Abstract
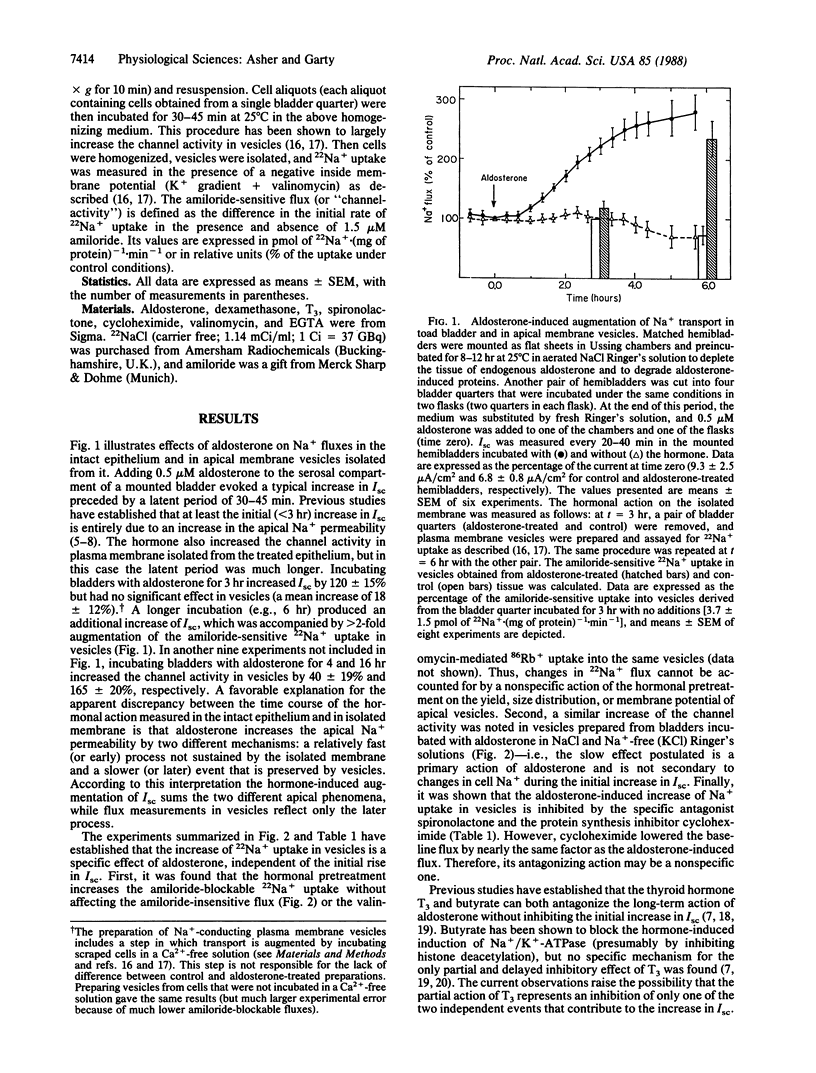
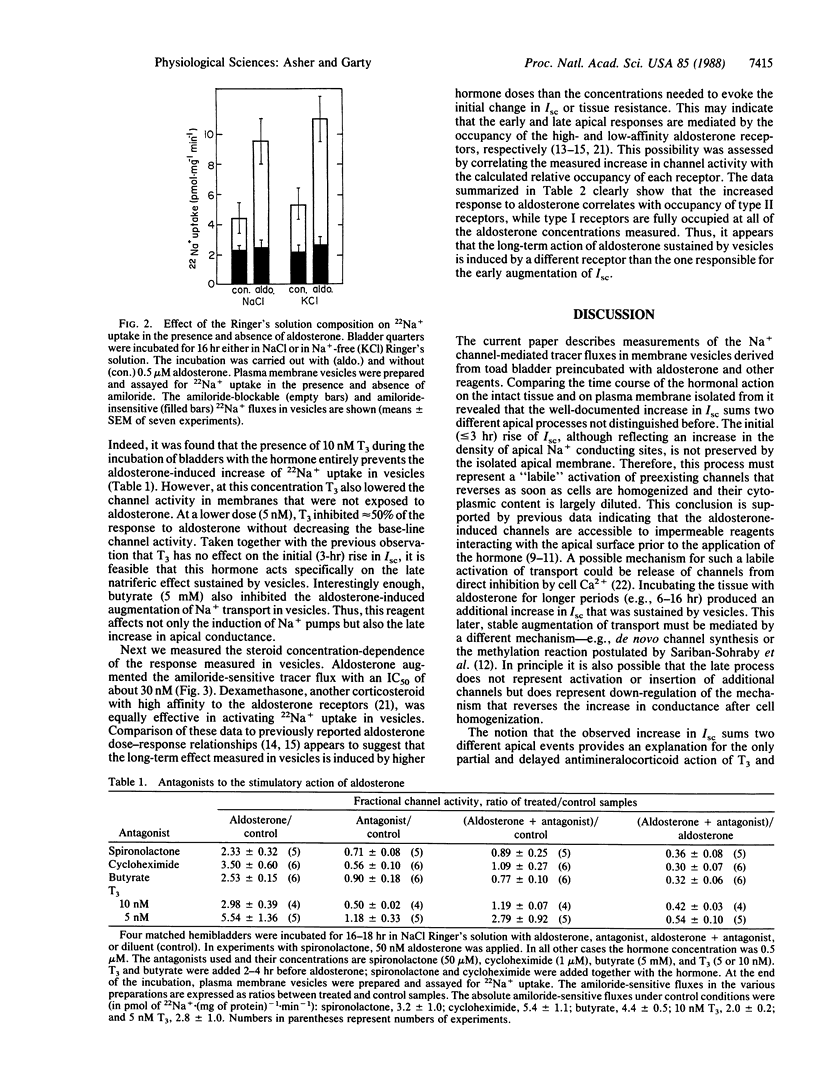
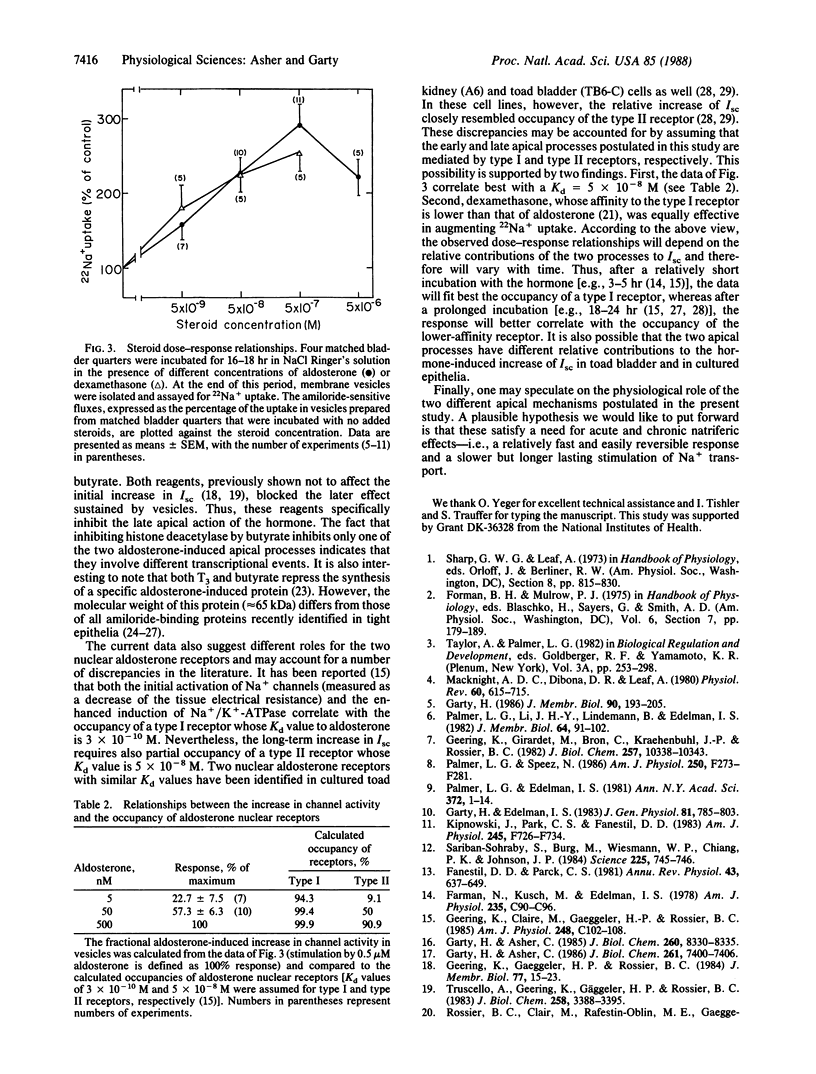
The aldosterone-induced augmentation of Na+ transport in toad bladder was analyzed by comparing the hormonal actions on the transepithelial short-circuit current and on the amiloride-sensitive 22Na+ uptake in isolated membrane vesicles. Incubating bladders with 0.5 microM aldosterone for 3 hr evoked more than a 2-fold increase of the short-circuit current (because of the activation or insertion of apical amiloride-blockable channels) but had no effect on the amiloride-sensitive Na+ transport in apical vesicles derived from the treated tissue. A longer incubation (e.g., 6 hr) produced an additional augmentation of the short-circuit current, which was accompanied by about a 3-fold increase of the channel activity in isolated membranes. The stimulatory effect of aldosterone sustained in vesicles was inhibited by the antagonist spironolactone (present at 1000-fold excess) and the protein synthesis inhibitor cycloheximide (1 microM). In addition, triiodothyronine and butyrate, previously reported to partly inhibit the aldosterone-induced increase in short-circuit current, blocked the hormonal effect in vesicles. It is suggested that aldosterone elevates the apical Na+ permeability of target epithelia by two different mechanisms: a relatively fast effect (less than or equal to 3 hr), which is insensitive to triiodothyronine or butyrate and is not sustained by the isolated membrane, and a slower or later (greater than 3 hr) response blocked by these reagents, which is preserved by the isolated membrane. The data also indicate that these processes are mediated by different nuclear receptors.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbry P., Chassande O., Vigne P., Frelin C., Ellory C., Cragoe E. J., Jr, Lazdunski M. Purification and subunit structure of the [3H]phenamil receptor associated with the renal apical Na+ channel. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4836–4840. doi: 10.1073/pnas.84.14.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanestil D. D., Park C. S. Steroid hormones and the kidney. Annu Rev Physiol. 1981;43:637–649. doi: 10.1146/annurev.ph.43.030181.003225. [DOI] [PubMed] [Google Scholar]
- Farman N., Kusch M., Edelman I. S. Aldosterone receptor occupancy and sodium transport in the urinary bladder of Bufo marinus. Am J Physiol. 1978 Sep;235(3):C90–C96. doi: 10.1152/ajpcell.1978.235.3.C90. [DOI] [PubMed] [Google Scholar]
- Garty H., Asher C. Ca2+-dependent, temperature-sensitive regulation of Na+ channels in tight epithelia. A study using membrane vesicles. J Biol Chem. 1985 Jul 15;260(14):8330–8335. [PubMed] [Google Scholar]
- Garty H., Asher C. Ca2+-induced down-regulation of Na+ channels in toad bladder epithelium. J Biol Chem. 1986 Jun 5;261(16):7400–7406. [PubMed] [Google Scholar]
- Garty H., Asher C., Yeger O. Direct inhibition of epithelial Na+ channels by a pH-dependent interaction with calcium, and by other divalent ions. J Membr Biol. 1987;95(2):151–162. doi: 10.1007/BF01869160. [DOI] [PubMed] [Google Scholar]
- Garty H., Edelman I. S. Amiloride-sensitive trypsinization of apical sodium channels. Analysis of hormonal regulation of sodium transport in toad bladder. J Gen Physiol. 1983 Jun;81(6):785–803. doi: 10.1085/jgp.81.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garty H. Mechanisms of aldosterone action in tight epithelia. J Membr Biol. 1986;90(3):193–205. doi: 10.1007/BF01870126. [DOI] [PubMed] [Google Scholar]
- Geering K., Claire M., Gaeggeler H. P., Rossier B. C. Receptor occupancy vs. induction of Na+-K+-ATPase and Na+ transport by aldosterone. Am J Physiol. 1985 Jan;248(1 Pt 1):C102–C108. doi: 10.1152/ajpcell.1985.248.1.C102. [DOI] [PubMed] [Google Scholar]
- Geering K., Gaeggeler H. P., Rossier B. C. Effects of thyromimetic drugs on aldosterone-dependent sodium transport in the toad bladder. J Membr Biol. 1984;77(1):15–23. doi: 10.1007/BF01871096. [DOI] [PubMed] [Google Scholar]
- Geering K., Girardet M., Bron C., Kraehenbühl J. P., Rossier B. C. Hormonal regulation of (Na+,K+)-ATPase biosynthesis in the toad bladder. Effect of aldosterone and 3,5,3'-triiodo-L-thyronine. J Biol Chem. 1982 Sep 10;257(17):10338–10343. [PubMed] [Google Scholar]
- Kipnowski J., Park C. S., Fanestil D. D. Modification of carboxyl of Na+ channel inhibits aldosterone action on Na+ transport. Am J Physiol. 1983 Dec;245(6):F726–F734. doi: 10.1152/ajprenal.1983.245.6.F726. [DOI] [PubMed] [Google Scholar]
- Kleyman T. R., Yulo T., Ashbaugh C., Landry D., Cragoe E., Jr, Karlin A., Al-Awqati Q. Photoaffinity labeling of the epithelial sodium channel. J Biol Chem. 1986 Feb 25;261(6):2839–2843. [PubMed] [Google Scholar]
- Kusch M., Farman N., Edelman I. S. Binding of aldosterone to cytoplasmic and nuclear receptors of the urinary bladder epithelium of Bufo marinus. Am J Physiol. 1978 Sep;235(3):C82–C89. doi: 10.1152/ajpcell.1978.235.3.C82. [DOI] [PubMed] [Google Scholar]
- Macknight A. D., DiBona D. R., Leaf A. Sodium transport across toad urinary bladder: a model "tight" epithelium. Physiol Rev. 1980 Jul;60(3):615–715. doi: 10.1152/physrev.1980.60.3.615. [DOI] [PubMed] [Google Scholar]
- Palmer L. G., Edelman I. S. Control of apical sodium permeability in the toad urinary bladder by aldosterone. Ann N Y Acad Sci. 1981;372:1–14. doi: 10.1111/j.1749-6632.1981.tb15453.x. [DOI] [PubMed] [Google Scholar]
- Palmer L. G., Li J. H., Lindemann B., Edelman I. S. Aldosterone control of the density of sodium channels in the toad urinary bladder. J Membr Biol. 1982;64(1-2):91–102. doi: 10.1007/BF01870771. [DOI] [PubMed] [Google Scholar]
- Palmer L. G., Speez N. Stimulation of apical Na permeability and basolateral Na pump of toad urinary bladder by aldosterone. Am J Physiol. 1986 Feb;250(2 Pt 2):F273–F281. doi: 10.1152/ajprenal.1986.250.2.F273. [DOI] [PubMed] [Google Scholar]
- Pratt R. D., Johnson J. P. Thyroid hormone. Aldosterone antagonism in cultured epithelial cells. Biochim Biophys Acta. 1984 Dec 11;805(4):405–411. doi: 10.1016/0167-4889(84)90024-7. [DOI] [PubMed] [Google Scholar]
- Rossier B. C., Claire M., Rafestin-Oblin M. E., Gaeggeler H. P., Geering K. Effects of thyroid hormones and aldosterone on mineralocorticoid binding sites in the toad bladder. J Membr Biol. 1984;77(1):25–32. doi: 10.1007/BF01871097. [DOI] [PubMed] [Google Scholar]
- Sariban-Sohraby S., Burg M., Wiesmann W. P., Chiang P. K., Johnson J. P. Methylation increases sodium transport into A6 apical membrane vesicles: possible mode of aldosterone action. Science. 1984 Aug 17;225(4663):745–746. doi: 10.1126/science.6463652. [DOI] [PubMed] [Google Scholar]
- Truscello A., Geering K., Gäggeler H. P., Rossier B. C. Effects of butyrate on histone deacetylation and aldosterone-dependent Na+ transport in the toad bladder. J Biol Chem. 1983 Mar 10;258(5):3388–3395. [PubMed] [Google Scholar]
- Truscello A., Gäggeler H. P., Rossier B. C. Thyroid hormone antagonizes an aldosterone-induced protein: a candidate mediator for the late mineralocorticoid response. J Membr Biol. 1986;89(2):173–183. doi: 10.1007/BF01869713. [DOI] [PubMed] [Google Scholar]
- Watlington C. O., Perkins F. M., Munson P. J., Handler J. S. Aldosterone and corticosterone binding and effects on Na+ transport in cultured kidney cells. Am J Physiol. 1982 Jun;242(6):F610–F619. doi: 10.1152/ajprenal.1982.242.6.F610. [DOI] [PubMed] [Google Scholar]



