Abstract
Pain catastrophizing is an important variable in the context of acute and chronic pain. The neurophysiological correlates of pain catastrophizing, however, have not been rigorously evaluated. We examined the relationship between trait pain catastrophizing and morning salivary cortisol levels before and following a 45-minute laboratory pain testing session in healthy, pain-free (n=22) and temporomandibular disorder (TMD) participants (n=39). We also examined whether TMD patients evidenced generalized hyperalgesia and hypercortisolism. Pain catastrophizing was associated with a flattened morning salivary cortisol profile in the context of pain testing, irrespective of pain status. Cortisol profiles did not differ between healthy and TMD participants. TMD was associated with mechanical hyperalgesia only at the masseter. These data are the first to show an association between pain catastrophizing and elevated salivary cortisol profiles in the context of standardized experimental pain testing. These findings in both healthy individuals and those with chronic orofacial pain suggest that aberrant adrenocortical responses to pain may serve as a neurophysiologic pathway by which pain catastrophizing enhances vulnerability for development of chronic pain and maintains and/or exaggerates existing pain and associated morbidity.
Perspective
Neurophysiological mechanisms by which pain catastrophizing is related to acute and chronic pain recently have come under empirical study. Understanding of these mechanisms has the unique potential to shed light on key central nervous system factors that mediate catastrophizing-pain relations and therapeutic benefits associated with changes in catastrophizing and related cognitive processes.
Keywords: pain catastrophizing, pain threshold, temporomandibular disorders, HPA, pressure pain, thermal pain
Temporomandibular joint disorders (TMD) affect an estimated 12% of the population38, can promote pain and functional impairment, hinder quality of life56, and often are accompanied by depressive symptoms42,64,65. The onset and/or aggravation of TMD often covaries with the occurrence of environmental stressors, and is highly prevalent in other “stress-related” or “idopathic” somatic disorders, such as fibromyalgia1,11. Recent conceptualizations emphasize the multifactorial nature of TMD and jointly consider neurophysiological and psychosocial factors11. In the present study, we examined two such factors that we believe are implicated in the onset, maintenance and/or exacerbation of TMD: aberrant neuroendocrine responses to pain and pain-related catastrophizing.
There is growing consensus that altered basal and stress-induced HPA activity may exist in painful idiopathic conditions such as fibromyalgia3,4,55 and irritable bowel syndrome13. Comparatively, there exists a relative dearth of research that has evaluated the integrity of the HPA axis in TMD, although some findings do suggest that TMD patients (relative to healthy controls) exhibit altered HPA dynamics. For instance, TMD patients exhibited a more robust cortisol response than healthy controls to psychological stress30 and have been shown to exhibit 30–50% greater daytime plasma cortisol levels than matched healthy controls34. To our knowledge, no studies have systematically examined cortisol profiles in the context of standardized laboratory pain testing in individuals with TMD.
Pain catastrophizing has emerged as a critically important psychosocial construct in the context of acute and chronic painfor reviews, see 17,31,45,52. In TMD, catastrophizing has been linked to self-reported clinical pain, activity interference, negative mood, greater clinical exam findings and increased health care utilization56,57. Although there has been speculation concerning altered HPA axis responses to pain as a potential neurophysiological correlate of catastrophizing17, whether this is the case has scantly been examined and has yielded conflicting results. In a recent study among healthy participants, little correlation was observed between pain catastrophizing and plasma cortisol responses to standardized laboratory pain testing19. However, Johansson et al.29 reported that less diurnal cortisol variability among back pain patients scheduled for lumbar disc surgery was associated with the tendency to catastrophize about pain, suggesting compromised HPA integrity among certain individuals with persistent pain. We hypothesize that an exaggerated cortisol response to pain might be readily observed in a sample of TMD patients, for which pain is a particularly salient stimulus, versus a sample of healthy individuals, for which pain may be significantly less salient and disease processes have not yet yielded measureable influence on HPA function.
In the present study, TMD and healthy participants underwent standardized mechanical, heat and cold pain testing19. Our chief hypothesis was that associations between catastrophizing and cortisol responses to pain testing would be strongest for TMD versus healthy participants. We also examined whether TMD patients exhibited a more robust cortisol response to pain testing than healthy controls, irrespective of pain catastrophizing levels. Finally, there is evidence that TMD might be characterized by generalized hyperalgesia5,40,48,62. Hence, we examined whether TMD patients were characterized by lower pressure pain thresholds at masseter and “unaffected” extracranial sites (i.e., trapezius), lower heat pain thresholds, and greater suprathreshold cold pain ratings than healthy controls. Portions of these data were presented in poster/abstract form at the 2009 annual meeting of the American Pain Society46.
Materials and Methods
Participants
We recruited TMD patients (n = 39) from a dental school-based, tertiary care, orofacial pain clinic and media advertisements for a larger prospective study concerning sleep disturbance and TMD pain and function. To be eligible, TMD patients had to receive a primary myofascial TMD diagnosis based on published Research Diagnostic Criteria (RDC)15. All TMD diagnostics were conducted by an experienced dentist who has completed formal training in RDC procedures and undergoes periodic reliability calibration. Additional major eligibility criteria for TMD patients included: typical pain severity > 2 out of 10 and minimum symptom duration ≥ 6 months. We excluded patients reporting primary pain conditions or serious medical disorders other than TMD, current alcohol or drug abuse problems, and use of narcotics, antidepressants, anticonvulsants, or muscle relaxants within two weeks of study participation. Healthy controls (n = 22) were recruited from fliers posted at a major teaching hospital and medical school. Major eligibility criteria for healthy controls included an absence of: significant medical/psychiatric history within the prior 6 months, and lifetime history of Raynaud's disease, bipolar or psychotic disorder, recurrent major depression, substance abuse disorder, use of antidepressant medications within the past 6 months and history of chronic pain (i.e., lifetime history of persistent pain for ≥ 6 months).
Apparatus and Measures
Salivary Cortisol
Salivary samples were collected immediately prior to the start of pain testing, immediately following the pain testing procedures and 20-minutes following the pain procedures by having participants chew on a cotton role (Salivettes; Sarstedt, Numbrecht, Germany) for 30 seconds. Samples were centrifuged until a clear, low-viscosity supernatant emerged. Supernatants were then collected and stored at −30 °C until assay. Assays were performed in duplicate using a commercially available enzyme immunoassay (EIA) kit (DSL, Webster, TX, USA). This assay has a sensitivity of 0.11 μg/dl. The intra- and inter-assay coefficients of variation were < 10%.
Psychophysical Pain Testing
Pressure Pain Threshold (PPTh)
A somedic algometer (Sollentuna, Sweden) was used to assess responses to mechanical pressure using a 0.502-cm2 probe covered with 1-mm polypropylene material. Pressure was increased in a graded fashion at a rate of 30 kPa/sec until the participant reported pain threshold, defined as first felt pain. Once threshold was determined, application of pressure was terminated. PPTh was assessed bilaterally at masseter, forearm and trapezius muscle sites, over three trials, separated by at least 1 minute.
Heat Pain Threshold (HPTh)
A computer-controlled Medoc Thermal Sensory Analyzer (TSA-2001, Ramat Yishai, Israel), a peltier-element-based stimulator with a 30-mm2 surface area was used to deliver contact heat stimuli. HPTh was assessed three times on the left ventral forearm using an ascending limits method. The temperature increased from 32 ° C at baseline with a 0.5° C/sec rate of temperature rise19 until the participant pressed a button indicating when they first felt a painful sensation. The temperature of the thermode at the moment of the button press was recorded. Between trials, the positioning of the thermode was moved slightly up the arm to avoid overlapping the testing site. A 30-sec inter-stimulus interval was rigorously maintained. A maximum temperature of 52° C was used to prevent possible tissue damage. HPTh was defined as the average readings across all trials.
Cold Pain Ratings (CPR)
Cold pressor pain was assessed by having the subjects immerse their hand up to the wrist in 4°C water for 45 seconds. This procedure alternated right and left hand submersions across four consecutive trials (two trial each hand) with a 2-minute inter-stimulus interval between each trial. The water temperature was maintained (±0.1 °C) by a refrigeration unit (Neslab, Portsmouth, NH), and was constantly circulated to prevent local warming around the submerged hand. The participant was prompted to rate their pain on a 0 (no pain at all) to100 (extreme pain) scale at 20 seconds post-submersion. They were instructed to remove their hand from the water following this brief procedure (or at any time if intolerable). Cold water immersion was repeated twice and ratings were averaged together.
Psychosocial Questionnaires
Pain Catastrophizing Scale (PCS)51
The PCS is a 13-item self-report questionnaire used to assess the degree to which individuals typically catastrophize about their pain. The questionnaire is considered a comprehensive assessment of the catastrophizing construct and includes items designed to tap magnification, helplessness and rumination dimensions. Participants are instructed to indicate the degree to which each item describes them in relation to their recalled pain experience on a 0 (not at all) to 4 (very much) scale. Possible scores range from 0 to 52 with higher scores indicating greater catastrophizing. The questionnaire has been validated in patient and non-patient samples and the factor structure appears to be invariant across participant sex and patient/non-patient status7,43,44,51. It is important to note that we employed the original version of the PCS, which assesses a trait-like tendency to catastrophize about pain.
Brief Symptom Inventory (BSI)8
The BSI is a 53-item self-report questionnaire that taps the participant's degree of psychological distress over the past two weeks across a number of symptoms domains. Responses are given on a 0 (not at all) to 3 (very much) scale. Possible scores range from 0 to 3 with higher scores indicating greater levels of distress. Here, we utilized the Depression subscale of the BSI (BSI-D) because of the well-known conceptual and empirical overlap between pain catastrophizing and depressive symptoms. It is important to note that results were virtually identical using the General Severity Index (GSI) of the BSI, which is a composite index of a participant's overall level of distress collapsed across each symptom dimension (we observed a zero-order correlation coefficient (r) of .78 between the GSI and BSI-D).
Procedures
All procedures were approved by the appropriate Institutional Review Boards and written informed consent was obtained for all study participants. All procedures took place in a university hospital-based General Clinical Research Center (GCRC). All participants were enrolled in a larger study aimed at characterizing associations between objective polysomnography (PSG) sleep indexes and pain sensitivity. The analyses of the present investigation are based on next-morning procedures following an adaptation evening of sleep in which all participants were allotted an uninterrupted 8-hr period for sleep that was structured around participants' habitual sleep-wake times, which were ascertained from a 2-week daily sleep diary. Forty minutes after final awakening participants were escorted to a nearby research laboratory for pain testing procedures. Participants were seated comfortably in an upright reclining chair and the procedures were explained to them. The pre-pain testing saliva sample was obtained just prior to pain testing (M = 52 minutes post-awakening). Mechanical and thermal testing procedures were conducted in a randomized order across participants. In all cases, the cold pain testing procedures were conducted last in the pain testing battery sequence. As in prior studies conducted by our laboratory19, the pain testing procedures lasted approximately 45 minutes on average. Immediately and 20-min post-pain testing, saliva samples were again obtained. Cortisol typically follows a diurnal pattern, with an early morning peak approximately 40 minutes post-awakening, followed by a decline in levels throughout the day. Hence, our cortisol assessment occurred following the morning peak, and during the diurnal morning cortisol decline. Hence, effects of pain status and/or pain catastrophizing on cortisol patterns in the context of the experimental pain testing would appear as a flattened cortisol profile, or put otherwise, a diminished morning cortisol decline. Peak salivary free cortisol concentrations have been observed at approximately 30 minutes post-cold pressor pain54, so we feel that our 20-minute post-pain testing battery recovery assessment was likely to capture peak pain-induced cortisol levels. The PCS and BSI were completed as part of larger packet of questionnaires provided upon study entry. Importantly, the tightly controlled inpatient environment afforded by the GCRC assured that all participants had not smoked, eaten, ingested caffeinated or calorie-rich beverages or exercised prior to obtaining saliva samples. Hence, our salivary cortisol measurements were unlikely affected by such potential confounds27,36.
Data Analysis
All statistical analyses were conducted using Statistical Analysis Software version 9.2 (SASv9.2). Differences in demographics, pain catastrophizing and depressive symptoms between TMD and healthy participants were examined using t-tests and χ2 analyses as appropriate. We conducted a series of linear mixed-effects analyses using SAS PROC MIXED to characterize the cortisol response to pain testing in relation to PCS scores, pain status and their interaction. Within-subject nested variables included salivary cortisol values (μg/dl) at baseline and immediately and 20-minutes post-pain testing. These variables were represented by a three-level `Time' factor. Between-subjects variables included Pain Status (TMD, healthy), PCS (continuous), and 2- and 3-way interactions of Time, Pain Status and PCS. To determine whether TMD and/or pain catastrophizing were associated with cortisol responses to pain testing above and beyond depression symptoms, BSI-D scores were included as a between-subject variable in the model.
We subsequently examined whether TMD patients exhibited generalized hyperalgesia. Because we observed no laterality effects for PPTh at masseter or trapezius muscle sites, PPTh values at left and right sites were averaged to create a single PPTh index for the masseter and trapezius. To do so, 2 Pain Status (TMD, healthy) ANOVAs were conducted on PPTh, HPTh and cold pain ratings. Lastly, in light of prior established empirical relations between trait pain-related catastrophizing and laboratory pain testing52, we examined partial correlations between pain catastrophizing and PPTh, HPTh and cold pain ratings (partialling variance attributable to BSI-D).
Results
Sample Demographic and Psychosocial Characteristics by Pain Status
Sample characteristics are provided in Table 1. TMD and healthy participants were closely matched on sex [χ2(1) = 2.28, p = .13], occupational status [χ2(4) = 5.13, p = .27], education [χ2(3) = 1.89, p = .60] and Body Mass Index [t(59) < 1]. TMD and healthy controls differed in marital status [χ2(1) = 7.06, p < .01], such that a greater percentage of TMD patients reported being married at the time of study participation. A greater percentage of TMD patients were Caucasian than healthy controls [χ2(3) = 9.38, p < .05], and those with TMD were significantly older than healthy controls, [t(59) = 2.89, p < .01]. None of these demographic factors were related significantly with PCS scores, cortisol, PPTh indexes, HPTh or cold pain ratings, so were not considered covariates in subsequent analyses.
Table 1.
Sample characteristics.
| Characteristic | TMD (n = 39) | Healthy (n = 22) |
|---|---|---|
| TMD Duration (in months; Mean, SD) | 110.26 (103.04) | |
| Age (Mean, SD)a | 33.79 (12.00) | 25.91 (5.76) |
| Sex (% Female) | 82.1 | 95.5 |
| Ethnicity | ||
| Non-Hispanic White | 79.5 | 40.9 |
| African-American | 7.7 | 27.3 |
| Asian-American | 7.7 | 22.7 |
| Multi-Racial | 5.1 | 4.5 |
| Education (%) | ||
| High School or Less | 5.1 | N/A |
| Some College | 17.9 | 27.3 |
| College Graduate or Greater | 77 | 72.7 |
| Occupational Status (% Employed) | 94.9 | 100 |
| Marital Status (% Married)a | 14 | 1 |
| BMI (Mean, SD) | 24.66 (5.08) | 23.54 (4.24) |
| PCS (Mean, SD) a | 14.05 (8.81) | 8.91 (6.77) |
| BSI-D (Mean, SD) a | .33 (.34) | .08 (.17) |
Note. BMI = Body Mass Index; PCS = Pain Catastrophizing Scale total scores; BSI-D = Brief Symptom Inventory – Depression subscale scores. Subscript indicates a statistically significant group difference at p < .05.
Relative to healthy controls, participants with TMD reported higher PCS [t(59) = 2.36, p < .05] and BSI-D scores [t(59) = 3.32, p < .01]. It is important to note even though the TMD patients had greater depressive symptoms, mean BSI-D scores (M = .23, SD = .31) were well within the normal range based on normative data8. Again, BSI-D scores were utilized as a covariate in all subsequent analyses focused on pain catastrophizing given a priori conceptual and empirical links between these constructs.
Pain Status and Pain Catastrophizing Effects on Salivary Cortisol Responses to Pain Testing
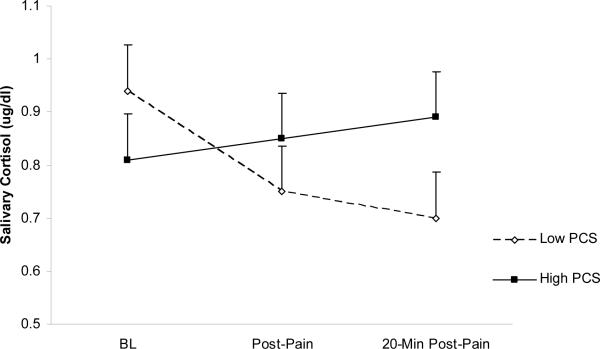
Linear mixed-effects analyses yielded a significant main effect of Time [F(2, 114) = 7.04, p < .001], suggesting that all subjects (irrespective of pain status and variations in pain catastrophizing) exhibited ed a significant morning cortisol decline, even in the context of pain testing. Contrary to our hypothesis, a significant Pain Status × PCS × Time effect failed to emerge [F(2, 114) < 1]. Investigation of 2-way interactions revealed a nonsignificant Pain Status × Time effect [F(2, 114) < 1], suggesting that cortisol responses to pain testing did not differ across Pain Status. However, a significant PCS × Time effect emerged [F(2, 114) = 4.18, p = .018]. Inspection of marginal means, displayed graphically in Figure 1, revealed that higher PCS scores (i.e., +1 SD) were associated with a blunted rate of cortisol decline from pre- to 20-minutes post-pain testing. A follow-up comparison confirmed this interpretation: a significant PCS × Time effect was noted for cortisol change from pre- to 20 minutes post-pain testing [t(114) = 3.26, p < .05]. These findings suggest that a tendency to catastrophize about pain is associated with flattened morning cortisol declines in the context of standardized laboratory pressure and thermal pain stimulation. It is noteworthy that no pain index was associated with cortisol (p's > .10), suggesting a dissociation between the markers of pain sensitivity assessed in the present study and adrenocortical responses to the pain testing.
Figure 1.
Marginal means (SE) of salivary cortisol values (μg/dl) at hypothetical values of high (+1 SD) and low (−1 SD) Pain Catastrophizing Scale (PCS) scores.
Pain Status Effects on Pain Indexes
To examine whether TMD patients evidenced a pattern of generalized hyperalgesia, we conducted a series of ANOVAs with Pain Status (healthy, TMD) as the between-groups factor (see Table 2 for Means and SDs). We observed no group differences in PPTh at the trapezius site [F(1, 58) < 1] and HPTh [F(1, 58) = 1.45, p = .23]. We also failed to observe group differences on cold pain ratings, [F(1, 58) < 1]. However, TMD participants evidenced lower PPTh at the masseter site than healthy, pain-free participants [F(1, 58) = 5.00, p < .05]. These findings are not supportive of generalized hyperalgesia in the context of TMD. Not surprisingly, participants with TMD had lower pain thresholds at the site of pain, and the magnitude of this effect was quite robust (d = .60). Entering BSI-D scores as a covariate did not alter these findings.
Table 2.
Mean (SD) PPTh, HPTh and CPR values by pain status.
| PPTh Massetera | PPTh Forearm | PPTh Trapezius | HPTh | CPR (0–100) | |
|---|---|---|---|---|---|
| TMD | 136.70 (50.01) | 247.74 (98.52) | 374.25 (158.92) | 43.42 (2.83) | 68.41 (26.73) |
| Healthy | 170.13 (62.62) | 267.79 (92.14) | 391.69 (139.47) | 42.43 (3.48) | 72.49 (23.68) |
Note. PPTh = Pressure Pain Threshold; HPTh = Heat Pain Threshold. CPR = Cold Pressor Pain Ratings. Subscript indicates a statistically significant group difference atp < .05.
Associations of Pain Catastrophizing and Pain Indices
To examine associations between PCS scores and pain indices, we computed partial correlation coefficients (rpartial) between PCS scores and pain indices with variance attributable to BSI-D scores partialled out. PCS scores were not significantly correlated with PPTh at masseter (rpartial = .06, p = .64), forearm (rpartial = .05, p = .66) or trapezius (rpartial = −.08, p = .52) sites, nor with cold pain ratings (rpartial = .12, p = .37). PCS scores also were not significantly correlated with HPTh, although the pattern of these data suggest that greater PCS scores were related to lower levels of HPTh (rpartial = −.23, p = .07).
Discussion
The principal aim of the present study was to examine whether pain catastrophizing is associated with aberrant adrenocortical responses to standardized pain testing, and among TMD patients in particular. We also were interested in whether TMD patients evidenced a stronger pattern of adrenocortical activity during painful stimulation than healthy controls and, lastly, whether TMD patients were characterized by generalized hyperalgesia. Although hypotheses were not fully supported, some noteworthy findings emerged.
Pain catastrophizing was not differentially associated with salivary cortisol profiles in the context of pain testing across TMD and healthy participants. However, we did observe that pain catastrophizing was associated with exaggerated cortisol responses to pain testing when collapsed across participant pain status. Put more appropriately in the context of the current study design, higher levels of catastrophizing were associated with a flattened or elevated morning cortisol profile in the context of acute pain. These are the first data to our knowledge that show a relationship between catastrophizing and salivary cortisol profiles in the context to standardized pain testing. These findings build on prior research suggesting that pain catastrophizing might yield maladaptive neurophysiological responses to painful experiences. For instance, positive associations between state catastrophizing and interleukin-6 (IL-6) responses to highly similar laboratory-based quantitative sensory testing methods were recently noted19. Moreover, catastrophizing has been associated with amplified activation of neural regions involved in the processing and regulation of affective elements of pain26,49.
It is noteworthy that relations between catastrophizing and cortisol responses to pain emerged in patients and non-patients alike. These data suggest that pain catastrophizing may serve as a risk factor for the development of persistent pain as well as aggravate and/or maintain existing chronic pain via HPA pathways. Aberrant HPA axis integrity has been associated with worse post-operative pain outcomes, including pain severity23. Moreover, hypo- and hypercortisolism have been associated with impaired inhibition of cellular pro-inflammatory cytokine proliferation47. This is particularly noteworthy in light of the fact that inflammation enhances central sensitization12,39,53,63, thereby putatively increasing vulnerability for transition from acute injury to a state of chronic, unremitting pain, or exacerbation and maintenance of existing pain. With emerging (albeit limited) evidence that pain catastrophizing may be related both to exaggerated cortisol levels29 and IL-6 activity19, it becomes possible to speculate that catastrophizing may be associated with a heightened state of glucocorticoid resistance at the cellular level, carrying the potential to account for associations between catastrophizing and clinical pain as well as pain-related functional outcomes, including sleep disturbance, fatigue and reduced activity levels21. Although admittedly speculative, whether such processes play a role in shaping pain-related outcomes in association with catastrophizing represents a potentially intriguing direction for future research.
Pain catastrophizing was not associated with pressure and thermal pain threshold or cold pressor pain ratings, irrespective of pain status. Although it has been suggested that catastrophizing accounts for between 7 and 31% of variance in clinical pain52, the literature has been rather mixed with respect to associations between pain catastrophizing and experimental pain sensitivity. It is important to keep in mind that all experiments must inform patients that they have control over any painful procedures through studies require informed consent. This likely limits the degree that catastrophizing processes ensue in a laboratory setting. Another intriguing idea is that trait-like assessments of pain catastrophizing may not capture responses to experimental pain testing as readily as state-like or situation-specific measures14,16,28. Indeed, trait measures of pain catastrophizing instruct participants to base responses on recall of prior painful experiences which may have little or nothing to do with the type, duration or intensity of laboratory pain. Situation-specific measures catastrophizing heed participants to base their responses on the laboratory pain experience. This approach has the benefit of standardizing the referent and minimizing potential influence of recall bias37. State assessments of catastrophizing appear to correlate more robustly with experimental pain than trait measures in healthy14,16,18,19,28 and in some clinical samples (Campbell CM, Manuscript Under Review), and have been linked to exaggerated IL-6 in the context of acute pain stimulation19.
It is noteworthy that we obtained a significant association between pain catastrophizing and cortisol responses to pain despite observing no association between catastrophizing and experimental pain sensitivity, or between any index of pain sensitivity and cortisol. It is possible that we failed to assess a crucial dimension of the catastrophizing-pain interface, such as pain-evoked distress, or hypervigilance to pain-related sensory and affective information61. For instance, it is possible that the sensory pain experience per se does not drive the catastrophizingcortisol association. Instead, it might be the affective constituent of pain that is exaggerated for pain catastrophizers41, thereby triggering a more robust stress response. Unfortunately, our data cannot address this hypothesis. We also failed to observe an association between our indexes of pain sensitivity and cortisol. This finding is not inconsistent with prior research. For instance, a recent study reported correlations between “state” catastrophizing and IL-6 responses to acute pain stimulation in the absence of an association between pain itself and IL-619. Moreover, two studies6,22 that examined IL-6 levels post-operatively failed to show an association between IL-6 levels and self-reported levels of post-operative pain; despite a disassociation between IL-6 and pain, these studies observed robust increases in IL-6 following surgery. It seems clear that an important area for future research is to isolate possible mechanisms by which pain and pain catastrophizing shape neurophysiological responses to pain. Thus far, it appears that a conceptual model linking catastrophizing to exaggerated neurophysiological responses to pain via increased pain per se is not a viable one.
TMD patients had lower pressure pain thresholds at the affected anatomical site than healthy controls. This finding enhances confidence that TMD diagnoses in the present study were accurate. However, evidence for generalized hyperalgesia among TMD participants was not observed, which is at odds with some prior investigations but consistent with others48. A plethora of factors appear to moderate pain sensitivity, and not taking them into consideration may have diminished our ability to detect generalized hyperalgesia in TMD patients, which is a highly heterogeneous disorder. One such genetic factor that highlights the heterogeneity in TMD are the single nucleotide polymorphisms (SNP) of the catechol-o-methyltransferase (COMT) genotype. A “pain sensitive” haplotype of this SNP enhances pain sensitivity9,10,66, for exceptions, see 32,33 and has been identified as a likely genetic determinant of transition to TMD8,10. George and colleagues24,25 have shown that a pain sensitive COMT diplotype moderate relations between catastrophizing and clinical pain report.
Recent work by our group suggests that sleep disorders are another important and prevalent moderator of alterations in generalized pain sensitivity in TMD50. We found that the diagnosis of primary insomnia (26% of TMD sample) was associated with generalized hyperalgesia in TMD, whereas sleep apnea diagnosis (28% of TMD sample) was associated with generalized hypoalgesia. We also found that reduced sleep efficiency is associated with impaired pain inhibitory function in TMD20. Failing to take into consideration potential moderating factors might have restricted our power to detect between-groups differences in responses to pain testing.
Some study limitations warrant mention. First, we conducted pain testing and obtained salivary samples for cortisol measurement in the morning during the diurnal cortisol decline. Although this may have limited our ability to detect pain status differences in salivary cortisol responses to pain testing, we nonetheless detected a significant association between pain catastrophizing and salivary cortisol responses. This may speak to the robust effect of catastrophizing on HPA responses to pain. Conversely, it is possible that the effect observed in the present investigation can be attributable to Type I error. Interestingly, a recent study revealed that absolute pre-stress levels of salivary cortisol are greater in the morning than in the afternoon or evening, where as salivary cortisol responsiveness to psychological stress does not appear to differ across morning and afternoon sessions35. Nonetheless, these findings require replication and extension.
Second, and not uncommonly, we did not include a no-pain control condition. Hence, associations between catastrophizing and salivary cortisol profiles might have had little to do with pain testing, instead representing an association with some “third” variable. However, it is well-recognized that cold pressor pain stimulates HPA response at both pituitary and adrenocortical levels2. Moreover, at present, there exists little empirical or theoretical reason to posit that catastrophizing would be associated with tonic exaggerations in HPA activity that are independent of pain exposure, particularly among healthy pain-free participants. Third, we do not have data that attest to the mechanism(s) by which pain catastrophizing is associated with adrenocortical activity. Even more problematic is that our cross-sectional approach does not allow for causal statements. The process of catastrophizing during pain exposure might enhance the subjective experience of pain, thereby affecting neurophysiological processes. Conversely, exaggerated neurophysiological responses to pain might cause an individual to catastrophize. Potential “third” variable mediation of catastrophizing and exaggerated HPA responses to pain also cannot be ruled out. Lastly, we did not include measures of other negative pain cognitive process variables, such as fear of pain or pain anxiety. Hence, we can only tentatively speak to the specificity of the findings with respect to catastrophizing.
These limitations notwithstanding, these findings suggest an intriguing association between pain catastrophizing and exaggerated HPA activity in the context of acute standardized laboratory pain testing. These data point to a potential neurophysiological pathway by which catastrophizing may confer risk not only for the maintenance and/or exacerbation of existing TMD pain, but for the development or onset of persistent pain. Bridging these findings with those indicating exaggerated inflammation associated with catastrophizing19 may yield novel insights into the processes by which pain catastrophizing shapes pain-related outcomes. Clinically, it has been well-documented that reductions in pain catastrophizing in the context of cognitive-behavioral therapy for TMD play a critical role in shaping immediate and longer-term outcomes58–60. It is a distinct possibility that cognitive-behavioral interventions not only affect cognitive processes that an individual brings to bear on painful encounters, but restores HPA function and crosstalk between neuroendocrine and immune pathways4. The study of the neurophysiological basis of pain catastrophizing is in its infancy, but these and other findings suggest that this promises to be an exciting and important area of investigation in the coming years.
Acknowledgements
This work was supported by Grants K23NS047168 from the National Institute of Neurological Disorder and Stroke (MTS), R01AR054871 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (MTS), the Johns Hopkins General Clinical Research Center (M01-RR002719), R24AT004641 from the National Center for Complementary and Alternative Medicine (JAH), and postdoctoral fellowship Grant T32MH75884 from the National Institute of Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aaron LA, Burke MM, Buchwald D. Overlapping conditions among patients with chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med. 2000;160:221–227. doi: 10.1001/archinte.160.2.221. [DOI] [PubMed] [Google Scholar]
- 2.al'Absi M, Petersen KL, Wittmers LE. Adrenocortical and hemodynamic predictors of pain perception in men and women. Pain. 2002;96:197–204. doi: 10.1016/s0304-3959(01)00447-x. [DOI] [PubMed] [Google Scholar]
- 3.Bennett RM. Adult growth hormone deficiency in patients with fibromyalgia. Curr Rheumatol Rep. 2002;4:306–312. doi: 10.1007/s11926-002-0039-4. [DOI] [PubMed] [Google Scholar]
- 4.Bonifazi M, Suman LA, Cambiaggi C, Felici A, Grasso G, Lodi L, Mencarelli M, Muscettola M, Carli G. Changes in salivary cortisol and corticosteroid receptor-alpha mRNA expression following a 3-week multidisciplinary treatment program in patients with fibromyalgia. Psychoneuroendocrinology. 2006;31:1076–1086. doi: 10.1016/j.psyneuen.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Bragdon EE, Light KC, Costello NL, Sigurdsson A, Bunting S, Bhalang K, Maxiner W. Group differences in pain modulation: Pain-free women compared to pain-free men and to women with TMD. Pain. 2002;96:227–237. doi: 10.1016/S0304-3959(01)00451-1. [DOI] [PubMed] [Google Scholar]
- 6.Buvanendran A, Kroin JS, Berger RA, Hallab NJ, Saha C, Negrescu C, Moric M, Caicedo MS, Tuman KJ. Upregulation of prostaglandin E2 and interleukins in the central nervous system and peripheral tissueduring and after surgery in humans. Anesthesiology. 2006;104:403–410. doi: 10.1097/00000542-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 7.D'Eon JL, Harris CA, Ellis JA. Testing Factorial Validity and Gender Invariance of the Pain Catastrophizing Scale. J Behav Med. 2004;27:361–372. doi: 10.1023/b:jobm.0000042410.34535.64. [DOI] [PubMed] [Google Scholar]
- 8.Derogatis LR, Meliseratos N. The Brief Symptom Inventory: An introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- 9.Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MD, Makarov SS, Maxiner W. Genetic basis for individual variations I pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 10.Diatchenko L, Nackley AG, Slade GD, Bhalang K, Belfer I, Max MB, Goldman D, Maxiner W. Catechol-O-methyltransferase gene polymorphisms are associated with multiple pain-evoking stimuli. Pain. 2006;125:216–224. doi: 10.1016/j.pain.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 11.Diatchenko L, Nackley AG, Slade GD, Fillingim RB, Maxiner W. Idiopathic pain disorders – pathways of vulnerability. Pain. 2006;123:226–230. doi: 10.1016/j.pain.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 12.Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience. 2008;152:521–525. doi: 10.1016/j.neuroscience.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinan TG, Quigley EM, Ahmed SM, Scully P, O'Brien S, O'Mahony L, O'Mahony S, Shanahan F, Keeling PW. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: Plasma cytokines as a potential biomarker? Gastroenterology. 2006;130:304–311. doi: 10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 14.Dixon KE, Thorn BE, Ward LC. An evaluation of sex differences in psychological and physiological responses to experimentally-induced pain: a path analytic description. Pain. 2004;112:188–196. doi: 10.1016/j.pain.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Dworkin SF, LeResche L. Research diagnostic criteria for tempormandibular disorders. J Craniomandib Disord. 1992;6:301–355. [PubMed] [Google Scholar]
- 16.Edwards RE, Bingham CO, III, Bathon J, Haythornthwaite JA. Catastrophizing and pain in arthritis, fibromyalgia, and other rheumatic diseases. Arthitis and Rheum. 2006;55:325–332. doi: 10.1002/art.21865. [DOI] [PubMed] [Google Scholar]
- 17.Edwards RR, Campbell CM, Fillingim RB. Catastrophizing and Experimental Pain Sensitivity: Only In Vivo Reports of Catastrophic Cognitions Correlate With Pain Responses. J Pain. 2005;6:338–339. doi: 10.1016/j.jpain.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Edwards RR, Smith MT, Stonerock G, Haythornthwaite JA. Pain-related catastrophizing in healthy women is associated with greater temporal summation of and reduced habituation to thermal pain. Clin. J. Pain. 2006;22:730–737. doi: 10.1097/01.ajp.0000210914.72794.bc. [DOI] [PubMed] [Google Scholar]
- 19.Edwards RR, Kronfli T, Haythornthwaite JA, Smith MT, McGuire L, Page GG. Association of catastrophizing with interleukin-6 responses to acute pain. Pain. 2008;140:135–144. doi: 10.1016/j.pain.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards RR, Grace E, Peterson S, Klick B, Haythornthwaite JA, Smith MT. Sleep continuity and architecture: Associations with pain-inhibitory processes in patients with temporomandibular joint disorder. Eur J Pain Jan. 2009 doi: 10.1016/j.ejpain.2008.12.007. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elenkov IJ, Lezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2006;12:255–269. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- 22.Feng Y, Ju H, Yang B, An H. Effects of a selective cyclooxygenase-2 inhibitor on postoperativeinflammatory reaction and pain after total knee replacement. J Pain. 2008;9:45–52. doi: 10.1016/j.jpain.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Geiss A, Rohleder N, Kirschbaum C, Steinbach K, Bauer HW, Anton F. Predicting the failure of disc surgery by a hypofunctional HPA axis: Evidence from a prospective study on patients undergoing disc surgery. Pain. 2005;114:104–117. doi: 10.1016/j.pain.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 24.George SZ, Wallace MR, Wright TW, Moser MW, Greenfield WH, III, Sack BK, Herbstman DM, Fillingim RB. Evidence for a biopsychosocial influence on shoulder pain: Pain catastrophizing and catechol-O-methyltransferase (COMT) diplotype predict clinical pain ratings. Pain. 2008;136:53–61. doi: 10.1016/j.pain.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George SZ, Dover GC, Wallace MR, Sack BK, Herbstman DM, Aydog E, Fillingim RB. Biopsychosocial influence on exercise-induced delayed onset muscle soreness at the shoulder: Pain catastrophizing and catechol-O-methyltransferase (COMT) diplotype predict pain ratings. Clin J Pain. 2008;24:793–801. doi: 10.1097/AJP.0b013e31817bcb65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gracely RH, Geisser ME, Giesecke T, Grant MAB, Petzke F, Williams DA, Clauw DJ. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127:835–843. doi: 10.1093/brain/awh098. [DOI] [PubMed] [Google Scholar]
- 27.Hanson AM, Garde AH, Persson R. Sources of biological and methodological variation in salivary cortisol and their impact on measurement among healthy adults: A review. Scan J Clin Lab Invest. 2008;68:448–458. doi: 10.1080/00365510701819127. [DOI] [PubMed] [Google Scholar]
- 28.Hirsh AT, George SZ, Bialosky JE, Robinson ME. Fear of Pain, Pain Catastrophizing, and Acute Pain Perception: Relative Prediction and Timing of Assessment. J Pain. 2008;9:806–812. doi: 10.1016/j.jpain.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansson AC, Gunnarsson LG, Linton SJ, Bergvist L, Stridsberg M, Nilsson O, Cornefjord M. Pain, disability and coping reflected in the diurnal cortisol variability in patients scheduled for lumbar disc surgery. Eur J Pain. 2008;12:633–640. doi: 10.1016/j.ejpain.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Jones DA, Rollman GB, Brooke RI. The cortisol response to psychological stress in temporomandibular dysfunction. Pain. 1997;72:171–182. doi: 10.1016/s0304-3959(97)00035-3. [DOI] [PubMed] [Google Scholar]
- 31.Keefe FJ, Rumble ME, Scopio CD, Girdano LA, Perri LCM. Psychological aspects of persistent pain: Current state of the science. J Pain. 2004;5:195–211. doi: 10.1016/j.jpain.2004.02.576. [DOI] [PubMed] [Google Scholar]
- 32.Kim H, Neubert JK, San Miguel A, Xu K, Krishnaraju RK, Iadarola MJ, Goldman D, Dionne RA. Genetic influence on variability in human acute experimental pain sensitivity associated with gender, ethnicity and psychological temperament. Pain. 2004;109:488–496. doi: 10.1016/j.pain.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 33.Kim H, Lee H, Rowan J, Brahim J, Dionne RA. Genetic polymorphisms in monoamine neurotransmitter systems show only weak association with acute post-surgical pain in humans. Mol Pain. 2006;2:24. doi: 10.1186/1744-8069-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korsun A, Young EA, Singer K, Carlson NE, Brown MB, Crofford L. Basal circadian cortisol secretion in women with temporomandibular disorders. J Dent Res. 2002;81:279–283. doi: 10.1177/154405910208100411. [DOI] [PubMed] [Google Scholar]
- 35.Kudielka BM, Schommer NC, Hellhammer DH, Kirschbaum C. Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology. 2004;29:983–992. doi: 10.1016/j.psyneuen.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 36.Kudielka BM, Hellhammer DH, Wust S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrin. 2009;34:2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Lefebvre JC, Keefe FJ. Memory for pain: The relationship of pain catastrophizing to the recall of daily rheumatoid arthritis pain. Clin J Pain. 2002;18:56–63. doi: 10.1097/00002508-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 38.LeResche L. Epidemiology of temporomandibular disorders: implications for the investigation of etiologic factors. Crit Rev Oral Biol Med. 1997;8:291–305. doi: 10.1177/10454411970080030401. [DOI] [PubMed] [Google Scholar]
- 39.Marchand F, Perretti M, McMahon SB. Role of the immune system in pain. Nat Rev Neurosci. 2005;6:521–532. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 40.Maxiner W, Fillingim R, Sigurdsson A, Kincaid S, Silva S. Sensitivity of patients with painful temporomandibular disorder to experimentally-evoked pain: Evidence for altered temporal summation of pain. Pain. 1998;76:71–81. doi: 10.1016/s0304-3959(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 41.Michael ES, Burns JW. Catastrophizing and Pain Sensitivity Among Chronic Pain Patients: Moderating Effects of Sensory and Affect Focus. Annals Behav Med. 2004;27:185–194. doi: 10.1207/s15324796abm2703_6. [DOI] [PubMed] [Google Scholar]
- 42.Nifosi F, Violato E, Pavan C, Sifari L, Novello G, Guarda Nardini L, Manfredini D, Semenzin M, Pavan L, Marini M. Psychopathology and clinical features in an Italian sample of patients with myofascial and temporomandibular joint pain: Preliminary Data. Int J Psychiatry Med. 2007;37:283–300. doi: 10.2190/PM.37.3.f. [DOI] [PubMed] [Google Scholar]
- 43.Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O'Neill E. Factor structure, reliability, and validity of the pain catastrophizing scale. J Behav Med. 1997;20:589–605. doi: 10.1023/a:1025570508954. [DOI] [PubMed] [Google Scholar]
- 44.Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L. The Pain Catastrophizing Scale: Further psychometric evaluation with adult samples. J Behav Med. 2000;23:351–365. doi: 10.1023/a:1005548801037. [DOI] [PubMed] [Google Scholar]
- 45.Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: A critical review. Expert Rev Neurother. 2009;9:745–758. doi: 10.1586/ERN.09.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quartana PJ, Smith M, Edwards R, Klick B, Beunavery L, Haythornthwaite J. Pain catastrophizing and cortisol resposnes to laboratory pain testing among temporomandibular disorder and healthy, pain-free participants. J Pain. 2009;10:S33. doi: 10.1016/j.jpain.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raison CL, Miller AH. When not enough is too much: The role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 48.Sarlani E, Greenspan JD. Evidence for generalized hyperalgesia in temporomandibular disorder patients. Pain. 2003;102:221–226. doi: 10.1016/S0304-3959(03)00095-2. [DOI] [PubMed] [Google Scholar]
- 49.Seminowicz DA, Davis KD. Cortisol responses to pain in healthy individuals depends on pain catastrophizing. Pain. 2006;120:297–306. doi: 10.1016/j.pain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 50.Smith MT, Wickwire EM, Grace EG, Edwards RR, Buenaver LF, Peterson S, Klick B, Haythornthwaite JA. Sleep disorders and their association with laboratory pain sensitivity in temporomandibular joint disorder. Sleep. 2009;32:779–790. doi: 10.1093/sleep/32.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 52.Sullivan MJL, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, Lefebvre JC. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17:52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 53.Summer GJ, Romero-Sandoval EA, Bogen O, Dina OA, Khaser SG, Levine JD. Proinflammatory cytokines mediating burn-injury pain. Pain. 2008;19:98–107. doi: 10.1016/j.pain.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki K, Kenji M, Minakucki H, Yatani H, Clark GT, Matsuka Y, Kuboki T. Responses of the hypothalamic-pituitary-adrenal axis and pain threshold changes in the orofacial region upon cold pressor stimulation in normal volunteers. Arch Oral Biol. 2007;52:797–802. doi: 10.1016/j.archoralbio.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 55.Torpy DJ, Papanicolaou DA, Lotsikas AJ, Wilder RL, Chrousos GP, Pillemer SR. Responses of the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis to interleukin-6: A pilot study in fibromyalgia. Arthritis Rheum. 2000;43:872–880. doi: 10.1002/1529-0131(200004)43:4<872::AID-ANR19>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 56.Turner JA, Dworkin SF, Mancl L, Huggins KH, Truelove EL. The role of beliefs, catastrophizing, and coping in the functioning of patients with temporomandibular disorders. Pain. 2001;92:41–51. doi: 10.1016/s0304-3959(00)00469-3. [DOI] [PubMed] [Google Scholar]
- 57.Turner JA, Brister H, Huggins K, Mancl L, Aaron LA, Truelove EL. Catastrophizing is associated with clinical examination findings, activity interference, and health care use among patients with temporomandibular disorders. J Orofac Pain. 2005;19:291–300. [PubMed] [Google Scholar]
- 58.Turner JA, Mancl L, Aaron LA. Brief cognitive-behavioral therapy for temporomandibular disorder pain: Effects on daily electronic outcome and process measures. Pain. 2005;117:377–387. doi: 10.1016/j.pain.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 59.Turner JA, Mancl L, Aaron LA. Short- and long-term efficacy of brief cognitive-behavioral therapy for patients with chronic temporomandibular disorder pain: A randomized, controlled trial. Pain. 2006;13:181–194. doi: 10.1016/j.pain.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 60.Turner JA, Holtzman S, Mancl L. Mediators, moderators, and predictors of therapeutic change in cognitive-behavioral therapy for chronic pain. Pain. 2007;127:276–286. doi: 10.1016/j.pain.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 61.Vancleef LMG, Peters ML. Pain Catastrophizing, but not Injury/Illness Sensitivity or Anxiety Sensitivity, Enhances Attentional Interference by Pain. J Pain. 2006;7:23–30. doi: 10.1016/j.jpain.2005.04.003. 2006. [DOI] [PubMed] [Google Scholar]
- 62.Vierck CJ. Mechanisms underlying development of spatially disturbed chronic pain (fibromyalgia) Pain. 2006;124:242–263. doi: 10.1016/j.pain.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 63.Watkins LR, Maier SF. The pain of being sick: Implications of immune-to-brain communication for understanding pain. Ann Rev Psychol. 2000;51:29–57. doi: 10.1146/annurev.psych.51.1.29. [DOI] [PubMed] [Google Scholar]
- 64.Yap AU, Chua EK, Dworkin SF, Tan HH, Tan KB. Multiple pains and psychosocial functioning/psychologic distress in TMD patients. In J Prosthodont. 2002;15:461–466. [PubMed] [Google Scholar]
- 65.Yap AU, Chua EK, Hoe JK. Clinical TMD, pain-related disability and psychological status of TMD patients. J Oral Rehabil. 2002;29:374–380. doi: 10.1046/j.1365-2842.2002.00822.x. [DOI] [PubMed] [Google Scholar]
- 66.Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]