Abstract
In male hamsters mating behavior is dependent on chemosensory input from the main olfactory and vomeronasal systems, whose central pathways contain cell bodies and fibers of gonadotropin-releasing hormone (GnRH) neurons. In sexually-naïve males, vomeronasal organ removal (VNX), but not main olfactory lesions, impairs mating behavior. Intracerebroventricular (icv)-GnRH restores mating in sexually-naïve VNX- males and enhances medial amygdala (Me) immediate early gene activation by chemosensory stimulation. In sexually-experienced males, VNX does not impair mating and icv-GnRH suppresses Me activation. Thus, the main olfactory system is sufficient for mating in experienced-VNX males, but not in naïve-VNX males. We investigated the possibility that GnRH enhances main olfactory input to the amygdala in naïve-VNX males using icv-GnRH and pharmacological stimulation (bicuculline/D,L homocysteic acid mixture) of the main olfactory bulb (MOB). In sexually-naive intact-males there was a robust increase of Fos-protein expression in the anteroventral medial amygdala (MeAv) with MOB stimulation, but no effect of GnRH. There was no effect of stimulation or GnRH in posterodorsal medial amygdala (MePd). In naïve-VNX animals, GnRH increased Fos in MeAv and MePv. Only combined MOB stimulation and icv-GnRH produced a significant increase in Fos in the dorsal (reproduction-related) portion of MeP (MePd). When the animals were sexually-experienced before VNX, a condition in which GnRH does not enhance mating, icv-GnRH combined with MOB stimulation suppressed Fos expression in MePd. This suggests a more selective effect of GnRH on olfactory input in MePd than elsewhere in medial amygdala of VNX males.
Keywords: medial amygdala, vomeronasal organ, gonadotropin-releasing hormone, hamster, olfatory bulb, Fos
1. Introduction
Chemosensory signals received during social encounters, and mating, activate both the vomeronasal (VN) and the main olfactory sensory pathways. These regions also contain cell bodies and fibers of gonadotropin-releasing hormone (GnRH) neurons (Jennes and Stumpf, 1980; Lehman et al., 1987; Jennes et al., 1988; Silverman, 1988), suggesting a possible interaction between chemosensory input and GnRH. GnRH is traditionally known to cause release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary gland into the body’s blood supply. However, there is also evidence that GnRH may act within the brain, possibly in conjunction with vomeronasal and main olfactory input, to affect reproductive behavior (e.g., Meredith, 1998; see below). Here we investigate a potential mechanism by which intracerebral GnRH might facilitate main olfactory input to the medial amygdala.
1.1 Chemosensory pathways
The vomeronasal organ (VNO) receives chemosensory signals and sends information on these signals to the accessory olfactory bulb (AOB). In turn, the AOB projects strongly to the anterior medial amygdala (MeA), which projects heavily to the posterior medial amygdala (MeP). These areas project directly and indirectly to the medial preoptic area (MPOA) and hypothalamus, areas important for organizing reproductive and social behavior (Powers et al., 1987; Swanson, 2000; Canteras, 2002; see Figure 1). Male and female conspecific chemosensory stimuli activate neurons in the medial amygdala in characteristic spatial patterns in male hamsters and mice (demonstrated by immediate-early gene expression: Meredith and Westberry, 2004; Choi et al., 2005; Samuelsen and Meredith, 2009). Following VNO removal (VNX) in sexually-naïve males, mating behavior is seriously impaired or eliminated (Meredith, 1986; Fernandez-Fewell and Meredith, 1995; Westberry and Meredith, 2003a,b) and medial amygdala activation by conspecific chemosensory stimuli (e.g., hamster vaginal fluid) is dramatically reduced (Westberry and Meredith, 2003b) or completely lost (Fernandez-Fewell and Meredith, 1994; Fewell and Meredith, 2002; Samuelsen and Meredith, in press; Westberry and Meredith, unpublished).
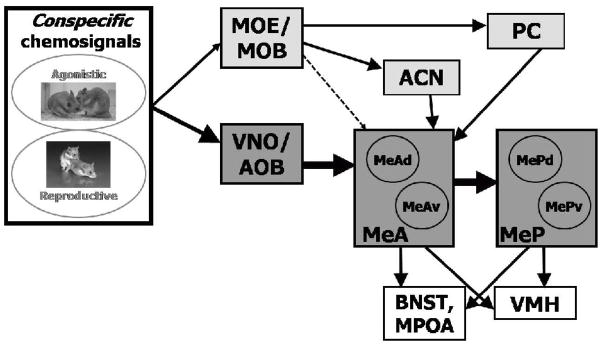
Figure 1. Activation of the main and accessory olfactory systems by different types of stimuli.
MOE/MOB = main olfactory epithelium/bulb, VNO/AOB = vomeronasal organ/accessory olfactory bulb, PC = piriform cortex, ACN = anterior cortical nucleus of the amygdala, MeA = anterior medial amygdala, MeAv, d = anterior medial amygdala ventral, dorsal portion, respectively, MeP = posterior medial amygdala, MePv, d = posterior medial amygdala ventral, dorsal portion, respectively. Conspecific, reproduction-related stimuli activate MeA and MePd.
Main olfactory information passes from the main olfactory bulb to the piriform cortex (Pir Ctx) and the anterior cortical nucleus of the amygdala (ACN; among other regions), which connect to MeA (Coolen and Wood, 1998; see Figure 1). There are also sparser projections directly from the main olfactory bulb to MeA (Lehman and Winans, 1982; Kang et al., 2009). Thus, the vomeronasal and main olfactory systems converge in the amygdala and can drive some of the same cells (Licht and Meredith, 1987; Case and Meredith, unpublished). We hypothesize that GnRH may facilitate this convergence by increasing main olfactory input to the central vomeronasal pathway.
1.2 GnRH
Gonadotropin-releasing hormone (GnRH) is a hypothalamic hypophysiotropic decapeptide that is important for mating in males and females. Approximately 50% of GnRH neurons in the brain project to the median eminence, which sends GnRH to the pituitary gland (Lehman et al., 1987; Silverman et al., 1987). GnRH receptors and fibers are found throughout the brain and in many regions involved in chemosensory processing and mating behavior, such as: the MPOA, mediobasal hypothalamus, ACN, nervus terminalis (NT), piriform cortex (among other main olfactory regions), including some fibers in the medial amygdala (Lehman et al., 1987; Silverman et al., 1987; Jennes et al., 1988). Because GnRH levels are altered transiently by pheromonal input and GnRH is located in regions that receive and interpret chemosignals, it has been suggested that the GnRH system may interact with chemosensory systems in the CNS. For instance, GnRH can excite or inhibit MPOA neurons (Pan et al., 1988), facilitate or suppress chemosensory responses in the amygdala (Westberry and Meredith, 2003a,b); and stimulation of GnRH receptors can cause short term facilitation of sexual behavior (Dorsa et al., 1980; Sakuma and Pfaff, 1983). A second form of the GnRH peptide and receptor, GnRH-II, is located in the midbrain in many mammals, including primates, but not mice or rats (Millar, 2003; Millar et al., 2008). However, the GnRH-II neurons are not in direct communication with chemosensory pathways and are probably not involved in the systems explored here.
1.3 VNO-triggered GnRH release in the brain
Levels of intracerebral GnRH increase following natural chemosensory stimulation via activation of the vomeronasal organ. Increases in circulatory luteinizing hormone (LH), follicle stimulating hormone (FSH) and a subsequent increase in gonadal steroids are triggered by opposite-sex chemosensory stimuli in many species (Wysocki et al., 1983; Coquelin et al., 1984; Pfeiffer and Johnston, 1994; Anand et al., 2002, 2004; Richardson et al., 2004; Murata et al., 2009). LH secretion by the pituitary gland is triggered by GnRH released into portal vessels the median eminence, which is also the case for chemosensory-evoked secretion (Anand et al., 2002). In male mice, the LH increase occurs within 5 minutes of female chemosensory exposure (Coquelin et al., 1984), implying a rapid intracerebral release of GnRH. Both male mice and hamsters show a significant increase in testosterone within 30 min. of exposure to a chemosignal (Wysocki et al., 1983; Pfeiffer and Johnston, 1994; Anand et al., 2002). Vomeronasal organ removal (VNX) eliminates these hormonal responses to female chemosignals, regardless of whether the male has had prior sexual experience; although VNX does not eliminate hormonal responses to direct contact with females in experienced males (Coquelin et al., 1984; Pfeiffer and Johnston, 1994).
1.4 GnRH effects on mating behavior and on medial amygdala responses to natural chemosensory stimulation
Damage to the vomeronasal organ (VNX; Meredith, 1986), but not main olfactory input (Fernandez-Fewell and Meredith, 1998), severely impairs or eliminates mating in sexually-naïve male hamsters. Damage to both systems peripherally or by removal of the main olfactory bulb (MOB) eliminates mating regardless of experience (Murphy and Schneider, 1970; Winans and Powers, 1977). Thus, chemosensory input is always essential for mating. Vomeronasal input is necessary and sufficient for mating behavior in the absence of experience or exogenous GnRH. In other words, experienced male hamsters can use main olfactory input to maintain mating after vomeronasal organ removal (VNX); and in naïve-VNX animals main olfactory input is sufficient if the animals are given additional intracerebral GnRH 30 min before behavioral testing. Therefore, two circumstances in which mating is restored after VNX are: sexual experience before VNX and icv-GnRH after VNX. We conclude that mating (before VNX) allows males to learn the olfactory characteristics of females, perhaps by association between main olfactory input and vomeronasal input (Meredith, 1998). Main olfactory input may contribute to chemosensory facilitation of mating under normal conditions (Fernandez-Fewell and Meredith, 1998), but it is not necessary for mating in males with an intact vomeronasal pathway (Powers and Winans, 1973; Fernandez-Fewell and Meredith, 1998).
We and others have previously demonstrated a facilitatory action of GnRH on reproductive behavior in a variety of circumstances (Moss and McCann, 1973; Dorsa and Smith, 1980; Moss and Dudley, 1989; Meredith and Howard, 1992; Meisel and Sachs, 1994; Pfaff et al., 1994; Fernandez-Fewell and Meredith, 1995; Westberry and Meredith, 2003a,b). Antagonists of the pituitary-type GnRH-Receptor do not always block reproductive behavior in intact animals (e.g., Zadina et al., 1981), perhaps because behavioral effects of GnRH are mediated by a different receptor type, or conformation (Millar et al., 2008). GnRH and an analog that does not elicit LH release (AcLHRH5–10) injected intracerebroventricularly (icv) restore mating behavior in sexually-naïve VNX males (Fernandez-Fewell and Meredith, 1995; Westberry and Meredith, 2003b). The present experiments were designed to investigate a potential mechanism for this phenomenon.
Because chemosensory input is necessary for mating behavior in male hamsters, we hypothesized that an important action of GnRH in naïve-VNX males might be to facilitate main olfactory input to the medial amygdala. The medial amygdala (Me) is the first brain area where central olfactory and vomeronasal pathways converge (Winans and Scalia, 1970; Davis et al., 1978; see Figure 1), and is critical for mating behavior (Lehman and Winans, 1982; Powers et al, 1987; Coolen and Wood, 1997). Female chemosignals activate the medial amygdala, and the medial preoptic area (MPOA) downstream, more strongly in experienced-VNX males than in naïve-VNX males (Fewell and Meredith, 2002). Thus, mating in experienced-VNX males may be possible because main olfactory input to the amygdala is more effective after experience.
Within the medial amygdala, conspecific and heterospecific chemosignals activate IEG protein expression in MeA and MeP differently; and stimuli with different social significance also activate specific portions (e.g., dorsal, ventral) of MeA and MeP. For example, the dorsal subdivision of MeP (MePd) is specifically activated by conspecific reproductive chemosignals in hamsters, mice and rats (Baum and Everett, 1992; Fernandez-Fewell and Meredith, 1994; Kollack-Walker and Newman, 1997; Choi et al., 2005; Samuelsen and Meredith, 2009) and connects with reproductive circuits in the hypothalamus in rodents (Swanson, 2000; Canteras, 2002; Choi et al., 2005). The effect of GnRH on chemosensory responses in these subregions has not previously been reported.
In sexually-naïve VNX male hamsters the increased immediate-early gene (IEG) expression in the medial amygdala in response to hamster vaginal fluid (HVF; a conspecific stimulus that contains female pheromones), is significantly enhanced by icv-GnRH (Westberry and Meredith, 2003b). In VNO-intact males there is no enhancement and in sexually-experienced VNX-males, GnRH suppresses medial amygdala activation by HVF exposure (Westberry and Meredith, 2003b).
1.5 Present experiments
The goal of the present experiments was to test the hypothesis that in the absence of vomeronasal (VN) system, neuronal response to main olfactory system input in regions along the VN sensory pathway would be enhanced by icv-GnRH. By using artificial stimulation we could investigate this hypothesis without the possible confounding effect of feedback from neural or behavioral responses to particular odors. We supplied the GnRH exogenously because VNX animals lack chemosensory driven GnRH-release and IEG response in the medial amygdala.
In male hamsters, we injected a bicuculline/D, L-homocysteic acid mixture (or saline) into the main olfactory bulb (MOB) and combined this treatment with either icv-GnRH or icv-saline. The combination of a glutamate agonist and GABA antagonist stimulates output neurons of the olfactory bulb, while suppressing local inhibitory interneurons, resulting in an increase in Fos protein expression in downstream brain areas.
These experiments are based in part on previous findings that icv-GnRH facilitates mating in sexually-naïve VNX-males, but not in sexually-experienced VNX-males or in sexually-naïve intact-males. Therefore, we tested animals of each of those three conditions and conducted a mating test after treatment and just prior to perfusion (at too short a latency to affect Fos protein expression). This mating test was to confirm that pharmacological stimulation of the MOB did not alter the general behavioral effects of GnRH on mating, as observed in the animals on whose performance our hypothesis was based.
Changes in main olfactory input by GnRH in naïve-VNX animals were evaluated by comparing naïve-intact to naïve-VNX animals. The effect of sexual experience before VNX was evaluated by comparing naive-VNX to experienced-VNX animals. We hypothesized that icv-GnRH would produce a general increase in olfactory input to medial amygdala, as measured by an increase in Fos protein expression.
2. Results
Results from the different groups of animals revealed a more specific action of GnRH on main olfactory input to subregions of medial amygdala than anticipated. Because different subregions of the anterior (MeA) and posterior (MeP) portions of the medial amygdala respond differently to stimuli with different social the implications (Choi et al., 2005; Meredith et al., 2008) we examined the dorsal and ventral subdivisions of MeA (MeAd, MeAv) and MeP (MePd, MePv). For comparison with previous reports that did not subdivide the areas, we also analyzed MeA as a whole (MeAtotal) and MeP as a whole (MePtotal).
In a preliminary analysis for each experiment, counts from the two sides in MOB-stimulated animals were compared to confirm that activation was higher on the stimulated side. All further analyses proceeded with counts for the MOB-implanted side. For each experiment, animals receiving the drug-cocktail had significantly more Fos expression on the stimulated side than the unstimulated side, in one or more of the areas: MeAtotal, MePtotal, or Pir Ctx, and one or more of the subdivisions (MeAd, MeAv, MePd, MePv) by 2-way repeated measures ANOVA with side (stimulated vs. unstimulated) and treatment (GnRH vs. saline) as factors. In the absence of MOB stimulation there were no main effects of GnRH, or interactions and no difference between sides (animals with saline injections into the implanted MOB).
In the main analysis of experiments 1–3 we analyzed Fos expression in the medial amygdala and olfactory cortex on the MOB-implanted side only, by 2-way ANOVA with stimulation (MOB-stimulation vs. saline injection) and treatment (icv-GnRH vs. icv-saline) as factors. For each experiment there were four groups (n=6 in each group): 1) icv-GnRH and MOB-stimulation; 2) icv-GnRH and MOB-unstimulated (control; injected with saline); 3) icv-saline and MOB-stimulation; and 4) icv-saline and MOB-unstimulated (control; injected with saline).
2.1 Experiment 1: Naïve-Intact Males
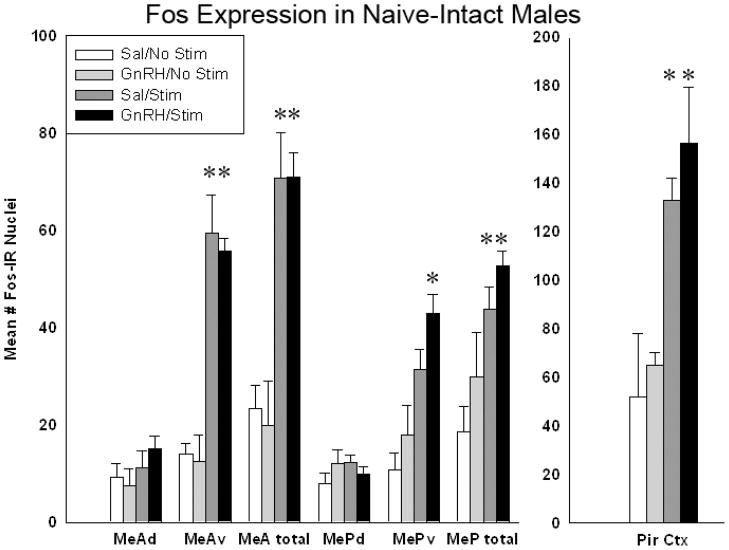
In the preliminary analysis of implanted and unimplanted sides for effects of stimulation (see above), there was a significant main effect of stimulation; in MeAtotal (p = 0.001; F(1,23)= 53.69;), MePtotal (p= 0.0043; F(1,23)= 16.74) and Pir Ctx (p =0.004; F(1,23)= 14.74). (Data for this preliminary analysis are not shown graphically.) Fos expression in the medial amygdala of the implanted/stimulated sides was then analyzed for each of the four groups listed above. Pharmacological stimulation of the MOB yielded significantly more Fos expression in MeA and MeP than saline injection, mainly due to the activation of the ventral subdivisions of each nucleus (see Figure 3). A 2-way ANOVA comparing treatment (GnRH or saline) and MOB stimulation (drug or saline) revealed a significant main effect of stimulation in the whole area of MeA (MeAtotal; p<.001; F(1,23)= 27.738), and the whole area of MeP (MePtotal; p<.001; F(1,23)= 20.248). There were no significant effects of treatment (GnRH vs. saline) or any significant interactions (see Figure 3). Thus, GnRH did not significantly increase Fos expression above the levels seen in animals with icv saline injection.
Figure 3.
In sexually-naïve intact males chemical stimulation of the MOB yielded significantly more Fos expression in MeA, MeP, the ventral subdivisions of each nucleus (MeAv/MePv) and in the piriform cortex (Pir). (* = significantly more than control, Holm-Sidak posthoc tests after 2-way ANOVA, p<.001). Analysis of the IEG expression in the accessory olfactory bulbs indicates that the strong effect of stimulation in the medial amygdala is not due to cross-stimulation of the AOB. The cannulated bulbs of the stimulated and unstimulated animals did not have significantly different amounts of Fos expression in the AOB, data not shown.
There were also significant main effects of stimulation within the ventral subdivisions of MeA and MeP (MeAv: p<.001; F(1,23)= 38.079; MePv: p<.001; F(1,23)= 21.878) (see Figure 3). Again there were no significant effects of GnRH treatment and no interactions. There were no significant effects found within the dorsal subdivisions (MeAd, MePd).
To examine the effect of MOB stimulation on central main-olfactory projection areas, Fos expression was also analyzed in the piriform cortex (Pir Ctx) and the anterior cortical nucleus of the amygdala (ACN, not shown). In Pir Ctx, stimulation increased Fos expression, but GnRH did not (2-way ANOVA as above, p<.001; F(1,23)= 59.916). There were no significant interactions in Pir Ctx and no significant differences in ACN (data not shown).
Because we saw an increase in Fos due to stimulation alone, we analyzed the MOBs to investigate if this effect was due to inadvertent activation of the VN system via the accessory olfactory bulbs. There were no significant differences in Fos expression in the AOBs, between the cannulated bulbs of the MOB-stimulated animals compared to the saline-injected, unstimulated animals (data not shown). Thus, the strong effect of stimulation in the medial amygdala is unlikely to be due to cross-stimulation of the AOB.
Mating Test
In the 5 min. mate test administered immediately prior to perfusion naïve-intact males that received icv-GnRH and MOB stimulation did not reach criterion (see Experimental Procedure), but all other animals (GnRH and no stim, saline and stim, saline and no stim) did reach mating test criterion (5 intromissions in 5 minutes; see Experimental Procedure).
2.2 Experiment 2: Naïve-VNX Males
Naive-VNX males have a demonstrated mating deficit, but mate normally after icv-GnRH (Meredith and Howard, 1992; Westberry and Meredith, 2003a,b). In the preliminary analysis of implanted and unimplanted sides for effects of stimulation (data not shown graphically), there was a significant main effect of stimulation in MeAtotal (p = 0.031; F(1,23)= 6.32;).
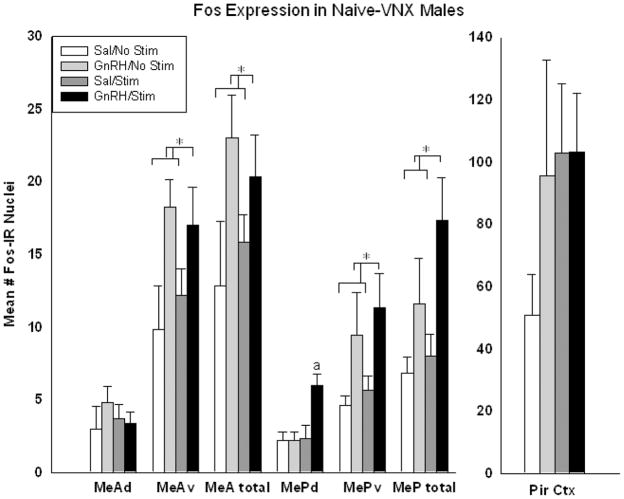
Fos expression in the medial amygdala of the implanted/stimulated sides only was then analyzed for each area listed above. Icv-GnRH yielded significantly more Fos expression than icv-saline in MeAtotal and MePtotal, again due largely to increases in activation of the ventral subdivisions of each nucleus. 2-way ANOVAs comparing treatment (GnRH or saline) and MOB stimulation (drug or saline) revealed a significant main effect of treatment, but not stimulation, in MeAtotal (p=.027; F(1,23)= 5.784) and MePtotal (p= .008; F(1,23)= 9.057) (see Figure 4).
Figure 4.
In sexually-naïve VNX males there was significantly more Fos expression in the GnRH-treated animals compared to saline (*= statistically significant main effect within area, 2-way ANOVA, p=.005). There was an additional enhancement of Fos expression in MePd of animals that were treated with GnRH and stimulated (a = statistically significant over all other groups within MePd, Holm-Sidak post-hoc analysis, p=.002).
There were also significant main effects of treatment within the ventral subdivisions of MeA and MeP (MeAv: p= .012; F(1,23)= 7.725; MePv: p= .016; F(1,23)= 7.131). In other words, icv-GnRH increased Fos expression significantly above levels for animals treated with icv-saline, regardless of MOB stimulation. Within the dorsal portion of MeP (MePd) there were significant main effects of treatment (p= .025; F(1,23)= 6.007), stimulation (p= .017; F(1,23)= 6.913), AND a significant interaction of GnRH and stimulation (p=.025; F(1,23)= 6.007), indicating a significant additional enhancement by the combination. There were no significant effects found in the dorsal portion of MeA (MeAd), and there were no significant interactions in any other brain region analyzed (see Figure 4).
To examine stimulation of the main olfactory system, Fos expression was also analyzed in the piriform cortex (Pir Ctx) and anterior cortical nucleus of the amygdala (ACN, not shown). In both regions, Fos expression was greater after stimulation and also after GnRH, but neither effect reached significance.
Mating Test
In a 5 min. mate test administered immediately prior to perfusion naïve-VNX males with GnRH reached criterion for mating (see Experimental Procedure), regardless of whether they received MOB stimulation or not. Naïve-VNX males that received icv-saline did not reach criterion for mating. These results match those previously seen in behavior (Meredith and Howard, 1992; Fernandez-Fewell and Meredith, 1994; Westberry and Meredith 2003a,b).
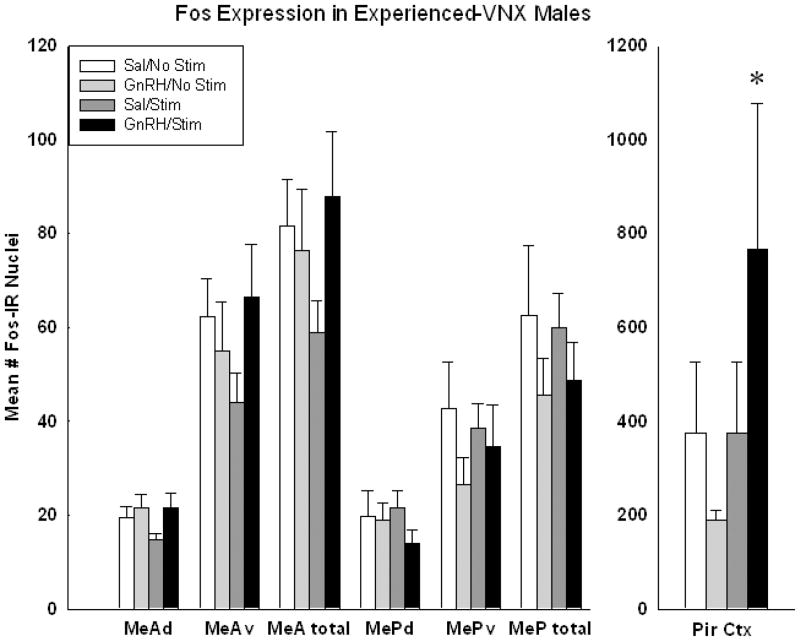
2.3 Experiment 3: Experienced VNX Males
Experienced-VNX males have no known mating deficit and may show a suppression of mating behavior after icv-GnRH (Meredith and Howard, 1992). In the preliminary analysis of implanted and unimplanted sides for effects of stimulation (data not shown graphically), there was a significant main effect of MOB stimulation in MePtotal (p = 0.016; F(1,23)= 9.15) and in Pir Ctx (p =0.028; F(1,23)= 7.16), and a significant interaction between stimulation and drug in MePtotal (p=.016; F(1,23)= 9.147). Within the GnRH-treated group, Fos expression was higher on the stimulated side than on the unstimulated side for MePtotal (p<0.003) and for Pir Ctx (p=.022; Holm-Sidak post-hoc analysis). Within the icv-saline-treated animals, there was also significantly more Fos on the stimulated side in MePtotal (p= 0.016).
There was a significant main effect of MOB stimulation also in MePv (p=.034; F(1,23)=6.499) with a significant interaction between stimulation and drug (p=.034; F(1,23)=6.499), and with the stimulated sides displaying more Fos than the unstimulated sides within the GnRH stimulated animals (p=0.005).
The only region or sub-region where there was a significant main effect of drug (GnRH/saline) was in MePd of MOB-stimulated animals (p=.043; F(1,23)=5.853). In this group, icv-GnRH treatment resulted in significantly less Fos than icv-saline treatment.
For the main analysis of Fos expression on the stimulated/implanted sides only, there were no significant differences in any region counted.
To examine stimulation of the main olfactory system, Fos expression was also analyzed in the piriform cortex (Pir Ctx) and anterior cortical nucleus of the amygdala (ACN, not shown). There was a significant interaction of GnRH and MOB stimulation in Pir Ctx: GnRH increased activation when combined with MOB stimulation (p=.002; F(3,39)= 2.565; see Figure 5). There were no significant differences in ACN (data not shown).
Figure 5.
In sexually-experienced VNX males there is an overall increase in Fos expression in all groups, consistent with enhanced access of olfactory information to medial amygdala. The depression seen in MePd with GnRH and stimulation was not significant in this analysis. In piriform cortex (Pir) there was significant effect of GnRH with more Fos expression in animals that also received stimulation (* = statistically significant main effect, 2-way ANOVA, p<.05).
Mating Test
All sexually-experienced VNX animals reached criterion (see Experimental Procedure) in the 5 min. mating test administered just prior to perfusion.
2.4 Normalized MePd data
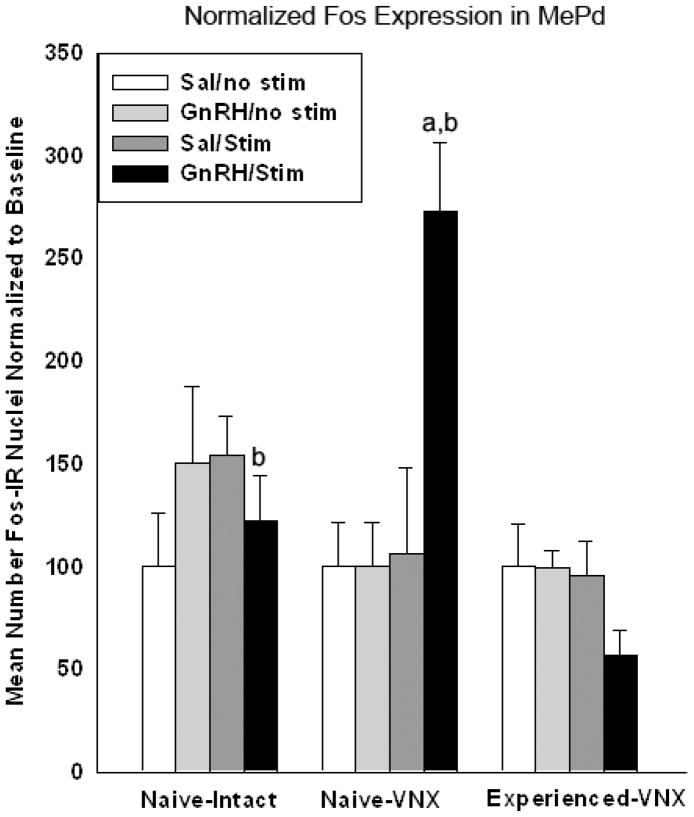
In order to compare the results in MePd from all 3 experiments, the data were normalized to the baseline level for each experiment (see Experimental Procedure). The mean cell-count for the control groups used to normalize the MePd data are as follows: 7.83 for naïve-intact, 2.2 for naïve-VNX, and 21.9 for experienced-VNX. A 3-way ANOVA was conducted comparing animal group (naïve-intact, naïve-VNX, and experienced-VNX), treatment (icv-GnRH or -saline), and simulation (MOB-stimulation or -saline). There was a statistically significant effect of animal group (p<.001; F(2,59)= 14.056) and stimulation (p<.001; F(1,59)= 15.756). Within the animals that received MOB stimulation, there was a significant interaction between animal group and treatment (p<.001). Within the animals that received icv-GnRH and MOB stimulation, there is a significant effect of animal group (p<.05, Holm-Sidak post-hoc). Of all of the animals that received GnRH and MOB stimulation: the naïve-VNX males yielded significantly more Fos than the naïve-intact and experienced VNX males, while the naïve-intact males displayed significantly more Fos expression than the experienced-VNX males (all relative to baseline; see Figure 6). There may be a suppression of Fos expression within the experienced-VNX males by GnRH and MOB stimulation. However, this difference did not reach significance in this analysis.
Figure 6.
Fos expression in the posterodorsal division of the medial amygdala (MePd) is here normalized to the baseline (icv-saline/MOB-saline control group) within each experiment. MePd is known to be involved in responses to chemosensory input relevant to reproductive behavior. Among the animals that received icv-GnRH and MOB-stimulation, there was a statistically significant effect of combined MOB-stimulation and GnRH between groups of animals (p<.05; Naïve-VNX>Naïve-intact>Experienced-VNX (3-way ANOVA with Holm-Sidak post-hoc analysis). a= significantly greater than icv-GNRH Naïve-intact group. b= significantly greater than icv-GNRH experienced-VNX group.
3. Discussion
Our results did not entirely support our original hypothesis: that there would be a GnRH-dependent general increase in olfactory input to the medial amygdala. However, there does appear to be a selective effect of GnRH in sexually-naive VNX males to increase MOB-stimulated Fos activation within the posterior dorsal subdivision (MePd), a region indicated as important for reproductive behavior. MePd connects with reproductive circuits in the hypothalamus in rodents (Swanson, 2000; Canteras, 2002; Choi et al., 2005) and is known to be activated by mating and by conspecific reproductive chemosignals in male hamsters, mice and rats (Baum and Everett, 1992; Fernandez-Fewell and Meredith, 1994; Kollack-Walker and Newman, 1997; Choi et al., 2005; Samuelsen and Meredith, 2009). Thus, it is meaningful that a selective effect of GnRH on main olfactory input may exist in MePd.
This result was obtained with artificial stimulation of the olfactory input, where the spatiotemporal pattern of input presumably carries no information relevant for reproductive behavior. A selective enhancement of olfactory system input by GnRH in MePd should therefore be a property of the location rather than the consequence of a particular pattern of input. Posterior medial amygdala appears to be unactivated by artificial patterns of input, at least for the vomeronasal system (Meredith and Westberry, 2004). A similar enhancement of Fos response in MePd was not seen in naïve-intact animals or in experienced-VNX animals. Additionally, Fos activity in experienced-VNX animals was low in this region, as in all regions for naïve-VNX animals. A neuromodulatory effect of GnRH at low background levels could be important for natural chemosensory stimulation. These Fos results with artificially stimulated input are consistent with previous observations on the effects of GnRH (see below).
3.1 MePd response is consistent with the behavioral effect of GnRH
In sexually-naïve VNX males, in which GnRH restores mating behavior, GnRH enhanced the MOB-stimulated activation in MePd, in addition to increasing Fos expression in ventral subdivisions of MeA and MeP. In sexually-naïve intact males, in which GnRH has no effect on mating, MOB stimulation produced robust activation of the ventral parts of MeA and MeP, but no significant activation in MePd and no extra effect of GnRH. In sexually-experienced VNX males, which also do not require icv-GnRH for mating, there was a decrease in Fos expression in MePd in males with both MOB stimulation and GnRH, but no general effect of GnRH.
The naïve-VNX group is the only group where GnRH facilitates mating (Fernandez-Fewell and Meredith, 1995). Thus, the GnRH-enhanced activation of the reproductively-relevant subdivision of the medial amygdala (MePd) seen here is consistent with the behavioral results in previous studies. Although the depression of activation in MePd of experienced-VNX males treated with GnRH and MOB stimulation is not statistically significant here, it is consistent with of a depression in mating behavior in hamsters with (repeated) icv-GnRH (Meredith and Howard, 1992) and aging monkeys with GnRH injections (Phoenix and Chambers, 1990).
3.2 GnRH effects on responses to artificial and natural chemosensory stimulation are consistent
The results seen here are also generally consistent with the effect of icv-GnRH on chemosensory-stimulated Fos expression in the medial amygdala. Naïve-VNX male hamsters show a significant enhancement of Fos expression in the medial amygdala with GnRH and HVF-exposure, as with pharmacological MOB stimulation here. Experienced-VNX males show a significant depression of medial amygdala activation with GnRH and HVF, and naïve-intact males showed no effect of GnRH on HVF-activation of Me (Westberry and Meredith, 2003b). In those experiments, Fos expression in dorsal and ventral subdivisions of MeA and MeP was not recorded separately so we cannot compare effects in MePd directly with the present results. It is clear that there was a greater response in the medial amygdala to the natural chemosensory stimulus than, here, to the pharmacological stimulation of MOB.
3.3 Comparison of normalized MePd response in different experiments
Differences in baseline Fos expression (animals that received icv-saline and MOB -saline) between the three experiments reported here make comparisons between the effects of GnRH difficult. After normalization for baseline, the differences in GnRH effects are more apparent (see Figure 6). There was a difference in Fos expression among the animals that received GnRH and MOB stimulation, in that naïve-VNX males showed the greatest change, relative to baseline, more than naïve-intact and experienced-VNX animals, while naïve-intact males showed a significantly greater effect than experienced-VNX males. Thus, the effect of GnRH on main olfactory input appears to be different depending on VNO status and sexual experience.
Differences in background are not necessarily random. Removal of vomeronasal organs must reduce the background sensory input and presumably accounts in part for the reduction in control-level Fos expression in naïve-VNX animals here and elsewhere (Westberry and Meredith 2003b, Samuelsen and Meredith, in press). Sexual experience has also been shown to alter baseline IEG expression in many regions (Can et al., 2007), including the caudal portion of MePd in hamsters (Kollack-Walker and Newman, 1997).
3.5 Timing and site of the MOB stimulation
The rostro-lateral location of the drug injection would produce a spatially nonuniform output from the MOB (but it would be difficult or impossible to activate the entire MOB without also activating AOB). It is possible that this spatially restricted output resulted in a spatially non-uniform input to medial amygdala (whether direct or via olfactory amygdala), but this not intrinsically different from the pattern of MOB output from natural chemosensory stimulation, which is also spatially non-uniform (Leon and Johnson, 2009).
Recent evidence suggests a more important influence on mating than previously thought, for main olfactory input in mice (Pankevich et al., 2006). The robust activation of medial amygdala by MOB stimulation in naïve-intact hamsters that we demonstrate here may reflect a larger main olfactory input than previously considered in this species too. The main olfactory input appears to be considerably reduced in naïve-VNX or experienced VNX males here. The possibility that main olfactory input to the medial amygdala may depend in part on an intact vomeronasal projection could explain the loss of driving from MOB in VNX animals here, and in mice after VNX (Samuelsen and Meredith, in press)
3.6 Effect of pattern of MOB stimulation
We know from other work (Meredith and Fewell, 2001; Meredith and Westberry, 2004) that artificial stimulation in these sensory systems is not equivalent to natural stimulation by conspecific chemosensory stimuli. For example, the unnatural spatio-temporal pattern of input from electrical stimulation of VNO activates MeA, but not MeP (Meredith and Westberry, 2004). Artificial MOB stimulation here could not mimic all the effects of natural stimulation but our purpose was to test whether any main olfactory input arriving at medial amygdala could be potentiated by GnRH, regardless of its source or spatiotemporal effect in MOB (see above). In the amygdala the spatial patterns of Fos expression across treatment groups were different for VNX and intact animals. For intact animals there was a significant effect of stimulation, regardless of GnRH. However, the Fos pattern seen in the medial amygdala was different than that after natural female chemosignals (e.g., hamster vaginal fluid; Fiber et al., 1993; Fernandez-Fewell and Meredith, 1994; Fewell and Meredith, 2002; Westberry and Meredith, 2003b), and with activation also biased towards MeA rather than MeP.
In the naïve-VNX males, there was a significant main effect of GnRH regardless of stimulation, suggesting an effect of vomeronasal organ removal on the substrate for GnRH action. Experienced-VNX animals had more Fos expression throughout the medial amygdala than naïve-VNX animals, but because the tissue was processed separately between the 3 experiments, we cannot attribute this to an effect of experience. Pharmacological MOB stimulation did produce significant activation in VNX animals, when the stimulated side of the brain was compared to the unstimulated side (see Results), but it was less effective in both experienced-VNX and naïve-VNX animals than the same type of stimulation in intact animals. Possibly the MOB input normally converges with a background VN activation which is absent in VNX animals. The artificial MOB stimulation here produced less robust Fos expression than chemosensory stimulation (without GnRH) in experienced-VNX males of a previous experiment (Westberry and Meredith, 2003b).
In intact animals, activation by artificial MOB stimulation was predominantly in the ventral parts of the medial amygdala (MeAv, MePv). Activation of these areas is characteristic of chemosensory stimuli from other males (potential competitors) and, in mice, from cat stimuli (potential predator; Choi et al., 2005; Meredith et al., 2008; Samuelsen and Meredith, 2009). However, we do not yet know whether similar combinations of cells were activated within these regions and should not expect that activation of MeAv and/or MePv by artificial stimulation would have any particular “meaning” for the animal. Animals were stimulated in an otherwise empty cage and there was no obvious aversive (or any) behavioral response.
3.7 Potential sources of GnRH affecting chemosensory response (and behavior)
Our experiments provide no evidence on the origin or site of action of GnRH naturally released in response to chemosensory input. However, there are a limited number of possibilities. Neurons producing mammalian GnRH (GnRH-I) are scattered through the ventral forebrain from the olfactory bulbs to the hypothalamus. The GnRH neurons controlling hormone secretion in rats and hamsters are mainly in the preoptic area, projecting to the median eminence, where GnRH is released into the portal blood, leading to the pituitary. However, many preoptic GnRH neurons have axons that end in other brain areas (Lehman et al., 1987; Silverman et al., 1987; Jennes et al., 1988), including medial amygdala and other areas along the central chemosensory pathways. Some species, including humans (Stopa et al., 1991), have GnRH neurons located in the medial amygdala, but in hamsters and rats the GnRH fibers in medial amygdala originate mainly in scattered cell bodies in ventral medial preoptic area (Lehman et al., 1987; Silverman, 1988). If naturally released GnRH has an effect in the amygdala (as exogenous GnRH does; Westberry and Meredith, 2003b) it would likely come from this source.
The medial amygdala projects centrally to the bed nucleus of the stria terminalis (BNST) and medial preoptic area (MPOA; Coolen and Wood, 1998). The hormonal response to chemosensory input includes an increase in LH, implicating GnRH release from MPOA axons in the median eminence (Anand et al., 2002). Electrical stimulation of the vomeronasal organ can activate Fos expression in a few MPOA GnRH neurons in hamsters (Meredith and Fewell, 2001). The normal chemosensory-system input presumably arrives via the accessory olfactory bulb and amygdala, and not via the central NT projection (Wirsig-Wiechmann, 1993). Thus, GnRH neurons in the MPOA are potential targets of chemosensory input from medial amygdala, and are also potential sources of GnRH released at the median eminence and into medial amygdala.
They may also connect to non-GnRH neurons projecting to the medial amygdala, resulting in an indirect GnRH influence there (Boehm et al., 2005). However, Yoon et al. (2005), using MPOA injection of a virus carrying a trans-synaptic retrograde tracer, concluded that no GnRH neurons receive input from medial amygdala or other parts of the vomeronasal pathway. However, GnRH could be released from axon terminals by axo-axonal synapses from the medial amygdala or elsewhere, without stimulating GnRH cell-bodies directly and without picking up tracers that are transported only retrogradely. The lack of Fos response in GnRH neurons of males after natural chemosensory input sufficient to produce an LH and testosterone increase would be consistent with this possibility (Rajendren and Moss, 1993; Meredith and Fewell, 2001). Whatever the site of the chemosensory input, the physiology clearly indicates a release of LH following VN input, presumably preceded by a release of GnRH at the median eminence and maybe elsewhere (see Introduction).
3.8 Potential sites of GnRH action
We do not know where icv-injected GnRH acts to facilitate mating or enhance activation of medial amygdala in naïve-VNX male hamsters. Sites of action in the MPOA, medial amygdala and in the olfactory and accessory olfactory bulbs would all be accessible to exogenous icv-GnRH. However, the relatively high concentration that is effective by this route requires more than 15 min to become effective (Meredith, 1998). Thus, the effective site of action may be remote from the injection site in the dorsal lateral ventricle. The amygdala is one such site. Intranasal GnRH is not effective (Fernandez-Fewell and Meredith, 1995), so a peripheral site of action is unlikely. A site of GnRH action peripheral to the MOB would also be unlikely to influence medial amygdala activation resulting from stimulation at the olfactory bulb in our experiments. GnRH fibers in the main olfactory bulb are mainly in the outer layers, close to the afferent synapses from the olfactory epithelium. Those in the medial amygdala are mainly in the superficial zone close to the incoming fibers from the AOB (Lehman et al., 1987), and MOB (Kang et al., 2009). GnRH could act at the afferent synaptic connections for both structures (a possibility that can be tested in vitro), but as yet we have no evidence that it does.
Regardless of the small effect of MOB stimulation, there is still some indication of an additional stimulus-dependent effect of GnRH in MePd (see Figure 6). If GnRH is influencing main olfactory input to the medial amygdala, it is likely doing it at the level of MePd. Previous studies have found differential effects in the medial amygdala with chemosensory stimuli of different social implications (Swanson, 2000; Canteras, 2002: Choi et al., 2005). MePd is selectively activated during reproductive behavior (Fernandez-Fewell and Meredith, 1994; Heeb and Yahr, 1996; Kollack-Walker and Newman, 1997) and by reproductive chemosensory stimuli alone (Fernandez-Fewell and Meredith, 1994; Bressler and Baum, 1996; Heeb and Yahr, 1996; Choi et al., 2005). It is possible that GnRH is capable of acting to restore a mating deficit involving chemosensory mechanisms because it affects the specific subnucleus activated by reproductively-relevant, chemosensory stimuli.
4. Experimental Procedures
4.1 Experimental Animals
Adult (2–3 month) male golden hamsters (Mesocricetus auratus), from Charles River Laboratories used in these experiments were maintained on a long photoperiod (a partially reversed-14L/10D light cycle). The animals were group-housed before surgery and individually house after, in clear plastic cages (44 cm × 21 cm × 18 cm) containing bedding, with food and water ad libitum. Experiments 1–3 used intact naive males, naive males with vomeronasal lesions (VNX), and sexually experienced VNX-males, respectively. Each of these groups of subjects was further divided in to four sub-groups to investigate the effects of pharmacological stimulation or saline injection of the main olfactory bulb, and the effects of icv-injection of GnRH or saline on Fos expression. All experimental procedures were approved by the Florida State University Institutional Animal Care and Use Committee.
4.2 Experience Protocol
For the experienced-VNX group, inexperienced male hamsters between 2 and 3 months of age were placed in a clean cage and provided with a naturally cycling behaviorally receptive female and observed mating for 1 h. If males did not achieve three ejaculations they were presented with another receptive female for up to 1 additional hour. Animals were returned to their home cage and considered sexually experienced after this one exposure (Meredith, 1986). All of the males achieved three ejaculations with this procedure in the first hour. Vomeronasal organs were removed 3–9 days following experience.
4.3 VNO Removal
For all VNX animals, a midline incision through the palate exposed the bony VN capsules. The natural openings in the palatal bones were extended rostrally and across the midline with a dental drill to form a ‘U’ shaped groove connecting the two natural openings. Forceps were used to break the medial palatine process of the maxillary bones to disconnect the capsules at the caudal end. The capsules were then separated by pressing into the midline suture with a scalpel. The final, anterior, connection with the palate was broken by drilling rostro-dorsally on the midline anterior to the U-groove. Each capsule containing one VNO was then removed with small forceps, separately. The palatal incision was closed with 3–4 sutures and sealed with cyanoacrylate adhesive. Experiments were conducted 3–7 days following VNX surgery. The noses of the VNX animals were collected and post-fixed separately and later decalcified in 0.3M EDTA and sectioned on a cryostat for verification of complete VNO lesions. Serial sections through the VNO region were examined and animals with incomplete lesions were eliminated from the study.
4.4 Intracerebroventricular injections
Guide tubes (28 gage, Plastics One) were implanted in the right lateral cerebral ventricle (for the VNX animals this was performed immediately following vomeronasal organ removal), at the following coordinates (in mm) from Bregma: −0.30 AP, −2.10 ML, and 3.40 DV. After an additional 3–5 days animals were given icv injections of either GnRH or saline, and MOB injections of either bicuculline/homocysteic acid mix or saline. Thirty minutes prior to MOB stimulation GnRH (50 ng in 2 μl) or saline vehicle (2 μl) was pressure injected through a 33 gauge cannula inserted into the guide tube of the implanted freely-moving animals. Injection of a 10 fold lower dose of GnRH (icv) or the same dose at 15 min before introduction of a female, did not result in significant behavioral enhancement in the earlier experiments, nor did a higher dose delivered intranasally or subcutaneously (Fernandez-Fewell and Meredith, 1995; Meredith, 1998). The animal was momentarily restrained by hand. The cap on the guide tube was removed and replaced with the injection cannula. The cannula was first filled to the tip after being connected to a long tube containing the solution to be injected and attached to a Picospritzer (General Valve Co.). The cannulae were individually calibrated before each use by adjusting the duration of the Picospritzer air pulse at standard pressure until 2 mg of distilled water (2 μl) was ejected onto a filter paper placed on the pan of an analytical balance. For GnRH and saline injections, the full dose was pressure injected, in three pulses spaced 30 s apart, starting 30 min before MOB stimulation. The cannula was left in place for 90 s after the last pulse to allow the injected material to diffuse away from the tip of the cannula, reducing the amount sucked back up into the guide tube as the cannula was withdrawn. A cap with wire stylet (dummy cannula) was used to seal the guide tube before and after use. Fresh cannulae were used for each experiment.
4.5 MOB injections and mating test
Immediately prior to the implantation of the ventricular cannulae, guide tubes (28 gage, Plastics One) were implanted into the right rostro-lateral olfactory bulb, using the fronto-nasal suture (above the cribriform plate) as a guide, approximately: −7.6 mm (anterior to) bregma, 1.4 mm lateral from the midline and 1.7 mm ventral to the surface of the bulb, aimed at the mitral layer in the rostral lateral MOB. These coordinates were chosen as a result of preliminary experiments that used stimulating electrodes (Blake, 2009) and to place the tip of the guide tube in close proximity to the mitral cell layer, in order to distribute the drug cocktail to the external plexiform (EPL) and granule cell layers, where Mitral/tufted output cells and inhinbitory interneurons are located. Initial ink injections and Fos expression in stimulated bulbs confirmed that the drug spread to these layers, an area approximately 1.5 mm in diameter, stretching from the glomerular layer to the opposite side granule cell layer, but did not spread back to the AOB. Fos response in mitral cells is difficult to detect even when they are activated by chemosensory stimulation (Guthrie et al., 1993) so we did not make systematic Fos counts in MOB. The excitatory D,L homocysteic acid can depolarize mitral or tufted output cells via extraglomerular glutamate receptors (Aroniadou-Anderjaska et al., 1999; Sassoé-Pognetto et al., 2003) or after penetrating glomeruli. It would also excite granule cells and various types of juxtaglomerular cells. However, the GABA antagonist would prevent inhibition of the output cells by GABAergic inhibitory interneurons, which are the vast majority of interneurons synapsing on output cells (Shepherd and Greer, 2003).
During the experiment, 30 min. after the start of the icv injections, a 33gage cannula was inserted in the MOB guide tube in same manner as the ventricular cannulae (see above). The implanted MOB of each animal was injected with either saline vehicle or a cocktail containing bicuculline methiodide (100 μM; Sigma) and D,L homocysteic acid (20mM; Sigma) in 0.9% saline (2 μl) over 15 minutes with a Hamilton syringe. The cocktail used was similar to that used previously to stimulate the medial preoptic area in order to drive Fos expression in the Pons and locus ceruleus (Rizvi et al., 1996). After the injection, the animal was placed back in its home cage for 30 min before perfusion, to allow for detectable Fos protein production.
To assess the effects of this artificial stimulation and of GnRH on facilitation of mating, animals were then given a standard 5-min mating test immediately before perfusion at a short enough time interval to not affect Fos protein expression. Animals were considered to have intact mating behavior if they met the criterion of 5 intromissions within 5 minutes. The mate test began at 40 minutes after the start of the MOB injection and animals were injected for perfusion upon completion of the mate test (approx 45 min after the start of the MOB infusion).
4.6 Fos ICC
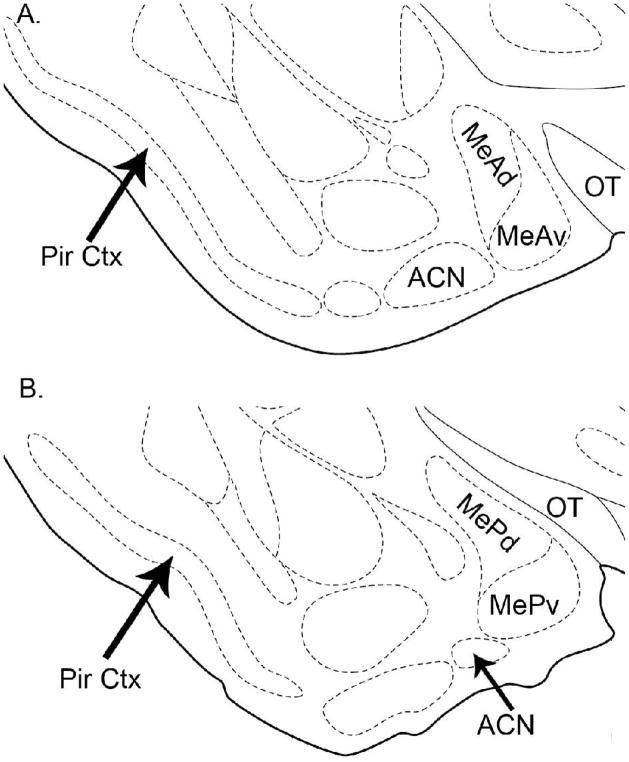
Animals were deeply anesthetized with sodium pentobarbital and perfused through the heart with 0.1 M phosphate buffer (PB; pH 7.4) followed by 4% paraformaldehyde. Brains were removed and post-fixed 12–16 h, cryoprotected in 30% sucrose, then sectioned serially on a freezing microtome at 40-μm thickness. Free-floating coronal sections were then processed for Fos protein expression immunocytochemistry. Sections were washed twice for 10 min each in 0.1 M PB and then incubated in 1% H2O2 for 15 min. The sections were then washed twice for 10 min. each in 0.1 M PBS and incubated in a pre-primary solution of 0.2% Triton-X 100 and 5% normal goat serum in 0.1M PBS for 30min. The tissue was then placed into polyclonal rabbit anti c-fos primary antiserum (1:10,000, Santa Cruz Biotechnology, Inc.), for 20 h at room temperature. Sections were then washed twice for 10 min each in 0.1 M PBS and incubated in biotinylated goat anti-rabbit secondary antiserum (1:400, Vector Labs) for 2 h, followed by incubation in avidin–biotin–horseradish peroxidase complex (ABC; Vector) and diaminobenzidine (DAB). Tissue from each sub-group of animals was processed together within each experiment. The 3 experiments were run in sequence and although the processing described here was used for all experiments, tissues from different experiments were not processed together. Fos-positive nuclei were counted using computer image analysis software (Image-Pro Plus), in a single tissue section for each brain-area of interest, carefully selected to be at the same anatomical position in each animal. The areas of interest included central projections of the VN and main olfactory pathway such as the anterior (MeA) and posterior (MeP) medial amygdala and their dorsal and ventral subdivisions (MeAd/v/MePd/v), the anterior cortical nucleus of the amygdala (ACN) and piriform cortex (Pir Ctx). Two sections were counted, and averaged, for Pir Ctx, at the level of MeA and at the level of MeP. In each area Fos-positive nuclei were counted within the border of the neuroanatomical nucleus (see Figure 2), on both sides. The anatomical positions of the sections chosen for counting were previously selected as representative areas of each nucleus in which Fos expression is increased in mating animals (Fernandez-Fewell and Meredith, 1994).
Figure 2.
Schematic of areas of interest in all 3 experiments. A. The anterior medial amygdala (MeA) is subdivided into dorsal (MeAd) and ventral (MeAv) portions. B. The posterior medial amygdala (MeP) is subdivided into dorsal (MePd) and ventral (MePv) portions. The anterior cortical nucleus (ACN) and piriform cortex (Pir Ctx) were counted in both sections and averaged (see Experimental Procedures). Modified from Morin and Wood (2001). MeA sections correspond to Fig. 26, AP −1.2 mm from bregma; and MeP sections correspond to Fig. 28; AP −1.8 mm from bregma in the Morin and Wood atlas.
4.7 Statistical Analysis
To analyze the effectiveness of the pharmacological MOB stimulation, we ran a preliminary analysis. Fos counts for the guide tube-implanted and drug cocktail-stimulated side of the brain were compared to the unimplanted (and unstimulated) side of the brain for animals that received MOB stimulation with a 2-way repeated measures (RM) analysis of variance (ANOVA). Two factors used were: drug (icv-GnRH vs. icv-saline) and side (implanted and stimulated side vs. unimplanted and unstimulated side). In a similar analysis the implanted and unimplanted sides of the unstimulated animals (receiving MOB saline) were not significantly different (no main effects or interactions) for any of the animals analyzed: naïve-intact, naïve-VNX or experienced-VNX (data not shown).
With effective stimulation confirmed by this preliminary analysis for each experiment, we then proceeded with the main analysis. Fos counts from the drug-cocktail stimulated (or saline injected) side of each brain region were then analyzed with a two-way analysis of variance (ANOVA) comparing two factors: treatment (icv-saline vs. icv-GnRH) and MOB-stimulation (MOB saline vs. MOB stimulation). A significant main effect of treatment would indicate a significant difference in Fos expression due to icv-GnRH, regardless of MOB stimulation effects. Separate 2-way ANOVAs were used for each region: whole-area data (MeAtotal; MePtotal), the individual subdivisions (MeAd, MeAv, MePd, MePv), and the main olfactory projection areas (ACN, Pir). A significant main effect of MOB-stimulation would indicate a significant difference in Fos expression due to MOB stimulation regardless of GnRH treatment. A significant interaction would indicate that the effect of GnRH on Fos expression was significantly different in MOB-stimulated and -unstimulated animals.
In order to compare the results in MePd from all 3 experiments, the data were normalized to the baseline level for each experiment. Fos counts were normalized by dividing each animal’s MePd count (from the stimulated side) by the average MePd count for control animals (received icv- and MOB-saline) in that experiment. Data for all groups within an experiment are expressed as a percentage of the average MePd count for control animals in that experiment. Thus, the averages for control animals in all experiments are the same at 100%, but the variance within each control group is preserved as are differences between the groups receiving different treatments within an experiment and the variances for each treatment group. These data were analyzed by a 3-way ANOVA with factors: group (naïve-intact, naïve-VNX or experienced-VNX); treatment (icv GnRH or icv-saline) and MOB-stimulation (MOB-drug-cocktail or MOB-saline), with Holm-Sidak post-hoc tests.
Acknowledgments
The authors would like to acknowledge the support of the National Institute on Deafness and Other Communication Disorders Grants DC 005813 and T32 DC00044 and F31 DC07782.
Abbreviations
- ACN
anterior cortical nucleus of amygdala
- AOB
accessory olfactory bulb
- BNST
bed nucleus of the stria terminalis
- GnRH
gonadotropin-releasing hormone
- MeAd/v
anterior medial amygdala, dorsal and ventral subdivisions
- MePd/v
posterior medial amygdala, dorsal and ventral subdivisions
- MOE
main olfactory epithelium
- MOB
main olfactory bulb
- Pir
piriform cortex
- MPOA
medial preoptic area
- VMH
ventromedial hypothalamus
- VNO
vomeronasal organ
- VNX
vomeronasal organ removal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature References
- Anand S, Losee-Olson S, Turek FW, Horton TH. Differential regulation of luteinizing hormone and follicle stimulating hormone in male siberian hamsters by exposure to females and photoperiod. Endocrinol. 2002;143(6):2178–2188. doi: 10.1210/endo.143.6.8839. [DOI] [PubMed] [Google Scholar]
- Anand S, Turek FW, Horton TH. Chemosensory stimulation of luteinizing hormone secretion in male siberian hamsters (Phodopus sungorus) Biol Reprod. 2004;70:1033–1040. doi: 10.1095/biolreprod.103.019380. [DOI] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Ennis M, Shipley MT. Dendodenritic recurrent excitation in mitral cells of the rat olfactory bulb. J Neurophysiol. 1999;82:489–494. doi: 10.1152/jn.1999.82.1.489. [DOI] [PubMed] [Google Scholar]
- Blake CB. PhD Dissertation. Florida State University; 2009. Amygdala Response To Artificial Olfactory and Chemosensory Input: Modulation By Neurohormones. [Google Scholar]
- Baum MJ, Everitt MJ. Increased expression of c-fos in the medial preoptic area after mating in male rats: role of afferent inputs from the medial amygdala and midbrain central tegmental field. Neurosci. 1992;50(3):627–646. doi: 10.1016/0306-4522(92)90452-8. [DOI] [PubMed] [Google Scholar]
- Boehm U, Zou Z, Buck LB. Feedback loops link odor and pheromone signaling with reproduction. Cell. 2005;123:683–695. doi: 10.1016/j.cell.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Bressler SC, Baum MJ. Sex comparison of neuronal Fos immunoreactivity in the rat vomeronasal projection circuit after chemosensory stimulation. Neurosci. 1996;71:1063–1072. doi: 10.1016/0306-4522(95)00493-9. [DOI] [PubMed] [Google Scholar]
- Can A, Domjan M, Delville Y. Sexual experience modulates neuronal activity in male Japanese quail. Horm Behav. 2007;52(5):590–599. doi: 10.1016/j.yhbeh.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS. The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacol Biochem Behav. 2002;71(3):481–491. doi: 10.1016/s0091-3057(01)00685-2. [DOI] [PubMed] [Google Scholar]
- Choi GB, Dong H, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 Delineates a Pathway Mediating Innate Reproductive Behaviors from the Amygdala to the Hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. Integration of chemosensory and hormonal cues is essential for sexual behavior in the male Syrian hamster: role of the medial amygdaloid nucleus. Neurosci. 1997;78(4):1027–35. doi: 10.1016/s0306-4522(96)00629-x. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. J Comp Neurol. 1998;399:189–209. doi: 10.1002/(sici)1096-9861(19980921)399:2<189::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Coquelin A, Clancy AN, Macrides F, Noble EP, Gorski RA. Pheromonally induced release of luteinizing hormone in male mice: involvement of the vomeronasal system. J Neurosci. 1984;4:2230–2236. doi: 10.1523/JNEUROSCI.04-09-02230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BJ, Macrides F, Youngs WM, Schneider SP, Rosene DL. Efferents and centrifugal afferents of the main and accessory olfactory bulbs in the hamster. Brain Res Bull. 1978;3:59–72. doi: 10.1016/0361-9230(78)90062-x. [DOI] [PubMed] [Google Scholar]
- Dorsa DM, Smith ER. Facilitation of mounting behavior in male rats by intracranial injections of luteinizing hormone-releasing hormone. Regul Pept. 1980;1(2):147–155. doi: 10.1016/0167-0115(80)90017-8. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fewell GD, Meredith M. c-fos expression in vomeronasal pathways of mated or pheromone-stimulated male golden hamsters: contributions from vomeronasal sensory input and expression related to mating performance. J Neurosci. 1994;14:3643–3654. doi: 10.1523/JNEUROSCI.14-06-03643.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Fewell GD, Meredith M. Facilitation of mating behavior in male hamsters by LHRH and AcLHRH5–10: interaction with the vomeronasal system. Physiol Behav. 1995;57:213–221. doi: 10.1016/0031-9384(94)00276-b. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fewell GD, Meredith M. Olfactory contribution to Fos expression during mating in inexperienced male hamsters. Chem Senses. 1998;23(3):257–267. doi: 10.1093/chemse/23.3.257. [DOI] [PubMed] [Google Scholar]
- Fewell GD, Meredith M. Experience facilitates vomeronasal and olfactory influence on Fos expression in medial preoptic area during pheromone exposure or mating in male hamsters. Brain Res. 2002;941(1–2):91–106. doi: 10.1016/s0006-8993(02)02613-6. [DOI] [PubMed] [Google Scholar]
- Fiber JM, Adames P, Swann JM. Pheromones induce c-fos in limbic areas regulating male hamster mating behavior. Neuroreport. 1993;4(7):871–874. doi: 10.1097/00001756-199307000-00008. [DOI] [PubMed] [Google Scholar]
- Guthrie KM, Anderson AJ, Leon M, Gall C. Odor-induced increases in c-fos mRNA expression reveal an anatomical “unit” for odor processing in olfactory bulb. Proc Natl Acad Sci USA. 1993;90(8):3329–3333. doi: 10.1073/pnas.90.8.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeb MM, Yahr P. c-Fos immunoreactivity in the sexually dimorphic area of the hypothalamus and related brain regions of male gerbils after exposure to sex-related stimuli or performance of specific sexual behaviors. Neurosci. 1996;72:1049–1071. doi: 10.1016/0306-4522(95)00602-8. [DOI] [PubMed] [Google Scholar]
- Jennes L. The gonadotropin-releasing hormone immunoreactive system in mouse. Brain Res. 1986;386:351–353. doi: 10.1016/0006-8993(86)90172-1. [DOI] [PubMed] [Google Scholar]
- Jennes L, Dalati B, Conn PM. Distribution of gonadotropin releasing hormone agonist binding sites in the rat central nervous system. Brain Res. 1988;452(1–2):156–164. doi: 10.1016/0006-8993(88)90020-0. [DOI] [PubMed] [Google Scholar]
- Jennes L, Stumpf WE. LHRH-systems in the brain of the golden hamster. Cell Tissue Res. 1980;209(2):239–256. doi: 10.1007/BF00237629. [DOI] [PubMed] [Google Scholar]
- Kang N, Baum MJ, Cherry JA. A direct main olfactory bulb projection to the ‘vomeronasal’ amygdala in female mice selectively responds to volatile pheromones from males. Eur J Neurosci. 2009;29(3):624–634. doi: 10.1111/j.1460-9568.2009.06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollack-Walker S, Newman SW. Mating-induced expression of c-fos in the male Syrian hamster brain: role of experience, pheromones, and ejaculations. J Neurobiol. 1997;32:481–501. doi: 10.1002/(sici)1097-4695(199705)32:5<481::aid-neu4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Newman SW, Silverman AJ. Luteinizing hormone-releasing hormone in the vomeronasal system and terminal nerve of the hamster. Ann NY Acad Sci. 1987;519:229–240. doi: 10.1111/j.1749-6632.1987.tb36300.x. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Winans SS. Vomeronasal and olfactory pathways to the amygdala controlling male hamster sexual behavior: autoradiographic and behavioral analyses. Brain Res. 1982;240(1):27–41. doi: 10.1016/0006-8993(82)90641-2. [DOI] [PubMed] [Google Scholar]
- Leon M, Johnson BA. Is there a space-time continuum in olfaction? Cell Mol Life Sci. 2009 doi: 10.1007/s00018-009-0011-9. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht G, Meredith M. Convergence of main and accessory olfactory pathways onto single neurons in the hamster amygdala. Exp Brain Res. 1987;69(1):7–18. doi: 10.1007/BF00247024. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Sachs BD. The physiology of male sexual behavior. In: Neill JD, editor. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 3–104. [Google Scholar]
- Meredith M. Vomeronasal organ removal before sexual experience impairs male hamster mating behavior. Physiol Behav. 1986;36:737–743. doi: 10.1016/0031-9384(86)90362-8. [DOI] [PubMed] [Google Scholar]
- Meredith M. Vomeronasal, olfactory, hormonal convergence in the brain: cooperation or coincidence. Ann NY Acad Sci. 1998;855:349–361. doi: 10.1111/j.1749-6632.1998.tb10593.x. [DOI] [PubMed] [Google Scholar]
- Meredith M, Fewell G. Vomeronasal organ: electrical stimulation activates Fos in mating pathways and in GnRH neurons. Brain Res. 2001;922:87–94. doi: 10.1016/s0006-8993(01)03153-5. [DOI] [PubMed] [Google Scholar]
- Meredith M, Howard G. Intracerebroventricular LHRH relieves behavioral deficits due to vomeronasal organ removal. Brain Res Bull. 1992;29(1):75–79. doi: 10.1016/0361-9230(92)90011-l. [DOI] [PubMed] [Google Scholar]
- Meredith M, Samuelsen CL, Blake C, Westberry JM. Selective Response of Medial Amygdala Subregions to Reproductive and Defensive Chemosignals from Conspecific and Heterospecific Species. In: Hurst JL, Beynon RJ, Roberts SC, Wyatt TD, editors. Chemical Signals in Vertebrates. Springer; New York: 2008. pp. 367–378. [Google Scholar]
- Meredith M, Westberry JM. Distinctive responses in the medial amygdala to same-species and different-species pheromones. J Neurosci. 2004;24(25):5719–5725. doi: 10.1523/JNEUROSCI.1139-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar RP. GnRH II and type II GnRH receptors. Trends Endocrinol Metab. 2003;14(1):35–43. doi: 10.1016/s1043-2760(02)00016-4. [DOI] [PubMed] [Google Scholar]
- Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR. Gonadotropin releasing hormone receptors. Endocrine Rev. 2008;25:235–275. doi: 10.1210/er.2003-0002. [DOI] [PubMed] [Google Scholar]
- Moss RL, Dudley CA. Neural control of reproductive function. John Wiley & Sons; 1989. Luteinizing hormone-releasing hormone (LHRH): peptidergic signals in the neural integration of female reproductive behavior; pp. 485–499. [Google Scholar]
- Moss RL, McCann SM. Induction of mating behavior in rats by luteinizing hormone-releasing factor. Science. 1973;181(95):177–179. doi: 10.1126/science.181.4095.177. [DOI] [PubMed] [Google Scholar]
- Murata K, Wakabyashi Y, Kitago M, Ohara H, Watanabe H, Tamogami S, Warita Y, Yamagishi K, Ichikawa M, Takeuchi Y, Okamura H, Mori Y. Modulation of gonadotrophin-releasing hormone pulse generator activity by the pheromone in small ruminants. J Neuroendocrinol. 2009;21:346–350. doi: 10.1111/j.1365-2826.2009.01836.x. [DOI] [PubMed] [Google Scholar]
- Murphy MR, Schneider GE. Olfactory bulb removal eliminates mating behavior in the male golden hamster. Science. 1970;167:302–304. doi: 10.1126/science.167.3916.302. [DOI] [PubMed] [Google Scholar]
- Pankevich DE, Cherry JA, Baum MJ. Effect of vomeronasal organ removal from male mice on their preference for and neural Fos responses to female urinary odors. Behav Neurosci. 2006;120(4):925–936. doi: 10.1037/0735-7044.120.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan JT, Kow LM, Pfaff DW. Modulatory actions of luteinizing hormone-releasing hormone on electrical activity of preoptic neurons in brain slices. Neurosci. 1988;27:623–628. doi: 10.1016/0306-4522(88)90293-x. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Schwartz-Giblin S, McCarthy MM, Kow LM. Cellular and molecular mechanisms of female reproductive behaviors. In: Neill JD, editor. The physiology of reproduction. New York: Raven Press; 1994. pp. 107–220. [Google Scholar]
- Pfeiffer CA, Johnston RE. Hormonal and behavioral responses of male hamsters to females and female odors: roles of olfaction, the vomeronasal system, and sexual experience. Physiol Behav. 1994;55(1):129–138. doi: 10.1016/0031-9384(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Chambers KC. Sexual performance of old and young male rhesus macaques following treatment with GnRH. Physiol Behav. 1990;47(3):513–517. doi: 10.1016/0031-9384(90)90118-n. [DOI] [PubMed] [Google Scholar]
- Powers JB, Newman SW, Bergondy ML. MPOA and BNST lesions in male Syrian hamsters: differential effects on copulatory and chemoinvestigatory behaviors. Behav Brain Res. 1987;23:181–195. doi: 10.1016/0166-4328(87)90019-2. [DOI] [PubMed] [Google Scholar]
- Powers JB, Winans SS. Sexual behavior in peripherally anosmic male hamsters. Physiol Behav. 1973;10:361–368. doi: 10.1016/0031-9384(73)90323-5. [DOI] [PubMed] [Google Scholar]
- Rajendren G, Moss RL. The role of the medial nucleus of amygdala in the mating-induced enhancement of lordosis in female rats: the interaction with luteinizing hormone-releasing hormone neuronal system. Brain Res. 1993;617(1):81–86. doi: 10.1016/0006-8993(93)90616-u. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Nelson AL, Ahmed EI, Parfitt DB, Romeo RD, Sisk CL. Female pheromones stimulate release of luteinizing hormone and testosterone without altering GnRH mRNA in adult male Syrian hamsters (mesocricetus auratus) Gen Comp Endocrinol. 2004;138:211–217. doi: 10.1016/j.ygcen.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Murphy AZ, Shipley MT, Behbehani MM. Medial preoptic afferents to periaqueductal gray medullo-output neurons: a combined Fos and tract tracing study. J Neurosci. 1996;16:333–344. doi: 10.1523/JNEUROSCI.16-01-00333.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma Y, Pfaff DW. Modulation of the lordosis reflex of female rats by LHRH, its antiserum and analogs in the mesencephalic central gray. Neuroendocrinol. 1983;36:218–224. doi: 10.1159/000123459. [DOI] [PubMed] [Google Scholar]
- Samuelsen CL, Meredith M. The vomeronasal organ is required for the male mouse medial amygdala response to chemical-communication signals, as assessed by immediate early gene expression. Neurosci. doi: 10.1016/j.neuroscience.2009.09.030. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsen CL, Meredith M. Categorization of salient chemical signals in the medial amygdala. Brain Res. 2009;1263:33–42. doi: 10.1016/j.brainres.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoé-Pognetto M, Utvik JK, Camoletto P, Watanabe M, Stephenson FA, Bredt DS, Ottersen OP. Organization of postsynaptic density proteins and glutamate receptors in axodendritic and dendrodendritic synapses of the rat olfactory bulb. J Comp Neurol. 2003;463(3):237–248. doi: 10.1002/cne.10745. [DOI] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989;338(6211):161–164. doi: 10.1038/338161a0. [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Greer CA. Olfactory bulb. In: Shepard GM, editor. Synaptic organization of the brain. Oxford University Press; New York: 2003. pp. 159–204. [Google Scholar]
- Silverman AJ. The gonadotropin-releasing hormone (GnRH) neuronal systems: Immunocytochemistry. In: Nobil E, Niell J, et al., editors. The physiology of reproduction. Raven Press; NY: 1988. pp. 1283–1304. [Google Scholar]
- Silverman AJ, Jhamandas J, Renaud LP. Localization of luteinizing hormone-releasing hormone (LHRH) neurons that project to the median eminence. J Neurosci. 1987;7(8):2312–2319. [PMC free article] [PubMed] [Google Scholar]
- Stopa EG, Koh ET, Svendsen CN, Rogers WT, Schwaber JS, King JC. Computer-assisted mapping of immunoreactive mammalian gonadotropin-releasing hormone in adult human basal forebrain and amygdala. Endocrinol. 1991;128(6):3199–3207. doi: 10.1210/endo-128-6-3199. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886(1–2):113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Westberry JM, Meredith M. Pre-exposure to female chemosignals or intracerebral GnRH restores mating behavior in naive male hamsters with vomeronasal organ lesions. Chem Senses. 2003a;28:191–196. doi: 10.1093/chemse/28.3.191. [DOI] [PubMed] [Google Scholar]
- Westberry JM, Meredith M. The influence of chemosensory input and gonadotropin releasing hormone on mating behavior circuits in male hamsters. Brain Res. 2003b;974:1–16. doi: 10.1016/s0006-8993(03)02535-6. [DOI] [PubMed] [Google Scholar]
- Winans SS, Powers JB. Olfactory and vomeronasal deafferentation of male hamsters: histological and behavioral analyses. Brain Res. 1977;126(2):325–44. doi: 10.1016/0006-8993(77)90729-6. [DOI] [PubMed] [Google Scholar]
- Winans SS, Scalia F. Amygdaloid nucleus: new afferent input from the vomeronasal organ. Science. 1970;170(955):330–332. doi: 10.1126/science.170.3955.330. [DOI] [PubMed] [Google Scholar]
- Wirsig-Wiechmann CR. Nervus terminalis lesions: I. No effect on pheromonally induced testosterone surges in the male hamster. Physiol Behav. 1993;53:251–255. doi: 10.1016/0031-9384(93)90201-p. [DOI] [PubMed] [Google Scholar]
- Wray S, Grant P, Gainer H. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode Proc. Natl Acad Sci USA. 1989;86(20):132–136. doi: 10.1073/pnas.86.20.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki CJ, Katz Y, Bernhard R. Male vomeronasal organ mediates female-induced testosterone surges in mice. Biol Reprod. 1983;28(4):917–22. doi: 10.1095/biolreprod28.4.917. [DOI] [PubMed] [Google Scholar]
- Yoon H, Enquist LW, Dulac C. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell. 2005;123(4):669–82. doi: 10.1016/j.cell.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Zadina JE, Kastin AJ, Fabre LA, Coy DH. Facilitation of sexual receptivity in the rat by an ovulation-inhibiting analog of LHRH. Pharmacol Biochem Behav. 1981;15(6):961–964. doi: 10.1016/0091-3057(81)90062-9. [DOI] [PubMed] [Google Scholar]