV-Luc complexes at a w/w ratio 0.6:1 were used as a positive control, Fig. 2 shows that both poly(CBA-DAH) and poly(CBA-DAH-R) have significantly higher luciferase gene expression than bPEI at w/w ratios of 5:1, 10:1 and 20:1. Poly(CBA-DAH) and poly(CBA-DAH-R) mediated 11- and 16-fold higher luciferase expression, respectively, than bPEI. Poly(disulfide amine)s have defined structures during synthesis, containing disulfide bonds and tertiary amines in the main chain, and primary amines and guanidinium groups in the side chains. These features give poly(disulfide amine)s good stability and DNA binding ability in the extracellular oxidative environment and superior buffering capacity and DNA releasing ability in the intracellular reductive environment, thereby facilitating efficient gene expression. While primary myoblasts are progenitor cells that previously were difficult to transfect with many cationic polymers, the luciferase expression achieved in these experiments indicates that poly(disulfide amine)s can be optimal gene carriers for skeletal primary myoblasts.
Figure 2.
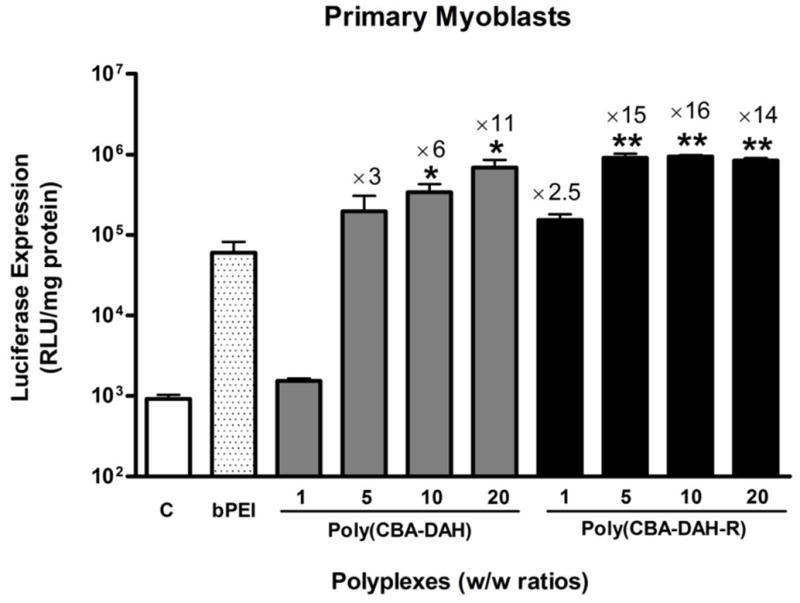
In vitro transfection efficiency of poly (CBA-DAH) and poly(CBA-DAH-R) transfected primary myoblasts at varying w/w ratios from 1:1 to 20:1 in the absence of serum. Control (C): non-treated cells. bPEI (25kDa)/pCMV-Luc at w/w ratio of 0.6:1 is positive control. Transfection results are expressed triplicate as the relative luminescent unit (RLU) of luciferase reporter gene expression normalized by total cell protein content in each well as mean values ± standard deviations (one-way ANOVA followed by Tukey/Bonferroni post-hoc correction for multiple comparison, compared to bPEI, *P<0.05, **P<0.001).
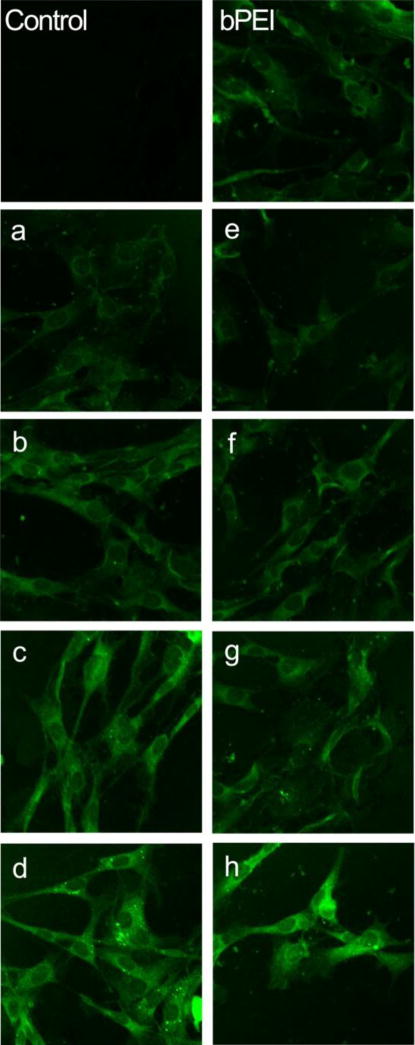
Reporter gene pCMV-GFP was transfected into primary myoblasts by poly(CBA-DAH) and poly(CBA-DAH-R) with the same w/w ratios mentioned above, and the GFP expression detected by confocal microscopy. As shown in Fig 3, GFP protein was visualized throughout the cytoplasm of primary myoblasts after 48 h of polymeric transfection. With the weight ratios increased from 1:1 to 20:1, the GFP expression in primary myoblasts increased with both poly(CBA-DAH) and poly(CBA-DAH-R). In addition, both poly(disulfide amine)s mediated stronger GFP expression signals than bPEI 25kDa (w/w 0.6:1). There was no significant GFP fluorescence detected in the untransfected primary myoblasts. These results are consistent with the luciferase transfection study, demonstrating that both poly(disulfide amine)s can induce higher transfection efficiency than bPEI 25kDa.
Figure 3.
Confocal images of GFP expression from polymer transfected primary myoblasts (× 200). Control: non-treated primary myoblasts. Positive control: bPEI (25kDa)/pCMV-GFP at w/w ratio of 0.6:1. a–d: poly(CBA-DAH)/pCMV-GFP transfected primary myoblasts at w/w ratio of 1:1, 5:1, 10:1 and 20:1. e–h: poly(CBA-DAH-R)/pCMV-GFP transfected primary myoblasts at w/w ratio of 1:1, 5:1, 10:1 and 20:1.
Using YOYO-1 interclated plasmid DNA, the cellular uptake of poly(disulfide amine)s/DNA complexes was estimated by flow cytometry. In Fig. 4, poly(disulfide amine)s/DNA complexes were efficiently taken up by primary myoblasts compared to untreated cells. The cellular uptake of polyplexes increased with the increasing weight ratios from 1:1 to 20:1 for both poly(CBA-DAH) and poly(CBA-DAH-R), shifting to the stronger fluorescent histogram area. Both poly(disulfide amine)s induced stronger cellular uptake signals than bPEI 25kDa (Figure 4a and 4b). The quantitative cellular uptake of polyplexes was represented as a percentage of cell counts in the R2 gated region (Figure 4c). At a w/w ratio of 5:1, both poly(CBA-DAH) and poly(CBA-DAH-R) induced high levels of cellular uptake. While cellular uptakes of 97% and 99% were measured for poly(CBA-DAH) and poly(CBA-DAH-R) at w/w ratios of 10:1 and 20:1, separately, only 87% cellular uptake was measured for bPEI 25kDa. These results demonstrate that both poly(disulfide amine)s can induce high levels of cellular uptake for primary myoblasts.
Figure 4.
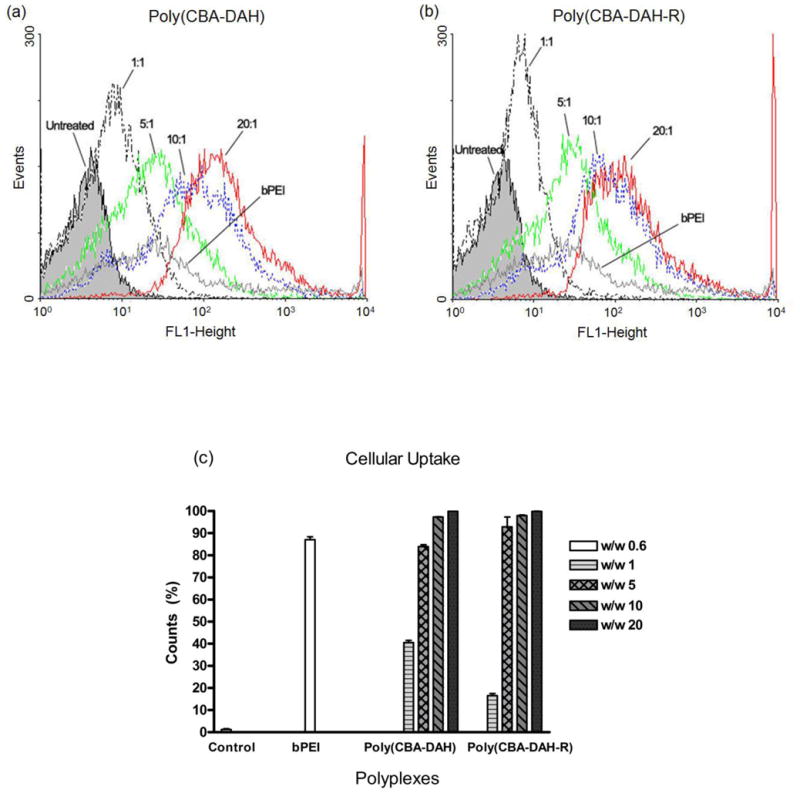
Flow cytometric analyses of cellular uptake assay. (a) Fluorescence histogram of poly(CBA-DAH)/pDNA transfected primary myoblasts. (b) Fluorescence histogram of poly(CBA-DAH-R)/pDNA transfected primary myblasts. (c) Cell count in R2 gated region, bPEI/pDNA at w/w ratio of 0.6:1 is positive control. Untreated primary myoblasts is the background control for calibration. Results were analyzed using WinMDI software version 2.9.
MTT assay showed that both poly(CBA-DAH) and poly(CBA-DAH-R) are less cytotoxic than bPEI 25kDa at w/w ratios of 5:1 and higher (Figure 5). While bPEI had 4% cell viability at a w/w ratio of 20:1, poly(CBA-DAH) and poly(CBA-DAH-R) had 95% and 82% cell viabilities, respectively, at the same w/w ratio. The main concern with bPEI 25kDa is its high cytotoxicity due to its non-degradability and intracellular accumulation. Poly(disulfide amine)s would alleviate these concerns because they are biodegraded to non-toxic small molecules through the cleavage of disulfide bonds and rapidly cleared from the body. Based on MTT assay, poly(disulfide amine)s have very low cytotoxicity for primary myoblasts. Taken together, these results demonstrate that poly(disulfide amine)s are the optimal polymer gene carriers for primary myoblasts due to high gene transfection efficiency and very low cytotoxicity. These carriers are I: therefore feasible to transfect VEGF165 into primary myoblasts.
Figure 5.
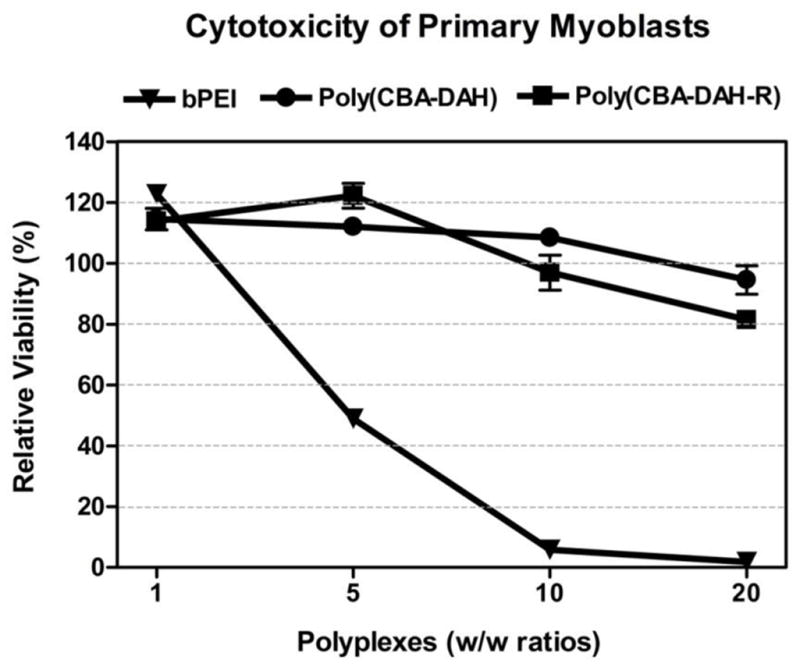
Relative cell viability of poly(CBA-DAH), poly(CBA-DAH-R) and bPEI 25kDa transfected primary myoblasts at varying w/w ratios of 1, 5,10 and 20:1. Cytotoxicity was measured by MTT assay and represented triplicate as mean values ± standard deviations.
3.3. VEGF expression study
Using ELISA assay, the VEGF165 protein time-course concentration profile of polymer mediated transfection of primary myoblasts can be determined. As shown in Fig. 6, poly(disulfide amine)s transfected primary myoblasts expressed VEGF165 for seven days. At 3 days post transfection, maximum levels of VEGF165 expression were reached at 430 and 891 pg/mL for poly(CBA-DAH) and poly(CBA-DAH-R), respectively. The untransfected cells had no significant VEGF165 expression. As the cells reached confluence after four days of culture in vitro, VEGF expression dropped due to cessation of cells growth. VEGF expression is expected to last longer in vivo because cells will continue to proliferate after transplantation in vivo. Although poly(CBA-DAH-R) induced higher VEGF expression than poly(CBA-DAH), both poly(disulfide amine)s can transfect a functional VEGF165 gene into primary myoblasts. Therefore, poly(disulfide amine)s primary myoblasts to be a cellular reservoir for exogenous functional gene expression and can induce angiogenic proliferation.
Figure 6.
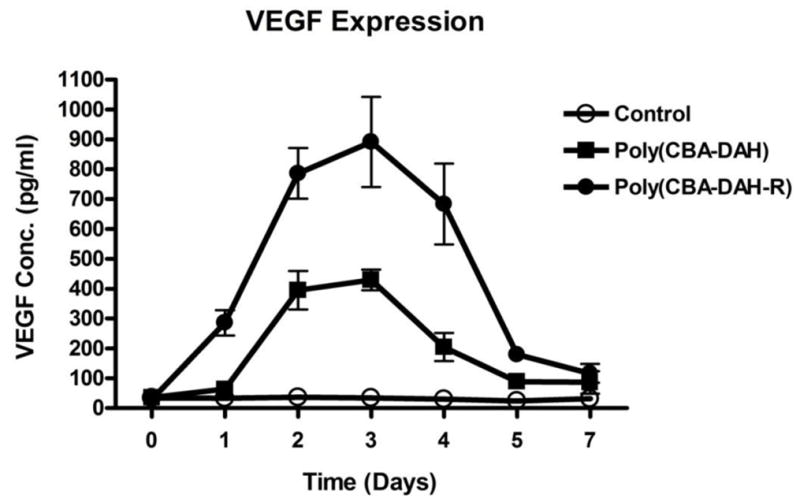
VEGF expression from primary myoblasts was measured by ELISA assay from day 0 (pre-transfected) to day 7 after polymeric transfection. Poly(CBA-DAH)/pCMV-VEGF165 and poly(CBA-DAH-R)/pCMV-VEGF165 complexes were formed at w/w ratio of 20:1 (1.0 μg/mL DNA). Control: untreated cells. All experiments were performed triplicate and represented as mean values ± standard deviations.
3.4. Polymer engineered primary myoblasts induced angiogenic proliferation measurement
To determine the angiogenic proliferation induced by polymer transfected primary myoblasts, in vitro time-course and co-culture endothelial cell proliferation assays were performed. Since HUVECs express VEGFR-1 (Flt-1) and VEGFR-2 (KDR/Flk-1), the proliferation of HUVEC is expected to be stimulated and enhanced by VEGF165 expressed from polymer transfected primary myoblasts. As shown in Fig. 7a, with 3 days incubation, HUVEC treated with culture medium from poly(disulfide amine)s/pCMV-VEGF165 transfected primary myoblasts demonstrated greater proliferation than control cells (HUVEC treated with medium from non-transfected primary myoblasts). With 5 days treatment, HUVEC proliferation was significantly accelerated. By using cell culture inserts, HUVEC and polymer transfected primary myoblasts were co-cultured, The results (Figure 7b) showed that the HUVEC proliferation induced by poly(CBA-DAH)/pCMV-VEGF165 and poly(CBA-DAH-R)/pCMV-VEGF165 transfected primary myoblasts were 128.4% and 124.2%, respectively. The HUVEC proliferation was significantly enhanced by VEGF165 expressed from poly(disulfide amine)s transfected primary myoblasts, higher than that of control cells (P < 0.05, one-way ANOVA followed by Tukey/Bonferroni post-hoc correction for multiple comparison). In both time-course and co-culture experiments, the HUVEC proliferation induced by poly(CBA-DAH) transfected primary myoblasts was higher than the HUVEC proliferation induced poly(CBA-DAH-R) transfected primary myoblasts.
Figure 7.
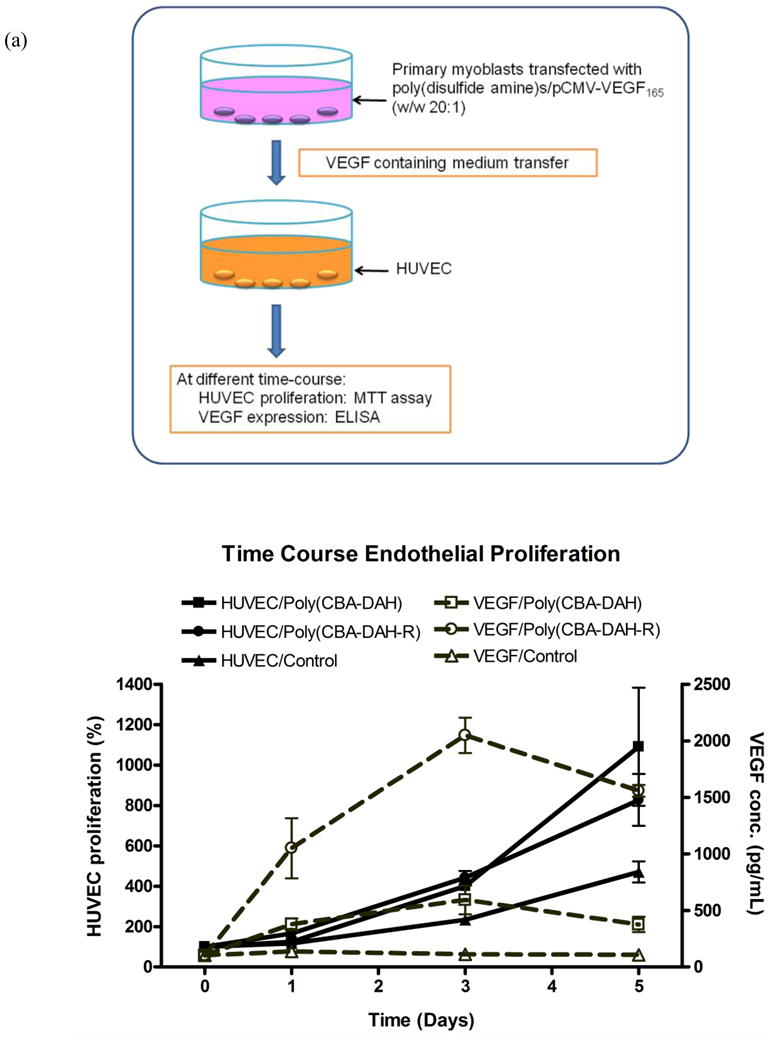
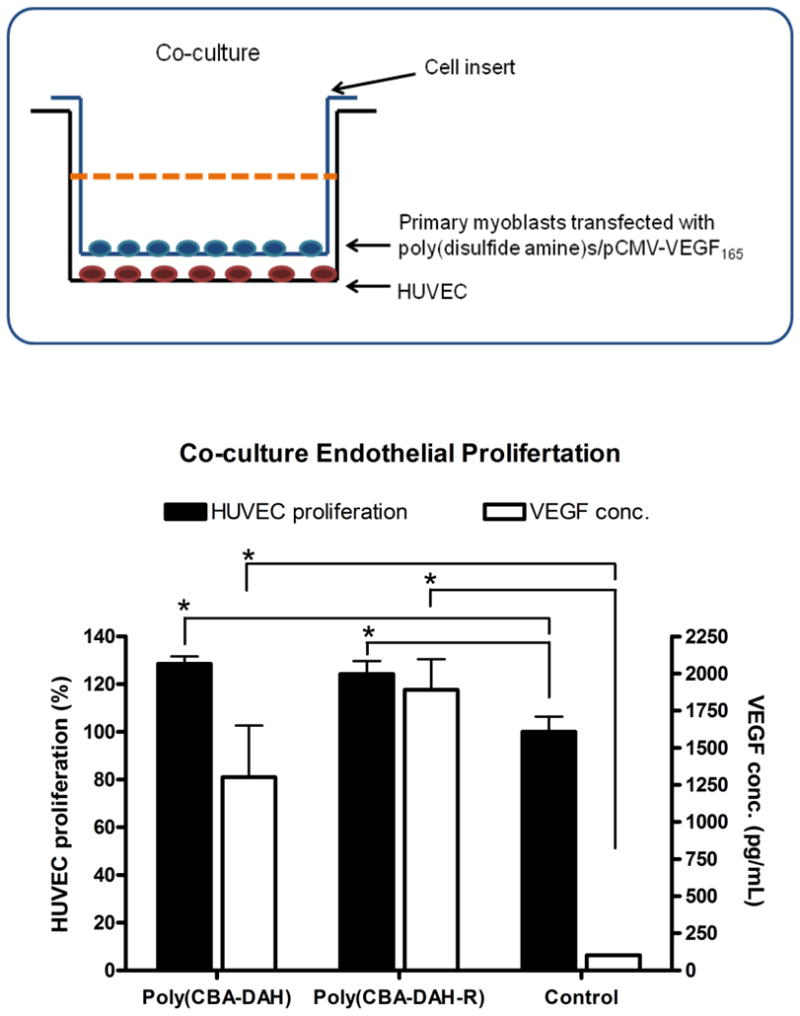
Polymer transfected primary myoblasts mediated HUVEC proliferation and VEGF expression levels. (a) The time-course endothelial proliferation assay. Primary myoblasts were transfected by poly(CBA-DAH)/pCMV-VEGF165 and poly(CBA-DAH-R)/pCMV-VEGF165 complexes (w/w ratio of 20:1, 1.0 μg/mL DNA). After transfection, culture medium from primary myoblasts were collected and transferred to HUVEC, whose proliferation was measured by MTT assay at 0, 1, 3, 5 days incubation (black line). At the same time point, VEGF165 concentration in HUVEC culture medium was determined by ELISA assay (dot grey line). HUVEC incubated with medium from non-transfected primary myoblasts was the control, whose cell proliferation was normalized as 100% at day 0. (b) The co-culture endothelial proliferation assay. Primary myoblasts were seeded in cell culture inserts (0.4 μm pore size for 12-well plates) and transfected with poly(CBA-DAH)/pCMV-VEGF165 and poly(CBA-DAH-R)/pCMV-VEGF165 complexes (w/w ratio of 20:1, 1.0 μg/mL DNA). After 4 h transfection, primary myoblasts and HUVEC (in 12-well plates) were co-cultured at 37°C for 4 days. HUVEC proliferation was measured by MTT assay (black bar). At the same time, VEGF165 concentration in co-culture system was measured by ELISA (white bar). HUVEC co-cultured with non-transfected primary myoblasts was the control, whose proliferation was normalized as 100%. All experiments were performed triplicate and represented as mean values ± standard deviations (one-way ANOVA followed by Tukey/Bonferroni post-hoc correction for multiple comparison, compared to the control, *P < 0.05).
At the same time, VEGF165 concentration in culture medium for both time-course and co-culture experiments was measured by ELISA kits (Figure 7a and 7b). In co-culture endothelial proliferation assay, the VEGF levels in HUVEC medium expressed from poly(CBA-DAH) and poly(CBA-DAH-R) transfected primary myoblasts were 1300.4 and 1889.5 pg/mL, respectively, which were higher than the VEGF level in control group (101.7 pg/mL, P < 0.05, one-way ANOVA followed by Tukey/Bonferroni post-hoc correction for multiple comparison). In time-course endothelial proliferation assay, the VEGF levels induced by both poly(disulfide amine)s/pCMV-VEGF165 transfected primary myoblast were also higher than that of control group. In both experiments, VEGF concentration expressed from poly(CBA-DAH-R) transfected primary myoblasts was higher than that of poly(CBA-DAH) transfected primary myoblasts. Despite the fact that poly(CBA-DAH-R) induced higher level of VEGF expression than poly(CBA-DAH), poly(CBA-DAH) transfected primary myoblasts can initiate higher level of HUVEC proliferation than poly(CBA-DAH-R), suggesting that an optimal VEGF concentration in medium is needed for angiogenic proliferation.
4. Discussion
Exogenous cell implantation into ischemic myocardium can repopulate post-infarction scar tissue and restore cardiac function [2, 5, 6]. Skeletal primary myoblasts are the only progenitor cells in the human body that feature contractile properties and that can differentiate and proliferate into functional myocardium within the infarcted heart [1–4]. Experimental studies verify the feasibility of primary myoblasts transplantation for the treatment of ischemic heart disease [37]. Morphologically, the injected primary myoblasts differentiate into multinucleated myotubes, expressing typical fast and slow myosin isoform [5, 38]. Functionally, left ventricular (LV) function and contractility are improved after myoblasts injection into damaged myocardial tissue[5, 39]. There are three hypotheses by which implanted myoblasts improve LV function: 1) the cells act as a scafford to strengthen the ventricular wall and limit post-infarct scar expansions; 2) mechanical links between grafted myoblasts and the extracellular matrix of native cardiomyocytes improve systolic LV function: 3) the transplanted myoblasts release paracrine factors that enhace survival of the native cardiomyocytes[2, 5, 6]. Although myoblasts do not convert into cardiomyocytes, primary myoblasts proliferate and form contractile myotubes and provide mechanical support to restore damaged myocardium [37]. In our study, a large population of primary myoblasts were successfully isolated and purified from a relatively small rat skeletal muscle biopsy in a 2–3 week- time frame. These primary myoblasts were identified by desmin immunostaining. These primary myoblasts can be used for both in vitro and in vivo studies.
Another characteristic of ischemic heart disease is the reduced blood supply to the ischemic myocardium. To promote neovasculature, therapeutic angiogenesis is required [15]. VEGF is the major growth factor involved in physiological angiogenesis[14]. VEGF is a secreted glycoprotein with at least four isoforms through alternative splicing of mRNA from a single gene. Each molecule contains 121, 165, 189, or 206 amino acids, with differences in their heparin binding capacity, ditermining their different angiogenic potency. VEGF165 is predominant among the four isoforms. It promotes endothelial proliferation and migration through binding to the VEGF receptors[15, 39]. VEGF also inhibits apoptosis due to ischemia and promotes angiogenesis in a variety of tissues, including bone, central nervous system, and liver[40]. Primary myoblasts have paracrine effects that can influence the surrounding myocardium. Primary myoblasts may be able to act as a cellular reservoir that releases cytokines and/or growth factors, e.g. VEGF165, stimulates therapeutic angiogenesis and enhances the survival of cardiomyocytes.
An ideal gene vector system should: (i) condense DNA into small size particles and protect DNA from nuclease degradation; (ii) ensure a high level of gene transfection and expression with low cytotoxicity and immunogenicity; (iii) demonstrate high gene loading capability and ease of manufacturing[1, 28]. To date, branched poly(ethylenimine) (bPEI Mw=25kDa) remains the mostly studied polymer carrier for gene delivery. bPEI 25kDa has relatively high gene transfection efficiency but also is associated with significant cytotoxicity because of its non-biodegradability, high charge density and tissue accumulation, which limit its clinical application [28, 41]. To circumvent these problems, many modifications have been made to PEI. One modification is to conjugate poly(ethylene glycol) (PEG) or other hydrolysable links, i.e. cholesterol, to low molecular weight PEI[42, 43]. Although cytotoxicity has been reduced through such modifications, so has gene transfection efficiency. Another modification has been the development of biodegradable polymers as gene vectors, such as poly(β-amino ester)s and poly(amido amine)s[29–31]. These polymers have lower cytotoxicity than bPEI 25kDa, but modest gene transfection efficiency. We previously synthesized and evaluated a series of poly(disulfide amine)s as gene carriers, including poly(CBA-DAH) and poly(CBA-DAH-R), which have higher transfection efficiency and much lower cytotoxicity than bPEI 25kDa in a variety of cell lines[34, 35]. Poly(disulfide amine)s are designed to meet the criteria for effective gene carriers. The primary amine and guanidinium groups in poly(disulfide amine)s can efficiently condense plasmid DNA into nanosized particles (~ 100 nm) as well as facilitate endocytosis of the polyplexes. The tertiary amine groups enable polymers superior buffering capacity of the polymers and efficient endosomal-lysosomal escape based on the “proton sponge hypothesis”[41]. Disulfide bonds are readily degraded via glutathione and thioredoxin reducatases in the cytoplasm, cleaving the polymers to non-toxic small molecules and releasing plasmid DNA.
In this study, we demonstrated that poly(disulfide amine)s are the ideal polymer gene carriers for primary myoblasts. The two cationic polymers which we studied demonstrated higher luciferase and GFP expression and much lower cytotoxicity than bPEI 25kDa in primary myoblasts. Flow cytometry indicated that both poly(disulfide amine)s mediate very high levels of cellular uptake of polyplexes. The optimal w/w ratio is 20:1 for poly(disulfide amine)s to transfect functional gene VEGF165 into primary myoblasts. VEGF165 protein expression can be detected for seven days under in vitro conditions. Polymer engineered primary myoblasts have the ability to stimulate angiogenic proliferation as shown in the endothelial cell proliferation assays. The time-course and co-culture experiments showed that HUVEC proliferation is statistically enhanced by VEGF165 secreted from polymer transfected primary myoblasts with 3–4 days of incubation. An interesting finding in our study is that while poly(CBA-DAH-R) mediated higher levels of VEGF165 expression than poly(CBA-DAH) in primary myoblasts, poly(CBA-DAH) transfected primary myoblasts induced higher levels of HUVEC proliferation than poly(CBA-DAH-R) transfected primary myoblasts. Previously, hemagglutinating virus of Japan using a liposome system has been used to transfect skeletal primary myoblasts, initiating neovascularization when the level of VEGF165 protein was 2.78 ± 0.2 ng/mL[44]. The long-term overexpression of VEGF for therapeutic angiogenesis may be limited by the formation of irregular blood vessels and hemangiomas[23–26]. Therefore, the concentration of VEGF, in the microenvironment instead of the total dose of VEGF, is more important in determining therapeutic efficacy and safety [23–26].
With regard to other potential reasons to genetically modify primary myoblasts, there has been accumulating evidence that implanted myoblasts may cause ventricular arrhythmias because engrafted myoblasts do not physically couple with host cardiac cells because of downregulation of two major junction proteins, connexin 43 and N-cadherin[2, 37]. Genetically transfecting these two genes into primary myoblasts may improve electrical impulse propagation between primary myoblasts and the native cardiac cells and thereby decrease arrhythmogenesis. Another difficulty with myoblasts transplantation has been cell death after injection[6]. Consequently, the transfection of anti-apoptotic genes such as bcl-2 or Akt into primary myoblasts would be a strategy to inhibit apoptosis enhance cell survival and optimize the therapeutic benefits of engrafted myoblasts. Based on our study, poly(disulfide amine)s are effective polymer carriers to transfect functional genes into primary myoblasts prior to cellular transplantation. We next plan to perform animal studies to evaluate the therapeutic effectiveness of our polymer engineered primary myoblasts system.
5. Conclusions
We have demonstrated that new poly(disulfide amine)s, poly(CBA-DAH) and poly(CBA-DAH-R), can be the effective polymer gene carriers for primary myoblasts, by mediating a high level of gene transfection and low cytotoxicity. These two cationic polymers can transfect a functional gene VEGF165 into primary myoblasts and mediate controlled protein expression. In addition, polymer engineered primary myoblasts can stimulate angiogenic proliferation under in vitro conditions.
Acknowledgments
This work is supported by National Institutes of Health (NIH) grant HL065477 (SWK) and HL071541 (DAB).
References
- 1.Haider HK, Elmadbouh I, Jean-Baptiste M, Ashraf M. Nonviral vector gene modification of stem cells for myocardial repair. Mol Med. 2008;14(1–2):79–86. doi: 10.2119/2007-00092.Haider. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menasche P. Skeletal myoblast transplantation for cardiac repair. Expert Rev Cardiovasc Ther. 2004;2(1):21–28. doi: 10.1586/14779072.2.1.21. [DOI] [PubMed] [Google Scholar]
- 3.Taylor DA. Cellular cardiomyoplasty with autologous skeletal myoblasts for ischemic heart disease and heart failure. Curr Control Trials Cardiovasc Med. 2001;2(5):208–210. doi: 10.1186/cvm-2-5-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ott HC, McCue J, Taylor DA. Cell-based cardiovascular repair--the hurdles and the opportunities. Basic Res Cardiol. 2005;100(6):504–517. doi: 10.1007/s00395-004-0558-z. [DOI] [PubMed] [Google Scholar]
- 5.Menasche P. Skeletal muscle satellite cell transplantation. Cardiovasc Res. 2003;58(2):351–357. doi: 10.1016/s0008-6363(02)00769-1. [DOI] [PubMed] [Google Scholar]
- 6.Menasche P. Skeletal myoblasts and cardiac repair. J Mol Cell Cardiol. 2008;45(4):545–553. doi: 10.1016/j.yjmcc.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function? Cell. 2001;105(7):829–841. doi: 10.1016/s0092-8674(01)00409-3. [DOI] [PubMed] [Google Scholar]
- 8.von Degenfeld G, Banfi A, Springer ML, Blau HM. Myoblast-mediated gene transfer for therapeutic angiogenesis and arteriogenesis. Br J Pharmacol. 2003;140(4):620–626. doi: 10.1038/sj.bjp.0705492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menasche P. Skeletal myoblasts as a therapeutic agent. Prog Cardiovasc Dis. 2007;50(1):7–17. doi: 10.1016/j.pcad.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 10.McCue JD, Swingen C, Feldberg T, Caron G, Kolb A, Denucci C, Prabhu S, Motilall R, Breviu B, Taylor DA. The real estate of myoblast cardiac transplantation: negative remodeling is associated with location. J Heart Lung Transplant. 2008;27(1):116–123. doi: 10.1016/j.healun.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagege AA, Marolleau JP, Vilquin JT, Alheritiere A, Peyrard S, Duboc D, Abergel E, Messas E, Mousseaux E, Schwartz K, Desnos M, Menasche P. Skeletal myoblast transplantation in ischemic heart failure: long-term follow-up of the first phase I cohort of patients. Circulation. 2006;114(1 Suppl):I108–113. doi: 10.1161/CIRCULATIONAHA.105.000521. [DOI] [PubMed] [Google Scholar]
- 12.Menasche P, Hagege AA, Scorsin M, Pouzet B, Desnos M, Duboc D, Schwartz K, Vilquin JT, Marolleau JP. Myoblast transplantation for heart failure. Lancet. 2001;357(9252):279–280. doi: 10.1016/S0140-6736(00)03617-5. [DOI] [PubMed] [Google Scholar]
- 13.Hagege AA, Carrion C, Menasche P, Vilquin JT, Duboc D, Marolleau JP, Desnos M, Bruneval P. Viability and differentiation of autologous skeletal myoblast grafts in ischaemic cardiomyopathy. Lancet. 2003;361(9356):491–492. doi: 10.1016/S0140-6736(03)12458-0. [DOI] [PubMed] [Google Scholar]
- 14.Lei Y, Haider H, Shujia J, Sim ES. Therapeutic angiogenesis. Devising new strategies based on past experiences. Basic Res Cardiol. 2004;99(2):121–132. doi: 10.1007/s00395-004-0447-x. [DOI] [PubMed] [Google Scholar]
- 15.Henry TD. Therapeutic angiogenesis. Bmj. 1999;318(7197):1536–1539. doi: 10.1136/bmj.318.7197.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18(1):4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 17.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 18.Haider H, Ye L, Jiang S, Ge R, Law PK, Chua T, Wong P, Sim EK. Angiomyogenesis for cardiac repair using human myoblasts as carriers of human vascular endothelial growth factor. J Mol Med. 2004;82(8):539–549. doi: 10.1007/s00109-004-0546-z. [DOI] [PubMed] [Google Scholar]
- 19.Ye L, Haider H, Jiang S, Ge R, Law PK, Sim EK. High efficiency transduction of human VEGF165 into human skeletal myoblasts: in vitro studies. Exp Mol Med. 2003;35(5):412–420. doi: 10.1038/emm.2003.54. [DOI] [PubMed] [Google Scholar]
- 20.Rissanen TT, Yla-Herttuala S. Current status of cardiovascular gene therapy. Mol Ther. 2007;15(7):1233–1247. doi: 10.1038/sj.mt.6300175. [DOI] [PubMed] [Google Scholar]
- 21.Marshall E. Gene therapy death prompts review of adenovirus vector. Science. 1999;286(5448):2244–2245. doi: 10.1126/science.286.5448.2244. [DOI] [PubMed] [Google Scholar]
- 22.Lehrman S. Virus treatment questioned after gene therapy death. Nature. 1999;401(6753):517–518. doi: 10.1038/43977. [DOI] [PubMed] [Google Scholar]
- 23.von Degenfeld G, Banfi A, Springer ML, Wagner RA, Jacobi J, Ozawa CR, Merchant MJ, Cooke JP, Blau HM. Microenvironmental VEGF distribution is critical for stable and functional vessel growth in ischemia. FASEB J. 2006;20(14):2657–2659. doi: 10.1096/fj.06-6568fje. [DOI] [PubMed] [Google Scholar]
- 24.Ozawa CR, Banfi A, Glazer NL, Thurston G, Springer ML, Kraft PE, McDonald DM, Blau HM. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest. 2004;113(4):516–527. doi: 10.1172/JCI18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carmeliet P. VEGF gene therapy: stimulating angiogenesis or angioma-genesis? Nat Med. 2000;6(10):1102–1103. doi: 10.1038/80430. [DOI] [PubMed] [Google Scholar]
- 26.Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation. 2000;102(8):898–901. doi: 10.1161/01.cir.102.8.898. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman AS. The origins and evolution of “controlled” drug delivery systems. J Control Release. 2008 doi: 10.1016/j.jconrel.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Mintzer MA, Simanek EE. Nonviral vectors for gene delivery. Chem Rev. 2009;109(2):259–302. doi: 10.1021/cr800409e. [DOI] [PubMed] [Google Scholar]
- 29.Lin C, Engbersen JF. Effect of chemical functionalities in poly(amido amine)s for non-viral gene transfection. J Control Release. 2008 doi: 10.1016/j.jconrel.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 30.Lynn DM, Anderson DG, Putnam D, Langer R. Accelerated discovery of synthetic transfection vectors: parallel synthesis and screening of a degradable polymer library. J Am Chem Soc. 2001;123(33):8155–8156. doi: 10.1021/ja016288p. [DOI] [PubMed] [Google Scholar]
- 31.Anderson DG, Lynn DM, Langer R. Semi-automated synthesis and screening of a large library of degradable cationic polymers for gene delivery. Angew Chem Int Ed Engl. 2003;42(27):3153–3158. doi: 10.1002/anie.200351244. [DOI] [PubMed] [Google Scholar]
- 32.Lim YB, Kim SM, Suh H, Park JS. Biodegradable, endosome disruptive, and cationic network-type polymer as a highly efficient and nontoxic gene delivery carrier. Bioconjug Chem. 2002;13(5):952–957. doi: 10.1021/bc025541n. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Wu D, Ma Y, Tang G, Wang S, He C, Chung T, Goh S. Novel poly(amino ester)s obtained from Michael addition polymerizations of trifunctional amine monomers with diacrylates: safe and efficient DNA carriers. Chem Commun (Camb) 2003;(20):2630–2631. doi: 10.1039/b309487a. [DOI] [PubMed] [Google Scholar]
- 34.Ou M, Wang XL, Xu R, Chang CW, Bull DA, Kim SW. Novel biodegradable poly(disulfide amine)s for gene delivery with high efficiency and low cytotoxicity. Bioconjug Chem. 2008;19(3):626–633. doi: 10.1021/bc700397x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim TI, Ou M, Lee M, Kim SW. Arginine-grafted bioreducible poly(disulfide amine) for gene delivery systems. Biomaterials. 2009;30(4):658–664. doi: 10.1016/j.biomaterials.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banfi A, Springer ML, Blau HM. Myoblast-mediated gene transfer for therapeutic angiogenesis. Methods Enzymol. 2002;346:145–157. doi: 10.1016/s0076-6879(02)46054-5. [DOI] [PubMed] [Google Scholar]
- 37.Menasche P. Skeletal myoblasts for cardiac repair: Act II?*. J Am Coll Cardiol. 2008;52(23):1881–1883. doi: 10.1016/j.jacc.2008.07.066. [DOI] [PubMed] [Google Scholar]
- 38.Murry CE, Wiseman RW, Schwartz SM, Hauschka SD. Skeletal myoblast transplantation for repair of myocardial necrosis. J Clin Invest. 1996;98(11):2512–2523. doi: 10.1172/JCI119070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, Glower DD, Kraus WE. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4(8):929–933. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- 40.Arsic N, Zacchigna S, Zentilin L, Ramirez-Correa G, Pattarini L, Salvi A, Sinagra G, Giacca M. Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol Ther. 2004;10(5):844–854. doi: 10.1016/j.ymthe.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995;92(16):7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang DA, Narang AS, Kotb M, Gaber AO, Miller DD, Kim SW, Mahato RI. Novel branched poly(ethylenimine)-cholesterol water-soluble lipopolymers for gene delivery. Biomacromolecules. 2002;3(6):1197–1207. doi: 10.1021/bm025563c. [DOI] [PubMed] [Google Scholar]
- 43.Zhong Z, Feijen J, Lok MC, Hennink WE, Christensen LV, Yockman JW, Kim YH, Kim SW. Low molecular weight linear polyethylenimine-b-poly(ethylene glycol)-b-polyethylenimine triblock copolymers: synthesis, characterization, and in vitro gene transfer properties. Biomacromolecules. 2005;6(6):3440–3448. doi: 10.1021/bm050505n. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki K, Murtuza B, Smolenski RT, Sammut IA, Suzuki N, Kaneda Y, Yacoub MH. Cell transplantation for the treatment of acute myocardial infarction using vascular endothelial growth factor-expressing skeletal myoblasts. Circulation. 2001;104(12 Suppl 1):1207–212. doi: 10.1161/hc37t1.094524. [DOI] [PubMed] [Google Scholar]