Abstract
We have shown recently that cyclophosphamide (CTX) treatment induced a marked increase in the numbers of immature dendritic cells (DCs) in blood, coinciding with enhanced antigen-specific responses of the adoptively transferred CD8+ T cells. Because this DC expansion was preceded by DC proliferation in bone marrow (BM), we tested whether BM post CTX treatment can generate higher numbers of functional DCs. BM was harvested three days after treatment of C57BL/6 mice with PBS or CTX and cultured with GM-CSF/IL-4 in vitro. Compared with control, BM from CTX-treated mice showed faster generation and yielded higher numbers of DCs with superior activation in response to toll-like receptor (TLR) agonists. Vaccination with peptide-pulsed DCs generated from BM from CTX-treated mice induced comparable adjuvant effects to those induced by control DCs. Taken together, post CTX BM harbors higher numbers of DC precursors capable of differentiating into functional DCs, which be targeted to create host microenvironment riches in activated DCs upon treatment with TLR agonists.
Keywords: Dendritic cells, Bone marrow, Cyclophosphamide, Toll-like receptor ligands, TLR, Vaccination
Introduction
Cyclophosphamide (CTX) is a common anti-cancer chemotherapeutic agent used alone or in combination with other chemotherapeutic drugs for the treatment of several human malignancies [1; 2]. Recent studies have also demonstrated that preconditioning a recipient host with CTX-induced lymphodepletion regimen prior adoptive transfer of T cells can significantly improve the homeostasis-driven responses (activation, proliferation and functions) [3; 4] as well as the antigen-specific responses of the adoptively transferred T-cells upon vaccination with MHC class I or MHC class II peptides [5–8]. Even in absence of adoptive T cell transfer, CTX preconditioning regimen can also augment T-cell responses to active vaccination, including DC-based vaccination [8–18]. Mechanisms that have been suggested to underlie these beneficial effects of CTX-induced lymphodepletion to T cell responses include: 1) enhanced engraftment and survival of the transferred T cells by creation of an immunological “niche” [19]; 2) induction of survival cytokines [5; 7; 20; 21]; 3) elimination of regulatory CD4+CD25+ T cells [9; 21–29], and 4) depletion of endogenous cells that compete with the transferred T cells for cytokines “i.e. elimination of the cytokine sink” [5; 19; 30–37]. Recent studies would suggest, however, that these mechanisms might not be the principal means by which lymphodepletion augments adoptive immunotherapy [7; 21; 37; 38–40]. Therefore, understanding the precise mechanisms of how CTX alters the host microenvironment is of a great significance to improve the clinical application of this drug in cancer immunotherapy.
Recently, we have reported that CTX preconditioning increases the numbers of DCs in the peripheral blood from days 9–16 [7]. Moreover, the expanded DCs significantly contributed to the beneficial effects of CTX to adoptive T cell therapy since their depletion reduced the antigen-specific expansion of the adoptively transferred CD8+ T cells [7]. In line with our studies, previous studies also showed that CTX can induce myelomonocytosis, including DCs, where its enhanced anti-tumor effects associated with recruitment of a large pool of DCs in the peripheral and the tumor site [7; 21; 41–43]. Of note, we have found that CTX-induced DC expansion was preceded by proliferation of cells with DC phenotype (CD11c+CD11b+Ly6G−) in the BM early (2–3 days) after CTX treatment [43]. This observation is consistent with the capability of CTX to induce mobilization of hematopoietic stem cells from BM to circulation alone or in combination with granulocyte-colony stimulating factor (G-CSF) [42; 44; 45; 46; 47; 48; 49]. These hematopoietic stem cells harvested from peripheral blood of cancer patients treated with CTX gave rise to higher yield of DCs in vitro than their control counterpart [50; 51; 52; 53; 54]. These studies indicate to the important role of DCs in mediation of the mechanisms of CTX to T cell responses.
The capability of CTX to expand DCs in vivo led us to evaluate whether BM post CTX treatment has the capability to generate higher numbers of DCs and whether they can be respond to toll-like receptor (TLR) agonists in vitro and therefore benefit T cell responses in vivo. Our data showed that BM harvested from CTX-treated mice generated higher number of DCs with superior activation phenotype in response to stimulation with different TLR agonists as compared to their control counterpart. Additionally, DCs generated from CTX-treated mice were capable of inducing T-cell response in vivo. Taken together, our results indicate that post CTX BM harbors higher numbers of DC precursors that could be targeted in vivo to create a host environment riches in DCs that can be activated by TLR agonists to enhance the application of CTX in cancer immunotherapy.
Materials and Methods
Mice
Females B6.SJL (Ly5.1) and C57BL/6 (Ly5.2) mice (8-week old) were purchased from The Jackson Laboratory (Bar Harbor, ME). OT-1 T cell receptor (TCR) transgenic (Vα2/Vβ5) mice on Ly5.1 background were bred with B6.SJL mice to generate Ly5.1+/Ly5.1+ mice heterozygous for the OT-1 TCR transgene. Presence of the transgene was confirmed by flow cytometry with mAb specific for Vα2. All animals were housed under specific pathogen-free conditions at the Medical University of South Carolina in accordance with institutional and federal guidelines.
Antibodies and reagents
Anti-CD16/CD32, and FITC-, PE-, APC-, and cytochrome-conjugated mAbs, including anti-Ly5.1, anti-CD8, anti-CD11c, anti-CD11b, anti-CD40, anti-CD80, anti-CD86, CD8 and Ly6G were purchased from Pharmingen (San Diego, CA). SIINFEKL, an ovalbumin (OVA) MHC class-I peptide, was purchased from American Peptide Company, Inc. (Sunnyvale, CA). Peptide was dissolved in 10% dimethy sulfoxide (DMSO) (Sigma, St. Louis, MO) and diluted in PBS to the indicated concentrations. CTX was purchased from (Sigma, St. Louis, MO), stored at −70°C, and reconstituted in PBS before use. Murine recombinant GM-CSF and IL-4 cytokines were purchased from R&D systems (Minneapolis, MN).
Generation of DCs from BM cells
BM-derived DCs were generated as we previously described [55–57]. Briefly, BM was flushed from the femurs and tibias of mice and then depleted of red blood cells by lysis with ACK buffer (Biofluids, Camarillo, CA). Equal numbers of BM cells from PBS-treated and CTX-treated mice were suspended in complete RPMI and then supplemented with murine GM-CSF (10ng/mL) and murine IL-4 (10ng/mL) and cultured in 175 T flasks at 1 × 106 cells/ml. On day 4 of culture, complete RPMI medium containing the same amount of cytokines was added to increase the total volume by 50%. On day 7, non-adherent and loosely adherent DCs were harvested, washed twice and the phenotype of DCs (CD11c+CD11b+) were confirmed by the flow cytometry. In some experiments, generated DCs were harvested on day 4 of the culture for phonotypic and activation analysis.
Treatment of DCs with TLR agonists
Non-adherent cells were harvested on day 7 from BM cultures, washed twice with PBS and then counted with hemocytometer. Cells were seeded into 6-well plates and then treated with different TLR agonists, including pam3CysK4 (a TLR2/6 agonist; 50μg/ml; Sigma-Aldrich), polyinosinic:polycytidylic acid; poly(I:C) (a TLR3 agonist; 25μg/ml); Sigma-Aldrich, St. Louis, MO), lipopolysaccharide (LPS) (a TLR4 agonist, 10ng/ml; Sigma-Aldrich), imiquimod (1-(2-methylpropyl)-1 H-imidazo[4,5-c] quinolin-4-amine) (a TLR7/8 agonist; 10μg/ml; Coley Pharmaceutical Group), or CpG ODN (a TLR9 agonist; 4μg/ml) (Coley Pharmaceutical Group) for 24 hours.
Adoptive transfer of OT-1 T cells and vaccination
Spleens and lymph nodes from naïve OT-1 TCR transgenic mice were harvested under sterile conditions, homogenized, and washed in HBSS (Cellgro). Pooled cells were then passed over a CD8− selection column from R&D Systems (Minneapolis, MN) to collect the CD8+ T-cells. Naive CD8+Ly5.1+ OT-1 T cells (1.5 × 106) were adoptively transferred into naive congenic C57BL/6 Ly5.2+ recipient mice and monitored by flow cytometry with anti-Ly5.1 and anti-CD8 mAb. After adoptive transfer, this antigen-specific CD8+ T-cell population represented approximately 0.3% of cells in the lymphoid organs. For vaccination with DCs, the non-adherent population which generated from BMPBS or BMCTX were harvested on day 7 and stimulated with LPS for 24 hours followed by pulsing with 5 μg/ml of SIINFIKLE peptide for 2 hours at 37°C. One million DCs were injected intravenously (i.v.) into mice adoptively transferred 1 day before with 1×106 Ly5.1 OT-1 cells. Single intraperitoneal (i.p.) injection of PBS or 200μg/mouse poly(I:C) was performed immediately after DC vaccination. On day 7 of DC vaccination, mice were bled to analyze OT-1 cells numbers in the peripheral blood.
Flow cytometry
Fresh single-cell suspensions were prepared and 1 × 106 cells were treated with anti-CD16/CD32 for 5 min on ice. Cells were then stained with the indicated conjugated mAb, and incubated for 30 min on ice. These cells were washed twice and re-suspended in 0.3 mL of 0.5% BSA, 0.02% sodium azide solution. Cells were analyzed by flow cytometry using the Cell Quest software package (Becton Dickinson, San Jose, CA).
Analysis of gene expression of TLRs in DCs by real time PCR analysis
On day 7 of the culture, non-adherent cells were harvested, washed out twice with PBS and the total RNA was isolated from the cells using Stat-40 (Invitrogen, Carlsbad, CA). The RNA was then reverse transcribed to a single-stranded cDNA according to the manufacturer's protocol (Invitrogen). Real-time PCR was performed on a Gene Amp 7300 Sequence Detection System (PE Biosystems, Foster City, CA). The primer-pairs for the gene analysis are listed in Table 1. The sequences of TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, and TLR9 were designed as previously described in [58] and as shown in Table 1. For a given real-time PCR sample, the RNA expression level was calculated from cycle threshold value with the Rockit program. In our analysis, we normalized the results to a reference control gene, beta2-microglobin, and reported as the expression level as mean normalized expression.
Table 1.
Primers used in the analysis of mouse TLR gene expression
| Genbank No | Primer | Sequence (5' to 3') |
|---|---|---|
| NM_009735 | B2M F | 5'-TGTCTCACTGACCGGCCTGTAT-3' |
| B2M R | 5'-GTTCAGTATGTTCGGCTTCCCA-3' | |
| NM_030682 | Tlr1F | 5'- TTCCGTGATGCACAGCTCCTT-3 |
| Tlr1R | 5'-TCTGCTCGCCTGAGTTCTTCA-3' | |
| NM_011905 | Tlr2 F | 5'-CCAAGAGGAAGCCCAAGAAAG-3' |
| Tlr2 R | 5'-AGGCATCATAGCAAACGTCCC-3' | |
| NM_126166 | Tlr3 F | 5'-CTTGCGTTGCGAAGTGAAGAA-3' |
| Tlr3 R | 5'-CCAATTGTCTGGAAACACCCC-3' | |
| NM_021297 | Tlr4 F | 5'-AGCAGGTGGAATTGTATCGCC-3' |
| Tlr4 R | 5'-CCCATTCCAGGTAGGTGTTTCT-3' | |
| NM_016928 | Tlr5 R | 5' -ATATCCACCGAAGACTGCGATG-3' |
| Tlr5 R | 5' -AGTGACCGTGCACAGGATGAA-3' | |
| NM_011604 | Tlr6 F | 5'-GAATGTGACCCTCCAGCACAT-3' |
| Tlr6 R | 5'-AGTTTAACCGAGCACTTCCAGG-3' | |
| NM_133211 | Tlr7 F | 5'-CTGGAGTTCAGAGGCAACCATT-3' |
| Tlr7 R | 5'-GTTATCACCGGCTCTCCATAGAA-3' | |
| NM_133212 | Tlr8 F | 5'-GCCTCAGAGCCTCCAAGAGTTA-3' |
| Tlr8 R | 5'-CCAGCAAGTGAAGGTGAGGAA-3' | |
| NM_031178 | Tlr9 F | 5'-AGCTGAACATGAACGGCATCT-3' |
| Tlr9 R | 5'-TGAGCGTGTACTTGTTGAGCG-3' | |
| NM_205819 | Tlr11 F | 5'-CTTGCATTTCCTCTCCCTTGTG-3' |
| Tlr11 R | 5'-AGGTCAAGTGCACGAAGCTCA-3' | |
| NM_205823 | Tlr12 F | 5'-CCAAATACGGATGAGCCCAGA-3' |
| Tlr12 F | 5'-AGGAACAATACTGCCGGAGCA-3' | |
| NM_205820 | Tlr13 F | 5'-TTGTCACCTGCTCGGAAACCTA-3' |
| Tlr13 F | 5'-GCTGCTTAATGCCCTCTGCAT-3' |
Statistics
Numerical data obtained from each experiment were expressed as mean ± SD and the statistical differences between experimental and control groups were assessed using the Student's t-test. P values less than 0.05 were considered statistically significant.
Results
CTX treatment induces higher frequency of DCs in BM
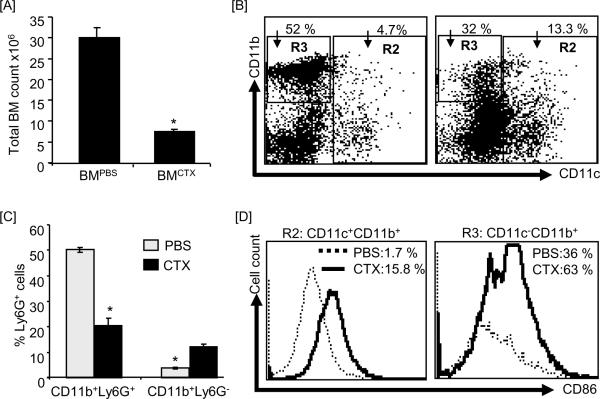
We have recently found that the increases in the numbers of DCs in the peripheral blood after treatment of B6 mice with CTX is preceded by a rapid (in 3 days) increase in the numbers of proliferating cells in BM expressing DC phenotype (CD11c+CD11b+) [43], indicating that post CTX therapy DC precursors in BM possess a high proliferation capacity. Therefore, we first determined the numbers of DCs in BM harvested 3 days after treatement with CTX or PBS. As expected, the total number of mononuclear cells in BM from CTX-treated mice was lower than in BM from PBS-treated mice (Fig. 1A) due to the CTX-induced lymphopenia as we previously reported [21]. Phenotypically DCs are characterized as CD11c+CD11b+, while other myeloid (macrophages and neutrophils) can be characterized as CD11c−CD11b+Ly6G+. We gated on the total CD11c+ populations (CD11c+CD11b+ + CD11c+CD11b−) shown in R2 Figure 1B and on CD11b+CD11c− population shown in R3 in Figure 1B. As compared to BM from PBS-treated mice, BM from CTX-treated mice showed higher numbers of the total CD11c+ (i.e. CD11c+CD11b+ added to CD11c+CD11b−; 13.3% versus 4.7%) and lower number of CD11c−CD11b+ cells (32% versus 52%) (Fig. 1B). Most of CD11c+ cells were CD11+CD11b−. Further analysis showed that BM from CTX-treated mice has higher numbers of CD11b+Ly6G− cells and lower numbers of CD11b−Ly6G+ cells as opposed to BM from PBS-treated mice (Fig. 1C). The absolute numbers of these populations were lower than in the control BM (data not shown) due to the presence of lymphopenia in CTX-treated mice (Fig. 1A). Because DCs differentiate from CD11c+CD11b−, CD11c+CD11b+, and CD11c−CD11b+ populations, these data suggest BM from CTX-treated mice harbors higher numbers of DC precursors than BM from PBS-treated mice. We then analyzed the expresion levels of the activation marker CD86 in cells gated in R2 and R3 of BM from PBS-treated mice and BM from CTX-treated mice. As shown in Figure 1D, the total CD11c+ populations (R2: CD11c+CD11b+ + CD11c+CD11b−) as well as the CD11c−CD11b+ population (R3) in BM from CTX-treated mice (bold lines) expressed higher levels of the costimulatory molecule CD86 than those in BM from PBS-treated mice (dashed lines).
Figure 1. CTX treatment induced an increased level of DCs in fresh BM.
C57BL/6 mice were i.p. treated with PBS (n=3 mice/group) or 4mg/mouse CTX (n=6 mice/group) and BM was harvested 3 days after treatments. BM single cell suspensions were prepared and stained with anti-CD11c, anti-CD11b, anti-Ly6G (Gr-1), and anti-CD86 mAbs. (A) Shows the total cell number of BM cells. (B) Shows the expression levels of CD11c and CD11b in the total BM. Cells are gated on CD11c+CD11b+ and CD11c+CD11b− (R2) populations and on CD11c−CD11b+ population (R3). (C) Shows the expression level of Ly6G in the total BM cells. (D) Shows the expression levels of CD86 on cells gated on R2 (gated on CD11c+CD11b+ + CD11c−CD11b+) and R3 (gated on CD11c−CD11b+). *, Statistically significant (P<0.05).
BM from CTX-treated mice can generate higher number of DC in vitro
Because BM from CTX-treated mice showed higher frequency of DC precursor (Fig. 1), we then asked whether it is capable of generating higher numbers of subsequent DCs in vitro as opposed to BM from PBS-treated mice. BM was collected 3 days after treatment of mice with PBS (n=3) or CTX (n=6). We chose day 3 because we have found previously that the numbers of proliferating DCs in BM was the highest at this time point after CTX treatment [43]. Equal numbers of BM from PBS-treated mice and BM from CTX-treated mice cells were cultured in the presence of GM-CSF and IL-4 as described in the Materials and Methods. To determine wheher DC precursors in BM from CTX-treated mice differentiate into DCs faster than those in BM from PBS-treated mice, we analyzed the total number of DCs and other myeloid cells in the non-adherent and adherent populations generated after 4 and 7 days of culturing BM from CTX-treated mice and BM from PBS-treated mice.
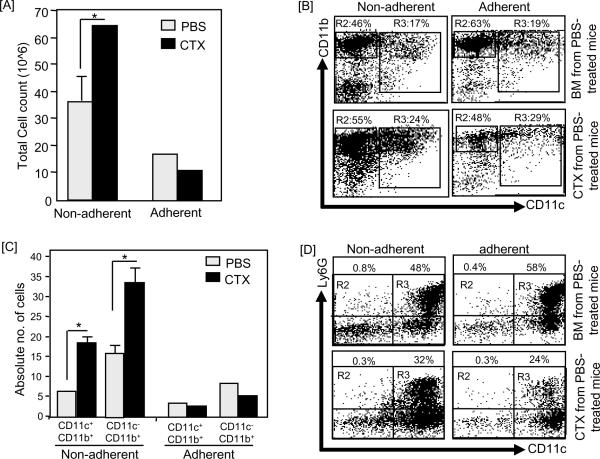
On day 4 of BM culture, the total numbers of the non-adherent cells harvested from BM from CTX-treated mice (Fig. 2A) were higher while the total numbers of the adherent cells were slightly lower as compared to those harvested from BM from PBS-treated mice. The relative numbers of cells expressing both CD11c and CD11b increased in BM from CTX-treated mice, which showed 24% CD11c+CD11b+ cells in the non-adherent population versus 17% in BM from PBS-treated mice; and 29% in the adherent population versus 19% in BM from PBS-treated mice. Although the numbers of CD11c−CD11b+ cells in the non-adherent populations of BM from CTX-treated mice increased from 46% to 55%, it was decreased in the adherent population from 63% to 48% (Fig. 2B). Interestingly, the absolute numbers of both CD11c+CD11b+ and CD11c−CD11b+ populations in BM from CTX-treated mice were significantly higher than those in control (Fig. 2C).
Figure 2. Non-adherent population harvested on day-4 culture of BM from CTX-treated mice showed higher number of CD11c+ cells.
C57BL/6 mice were i.p. treated with PBS (n=3 mice/group) or 4mg/mouse CTX (n=6 mice/group) and BM was harvested 3 days after treatments. BM single cell suspensions were prepared and cultured with 10ng/mL of GM-CSF and IL-4. On day 4, the non-adherent and adherent populations were harvested, counted and their phenotypic characterization was analyzed by flow cytometry after staining with anti-CD11c, anti-CD11b, and anti-Ly6G mAbs. (A) Shows the total cell number of the non-adherent and adherent populations. (B) Shows the expression levels of CD11b and CD11c in the non-adherent (left panel) and adherent (right panel) cells in BM from PBS-treated mice (upper panel) and BM from CTX-treated mice (lower panel). (C) Shows the absolute numbers of CD11c+ and CD11b+ cells in the non-adherent and adherent populations from BM from PBS-treated mice (upper panel) and BM from CTX-treated mice (lower panel). The absolute number of cells was calculated by multiplying the % cells with a certain phenotype by the total number of the non-adherent and adherent cells. (D) Shows the expression levels of CD11c and Ly6G in in the non-adherent (left panel) and adherent (right panel) in BM from PBS-treated mice (upper panel) and BM from CTX-treated mice (lower panel). *, Statistically significant (P<0.05).
As shown in Figure 1C, DCs (CD11c+CD11b+) in BM also expressed significant levels of the myeloid marker Ly6G, indicating to their immature phenotype. Because expression of Ly6G in DCs decreases during their differentaion [59; 60], we analyzed its expression in the non-adherent and adherent populations of BM from CTX-treated mice and BM from PBS-treated mice on day 4. Interstingly, the expression of this myleoid marker on CD11c+ cells in the non-adherent and adherent populations generated on day 4 from BM from CTX-treated mice was lower than those generated from BM from PBS-treated mice (Fig. 2D). In the non-adherent population generated from BM from CTX-treated mice, 24% CD11c+ cells expressed Ly6G as compared to 58% in BM from PBS-treated mice (Fig. 2D). In the adherent population of BM from CTX-treated mice, 32% CD11c+ cells expressed Ly6G as compared to 48% CD11c+ in BM from PBS-treated mice (Fig. 2D).
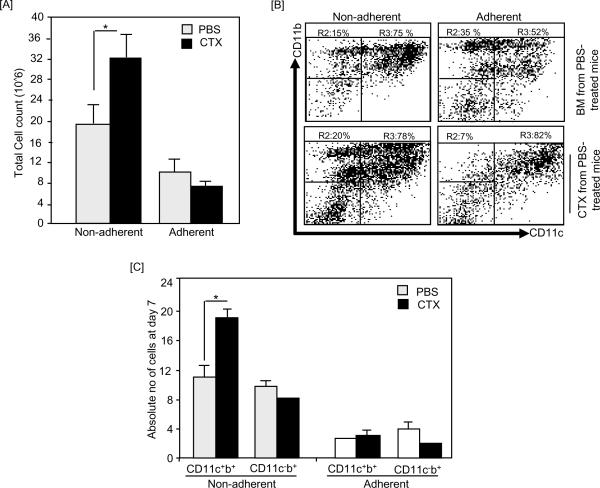
On day 7 of BM culture, the total numbers of the non-adherent cells harvested from BMCTX were higher with no significant alteration in the total numbers of the adherent cells (Fig. 3A). The relative numbers of CD11c+CD11b+ cells in BM from PBS-treated mice and BM from CTX-treated mice were higher than on day 4. In the non-adherent population, 78% and 75% of cellls were CD11c+CD11b+ in BM from CTX-treated mice and BM from PBS-treated mice, respectively (Fig. 3B). In the adherent population, BM from CTX-treated mice showed higher level CD11c+CD11b+ cells (82% versus 52% in control) but lower level of CD11c−CD11b+ cells (7% versus 35% in control) (Fig. 3B). Consistent with the relative numbers, the absolute numbers of CD11c+CD11b+ cells in In the non-adherent populations generated from BM from CTX-treated mice were higher than those generated from BM from PBS-treated mice (Fig. 3C). As expected, the expression level of both Ly6G on DCs on day 7 was very low (data not shown).
Figure 3. Non-adherent population of day 7 BM from CTX-treated mice culture showed higher number of CD11c+ cells.
C57BL/6 mice were i.p. treated with PBS (n=3 mice/group) or 4mg/mouse CTX (n=6 mice/group) and BM was harvested 3 days after treatments. BM single cell suspensions were prepared and cultured with 10ng/mL of GM-CSF and IL-4. On day 4, the non-adherent and adherent cells were harvested, counted and their phenotypic characterization was analyzed by flow cytometry. (A) Shows the total cell number of the non-adherent and adherent populations of BM from PBS-treated mice and BM from CTX-treated mice. (B) Shows the expression levels of CD11b and CD11c in the non-adherent (left panel) and adherent (right panel) cells of BM from PBS-treated mice (upper panel) and BM from CTX-treated mice (lower panel). (C) Shows the absolute numbers of CD11c+ and CD11b+ subsets in the non-adherent and adherent populations of BM from PBS-treated mice and BM from CTX-treated mice harvested on day 7. The absolute number of cells was calculated by multiplying the % cells with a certain phenotype by the total number of non-adherent and adherent cells. *, Statistically significant (P<0.05).
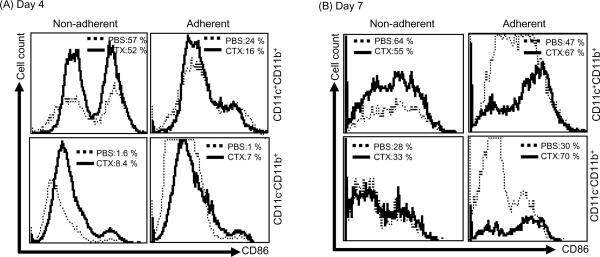
To get more insight into the quality of CD11c+CD11b+ and CD11c−CD11b+ cells generated from BM from CTX-treated mice as compared to BM from PBS-treated mice, we analyzed the activation phenotype of these cells by flow cytomtery after staining with mAb toward the costimulatory molecule CD86. Day 4 analysis of CD86 expression in CD11c+CD11b+ cells showed similar levels in the non-adherent population of BM from CTX-treated mice and control (57% and 52%, respectively) (Fig. 4A, upper panel), while the adherent population of BM from PBS-treated mice showed slightly higher expression of CD86 (24%) than those in BM from CTX-treated mice (16%) (Fig. 4A, lower panel). On day 7, however, CD11c+CD11b+ cells in the in non-adherent and adherent populations of BM from CTX-treated mice expressed lower and higher expression levels, respectively, of CD86 as compared to CD11c+CD11b+ cells of BM from PBS-treated mice (Fig. 4B, upper panel). CD11c−CD11b+ cells in the in the adherent, but not in the non-adherent, populations of BM from CTX-treated mice expressed higher expression level of CD86 as compared to CD11c−CD11b+ cells of BM from PBS-treated mice (Fig. 4B, lower panel).
Figure 4. Non-adherent cells from BM from CTX-treated mice showed higher activation phenotype.
C57BL/6 mice were i.p. treated with PBS (n=3 mice/group) or 4mg/mouse CTX (n=6 mice/group) and BM was harvested 3 days after treatments. BM single cell suspensions were prepared and cultured with 10ng/mL of GM-CSF and IL-4. On day 4, (A) and 7 (B), the non-adherent (left panel) and adherent (right panel) cells from BM of PBS- and CTX-treated mice were harvested and stained with anti-CD11c, anti-CD11b, and anti-CD86 mAbs and the expression level of the activation marker CD86 was analyzed by 3-color flow cytometry in CD11c+CD11b+ (upper panel) and CD11c−CD11b+ (lower panel) cells. *, Statistically significant (P<0.05).
CD11c+CD11b+ and CD11c−CD11b+ cells generated from BM from CTX-treated mice can respond to the stimulatory effects of TLR agonists
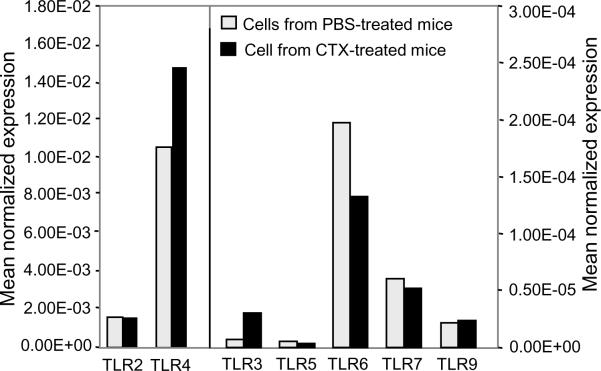
Although BM from CTX-treated mice generated higher numbers of DCs in vitro, these cells might be biologically nonfunctional. Therefore, we tested if these DCs can normally acquire activation phenotype in response to stimulation by potent stimuli such as TLR agonists as opposed to control DCs. First, we measured the gene expression levels of different TLRs in the non-adherent population (which contains >75% DCs; Fig. 3B), harvested on day 7 of BM culture. Real time PCR analysis showed that DCs generated from BM from CTX-treated mice and control expressed similar levels of TLRs, including TLR2, TLR4, TLR5, TLR6, TLR7, and TLR9 (Fig. 5). Of note, TLR4 showed the highest level as compared to the other TLRs.
Figure 5. DCs generated from BM from CTX-treated mice culture express simlar levels of different TLRs.
C57BL/6 mice were i.p. treated with PBS (n=3 mice/group) or 4mg/mouse CTX (n=6 mice/group) and BM was harvested 3 days after treatments. BM single cell suspensions were prepared and cultured with 10ng/mL of GM-CSF and IL-4. On day 7 of culture, the non-adherent cells were harvested and processed for the real-time PCR analysis. Results are expressed as normalized mean expression.
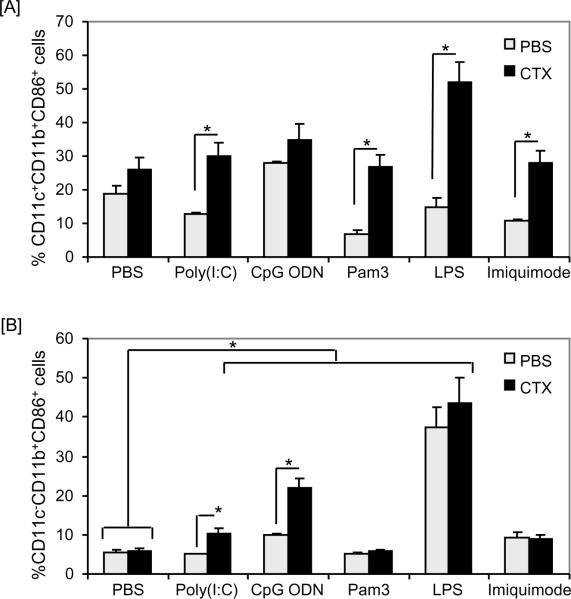
We then treated the non-adherent population generated on day 7 with the optimal concentrations of TLR agonists, which we selected in pilot experiments based on their activation of DCs without significant induction of cell death (data not shown). Equal numbers of non-adherent populations from BM from PBS- and CTX-treated mice, harvested on day 7 of BM culture, were stimulated with different TLR agonists in vitro for 24 hours. Because about 15–20% cells in the non-adherent population contains monocytes/macrophages (CD11c−CD11b+), we also analyzed the activation of these cells in response to the stimulatory effects of the tested TLR agonists. Based on CD86 expression, CD11c+CD11b+ (Fig. 6A) and CD11c−CD11b+ (Fig. 6B) cells generated from BM from CTX-treated mice showed higher activation phenotype as compared to those generated from BM from PBS-treated mice in response to treatments with different TLR agonists. Overall, the magnitude of CD86 expression in CD11c+CD11b+ cells (Fig. 6A) was higher than its expression in CD11c−CD11b+ cells (Fig. 6B). Of note, treatment with the TLR4 agonist LPS showed the highest effect on the activation of CD11c−CD11b+ cells generated from BM from CTX-treated mice (Fig. 6B). Similar profiles of CD80 and CD40 expression were also observed in both CD11c−CD11b+ and CD11c−CD11b+ cells (data not shown).
Figure 6. DCs generated from BM from CTX-treated mice can respond to stimulation with TLR agonists.
C57BL/6 mice were i.p. treated with PBS (n=3 mice/group) or 4mg/mouse CTX (n=6 mice/group) and BM was harvested 3 days after treatments. BM single cell suspensions were prepared and cultured with 10ng/mL of GM-CSF and IL-4. The non-adherent cells were harvested on day 7, counted, re-plated and treated for 24 hours with pam3CysK4 (a TLR2/6 agonist; 50μg/mL), poly(I:C) (a TLR3 agonist; 25μg/mL); LPS (a TLR4 agonist, 10ng/mL), imiquimode (a TLR7/8 agonist; 10μg/mL), or CpG ODN (a TLR9 agonist; 4μg/mL). (A) Shows the expression level of the activation marker CD86 on CD11c+CD11b+ and (B) shows the percentage of CD86 expression on CD11c−CD11b+ cells. *, Statistically significant (P<0.05).
DCs generated from BMCTX can prime CD8+ T-cells in vivo
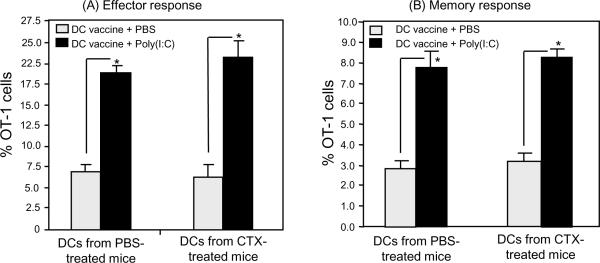
To further demonstrate the DCs generated from BM from CTX-treated mice are functional, we tested whether they can prime T cells in vivo. We used OT-1 TCR transgenic mouse model as we previously described [21; 56; 57; 61]. In this model, OT-1 CD8+ T cells, which can recognize the non-self MHC class-I OVA peptide SIINFEKL, are transferred into naïve congenic recipient mice followed by vaccination with SIINFEKL peptide in combination with the TLR3 agonist poly(I:C). DCs were generated from BM from PBS- and CTX-treated mice and harvested on day 7 and then activated with the TLR4 agonist LPS since it showed the highest stimulatory effects on DCs (Fig. 6). Cells were then washed and pulsed with 1μg/mL SIINFEKL peptide and used to vaccinate recipient mice adoptively transferred 1 day before with one million OT-1 cells. DC vaccine was co-administered with or without poly(I:C). The numbers of OT-I cells were analyzed in the recipient mice after 7 days of vaccination. As shown in Figure 7A, vaccination with DCs generated from BM from PBS- and CTX-treated mice induced similar adjuvant effects to the adoptively transferred T-cells as indicated by the comparable expansion of the OT-1 cells in the peripheral blood. The ability of DC vaccination to prime OT-1 cells was further augmented in both groups when it was combined with poly(I:C) administration (Fig. 7A). These results indicate that DCs from BM from CTX-treated mice can prime T cells in vivo. To confirm that effector T-cells generated after vaccination with DCs from BM from CTX-treated mice can differentiate into memory cells, vaccinated mice were boosted with OVA-peptide 30 days after priming and the number of memory OT-1 cells were measured in the peripheral blood after 3 days of boosting (as shown in Figure 7B). Boosting of mice primed with DCs generated from BM of PBS- or CTX-treated mice induced similar expansion of memory cells. These data indicate, under this experimental condition, that vaccination with DCs generated from BM of CTX-treated mice are biologically functional in vivo and can prime T cells to differentiate into effecter and memory responses.
Figure 7. DCs generated from BM from CTX-treated mice can prime T cells in vivo.
B6 mice (on Ly5.2 background) were treated with PBS and CTX as described in Figure 1 legend. After 3 days of treatment, BM was harvested and cultured with 10ng/mL of GM-CSF and IL-4. On day 7 of the culture, the non-adherent cells were harvested, counted and activated with 10ng/mL LPS for 24 hours. Cells were washed and pulsed with 5μg/mL of SIINFEKL peptide for 2 hours. Cells were then washed and injected through lateral tail vein into recipient B6 mice (on Ly5.2 background; n=4/group) adoptively transferred 1 day before with 1.5 × 106 cells of naïve OT-1 cells harvested from lymph nodes and spleen of B6 mice on Ly5.1 background. (A) Shows the numbers of effector OT-1 cells in the peripheral blood harvested 7 days after vaccination with DCs. (B) Shows the number of memory OT-1 cells in the peripheral blood harvested 3 days after s.c. revaccination with 100μg/mouse SIINFEKL 40 days after priming. *, Statistically significant (P<0.05).
Discussion
We have recently reported that single CTX treatment can induce a substantial expansion of DCs in the peripheral blood during the recovery from lymphopenia, peaking on day 12 [7]. In a subsequent study, we also observed higher numbers of proliferating cells with DC phenotype (CD11c+CD11b+) in the BM after 3 days of CTX treatment [43], indicating that post CTX BM is rich in DC precursors with higher tendency to differentiate into DCs in vivo. These observations led us in the present study to compare the capability of BM from CTX-treated and control mice to generate DCs in vitro in response to GM-CSF and IL-4. Our results showed the tendency of post CTX BM to give rise to a higher yeild of DCs in vitro. The kinetics of generation of DCs from BM of CTX-treated mice was more rapid than those of control BM, indicating that shorter time (about 4 days) might be sufficient to generate high numbers of DCs from BM harvested post chemotherapy. Our results are consistent with the previous studies, including ours, showing the ability of CTX to induce myelomonocytosis [7; 21; 42; 43] and mobilization of hematopoietic stem cells [44]. Previous studies also demonstrated higher yield of DCs from hematopoietic stem cells harvested from peripheral blood of cancer patients treated with CTX alone or in combination with G-CSF [50–54]. Importantly, our results showed that DCs generated from BM from CTX-treated mice can respond to the stimulatory effects of TLR agonists in vitro (Fig. 6) and can prime T cells in vivo (Fig. 7), indicating that they are biologically functional.
In our previous study, the kinetic analysis of proliferation of cells with CD11c+CD11b+ phenotype in BM indicated that the highest rate of these cells occured by day 3 after CTX treatment [43]. Therefore, we chose this time point in our studies. Additionaly previous studies showed that mobilization of hematopoietic stem cells from BM to circulation peaks at this time point post CTX treatment [42; 45; 62]. Interestingly, although CTX treatment induced a significant lymphopenia in BM, the relative numbers of DCs (CD11c+) were higher than in the steady state BM (Fig. 1A). Of note, DCs in BM from CTX-treated mice expressed higher activation phenotype as evidenced by the increased expression level of the costimulatory molecule CD86 (Fig. 1D). This rapid activation of DCs in fresh BM of CTX-treated mice is consistent with our previous studies showing the rapid activation of DCs in the spleen and liver 3 days after CTX treatment [21]. In line with this observation, treatment of B6 mice with sub-lethal body irradiation induced rapid increase in the numbers of activated DCs in lymph nodes [63]. Indeed, earlier studies have also reported that mouse interdigitating DCs isolated 3 days after CTX treatment showed an enhanced accessory function compared with the control DCs [64], and that follicular DCs harvested from lymph nodes 2–3 days post CTX treatment retained exogenous antigen for long time and were capable of inducing a better antibody responses [65]. Although it is not clearly known how DCs are activated after application of these lymphopenic regimens (CTX therapy and radiotherapy), we and others have correlated these activation with the systemic release of inflammatory cytokines after CTX treatment [7; 21; 63]. It has also been demonstrated that activation of DCs after the application of total body irradiation is mediated by LPS released due to microbial translocation after gut injury [63]. Taken together, it can be suggested that chemotherapy or radiotherapy can induce activation of DCs in different lymphoid compartments, including BM. This would explain why adoptive T cell transfer and vaccination following chemotherapy or radiotherapy results in a more robust anti-tumor immunity [7; 12; 63; 66–72]. It is still required, however, to determine the optimal time point after chemotherapy or radiotherapy at which DCs can be manipulated to show the most beneficial effects to antigen-specific T cell responses.
To understand whether BM from CTX-treated mice can generate functional DCs in vitro, we performed in vitro and in vivo experiments. When BM from control (steady state) and CTX-treated mice were cultured in vitro with GM-CSF and IL-4, we found on day 4 of the culture that BM from CTX-treated mice yielded higher number of non-adherent cells with higher levels of cells with the DC phenotype CD11c+CD11b+ as compared to those generated from control. This observation is consistent with our recent studies showing the presence of higher numbers of proliferating DCs in BM 3 days after CTX treatment measured by in vivo bromodeoxyuridine (BrdU) proliferation assay [43]. Interestingly, the increase in the total numbers of the non-adherent cells was coincided with a significant decrease in the total numbers of the adherent cells (Fig. 2B). Given that most of the non-adherent population on day 4 of BMCTX showed DC phenotype, it could be suggested that adherent cells in BM from CTX-treated mice loose their adherence and differentiate into DCs. This is consistent with the presence of lower levels of CD11b+Ly6G+ cells (neutrophils) and higher levels of CD11b+Ly6G− (monocytes) in fresh BM from CTX-treated mice than in control (Fig. 1B) as opposed to their counterparts. Furthermore, both non-adherent and adherent cells on day 4 of BM from CTX-treated mice culture showed lower numbers of CD11c+Ly6G+ cells and higher number of CD11c+Ly6G− cells (Fig. 3A). Given that DC progenitors and Ly6G+ monocytes are endowed with immediate DC precursor potential [73], the data suggest that post BM from CTX-treated mice has a tendency to rapidly generate higher number of DCs due to the increased levels of DC precursors.
Overnight treatment of DCs in the non-adherent and adherent populations of BM from CTX-treated mice with different TLR agonists induced higher levels of CD86 expression than in DCs of control (Fig. 6A). Among these TLR agonists, LPS showed the highest stimulatory effects on CD11c+CD11b+ and CD11c−CD11b+ cells. The hyper responsiveness of DCs to TLR4 agonist could be attributed to their expression of higher levels of TLR4 than the other TLRs (Fig. 5). Although activation of DCs after a single or combinatorial treatment with TLR agonists has been established [74], our studies would add another observation that post chemotherapy DCs might have a phenotype which is hyper-responsive to the stimulatory effects of TLR agonists. Further studies are required to understand the mechanisms underlying this observation.
Consistent with previous studies, including ours, that showed enhanced adjuvant effects of vaccination with DCs stimulated in vitro with TLR agonists [61; 75–77], vaccination with LPS-activated and peptide-pulsed DCs was able to induce a robust antigen-specific expansion of effector T cells, in particular after administration of poly(I:C) (Fig. 7A). These effector cells responded effectively to peptide revaccination (Fig. 7B). Vaccination with DCs generated from BM from CTX-treated mice were capable of inducing comparable T cell expansion to those of DCs generated from control (Fig. 6), indicating that DCs generated post chemotherapy can prime T cells in vivo. Although it is not clear why BM from CTX-treated mice DCs induced comparable CD8+ T cells expansion to those induced by control DCs even though they showed higher activation phenotype in vitro, it could be suggested that the LPS-induced hyper activation of DCs generated from BM from CTX-treated mice lead to a negative feedback mechanisms and anti-inflammatory signals such as up-regulation of MSK1/MSK2 and SOC1 pathways that have been found to be induced in DCs upon triggering of a strong TLR signaling [78–81]. Alternatively, it could be due to the low threshold of the T cell responses that can be achieved after DC-based vaccination in the OT-1 model, which is based on vaccination with the non-self tumor OVA antigen. Therefore, further studies are required to evaluate the potency DCs from BM of CTX-treated mice in a self tumor antigen model, in which threshold of T cell responses to DC-based vaccination requires a strong activation of DCs. Further studies are also required to optimize the responses of these DCs to activation with TLR agonists in vitro and in vivo so that they can be of more benefit to T cell responses.
In conclusion, our results indicate that post chemotherapy with CTX can rapidly create a host microenvironment in BM rich in proliferating DC precursors which are capable of differentiating into functional DCs. Our results would suggest that these cells could provide a platform for the manipulation of DCs in vivo post CTX therapy in the vaccination setting. These DCs can be targeted in vivo with factors that accelerate their mobilization (such as Flt3 ligand) differentiation and maturation (such as GM-CSF) or activation (such as TLR agonists). When manipulation of DCs in vivo with these factors is fine-tuned with active vaccination, it can significantly improve the application of CTX in cancer immunotherapy for the generation of effective antigen-specific responses.
Acknowledgments
Support: This work was supported by the National Institutes of Health Grant 1 R01 CA94856-01
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Jurado JM, Sanchez A, Pajares B, Perez E, Alonso L, Alba E. Combined oral cyclophosphamide and bevacizumab in heavily pre-treated ovarian cancer. Clin Transl Oncol. 2008;10:583–6. doi: 10.1007/s12094-008-0254-7. [DOI] [PubMed] [Google Scholar]
- [2].Rosell R, Moreno I, Maestre J, Olazabal A, Carles J, Barnadas A, Abad-Esteve A, Ribelles N, Canela M. Cyclophosphamide and ifosfamide combination as neoadjuvant chemotherapy for locally advanced nonsmall-cell lung cancer: a meta-analytic review. J Surg Oncol. 1990;45:124–30. doi: 10.1002/jso.2930450213. [DOI] [PubMed] [Google Scholar]
- [3].Saxton ML, Longo DL, Wetzel HE, Tribble H, Alvord WG, Kwak LW, Leonard AS, Ullmann CD, Curti BD, Ochoa AC. Adoptive transfer of anti-CD3-activated CD4+ T cells plus cyclophosphamide and liposome-encapsulated interleukin-2 cure murine MC-38 and 3LL tumors and establish tumor-specific immunity. Blood. 1997;89:2529–36. [PubMed] [Google Scholar]
- [4].Curti BD, Ochoa AC, Powers GC, Kopp WC, Alvord WG, Janik JE, Gause BL, Dunn B, Kopreski MS, Fenton R, Zea A, Dansky-Ullmann C, Strobl S, Harvey L, Nelson E, Sznol M, Longo DL. Phase I trial of anti-CD3-stimulated CD4+ T cells, infusional interleukin-2, and cyclophosphamide in patients with advanced cancer. J Clin Oncol. 1998;16:2752–60. doi: 10.1200/JCO.1998.16.8.2752. [DOI] [PubMed] [Google Scholar]
- [5].Bracci L, Moschella F, Sestili P, La Sorsa V, Valentini M, Canini I, Baccarini S, Maccari S, Ramoni C, Belardelli F, Proietti E. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin Cancer Res. 2007;13:644–53. doi: 10.1158/1078-0432.CCR-06-1209. [DOI] [PubMed] [Google Scholar]
- [6].Zhang S, Zhang HJ, Wang HY, Wang Q, Li WF, Yu L. CD4+ T cells tumor specific response exists in L615 leukemia mice: adoptive transfer in combination with cyclophosphamide. Exp Oncol. 2004;26:156–7. [PubMed] [Google Scholar]
- [7].Salem ML, Diaz-Montero CM, Al-Khami AA, El-Naggar SA, Naga O, Montero AJ, Khafagy A, Cole DJ. Recovery from cyclophosphamide-induced lymphopenia results in expansion of immature dendritic cells which can mediate enhanced prime-boost vaccination antitumor responses in vivo when stimulated with the TLR3 agonist poly(I:C) J Immunol. 2009;182:2030–40. doi: 10.4049/jimmunol.0801829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Koike N, Pilon-Thomas S, Mule JJ. Nonmyeloablative chemotherapy followed by T-cell adoptive transfer and dendritic cell-based vaccination results in rejection of established melanoma. J Immunother. 2008;31:402–12. doi: 10.1097/CJI.0b013e31816cabbb. [DOI] [PubMed] [Google Scholar]
- [9].Liu JY, Wu Y, Zhang XS, Yang JL, Li HL, Mao YQ, Wang Y, Cheng X, Li YQ, Xia JC, Masucci M, Zeng YX. Single administration of low dose cyclophosphamide augments the antitumor effect of dendritic cell vaccine. Cancer Immunol Immunother. 2007;56:1597–604. doi: 10.1007/s00262-007-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Taieb J, Chaput N, Schartz N, Roux S, Novault S, Menard C, Ghiringhelli F, Terme M, Carpentier AF, Darrasse-Jeze G, Lemonnier F, Zitvogel L. Chemoimmunotherapy of tumors: cyclophosphamide synergizes with exosome based vaccines. J Immunol. 2006;176:2722–9. doi: 10.4049/jimmunol.176.5.2722. [DOI] [PubMed] [Google Scholar]
- [11].Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels JP, Bieler JG, Emens LA, Reilly RT, Jaffee EM. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J Exp Med. 2005;201:1591–602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Teitz-Tennenbaum S, Li Q, Davis MA, Wilder-Romans K, Hoff J, Li M, Chang AE. Radiotherapy combined with intratumoral dendritic cell vaccination enhances the therapeutic efficacy of adoptive T-cell transfer. J Immunother. 2009;32:602–12. doi: 10.1097/CJI.0b013e3181a95165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rozkova D, Tiserova H, Fucikova J, Last'ovicka J, Podrazil M, Ulcova H, Budinsky V, Prausova J, Linke Z, Minarik I, Sediva A, Spisek R, Bartunkova J. FOCUS on FOCIS: combined chemo-immunotherapy for the treatment of hormone-refractory metastatic prostate cancer. Clin Immunol. 2009;131:1–10. doi: 10.1016/j.clim.2009.01.001. [DOI] [PubMed] [Google Scholar]
- [14].Berraondo P, Nouze C, Preville X, Ladant D, Leclerc C. Eradication of large tumors in mice by a tritherapy targeting the innate, adaptive, and regulatory components of the immune system. Cancer Res. 2007;67:8847–55. doi: 10.1158/0008-5472.CAN-07-0321. [DOI] [PubMed] [Google Scholar]
- [15].Ko HJ, Kim YJ, Kim YS, Chang WS, Ko SY, Chang SY, Sakaguchi S, Kang CY. A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res. 2007;67:7477–86. doi: 10.1158/0008-5472.CAN-06-4639. [DOI] [PubMed] [Google Scholar]
- [16].Song W, Levy R. Therapeutic vaccination against murine lymphoma by intratumoral injection of naive dendritic cells. Cancer Res. 2005;65:5958–64. doi: 10.1158/0008-5472.CAN-05-0406. [DOI] [PubMed] [Google Scholar]
- [17].Holtl L, Ramoner R, Zelle-Rieser C, Gander H, Putz T, Papesh C, Nussbaumer W, Falkensammer C, Bartsch G, Thurnher M. Allogeneic dendritic cell vaccination against metastatic renal cell carcinoma with or without cyclophosphamide. Cancer Immunol Immunother. 2005;54:663–70. doi: 10.1007/s00262-004-0629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ramanathapuram LV, Kobie JJ, Bearss D, Payne CM, Trevor KT, Akporiaye ET. alpha-Tocopheryl succinate sensitizes established tumors to vaccination with nonmatured dendritic cells. Cancer Immunol Immunother. 2004;53:580–8. doi: 10.1007/s00262-004-0499-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Klebanoff CA, Khong HT, Antony PA, Palmer DC, Restifo NP. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 2005;26:111–7. doi: 10.1016/j.it.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schiavoni G, Mattei F, Di Pucchio T, Santini SM, Bracci L, Belardelli F, Proietti E. Cyclophosphamide induces type I interferon and augments the number of CD44(hi) T lymphocytes in mice: implications for strategies of chemoimmunotherapy of cancer. Blood. 2000;95:2024–30. [PubMed] [Google Scholar]
- [21].Salem ML, Kadima AN, El-Naggar SA, Rubinstein MP, Chen Y, Gillanders WE, Cole DJ. Defining the ability of cyclophosphamide preconditioning to enhance the antigen-specific CD8+ T-cell response to peptide vaccination: creation of a beneficial host microenvironment involving type I IFNs and myeloid cells. J Immunother. 2007;30:40–53. doi: 10.1097/01.cji.0000211311.28739.e3. [DOI] [PubMed] [Google Scholar]
- [22].Awwad M, North RJ. Cyclophosphamide (Cy)-facilitated adoptive immunotherapy of a Cy-resistant tumour. Evidence that Cy permits the expression of adoptive T-cell mediated immunity by removing suppressor T cells rather than by reducing tumour burden. Immunology. 1988;65:87–92. [PMC free article] [PubMed] [Google Scholar]
- [23].Hoover SK, Barrett SK, Turk TM, Lee TC, Bear HD. Cyclophosphamide and abrogation of tumor-induced suppressor T cell activity. Cancer Immunol Immunother. 1990;31:121–7. doi: 10.1007/BF01742376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ikezawa Y, Nakazawa M, Tamura C, Takahashi K, Minami M, Ikezawa Z. Cyclophosphamide decreases the number, percentage and the function of CD25+ CD4+ regulatory T cells, which suppress induction of contact hypersensitivity. J Dermatol Sci. 2005;39:105–12. doi: 10.1016/j.jdermsci.2005.02.002. [DOI] [PubMed] [Google Scholar]
- [25].Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–44. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- [26].Hong SH, Yoon IH, Kim YH, Yang SH, Park MJ, Nam HY, Kim B, Kim Y, Park CS, Park CG. High-dose cyclophosphamide-mediated anti-tumor effects by the superior expansion of CD44(high) cells after their selective depletion. Immunobiology. 2009 doi: 10.1016/j.imbio.2009.01.010. [DOI] [PubMed] [Google Scholar]
- [27].Schabowsky RH, Madireddi S, Sharma R, Yolcu ES, Shirwan H. Targeting CD4+CD25+FoxP3+ regulatory T-cells for the augmentation of cancer immunotherapy. Curr Opin Investig Drugs. 2007;8:1002–8. [PubMed] [Google Scholar]
- [28].Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–8. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Motoyoshi Y, Kaminoda K, Saitoh O, Hamasaki K, Nakao K, Ishii N, Nagayama Y, Eguchi K. Different mechanisms for anti-tumor effects of low- and high-dose cyclophosphamide. Oncol Rep. 2006;16:141–6. [PubMed] [Google Scholar]
- [30].Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, Surh CD, Rosenberg SA, Restifo NP. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–12. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Finkelstein SE, Heimann DM, Klebanoff CA, Antony PA, Gattinoni L, Hinrichs CS, Hwang LN, Palmer DC, Spiess PJ, Surman DR, Wrzesiniski C, Yu Z, Rosenberg SA, Restifo NP. Bedside to bench and back again: how animal models are guiding the development of new immunotherapies for cancer. J Leukoc Biol. 2004;76:333–7. doi: 10.1189/jlb.0304120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schumacher TN, Restifo NP. Adoptive T cell therapy of cancer. Curr Opin Immunol. 2009;21:187–9. doi: 10.1016/j.coi.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–9. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Muranski P, Boni A, Wrzesinski C, Citrin DE, Rosenberg SA, Childs R, Restifo NP. Increased intensity lymphodepletion and adoptive immunotherapy--how far can we go? Nat Clin Pract Oncol. 2006;3:668–81. doi: 10.1038/ncponc0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gattinoni L, Powell DJ, Jr., Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–93. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wrzesinski C, Restifo NP. Less is more: lymphodepletion followed by hematopoietic stem cell transplant augments adoptive T-cell-based anti-tumor immunotherapy. Curr Opin Immunol. 2005;17:195–201. doi: 10.1016/j.coi.2005.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Proietti E, Greco G, Garrone B, Baccarini S, Mauri C, Venditti M, Carlei D, Belardelli F. Importance of cyclophosphamide-induced bystander effect on T cells for a successful tumor eradication in response to adoptive immunotherapy in mice. J Clin Invest. 1998;101:429–41. doi: 10.1172/JCI1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mihalyo MA, Doody AD, McAleer JP, Nowak EC, Long M, Yang Y, Adler AJ. In vivo cyclophosphamide and IL-2 treatment impedes self-antigen-induced effector CD4 cell tolerization: implications for adoptive immunotherapy. J Immunol. 2004;172:5338–45. doi: 10.4049/jimmunol.172.9.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lou Y, Wang G, Lizee G, Kim GJ, Finkelstein SE, Feng C, Restifo NP, Hwu P. Dendritic cells strongly boost the antitumor activity of adoptively transferred T cells in vivo. Cancer Res. 2004;64:6783–90. doi: 10.1158/0008-5472.CAN-04-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Radojcic V, Bezak KB, Skarica M, Pletneva MA, Yoshimura K, Schulick RD, Luznik L. Cyclophosphamide resets dendritic cell homeostasis and enhances antitumor immunity through effects that extend beyond regulatory T cell elimination. Cancer Immunol Immunother. 2009 doi: 10.1007/s00262-009-0734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sefc L, Psenak O, Sykora V, Sulc K, Necas E. Response of hematopoiesis to cyclophosphamide follows highly specific patterns in bone marrow and spleen. J Hematother Stem Cell Res. 2003;12:47–61. doi: 10.1089/152581603321210136. [DOI] [PubMed] [Google Scholar]
- [43].Salem ML, AL-Khami AA, EL-Naggar SA, Díaz-Montero CM, Chen Y, Cole DJ. Cyclophosphamide induces dynamic alterations in the host microenvironments resulting in a FLT3L-dependent expansion of dendritic cells. Submitted. 2009 doi: 10.4049/jimmunol.0902309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bashey A, Donohue M, Liu L, Medina B, Corringham S, Ihasz A, Carrier E, Castro JE, Holman PR, Xu R, Law P, Ball ED, Lane TA. Peripheral blood progenitor cell mobilization with intermediate-dose cyclophosphamide, sequential granulocyte-macrophage-colony-stimulating factor and granulocyte-colony-stimulating factor, and scheduled commencement of leukapheresis in 225 patients undergoing autologous transplantation. Transfusion. 2007;47:2153–60. doi: 10.1111/j.1537-2995.2007.01440.x. [DOI] [PubMed] [Google Scholar]
- [45].Szumilas P, Barcew K, Baskiewicz-Masiuk M, Wiszniewska B, Ratajczak MZ, Machalinski B. Effect of stem cell mobilization with cyclophosphamide plus granulocyte colony-stimulating factor on morphology of haematopoietic organs in mice. Cell Prolif. 2005;38:47–61. doi: 10.1111/j.1365-2184.2005.00329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Morrison SJ, Wright DE, Weissman IL. Cyclophosphamide/granulocyte colony-stimulating factor induces hematopoietic stem cells to proliferate prior to mobilization. Proc Natl Acad Sci U S A. 1997;94:1908–13. doi: 10.1073/pnas.94.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Barcew K, Karbicka A, Szumilas P, Marchlewicz M, Grzegrzolka R, Wiszniewska B, Machalinski B. Morphology of the bone marrow, spleen and liver during hematopoietic cell mobilization with cyclophosphamide in mice. Folia Histochem Cytobiol. 2008;46:501–9. doi: 10.2478/v10042-008-0063-y. [DOI] [PubMed] [Google Scholar]
- [48].Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111:187–96. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Levesque JP, Hendy J, Takamatsu Y, Williams B, Winkler IG, Simmons PJ. Mobilization by either cyclophosphamide or granulocyte colony-stimulating factor transforms the bone marrow into a highly proteolytic environment. Exp Hematol. 2002;30:440–9. doi: 10.1016/s0301-472x(02)00788-9. [DOI] [PubMed] [Google Scholar]
- [50].Gazitt Y, Akay C, Thomas C., 3rd No polarization of type 1 or type 2 precursor dendritic cells in peripheral blood stem cell collections of non-hodgkin's lymphoma patients mobilized with cyclophosphamide plus G-CSF, GM-CSF, or GM-CSF followed by G-CSF. Stem Cells Dev. 2006;15:269–77. doi: 10.1089/scd.2006.15.269. [DOI] [PubMed] [Google Scholar]
- [51].Vuckovic S, Kim M, Khalil D, Turtle CJ, Crosbie GV, Williams N, Brown L, Williams K, Kelly C, Stravos P, Rodwell R, Hill GR, Wright S, Taylor K, Gill D, Marlton P, Bradstock K, Hart DN. Granulocyte-colony stimulating factor increases CD123hi blood dendritic cells with altered CD62L and CCR7 expression. Blood. 2003;101:2314–7. doi: 10.1182/blood-2002-03-0973. [DOI] [PubMed] [Google Scholar]
- [52].Weiss AJ, Lackman RD. A comparison of human G-Csf and human Gm-Csf given concurrently with anti-cancer chemotherapy. Oncol Rep. 2002;9:945–50. [PubMed] [Google Scholar]
- [53].Morse MA, Vredenburgh JJ, Lyerly HK. A comparative study of the generation of dendritic cells from mobilized peripheral blood progenitor cells of patients undergoing high-dose chemotherapy. J Hematother Stem Cell Res. 1999;8:577–84. doi: 10.1089/152581699319731. [DOI] [PubMed] [Google Scholar]
- [54].Siena S, Di Nicola M, Bregni M, Mortarini R, Anichini A, Lombardi L, Ravagnani F, Parmiani G, Gianni AM. Massive ex vivo generation of functional dendritic cells from mobilized CD34+ blood progenitors for anticancer therapy. Exp Hematol. 1995;23:1463–71. [PubMed] [Google Scholar]
- [55].Rubinstein MP, Kadima AN, Salem ML, Nguyen CL, Gillanders WE, Cole DJ. Systemic administration of IL-15 augments the antigen-specific primary CD8+ T cell response following vaccination with peptide-pulsed dendritic cells. J Immunol. 2002;169:4928–35. doi: 10.4049/jimmunol.169.9.4928. [DOI] [PubMed] [Google Scholar]
- [56].Salem ML, Diaz-Montero CM, El-Naggar SA, Chen Y, Moussa O, Cole DJ. The TLR3 agonist poly(I:C) targets CD8+ T cells and augments their antigen-specific responses upon their adoptive transfer into naive recipient mice. Vaccine. 2009;27:549–57. doi: 10.1016/j.vaccine.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Salem ML, Kadima AN, Cole DJ, Gillanders WE. Defining the antigen-specific T-cell response to vaccination and poly(I:C)/TLR3 signaling: evidence of enhanced primary and memory CD8 T-cell responses and antitumor immunity. J Immunother. 2005;28:220–8. doi: 10.1097/01.cji.0000156828.75196.0d. [DOI] [PubMed] [Google Scholar]
- [58].Applequist SE, Wallin RP, Ljunggren HG. Variable expression of Toll-like receptor in murine innate and adaptive immune cell lines. Int Immunol. 2002;14:1065–74. doi: 10.1093/intimm/dxf069. [DOI] [PubMed] [Google Scholar]
- [59].Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- [60].Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Salem ML, El-Naggar SA, Kadima A, Gillanders WE, Cole DJ. The adjuvant effects of the toll-like receptor 3 ligand polyinosinic-cytidylic acid poly (I:C) on antigen-specific CD8+ T cell responses are partially dependent on NK cells with the induction of a beneficial cytokine milieu. Vaccine. 2006;24:5119–32. doi: 10.1016/j.vaccine.2006.04.010. [DOI] [PubMed] [Google Scholar]
- [62].Wright DE, Cheshier SH, Wagers AJ, Randall TD, Christensen JL, Weissman IL. Cyclophosphamide/granulocyte colony-stimulating factor causes selective mobilization of bone marrow hematopoietic stem cells into the blood after M phase of the cell cycle. Blood. 2001;97:2278–85. doi: 10.1182/blood.v97.8.2278. [DOI] [PubMed] [Google Scholar]
- [63].Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, Palmer DC, Boni A, Muranski P, Yu Z, Gattinoni L, Antony PA, Rosenberg SA, Restifo NP. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007;117:2197–204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Limpens J, Van Meijer M, Van Santen HM, Germeraad WT, Hoeben-Schornagel K, Breel M, Scheper RJ, Kraal G. Alterations in dendritic cell phenotype and function associated with immunoenhancing effects of a subcutaneously administered cyclophosphamide derivative. Immunology. 1991;73:255–63. [PMC free article] [PubMed] [Google Scholar]
- [65].Phipps RP, Mandel TE, Schnizlein CT, Tew JG. Anamnestic responses induced by antigen persisting on follicular dendritic cells from cyclophosphamide-treated mice. Immunology. 1984;51:387–97. [PMC free article] [PubMed] [Google Scholar]
- [66].Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, Rosenberg SA, Restifo NP. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wrzesinski C, Paulos CM, Gattinoni L, Palmer DC, Kaiser A, Yu Z, Rosenberg SA, Restifo NP. Hematopoietic stem cells promote the expansion and function of adoptively transferred antitumor CD8 T cells. J Clin Invest. 2007;117:492–501. doi: 10.1172/JCI30414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hwang LN, Yu Z, Palmer DC, Restifo NP. The in vivo expansion rate of properly stimulated transferred CD8+ T cells exceeds that of an aggressively growing mouse tumor. Cancer Res. 2006;66:1132–8. doi: 10.1158/0008-5472.CAN-05-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Gattinoni L, Ranganathan A, Surman DR, Palmer DC, Antony PA, Theoret MR, Heimann DM, Rosenberg SA, Restifo NP. CTLA-4 dysregulation of self/tumor-reactive CD8+ T-cell function is CD4+ T-cell dependent. Blood. 2006;108:3818–23. doi: 10.1182/blood-2006-07-034066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, Berzofsky JA, Leonard WJ. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–48. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wang LX, Li R, Yang G, Lim M, O'Hara A, Chu Y, Fox BA, Restifo NP, Urba WJ, Hu HM. Interleukin-7-dependent expansion and persistence of melanoma-specific T cells in lymphodepleted mice lead to tumor regression and editing. Cancer Res. 2005;65:10569–77. doi: 10.1158/0008-5472.CAN-05-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102:9571–6. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Naik SH. Demystifying the development of dendritic cell subtypes, a little. Immunol Cell Biol. 2008;86:439–52. doi: 10.1038/icb.2008.28. [DOI] [PubMed] [Google Scholar]
- [74].Krumbiegel D, Zepp F, Meyer CU. Combined Toll-like receptor agonists synergistically increase production of inflammatory cytokines in human neonatal dendritic cells. Hum Immunol. 2007;68:813–22. doi: 10.1016/j.humimm.2007.08.001. [DOI] [PubMed] [Google Scholar]
- [75].Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–76. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Schlosser E, Mueller M, Fischer S, Basta S, Busch DH, Gander B, Groettrup M. TLR ligands and antigen need to be coencapsulated into the same biodegradable microsphere for the generation of potent cytotoxic T lymphocyte responses. Vaccine. 2008;26:1626–37. doi: 10.1016/j.vaccine.2008.01.030. [DOI] [PubMed] [Google Scholar]
- [77].Warger T, Osterloh P, Rechtsteiner G, Fassbender M, Heib V, Schmid B, Schmitt E, Schild H, Radsak MP. Synergistic activation of dendritic cells by combined Toll-like receptor ligation induces superior CTL responses in vivo. Blood. 2006;108:544–50. doi: 10.1182/blood-2005-10-4015. [DOI] [PubMed] [Google Scholar]
- [78].Ananieva O, Darragh J, Johansen C, Carr JM, McIlrath J, Park JM, Wingate A, Monk CE, Toth R, Santos SG, Iversen L, Arthur JS. The kinases MSK1 and MSK2 act as negative regulators of Toll-like receptor signaling. Nat Immunol. 2008;9:1028–36. doi: 10.1038/ni.1644. [DOI] [PubMed] [Google Scholar]
- [79].Dai X, Sayama K, Yamasaki K, Tohyama M, Shirakata Y, Hanakawa Y, Tokumaru S, Yahata Y, Yang L, Yoshimura A, Hashimoto K. SOCS1-negative feedback of STAT1 activation is a key pathway in the dsRNA-induced innate immune response of human keratinocytes. J Invest Dermatol. 2006;126:1574–81. doi: 10.1038/sj.jid.5700294. [DOI] [PubMed] [Google Scholar]
- [80].Ivashkiv LB. A signal-switch hypothesis for cross-regulation of cytokine and TLR signalling pathways. Nat Rev Immunol. 2008;8:816–22. doi: 10.1038/nri2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Lang T, Mansell A. The negative regulation of Toll-like receptor and associated pathways. Immunol Cell Biol. 2007;85:425–34. doi: 10.1038/sj.icb.7100094. [DOI] [PubMed] [Google Scholar]