Abstract
Native predators are postulated to have an important role in biotic resistance of communities to invasion and community resilience. Effects of predators can be complex, and mechanisms by which predators affect invasion success and impact are understood for only a few well-studied communities. We tested experimentally whether a native predator limits an invasive species' success and impact on a native competitor for a community of aquatic insect larvae in water-filled containers. The native mosquito Aedes triseriatus alone had no significant effect on abundance of the invasive mosquito Aedes albopictus. The native predatory midge Corethrella appendiculata, at low or high density, significantly reduced A. albopictus abundance. This effect was not caused by trait-mediated oviposition avoidance of containers with predators, but instead was a density-mediated effect caused by predator-induced mortality. The presence of this predator significantly reduced survivorship of the native species, but high predator density also significantly increased development rate of the native species when the invader was present, consistent with predator-mediated release from interspecific competition with the invader. Thus, a native predator can indirectly benefit its native prey when a superior competitor invades. This shows the importance of native predators as a component of biodiversity for both biotic resistance to invasion and resilience of a community perturbed by successful invasion.
Keywords: Density-mediated effects, Invasive species, Keystone predator, Mosquitoes, Trait-mediated behavioral effects
Introduction
The hypothesis that enemies of an invasive species (including predators, parasites, or pathogens) contribute to biotic resistance of communities to invasion has been a prominent feature of invasion biology ever since Elton (1958), and has origins going back to Darwin (1859; Duncan and Williams 2002). Though it is intuitive that the presence of predators that can consume an invasive species could affect invasion success, theory in community ecology suggests that there are multiple possible mechanisms and quantitative effects of native predators on invasions (Leibold 1996; Chase et al. 2002; Shea and Chesson 2002; Noonberg and Byers 2005). In the simplest case, a native predator, along with populations of native prey that support it, may form a barrier to invasion, keeping an invasive species out of a community or limiting its invasion success (Baltz and Moyle 1993; Lodge 1993; Reusch 1998; Byers 2002; DeRivera et al. 2005; Nunez et al. 2008). This effect may derive solely from the lethal (density-mediated) effect of a predator on the invader, as in the case of native seed predators serving as a barrier to invasion by exotic conifers (Nunez et al. 2008). Alternatively, the barrier effect could result from a substantial, even dominant contribution from non-lethal (trait-mediated) effects (Werner and Peacor 2003; Preisser et al. 2005), if the presence of predators causes behavioral habitat avoidance by the invader. An example of such an effect occurs in invasive mink, which limit their movement to small islands when eagles are present (Salo et al. 2008). More subtle effects of native predators may occur when the presence of a native predator changes the impact of an invader on a community. Keystone predation, wherein a predator maintains greater diversity in a community by preying disproportionately on competitively dominant prey, thus releasing poorer competitors from interspecific competition (Paine 1966; Morin 1983; Leibold 1996; Smith 2006) can in principle ameliorate the potentially detrimental effects of competitive invasive species on native species, if the competitively superior invader is also more vulnerable to the native predator (Shea and Chesson 2002). Smith (2006) documented such an effect of native newts limiting the competitive impact of an invasive anuran on a native toad. Under such circumstances, the presence of a predator may contribute to the persistence of native species despite invasion. But native predators may also exacerbate the effects of invasion on natives, if invasion results in increased abundance of a native predator (a numerical response), which in turn contributes to decline and possible local elimination of native prey via apparent competition (Holt 1977; Noonberg and Byers 2005). Despite the range of possible effects of predators on invasions, and despite well-documented cases in which some predator effects on invasions have been demonstrated (Robinson and Wellborn 1988; Baltz and Moyle 1993; Reusch 1998; DeRivera et al. 2005; Smith 2006; Nunez et al. 2008; Salo et al. 2008) there have been few empirical investigations testing which of the types of predator effects and associated mechanisms operate in an invasion.
Container-dwelling mosquitoes provide an attractive model system in which to investigate the effects on native predators on invasions. Water-filled containers, including natural tree holes, bromeliads, and bamboos, and human-made containers such as discarded tires, flower pots, and cemetery vases, are larval habitats for mosquitoes in several genera, and have been focal habitats for several prominent invasive Aedes species (Lounibos 2002; Juliano and Lounibos 2005). Invasions of human-made containers and tree holes in North America (Livdahl and Willey 1991; O'Meara et al. 1995; Juliano 1998; Teng and Apperson 2000; Lounibos et al. 2001; Juliano et al. 2002, 2004) and South America (Braks et al. 2003, 2004) by Aedes albopictus (Skuse) have become a prominent model system for investigating how competitive interactions with invasive species may impact resident species (e.g., Livdahl and Willey 1991; Juliano 1998; Braks et al. 2004; Kesavaraju et al. 2008). A. albopictus is native to Asia and was introduced into North America during the 1980s (Hawley 1988; O'Meara et al. 1995). Since introduction, A. albopictus has spread rapidly in the southern US and in some urban areas in the north (Hawley 1988; O'Meara et al. 1995; Lounibos 2002). Females deposit desiccation-resistant eggs above the water line in containers (e.g., cemetery vases, discarded tires, natural tree holes); eggs hatch when flooded, except during winter diapause, and larvae develop in water-filled containers, feeding on microorganisms and detritus (Hawley 1988). Invasive Aedes albopictus encounter native Aedes triseriatus (Say), and naturalized Aedes aegypti (L.), Aedes japonicus (Theobald) and Culex pipiens L., and often can have strong interspecific competitive effects on field populations of other mosquitoes (Juliano 1998; Juliano et al. 2004; Braks et al. 2004; Costanzo et al. 2005; Armistead et al. 2008).
Invasion of human-made containers by A. albopictus has resulted in major declines in abundance of A. aegypti in southern North America (O'Meara et al. 1995; Lounibos 2002; Juliano et al. 2004; Juliano and Lounibos 2005), and more moderate declines in parts of South America (Lounibos 2002); probably due to superior interspecific competitive ability of A. albopictus (Juliano 1998; Braks et al. 2004). Human-made containers often lack major groups of predators that dominate in natural containers, such as tree holes (Kesavaraju et al. 2008) and this likely accentuates the role of interspecific competition in this invasion. Although A. albopictus have invaded natural tree holes in the southern USA, there is little evidence that the arrival of A. albopictus has had a strong negative impact on native A. triseriatus in southern tree holes (Lounibos et al. 2001; Kesavaraju et al. 2008), despite their competitive superiority in laboratory microcosm experiments (Livdahl and Willey 1991; Novak et al. 1993; Teng and Apperson 2000; Aliabadi and Juliano 2002; Bevins 2007; Yee et al. 2007). Competitive coexistence of A. albopictus and A. triseriatus in tree holes was predicted by Livdahl and Willey (1991) based on how these species perform in competition for resources derived from tree holes. However, tree holes are the natural habitat of predatory larvae of the mosquito Toxorhynchites rutilus (Coquillett) and the midge Corethrella appendiculata (Grabham) (Bradshaw and Holzapfel 1988; Lounibos et al. 2001). These predators are relatively rare in human-made containers where A. albopictus has had greater impact (Lounibos et al. 2001; Kesavaraju et al. 2008); and A. albopictus and C. appendiculata abundances are negatively associated (Kesavaraju et al. 2008). Thus, predator effects are an alternative hypothesis for the limited invasion impact of A. albopictus in tree holes (Lounibos et al. 2001; Griswold and Lounibos 2005a, b; Kesavaraju et al. 2007, 2008). In the laboratory, A. albopictus is more vulnerable than A. triseriatus to both C. appendiculata and T. rutilus, and this difference results from behavioral (Kesavaraju and Juliano 2004; Griswold and Lounibos 2005a, b; Kesavaraju et al. 2007) and size (Alto et al. 2009) differences that affect vulnerability. In controlled laboratory experiments, C. appendiculata can affect the outcome of competition between A. albopictus and A. triseriatus in a pattern consistent with keystone predation (Griswold and Lounibos 2005a, b). What remains unknown is: (1) whether this effect is important in open systems in nature, (2) whether C. appendiculata are more likely to be barriers to invasion or keystone predators favoring invader-native coexistence, and (3) whether effects of this predator in nature arise via lethal effects of predation on invader survivorship or non-lethal effects of behavioral avoidance by the invader of predator-dominated container habitats. We investigated these questions by conducting a field experiment testing the hypothesis that C. appendiculata predation is an important component of the resistance of natural tree holes to invasion and impact by A. albopictus.
Materials and methods
Study site
The experiment was conducted at the Florida Medical Entomology Laboratory (FMEL), Vero Beach, Florida (27.48°N, 80.38°W). The study site was an oak–palmetto hammock located immediately adjacent to the main laboratory. This site contains both natural tree holes and discarded tires that have been in the hammock 2–10 years. Prior to invasion by A. albopictus, tree holes at this site were dominated by A. triseriatus, and tires at this site contained both A. triseriatus and A. aegypti (Juliano 1998). A. albopictus arrived in Indian River County in 1990 (O'Meara et al. 1995) and became abundant in these tires in 1991 (L. P. Lounibos, unpublished data). The predatory midge C. appendiculata is common in tree holes at the FMEL site (Lounibos 1985). The predatory container-dwelling mosquito T. rutilus can be found at this site (Lounibos 1985), though its abundance has been low since drought and subsequent hurricanes in 2004 (unpublished data).
Organisms
A. triseriatus used in these experiments were first-generation progeny of individuals collected as immatures from the FMEL site. Immatures were sorted by species and reared to adulthood in the laboratory. Adults were provided with blood meals from restrained domestic chickens [housed and maintained in accordance with National Institutes of Health guidelines for animal care], and eggs were deposited on paper towels. C. appendiculata for this experiment were progeny of a colony maintained at FMEL, and frequently supplemented with field-collected individuals from a variety of locations.
Experimental setup
The experiment was run in July 2005 and July 2006. Experimental containers were used golf cart tires (outer diameter = 40.6 cm, inner diameter = 19.7 cm, width = 20.3 cm) placed on the forest floor at the study site. Tires were obtained from nearby golf courses, immersed in scalding water, scrubbed with a brush dipped in a dilute bleach solution, and thoroughly rinsed. Tires were arranged in eight spatial blocks, each with a central tire (not housing organisms) laying flat on the ground, and four experimental tires leaning against the central tire at an angle of ∼70°, each held in place with a wooden stake. Tires were placed into position 10–14 days before initiating the experiment and sealed inside plastic bags to eliminate colonization prior to the start of the experiment. Water for experiments was collected from mosquito-containing automobile tires at the FMEL site, sieved (0.2 mm) to remove macrobiota and detritus, and held at 4°C until used. Senescent live oak (Quercus virginiana) leaves were collected at the FMEL site, dried at 80°C for 48 h, and weighed into 2-g aliquots.
To begin the experiment, we warmed the water to ambient temperature. We hatched eggs of A. triseriatus by immersing them in water, and collected first-instar larvae 24 h later. We counted A. triseriatus larvae into batches of 100. We also collected newly molted fourth-instar C. appendiculata from rearing containers and counted them into groups of two or eight. Water (1 l) and leaves (2 g) were added to all tires, and experimental larvae were added to the four experimental tires in a block to create four treatments: control (no A. triseriatus or C. appendiculata larvae), competitor (100 A. triseriatus larvae only), low predator (100 A. triseriatus and two C. appendiculata larvae), and high predator (100 A. triseriatus and eight C. appendiculata larvae). Predator densities of two or eight per liter were chosen to represent low and median densities observed in natural treeholes at this site (L. P. Lounibos, unpublished data). A. albopictus were not added to the experiment, but instead, the existing population of A. albopictus at the site was then allowed to colonize the experimental tires. Containers were also open to colonization by other container-dwelling species from the site, including A. triseriatus.
The experiment lasted 27 days. Every 2 days we checked visually for eggs of T. rutilus (which are white and float on the surface, hence are highly visible in a water-filled tire). Every third day we siphoned out the contents of all tires, placed them in a white enamel pan, and removed all insect larvae except A. albopictus from control tires, and removed C. appendiculata and T. rutilus from competitor tires. Water from low predator and high predator tires was handled in the same way, but only T. rutilus larvae were removed. Water and larvae were then returned to the tires. Every ninth day (=period), we siphoned out the contents of all tires, and brought them to the laboratory for a complete census of all insects. At this time we determined the stage (larval instar or pupa) for all A. albopictus, A. triseriatus, and C. appendiculata. In conjunction with the censuses, we again removed all insect larvae from control tires, C. appendiculata and T. rutilus from competitor tires, and all T. rutilus larvae from low predator and high predator tires. Every ninth day we added another batch of 100 newly hatched A. triseriatus larvae to all treatments except controls, and additional groups of two and eight C. appendiculata larvae to low predator and high predator tires, respectively. We did not remove any of the originally added individuals of A. triseriatus and C. appendiculata. Because many of the C. appendiculata pupated or eclosed successfully from pupae by the end of the 9-day period (“Results”) this lack of removal likely had little effect on abundances of the predators across the periods.
After 27 days, we did another complete census of all insects in all tires, again determining stages of A. albopictus, A. triseriatus, and C. appendiculata. For each tire, we marked the water line, and stored the tire in a plastic bag at room temperature for 4 days. Because eggs are not reliably identifiable, we hatched Aedes eggs that had accumulated in the tires by filling each tire with a nutrient broth solution. After 24 h, we removed the hatching solution, collected hatchling larvae into rearing containers with food (yeast:lactalbumin 1:1) and raised them to the third instar for identification. Tires were dried for 4 days and then again filled with hatching solution, and the hatching-rearing-identification process repeated in an attempt to induce hatching of all viable eggs. The second flooding yielded <5% of the total hatch, hence we are confident that virtually all viable eggs were induced to hatch. We use the total number of A. albopictus larvae from the two hatches as our measure of oviposition into these tires.
Data analyses
All analyses were done as randomized block designs with treatment, year, and treatment–year interaction as fixed effects, and block nested in year as a random effect, using SAS 9.1, PROC GLM (SAS Institute 2003). For A. albopictus we analyzed all four treatments (control, competitor, low predator, high predator), but for A. triseriatus we excluded the controls because very few A. triseriatus colonized these replicates.
Abundances of A. albopictus and of A. triseriatus were analyzed as repeated measures multivariate ANOVAs, with the three 9-day sample periods as repeated measures. Abundances of both species in each sample were square root transformed to meet assumptions of normality and homogeneity of variances. Significant effects were further analyzed by pairwise multivariate comparisons with a Bonferroni adjustment for multiple tests. Standardized canonical coefficients (Scheiner 2001) were used to interpret which of the three sample periods contributed most to significant multivariate effects.
Numbers of viable eggs of A. albopictus present at the end of the experiment and mean instar as an indicator of developmental progress for both Aedes were analyzed as ANOVAs. To calculate mean instar, each individual was scored for instar (first to fourth, pupa = fifth). Numbers of A. albopictus were often zero in early samples, hence mean instar of A. albopictus was missing for a substantial number of early samples, which precluded using repeated measures on mean instar data. Instead we analyzed mean instar for A. albopictus and A. triseriatus recovered from a given tire across all three samples. For mean instars, because of missing observations, some F-tests were done using Satterthwaite's calculation of denominator mean square, resulting in noninteger df (SAS Institute 2003). Significant effects were further analyzed by Tukey's pairwise comparisons of least squares means.
Predictions
If native predators contribute to biotic resistance, we predict that addition of predators will result in less colonization by A. albopictus compared to treatments without predators. We further predict that the effect of predators on a population of A. albopictus will increase with predator number, and numbers of A. albopictus should be greater for low predation than for high predation replicates.
If native predators and competitors create a keystone effect, competitive effects of A. albopictus on A. triseriatus should be reduced in replicates with predators compared to replicates without predators. For those treatments with A. triseriatus added we therefore predict that numbers and mean instar of A. triseriatus should be greater in replicates with predators than in replicates without predators. We predict a similar effect on mean instar of A. albopictus.
If effects of the predator are density-mediated via predator-induced mortality only, we predict that number of eggs of A. albopictus in replicates with predators will be no different from that for replicates without predators. If, instead, trait-mediated behavioral habitat avoidance by A. albopictus contributes to predator effects, we predict that number of eggs of A. albopictus in replicates with predators should be lower than that for replicates without predators.
Results
Predators
C. appendiculata survived and developed well in the experimental tires. For the first 9 days of the experiment in both years, when only one C. appendiculata cohort was present, 67 ± 6% (mean ± SE) of the originally stocked larvae were recovered in the census at 9 days. Of these, 42% were pupae, indicating successful development, and suggesting that some of the missing individuals had eclosed from pupae and left the container. Thus, 67% survival represents a conservative minimum, and actual survival of predators was likely considerably higher. Only two C. appendiculata individuals (one in each year) and no T. rutilus individuals were recovered from control or competitor treatments during predator checks.
Colonization by A. albopictus
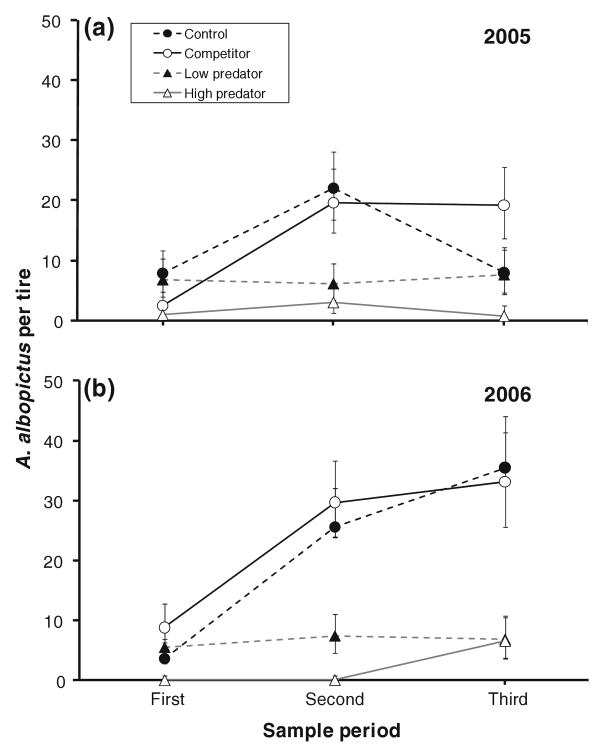
There was a significant effect of treatment on number of A. albopictus in tires (Table 1), but no significant effect of year or of year × treatment interaction (Table 1). Mean A. albopictus abundances were significantly greater for control and competitor treatments than for low predation and high predation treatments (Fig. 1). Control and competitor did not differ, and low predation and high predation did not differ (Fig. 1). Effects involving the repeated measure period were significant (Table 1), indicating that the temporal trajectory of A. albopictus colonization depended on treatment and year (Fig. 1). Numbers of A. albopictus increased from the first to the second sample period for control and competitor treatments in both years (Fig. 1). The significant sample–treatment–year interaction (Table 1) was primarily a product of variation in the trajectories between the second and third samples, with controls showing a decline in 2005 but an increase in 2006 (Fig. 1). For the two predator treatments, numbers of A. albopictus showed little temporal trend (Fig. 1).
Table 1.
Repeated measures multivariate ANOVA for abundances of Aedes albopictus and Aedes triseriatus immatures
| A. albopictus | A. triseriatus | |||||
|---|---|---|---|---|---|---|
| Source | dfn, dfd | F | P | dfn, dfd | F | P |
| Between subjects | ||||||
| Year | 1, 14 | 1.75 | 0.2071 | 1, 14 | 0.05 | 0.8211 |
| Block(Year) | 14 | 14 | ||||
| Treatment | 3, 42 | 16.25 | <0.0001 | 2, 28 | 7.79 | 0.0020 |
| Year × Treatment | 3, 42 | 1.05 | 0.3801 | 2, 28 | 1.73 | 0.1964 |
| Error | 42 | 28 | ||||
| Within subjects | ||||||
| Period | 2, 41 | 21.89 | <0.0001 | 2, 27 | 30.82 | <0.0001 |
| Period × Year | 2, 41 | 5.58 | 0.0072 | 2, 27 | 1.10 | 0.3479 |
| Period × Block(Year) | 28, 84 | 2.07 | 0.0057 | 28, 56 | 1.67 | 0.0509 |
| Period × Treatment | 6, 84 | 3.74 | 0.0024 | 4, 56 | 0.78 | 0.5425 |
| Period × Year × Treatment | 6, 84 | 2.22 | 0.0490 | 4, 56 | 0.53 | 0.7144 |
| Error(Period) | 84 | 56 | ||||
Multivariate F statistics derived from Pillai's trace (SAS Institute 2003)
Significant effects in bold
Fig. 1.
Numbers of invasive mosquito Aedes albopictus (least squares mean ± SE) recovered from experimental tires during three sample periods in a 2005, and b 2006. Treatments are defined by the combination of larvae added to each tire: no Aedes triseriatus or Corethrella appendiculata larvae (Control), 100 A. triseriatus larvae only (Competitor), 100 A. triseriatus and two C. appendiculata larvae (Low predator), and 100 A. triseriatus and eight C. appendiculata larvae (High predator). Repeated measures multivariate ANOVA (MANOVA) is reported in Table 1 and individual pairwise comparisons are reported in the text
Survival and development of A. triseriatus
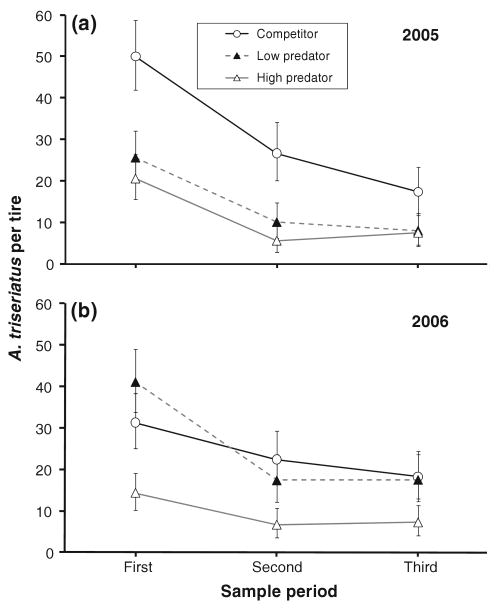
There was a significant treatment effect on number of surviving A. triseriatus (Table 1), but no year effect or year–treatment interaction (Table 1). The repeated measure period was significant (Table 1), indicating temporal changes in survival, but none of the interactions involving period was significant (Table 1), indicating treatment and year did not affect the temporal trajectory. Mean survival of A. triseriatus was greatest for the competitor treatment, and was reduced for both low predation and high predation treatments (Fig. 2). This trend was more obvious in 2005 than in 2006, but the lack of year–treatment interaction indicates that the treatment effect was not significantly dependent on year. Numbers of surviving A. triseriatus declined across sample periods (Fig. 2).
Fig. 2.
Numbers of native mosquito A. triseriatus (least squares mean ± SE) recovered from experimental tires during three sample periods in a 2005 and b 2006. The control treatment is absent because no A. triseriatus were stocked into the control tires (see “Materials and methods”), and very few A. triseriatus colonized the tires. Repeated measures MANOVA is reported in Table 1 and individual pairwise comparisons are reported in the text
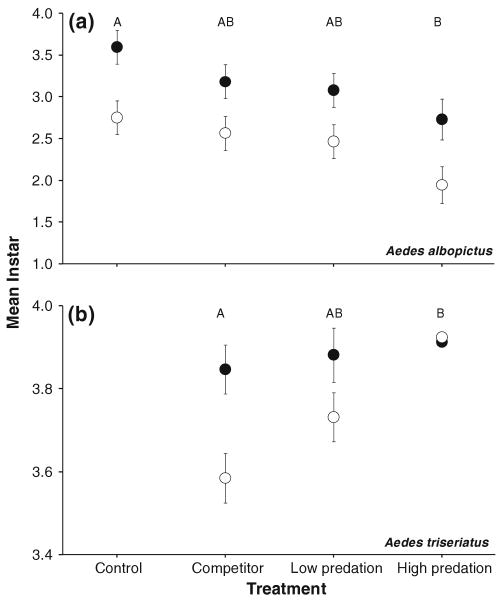
Mean instar of A. triseriatus was significantly affected only by treatment (F2,27 = 5.57, P = 0.0094), with high predation yielding significantly greater mean instar than did competitor (Fig. 3). Low predation was intermediate and statistically indistinguishable from the other two treatments (Fig. 3).
Fig. 3.
Developmental stages (least squares mean instar ± SE, across all samples from each year) for a A. albopictus and b A. triseriatus in 2005 (solid circles) and 2006 (open circles) from experimental tires with control, competitor, low and high density predators. ANOVA reported in text. For A. triseriatus in b, the control treatment is absent because no A. triseriatus were stocked into the control tires (see “Materials and methods”), and very few A. triseriatus colonized the tires. Treatments associated with the same letters (top of each graph) are not significantly different by Tukey's multiple comparisons
Development of A. albopictus
Treatment significantly (F3,39 = 5.01, P = 0.0049) affected mean instar of A. albopictus, but this effect was not consistent with release from competition via predation, as developmental progress was least in the high predation treatment (Fig. 3). Developmental progress was significantly (F1,14.35 = 12.95, P = 0.0028) greater in 2005 than in 2006 (Fig. 3). Year–treatment interaction was not significant (P ≫ 0.05).
Eggs of A. albopictus
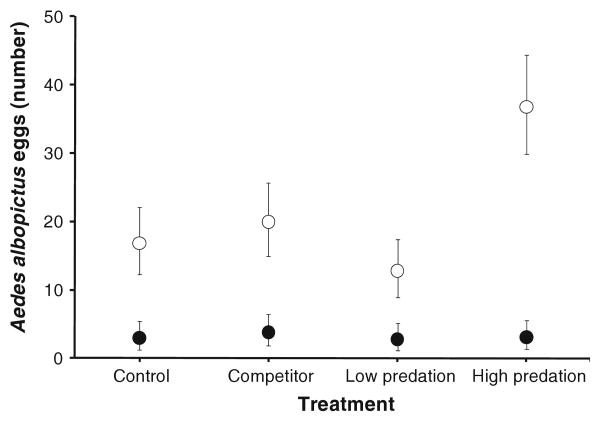
The overwhelming majority of individuals hatching from eggs in tires at the end of the experiment were A. albopictus (2005, one hundred and sixty-five A. albopictus, two A. triseriatus; 2006, nine hundred and five A. albopictus, three A. triseriatus, seven A. aegypti). Oviposition by the natural population of A. triseriatus was thus very rare. Hatching of A. albopictus from tires was significantly (F1,14 = 10.88, P = 0.0053) greater by a factor of 3–10 in 2006 than in 2005 (Fig. 4), but there were no significant effects of treatment or year–treatment interaction (P > 0.05).
Fig. 4.
Numbers of A. albopictus eggs (least squares mean ± SE) recovered at the end of the experiment in 2005 (solid circles) and 2006 (open circles). ANOVA reported in text
Discussion
This experiment clearly demonstrates that populations of predatory C. appendiculata contribute to the resistance of a container community to invasion by A. albopictus. Invasion of containers with predators is much lower than that of containers without predators. Further, the presence of native A. triseriatus alone had no effect on invasion by A. albopictus, indicating that competition from this native species is not an important impediment to invasion success. There was, however, no evidence in this experiment to support the prediction that increasing predator number (from two to eight) produces greater reduction of A. albopictus abundance. Further, predator effects on A. albopictus colonization do not appear to be due to trait-mediated avoidance of oviposition in habitats with predators. Egg abundances in all treatments were statistically indistinguishable, and we conclude that the effects of predatory C. appendiculata on populations of A. albopictus are direct results of the lethal effect of predation.
We also find strong evidence that the presence of this predator combined with a population of the native A. triseriatus creates conditions for keystone coexistence of invading A. albopictus and native A. triseriatus. Past laboratory studies of competition between A. albopictus and A. triseriatus (Livdahl and Willey 1991; Novak et al. 1993; Teng and Apperson 2000; Aliabadi and Juliano 2002; Bevins 2007; Yee et al. 2007) have suggested that invading A. albopictus are superior competitors under many circumstances. Despite this, field studies of south Florida tree holes have indicated that when C. appendiculata are present, A. triseriatus may not only persist following invasion by A. albopictus, but may remain numerically dominant (Lounibos et al. 2001; Kesavaraju et al. 2008). In our experiment, predatory C. appendiculata appeared to alleviate effects of competition, i.e., both interspecific competition from A. albopictus, and intraspecific competition, on development rate (as measured by mean instar) of A. triseriatus. However, although predatory C. appendiculata affected the development rate of A. albopictus, the effect was opposite of what we expect from a keystone predator effect reducing competition, so that mean instar of A. albopictus was less in treatments with C. appendiculata, rather than greater as we predicted. Given that we find evidence in mean instar of A. triseriatus of release from competition due to predator reductions of A. albopictus, why is there no similar effect on A. albopictus? We cannot answer this question with certainty, but it seems likely this effect arises because of continuous hatching of new A. albopictus eggs, and cropping of the A. albopictus population by C. appendiculata. Early instar A. albopictus in the predator treatments likely represent the most recently hatched individuals that have necessarily had only a brief time of exposure to predation. Older larvae that have been in the container long enough to attain second or third instar have a greater cumulative probability of death because they have been exposed longer. This situation would enrich the A. albopictus population at any single time with early instar larvae. This effect is likely absent in A. triseriatus because the larvae in the container were added in synchrony, at the beginning of a period, and because colonization via oviposition by A. triseriatus was very low (see “Results”, Eggs of A. albopictus). It is the synchrony of the cohorts of A. triseriatus that enables us to see the developmental effect of release from competition. An alternative explanation for decreased mean instar of A. albopictus is that its development was slowed due to reduced movement and foraging in response to predation cues (Kesavaraju et al. 2007), but this is unlikely because the native prey, A. triseriatus, shows an even stronger reduction in movement and foraging in the presence of C. appendiculata than does A. albopictus (Kesavaraju et al. 2007). Thus, if this behavioral effect were important we would expect to see reduced instar for the native as well, and we see instead the opposite: A. triseriatus develops more rapidly with predators present.
There are well-documented cases of native predators inhibiting invasion success (e.g., Robinson and Wellborn 1988; Baltz and Moyle 1993; Reusch 1998; Byers 2002; DeRivera et al. 2005; Salo et al. 2008). What has been rare is the demonstration that with invasion, native predators act as keystone predators contributing to persistence of natives despite increased competition from invaders (Smith 2006). Our system not only provides an example of such an effect but also, when combined with past behavioral studies of predation, suggests a likely mechanism for such effects. Previous laboratory behavioral studies have shown strong differences in vulnerability to C. appendiculata predation between A. triseriatus and A. albopictus (Griswold and Lounibos 2005a, b; Kesavaraju et al. 2007). The difference in vulnerability depends in part on differences in behavioral responses of native A. triseriatus and invasive A. albopictus to cues from predation (Kesavaraju et al. 2007). A. triseriatus strongly reduce foraging and movement in the presence of the predator, whereas A. albopictus shows less of a response (Kesavaraju et al. 2007). Further, even without predators present, A. triseriatus spends significantly less time foraging and moving than does A. albopictus (Kesavaraju et al. 2007). Thus, this system shows the typical patterns associated with a predation–competition tradeoff, with low activity and low foraging effort, along with reduced movement in the presence of predators, resulting in low risk of predation, but also low competitive ability (Morin 1983; Werner and Anholt 1993; Wellborn 2002).
One interpretation of the absence of behavioral avoidance of habitats with predators by A. albopictus is that this Asian species lacks evolutionary history with C. appendiculata and has not evolved behavioral avoidance of this predator. This interpretation is probably overly simple. Larval A. albopictus do show some behavioral responses (reduced movement and foraging) to predation cues from C. appendiculata, although the intensity of these responses is less than those of native A. triseriatus (Kesavaraju et al. 2007). North American A. albopictus originated in temperate Asia (Lounibos 2002) and are sympatric with other species of predatory Corethrella in temperate Japan, but the known Japanese Corethrella larvae are not container dwellers (Borkent 2008), hence it is likely that North American A. albopictus have little or no evolutionary history with predatory Corethrella. Avoidance of oviposition in habitats with predators appears to be common in some mosquito groups, particularly Culex and Culiseta (e.g., Kiflawi et al. 2003; Blaustein et al. 2005, reviewed by Juliano 2009) in which eggs laid on the water surface hatch shortly after oviposition, and cues perceived by ovipositing females provide a good indicator of predation risk to offspring. Most Aedes eggs are laid above the water, and hatch at a later time, often after a substantial delay. This situation may make current predator cues less reliable indicators of predation risk that will impinge on larvae hatching at some unknown time in the future, and oviposition avoidance by females less advantageous. Thus a phylogenetically constrained aspect of the life history of A. albopictus may contribute to the minimal importance of trait-mediated oviposition responses to risk of predation.
Previous work on Florida tree holes has shown that C. appendiculata can significantly reduce the abundance of its primary native prey, A. triseriatus (Lounibos 1983, 1985). Our results in this study show that this natural enemy of A. triseriatus can be an important contributor to both community resistance to invasion by a competing exotic species, and given invasion, to coexistence of the native and invader despite competitive superiority of the invader. When a community is invaded, it appears that the net effect of a native predator on a native prey species changes from clearly detrimental before invasion, to beneficial after invasion. This change in effect results from the indirect effect of the native predator on the native victim via predation on the invading competitor. Whether such a situation is general or widespread remains to be determined, but it does point out the importance of indirect and synergistic effects in communities. Further, this kind of effect in other communities that harbor rare, threatened, or economically valuable native species could influence strategies to enhance those native species' populations. Control of native predators might be one such strategy (e.g., Fisher et al. 2000; Roemer and Wayne 2003), but such control could result in communities that are more easily invaded, which could result in reduced likelihood of persistence by the native competitor. Such possible effects argue for an ecosystem-based approach to conservation and enhancement of target native species populations (Roemer and Wayne 2003); and that conservation (or restoration) of populations of predators may be useful for reducing the impacts of invasive species (as suggested by Salo et al. 2008).
Effects of predators on natural communities are likely to be more diverse than those observed here. The barrier and keystone effects of predatory C. appendiculata occur because the invader A. albopictus is the more vulnerable victim, along with being a superior competitor. Theory shows that the presence of predators can actually enhance invasion when it is the invader that is less competitive, but also less vulnerable (Noonberg and Byers 2005). More generally, theory (Leibold 1996) and experiment (Griswold and Lounibos 2005b) have shown that keystone predator effects on coexistence occur within an intermediate range of environmental productivity, so that we would expect the kinds of effects of predators seen in our experiment to be context dependent, and less likely in habitats with either very high or very low productivity. Patterns of distributions of the three principal species in this experiment are consistent with the expectations derived from this experiment, that predatory C. appendiculata contribute to invasion resistance and to coexistence of competitors in nature (Kesavaraju et al. 2008).
These results provide one more line of evidence for the positive effects of components of biodiversity, in this case the presence of a higher trophic level, on community stability and resilience in the face of an influx of non-native species (as suggested by Shea and Chesson 2002; Smith 2006; Salo et al. 2008). The presence of an intact assemblage of predator and prey in containers appears to act as a buffer that both limits the success of the invader and limits the impact of the invader on resident species. This effect occurs even within this relatively simple assemblage of container-dwelling aquatic invertebrates, which consists of perhaps ten to 20 species (Bradshaw and Holzapfel 1988; Lounibos 1985). The potential for such effects of biodiversity on invasions in more complex assemblages would seem to be greater. Our experiment does not enable us to evaluate the potential for additional effects on invasion success involving predators. For example, invasive species can have impacts on natives via apparent competition if the invasive enhances predator populations which in turn take a heavier predatory toll on native species (Noonberg and Byers 2005). Testing for such an effect requires sufficient time for a numerical response of predators (Chase et al. 2002), but our experiment was not run at a temporal scale that would enable us to test for this effect. Theory (Noonberg and Byers 2005) suggests that such an effect is less likely in the present case, with the invader more vulnerable to predation and a better competitor, but we cannot completely rule out an effect of apparent competition. What is clear is that our results provide strong evidence that a native enemy, normally detrimental to populations of a native species, can become a keystone predator that facilitates coexistence of the native with an invader when the ecological context changes due to invasion. Thus the presence of an enemy may become beneficial at the population level as the ecological context changes.
Acknowledgments
We thank R. Escher for his extensive efforts rearing A. triseriatus and C. appendiculata, and for other assistance in the laboratory, M. Reiskind, B. Kesavaraju, K. Smith, and J. Chase for informative discussion, and two anonymous referees for helpful comments. This research was supported by a grant from the National Institute of Allergy and Infectious Disease (R01-AI44793). Experiments comply with the current laws of the US.
Footnotes
Communicated by Steven Kohler.
Contributor Information
Steven A. Juliano, Email: sajulian@ilstu.edu, Behavior, Ecology, Evolution, and Systematics Section, School of Biological Sciences, Illinois State University, Normal, IL 61790-4120, USA.
L. Philip Lounibos, Email: lounibos@ufl.edu, Florida Medical Entomology Laboratory, University of Florida, Vero Beach, FL 32962, USA.
Naoya Nishimura, Email: nishimur@ufl.edu, Florida Medical Entomology Laboratory, University of Florida, Vero Beach, FL 32962, USA.
Krystle Greene, Email: kvansick@ufl.edu, Florida Medical Entomology Laboratory, University of Florida, Vero Beach, FL 32962, USA.
References
- Aliabadi BK, Juliano SA. Escape from gregarine parasites affects the competitive impact of an invasive mosquito. Biol Invasions. 2002;4:283–297. doi: 10.1023/A:1020933705556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto BW, Kesavaraju B, Juliano SA, Lounibos LP. Stage-dependent predation on competitors: consequences for the outcome of a mosquito invasion. J Anim Ecol. 2009;78:928–936. doi: 10.1111/j.1365-2656.2009.01558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armistead JS, Arias JR, Lounibos LP. Interspecific larval competition between Aedes albopictus and Aedes japonicus (Diptera: Culicidae) in northern Virginia. J Med Entomol. 2008;45:629–637. doi: 10.1603/0022-2585(2008)45[629:ilcbaa]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltz DM, Moyle PB. Invasion resistance to introduced species by a native assemblage of California stream fishes. Ecol Appl. 1993;3:246–255. doi: 10.2307/1941827. [DOI] [PubMed] [Google Scholar]
- Bevins SN. Timing of resource input and larval competition between invasive and native container-inhabiting mosquitoes (Diptera: Culicidae) J Vector Ecol. 2007;32:252–262. doi: 10.3376/1081-1710(2007)32[252:torial]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Blaustein L, Blaustein J, Chase J. Chemical detection of the predator Notonecta irrorata by ovipositing Culex mosquitoes. J Vector Ecol. 2005;30:299–301. [PubMed] [Google Scholar]
- Borkent A. The frog-biting midges of the world (Corethrellidae: Diptera) Zootaxa. 2008;1804:1–456. [Google Scholar]
- Bradshaw WE, Holzapfel CM. Drought and the organization of tree-hole mosquito communities. Oecologia. 1988;74:507–514. doi: 10.1007/BF00380047. [DOI] [PubMed] [Google Scholar]
- Braks MAH, Honório NA, Lourenço-de-Oliveira R, Juliano SA, Lounibos LP. Convergent habitat segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in southeastern Brazil and Florida, USA. J Med Entomol. 2003;40:785–794. doi: 10.1603/0022-2585-40.6.785. [DOI] [PubMed] [Google Scholar]
- Braks MAH, Honório NA, Lounibos LP, Lourenço-de-Oliveira R, Juliano SA. Interspecific competition between two invasive species of container mosquitoes, Aedes aegypti and Aedes albopictus (Diptera: Culicidae), in Brazil. Ann Entomol Soc Am. 2004;97:130–139. [Google Scholar]
- Byers JE. Physical habitat attribute mediates biotic resistance to non-indigenous species invasion. Oecologia. 2002;130:146–156. doi: 10.1007/s004420100777. [DOI] [PubMed] [Google Scholar]
- Chase JM, Abrams PA, Grover JP, Diehl S, Chesson P, Holt RD, Richards SA, Nisbet RM, Case TJ. The interaction between predation and competition: a review and synthesis. Ecol Lett. 2002;5:302–315. [Google Scholar]
- Costanzo KS, Kesavaraju B, Juliano SA. Condition-specific competition in container mosquitoes: the role of non-competing life-history stages. Ecology. 2005;86:3289–3295. doi: 10.1890/05-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. Murray; London: 1859. [PMC free article] [PubMed] [Google Scholar]
- DeRivera CE, Ruiz GM, Hines AH, Jivoff P. Biotic resistance to invasion: native predator limits abundance and distribution of an introduced crab. Ecology. 2005;86:3364–3376. [Google Scholar]
- Duncan RP, Williams PA. Ecology: Darwin's naturalization hypothesis challenged. Nature. 2002;417:608–6099. doi: 10.1038/417608a. [DOI] [PubMed] [Google Scholar]
- Elton C. The ecology of invasions by animals and plants. Wiley; New York: 1958. [Google Scholar]
- Fisher DO, Hoyle SD, Blomberg SP. Population dynamics and survival of an endangered wallaby: a comparison of four methods. Ecol Appl. 2000;10:901–910. [Google Scholar]
- Griswold MW, Lounibos LP. Does differential predation permit invasive and native mosquito larvae to coexist in Florida? Ecol Entomol. 2005a;30:122–127. doi: 10.1111/j.0307-6946.2005.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold MW, Lounibos LP. Competitive outcomes of aquatic container Diptera depend on predation and resource levels. Ann Entomol Soc Am. 2005b;98:673–681. doi: 10.1603/0013-8746(2005)098[0673:COOACD]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley WA. The biology of Aedes albopictus. J Am Mosq Control Assoc. 1988;4(Supplement):1–40. [PubMed] [Google Scholar]
- Holt RD. Predation, apparent competition, and the structure of prey communities. Theor Popul Biol. 1977;12:197–229. doi: 10.1016/0040-5809(77)90042-9. [DOI] [PubMed] [Google Scholar]
- Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology. 1998;79:255–268. [Google Scholar]
- Juliano SA. Species interactions among larval mosquitoes: context dependence across habitat gradients. Annu Rev Entomol. 2009;54:37–56. doi: 10.1146/annurev.ento.54.110807.090611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett. 2005;8:558–574. doi: 10.1111/j.1461-0248.2005.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, O'Meara GF, Morrill JR, Cutwa MM. Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes. Oecologia. 2002;130:458–469. doi: 10.1007/s004420100811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Lounibos LP, O'Meara GF. A field test for competitive effects of Aedes albopictus on Aedes aegypti in South Florida: differences between sites of coexistence and exclusion? Oecologia. 2004;139:583–593. doi: 10.1007/s00442-004-1532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B, Juliano SA. Differential behavioral responses to water-borne cues to predation in two container-dwelling mosquitoes. Ann Entomol Soc Am. 2004;97:194–201. doi: 10.1603/0013-8746(2004)097[0194:dbrtwc]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B, Alto BW, Lounibos LP, Juliano SA. Behavioural responses of larval container mosquitoes to a size-selective predator. Ecol Entomol. 2007;32:262–272. doi: 10.1111/j.1365-2311.2006.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B, Damal K, Juliano SA. Do natural container habitats impede invader dominance? Predator-mediated coexistence of invasive and native container-dwelling mosquitoes. Oecologia. 2008;155:631–639. doi: 10.1007/s00442-007-0935-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiflawi M, Blaustein L, Mangel M. Predation-dependent oviposition habitat selection by the mosquito Culiseta longiareolata: a test of competing hypotheses. Ecol Lett. 2003;6:35–40. [Google Scholar]
- Leibold MA. A graphical model of keystone predators in food webs: trophic regulation of abundance, incidence, and diversity patterns in communities. Am Nat. 1996;147:784–812. [Google Scholar]
- Livdahl TP, Willey MS. Prospects for an invasion: competition between Aedes albopictus and native Aedes triseriatus. Science. 1991;253:189–191. doi: 10.1126/science.1853204. [DOI] [PubMed] [Google Scholar]
- Lodge DM. Biological invasions: lessons for ecology. Trends Ecol Evol. 1993;8:133–136. doi: 10.1016/0169-5347(93)90025-K. [DOI] [PubMed] [Google Scholar]
- Lounibos LP. The mosquito community of treeholes in subtropical Florida. In: Frank JH, Lounibos LP, editors. Phytotelmata: terrestrial plants as hosts for aquatic insect communities. Plexus; Medford: 1983. pp. 223–246. [Google Scholar]
- Lounibos LP. Interactions influencing production of treehole mosquitoes in South Florida. In: Lounibos LP, Rey JR, Frank JH, editors. Ecology of mosquitoes: proceedings of a workshop. Florida Medical Entomology Laboratory; Vero Beach: 1985. pp. 65–77. [Google Scholar]
- Lounibos LP. Invasions by insect vectors of human diseases. Annu Rev Entomol. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, O'Meara GF, Escher RL, Nishimura N, Cutwa M, Nelson T, Campos R, Juliano SA. Testing predictions of displacement of native Aedes by the invasive Asian tiger mosquito Aedes albopictus in Florida, USA. Biol Invasions. 2001;3:151–166. [Google Scholar]
- Morin PJ. Predation, competition, and the composition of larval anuran guilds. Ecol Monogr. 1983;53:119–138. [Google Scholar]
- Noonberg EG, Byers JE. More harm than good: when invader vulnerability to predators enhances impact on native species. Ecology. 2005;86:2555–2560. [Google Scholar]
- Novak MG, Higley LG, Christianssen CA, Rowley WA. Evaluating larval competition between Aedes albopictus and A. triseriatus (Diptera, Culicidae) through replacement series experiments. Environ Entomol. 1993;22:311–318. [Google Scholar]
- Nunez MA, Simberloff D, Relva MA. Seed predation as a barrier to alien conifer invasions. Biol Invasions. 2008;10:1389–1398. [Google Scholar]
- O'Meara GF, Evans LF, Gettman AD, Cuda JP. Spread of Aedes albopictus decline of Ae aegypti (Diptera: Culicidae) in Florida. J Med Entomol. 1995;32:554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- Paine RT. Food web complexity and species diversity. Am Nat. 1966;100:65–75. [Google Scholar]
- Preisser EL, Bolnick DI, Benard MF. Scared to death? Behavioral effects dominate predator–prey interactions. Ecology. 2005;86:501–509. [Google Scholar]
- Reusch TBH. Native predators contribute to invasion resistance to the non-indigenous bivalve Musculista senhousia in southern California, USA. Mar Ecol Prog Ser. 1998;170:159–168. [Google Scholar]
- Robinson JV, Wellborn GA. Ecological resistance to the invasion of a freshwater clam, Corbicula fluminea: fish predation effects. Oecologia. 1988;77:445–452. doi: 10.1007/BF00377258. [DOI] [PubMed] [Google Scholar]
- Roemer GW, Wayne RK. Conservation in conflict: the tale of two endangered species. Conserv Biol. 2003;17:1251–1260. [Google Scholar]
- Salo P, Nordström M, Thomson RL, Korpimäki E. Risk induced by a native top predator reduces alien mink movements. J Anim Ecol. 2008;77:1092–1098. doi: 10.1111/j.1365-2656.2008.01430.x. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS user's guide: statistics, version 9 1. SAS Institute; Cary: 2003. [Google Scholar]
- Scheiner SM. MANOVA: multiple response variables and multispecies interaction. In: Scheiner SM, Gurevitch J, editors. Design and analysis of ecological experiments. 2nd. Oxford University Press; Oxford: 2001. pp. 99–115. [Google Scholar]
- Shea KP, Chesson P. Community ecology as a framework for biological invasions. Trends Ecol Evol. 2002;5:302–315. [Google Scholar]
- Smith KG. Keystone predators eastern newts, Notophthalmus viridescens reduce the impacts of an aquatic invasive species. Oecologia. 2006;148:342–349. doi: 10.1007/s00442-006-0370-y. [DOI] [PubMed] [Google Scholar]
- Teng HJ, Apperson CS. Development and survival of immature Aedes albopictus and Aedes triseriatus Diptera: Culicidae in the laboratory: effects of density, food, and competition on response to temperatures. J Med Entomol. 2000;37:40–52. doi: 10.1603/0022-2585-37.1.40. [DOI] [PubMed] [Google Scholar]
- Wellborn GA. Trade-off between competitive ability and antipredator adaptation in a freshwater amphipod species complex. Ecology. 2002;83:129–136. [Google Scholar]
- Werner EE, Anholt BR. Ecological consequences of the trade-off between growth and mortality rates mediated by foraging activity. Am Nat. 1993;142:242–272. doi: 10.1086/285537. [DOI] [PubMed] [Google Scholar]
- Werner EE, Peacor SD. A review of trait-mediated indirect interactions in ecological communities. Ecology. 2003;84:1083–1100. [Google Scholar]
- Yee DA, Kaufman MG, Juliano SA. The significance of ratios of detritus types and microorganism productivity to competitive interactions between aquatic insect detritivores. J Anim Ecol. 2007;76:1105–1115. doi: 10.1111/j.1365-2656.2007.01297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]