Abstract
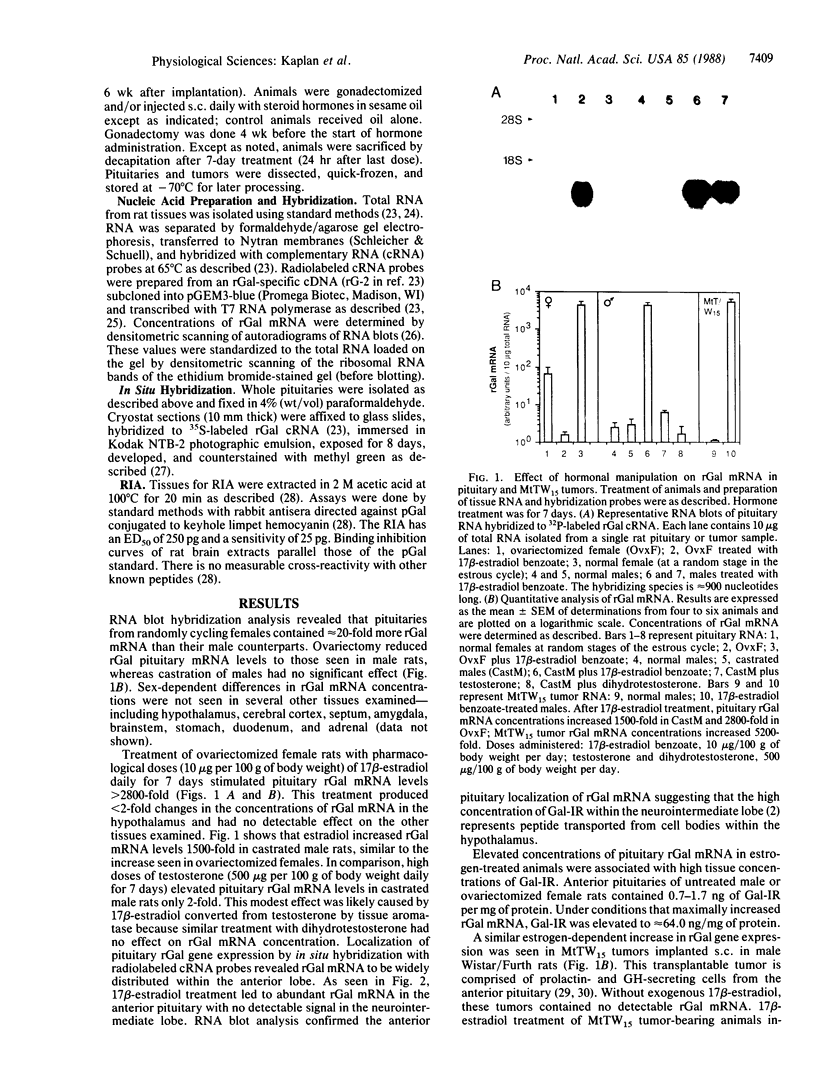
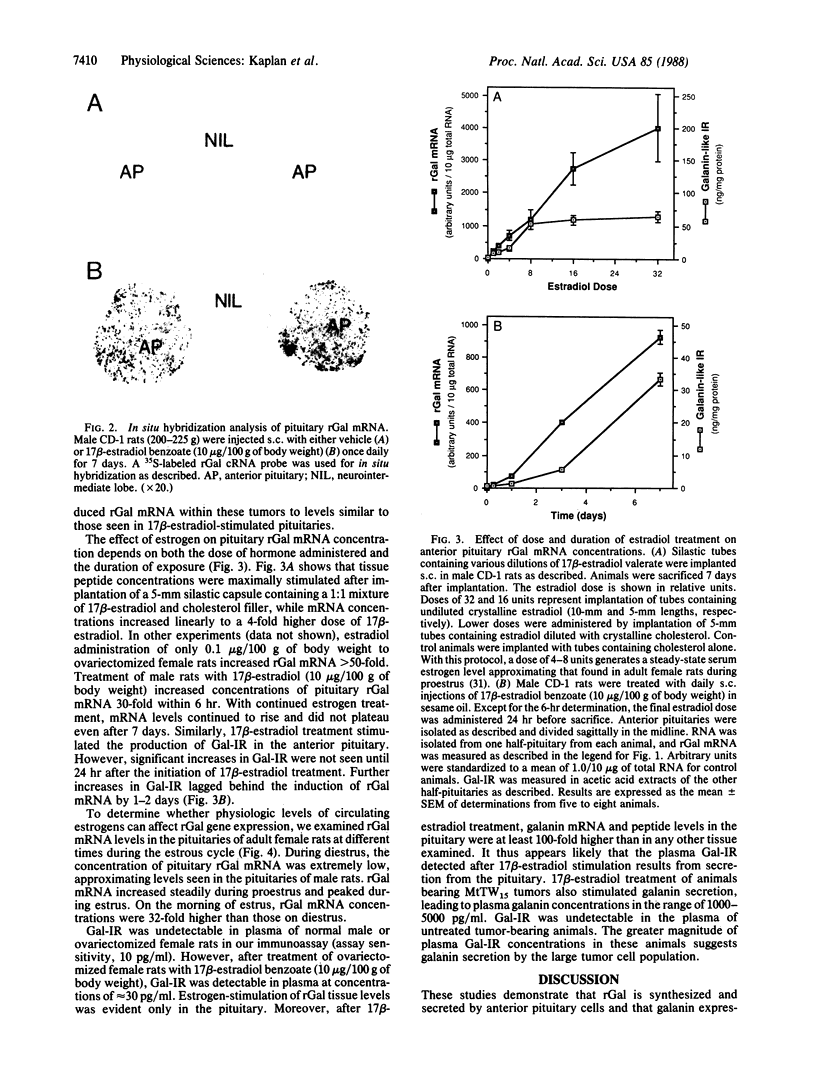
Galanin is a peptide widely distributed throughout vertebrate central and peripheral nervous systems. Although its precise physiologic role is unknown, it can stimulate the pituitary secretion of prolactin and growth hormone. We examined the control of rat galanin (rGal) gene expression in the anterior pituitary using RNA blot and in situ hybridization analyses and using specific RIA. Pituitaries of normal male and ovariectomized female rats contained little detectable rGal mRNA. Treatment of these animals with 17 beta-estradiol increased pituitary rGal mRNA up to 4000-fold. These increases depended on time and dose of estrogen administration and correlated with up to 50-fold increases in pituitary galanin-like immunoreactivity. Galanin-like immunoreactivity was detectable in the plasma of estrogen-treated animals. Pituitary levels of rGal mRNA in female rats varied greater than 30-fold during the estrous cycle, with a peak on estrus and a nadir on diestrus. Estrogen-induced rGal gene expression was also observed in transplantable MtTW15 prolactin- and growth hormone-containing tumors but not in neuronal tissues expressing this gene. These data demonstrate that rGal is a secreted product of rat anterior pituitary cells, where its gene expression is strongly affected by physiologic levels of circulating estrogen.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amiranoff B., Servin A. L., Rouyer-Fessard C., Couvineau A., Tatemoto K., Laburthe M. Galanin receptors in a hamster pancreatic beta-cell tumor: identification and molecular characterization. Endocrinology. 1987 Jul;121(1):284–289. doi: 10.1210/endo-121-1-284. [DOI] [PubMed] [Google Scholar]
- Bauer F. E., Adrian T. E., Yanaihara N., Polak J. M., Bloom S. R. Chromatographic evidence for high-molecular-mass galanin immunoreactivity in pig and cat adrenal glands. FEBS Lett. 1986 Jun 9;201(2):327–331. doi: 10.1016/0014-5793(86)80633-0. [DOI] [PubMed] [Google Scholar]
- Bauer F. E., Ginsberg L., Venetikou M., MacKay D. J., Burrin J. M., Bloom S. R. Growth hormone release in man induced by galanin, a new hypothalamic peptide. Lancet. 1986 Jul 26;2(8500):192–195. doi: 10.1016/s0140-6736(86)92490-6. [DOI] [PubMed] [Google Scholar]
- Cella S. G., Locatelli V., De Gennaro V., Bondiolotti G. P., Pintor C., Loche S., Provezza M., Müller E. E. Epinephrine mediates the growth hormone-releasing effect of galanin in infant rats. Endocrinology. 1988 Mar;122(3):855–859. doi: 10.1210/endo-122-3-855. [DOI] [PubMed] [Google Scholar]
- Clemens T. L., Garrett K. P., Zhou X. Y., Pike J. W., Haussler M. R., Dempster D. W. Immunocytochemical localization of the 1,25-dihydroxyvitamin D3 receptor in target cells. Endocrinology. 1988 Apr;122(4):1224–1230. doi: 10.1210/endo-122-4-1224. [DOI] [PubMed] [Google Scholar]
- Davis T. M., Burrin J. M., Bloom S. R. Growth hormone (GH) release in response to GH-releasing hormone in man is 3-fold enhanced by galanin. J Clin Endocrinol Metab. 1987 Dec;65(6):1248–1252. doi: 10.1210/jcem-65-6-1248. [DOI] [PubMed] [Google Scholar]
- Dunning B. E., Ahren B., Veith R. C., Böttcher G., Sundler F., Taborsky G. J., Jr Galanin: a novel pancreatic neuropeptide. Am J Physiol. 1986 Jul;251(1 Pt 1):E127–E133. doi: 10.1152/ajpendo.1986.251.1.E127. [DOI] [PubMed] [Google Scholar]
- Gabriel S. M., MacGarvey U. M., Koenig J. I., Swartz K. J., Martin J. B., Beal M. F. Characterization of galanin-like immunoreactivity in the rat brain: effects of neonatal glutamate treatment. Neurosci Lett. 1988 Apr 22;87(1-2):114–121. doi: 10.1016/0304-3940(88)90155-3. [DOI] [PubMed] [Google Scholar]
- Gharib S. D., Bowers S. M., Need L. R., Chin W. W. Regulation of rat luteinizing hormone subunit messenger ribonucleic acids by gonadal steroid hormones. J Clin Invest. 1986 Feb;77(2):582–589. doi: 10.1172/JCI112340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan L. M., Spindel E. R., Isselbacher K. J., Chin W. W. Tissue-specific expression of the rat galanin gene. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1065–1069. doi: 10.1073/pnas.85.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiyama H., Kato Y., Inoue T., Murakami Y., Ishikawa Y., Yanaihara N., Imura H. Central galanin stimulates pituitary prolactin secretion in rats: possible involvement of hypothalamic vasoactive intestinal polypeptide. Neurosci Lett. 1987 Mar 20;75(1):49–54. doi: 10.1016/0304-3940(87)90073-5. [DOI] [PubMed] [Google Scholar]
- Legan S. J., Coon G. A., Karsch F. J. Role of estrogen as initiator of daily LH surges in the ovariectomized rat. Endocrinology. 1975 Jan;96(1):50–56. doi: 10.1210/endo-96-1-50. [DOI] [PubMed] [Google Scholar]
- Malarkey W. B., Daughaday W. H. Divergent effects of medroxyprogesterone acetate on growth hormone and prolactin in the MStTW15 tumor bearing rat. Endocrinology. 1972 Apr;90(4):909–914. doi: 10.1210/endo-90-4-909. [DOI] [PubMed] [Google Scholar]
- Manabe T., Yoshimura T., Kii E., Tanaka Y., Ohshio G., Tobe T., Akaji K., Yajima H. Galanin-induced hyperglycemia: effect on insulin and glucagon. Endocr Res. 1986;12(1):93–98. doi: 10.1080/07435808609023655. [DOI] [PubMed] [Google Scholar]
- Meister B., Hulting A. L. Influence of coexisting hypothalamic messengers on growth hormone secretion from rat anterior pituitary cells in vitro. Neuroendocrinology. 1987 Nov;46(5):387–394. doi: 10.1159/000124849. [DOI] [PubMed] [Google Scholar]
- Melander T., Fuxe K., Härfstrand A., Eneroth P., Hökfelt T. Effects of intraventricular injections of galanin on neuroendocrine functions in the male rat. Possible involvement of hypothalamic catecholamine neuronal systems. Acta Physiol Scand. 1987 Sep;131(1):25–32. doi: 10.1111/j.1748-1716.1987.tb08201.x. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Kato Y., Koshiyama H., Inoue T., Yanaihara N., Imura H. Galanin stimulates growth hormone (GH) secretion via GH-releasing factor (GRF) in conscious rats. Eur J Pharmacol. 1987 Apr 29;136(3):415–418. doi: 10.1016/0014-2999(87)90316-5. [DOI] [PubMed] [Google Scholar]
- Nordström O., Melander T., Hökfelt T., Bartfai T., Goldstein M. Evidence for an inhibitory effect of the peptide galanin on dopamine release from the rat median eminence. Neurosci Lett. 1987 Jan 2;73(1):21–26. doi: 10.1016/0304-3940(87)90024-3. [DOI] [PubMed] [Google Scholar]
- Ottlecz A., Samson W. K., McCann S. M. Galanin: evidence for a hypothalamic site of action to release growth hormone. Peptides. 1986 Jan-Feb;7(1):51–53. doi: 10.1016/0196-9781(86)90060-4. [DOI] [PubMed] [Google Scholar]
- Rökaeus A., Brownstein M. J. Construction of a porcine adrenal medullary cDNA library and nucleotide sequence analysis of two clones encoding a galanin precursor. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6287–6291. doi: 10.1073/pnas.83.17.6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu A., Crowley W. R., Tatemoto K., Balasubramaniam A., Kalra S. P. Effects of neuropeptide Y, NPY analog (norleucine4-NPY), galanin and neuropeptide K on LH release in ovariectomized (ovx) and ovx estrogen, progesterone-treated rats. Peptides. 1987 Sep-Oct;8(5):921–926. doi: 10.1016/0196-9781(87)90081-7. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., McKnight G. S., Shapiro D. J., Sullivan D., Palacios R. Hormonal regulation of ovalbumin synthesis in the chick oviduct. Recent Prog Horm Res. 1975;31:175–211. doi: 10.1016/b978-0-12-571131-9.50009-8. [DOI] [PubMed] [Google Scholar]
- Servin A. L., Amiranoff B., Rouyer-Fessard C., Tatemoto K., Laburthe M. Identification and molecular characterization of galanin receptor sites in rat brain. Biochem Biophys Res Commun. 1987 Apr 14;144(1):298–306. doi: 10.1016/s0006-291x(87)80510-7. [DOI] [PubMed] [Google Scholar]
- Silvestre R. A., Miralles P., Monge L., Moreno P., Villanueva M. L., Marco J. Effects of galanin on hormone secretion from the in situ perfused rat pancreas and on glucose production in rat hepatocytes in vitro. Endocrinology. 1987 Jul;121(1):378–383. doi: 10.1210/endo-121-1-378. [DOI] [PubMed] [Google Scholar]
- Silvestre R. A., Miralles P., Monge L., Villanueva M. L., Marco J. Inhibitory effect of galanin on pancreatic polypeptide release by the perfused rat pancreas. Life Sci. 1987 May 4;40(18):1829–1833. doi: 10.1016/0024-3205(87)90094-4. [DOI] [PubMed] [Google Scholar]
- Sinha D. K., Meites J. Direct effects of a hypothalamic extract on hormone secretion by a pituitary mammo-somatotropic tumor. Endocrinology. 1967 Jan;80(1):131–134. doi: 10.1210/endo-80-1-131. [DOI] [PubMed] [Google Scholar]
- Tata J. R. The expression of the vitellogenin gene. Cell. 1976 Sep;9(1):1–14. doi: 10.1016/0092-8674(76)90047-7. [DOI] [PubMed] [Google Scholar]
- Tatemoto K., Rökaeus A., Jörnvall H., McDonald T. J., Mutt V. Galanin - a novel biologically active peptide from porcine intestine. FEBS Lett. 1983 Nov 28;164(1):124–128. doi: 10.1016/0014-5793(83)80033-7. [DOI] [PubMed] [Google Scholar]
- Vrontakis M. E., Peden L. M., Duckworth M. L., Friesen H. G. Isolation and characterization of a complementary DNA (galanin) clone from estrogen-induced pituitary tumor messenger RNA. J Biol Chem. 1987 Dec 15;262(35):16755–16758. [PubMed] [Google Scholar]
- de Weille J., Schmid-Antomarchi H., Fosset M., Lazdunski M. ATP-sensitive K+ channels that are blocked by hypoglycemia-inducing sulfonylureas in insulin-secreting cells are activated by galanin, a hyperglycemia-inducing hormone. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1312–1316. doi: 10.1073/pnas.85.4.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]








