Abstract
The forebrain circuits involved in singing and audition (the ‘song system’) in songbirds exhibit a remarkable capacity to synthesize and respond to steroid hormones. This review considers how local brain steroid production impacts the development, sexual differentiation, and activity of song system circuitry. The songbird forebrain contains all of the enzymes necessary for the de novo synthesis of steroids - including neuroestrogens - from cholesterol. Steroid production enzymes are found in neuronal cell bodies, but they are also expressed in pre-synaptic terminals in the song system, indicating a novel mode of brain steroid delivery to local circuits. The song system expresses nuclear hormone receptors, consistent with local action of brain-derived steroids. Local steroid production also occurs in brain regions that do not express nuclear hormone receptors, suggesting a non-classical mode-of-action. Recent evidence indicates that local steroid levels can change rapidly within the forebrain, in a manner similar to traditional neuromodulators. Lastly, we consider growing evidence for modulatory interactions between brain-derived steroids and neurotransmitter/neuropeptide networks within the song system. Songbirds have therefore emerged as a rich and powerful model system to explore the neural and neurochemical regulation of social behavior.
1. Introduction
The role of circulating sex steroids in the regulation of birdsong has been reviewed extensively elsewhere (Bottjer and Johnson, 1997; Schlinger, 1997; Schlinger and Brenowitz, 2002). In this review, we evaluate the evidence that brain-derived steroids, or ‘neurosteroids’, influence the formation and activity of brain circuits that are involved in birdsong. The capacity of the avian forebrain to produce its own supply of steroids de novo has recently emerged, and we present evidence that forebrain steroid production is linked to the mechanisms of both singing and audition. We consider evidence that the enzymes that synthesize neurosteroids from cholesterol are expressed in or near brain regions involved in song production and audition. We then describe the way that some song system circuits express classical steroid receptors, while others do not, suggesting that locally-produced neurosteroids can exert actions via both classical and non-classical steroid receptors. We follow with evidence that neurosteroid levels are subject to dynamic fluctuations in the avian forebrain when measured directly, and that steroids can exert actions on neurons in the avian brain, within both long-term (days-weeks) and acute (seconds-minutes) timescales. Lastly, we summarize recent studies indicating that neurosteroids have the capacity to interact with neurotransmitters within the avian forebrain, and we discuss the implications for neurosteroid regulation of birdsong.
A fundamental theme of this review is therefore that the songbird brain has the capacity to generate and respond to changing neurosteroid levels on a variety of timescales. As we describe, in addition to the well-characterized long-term effects of steroids on the song system, there is now growing evidence for acute changes in neurosteroid levels within the song system. Neuroanatomical and neurochemical studies together indicate that steroids can have substantially localized actions within the forebrain circuits involved in singing and audition in songbirds. Thus, although it is now widely appreciated that seasonal and developmental changes in gonadal and adrenal steroid production can lead to dramatic changes in songbird brain function and behavior, recent findings now shed light on the avian brain as not only a target, but also as a source for rapidly fluctuating steroid levels in response to environmental cues.
The primary focus of this review is on the actions and regulation of brain-derived androgens and estrogens in the songbird brain because the most complete description of steroid action in the songbird brain is for estrogens and androgens and their associated receptors (e.g., Gahr, 2001; Schlinger and Brenowitz, 2002). Nevertheless, there is good evidence for neural expression and regulation of progesterone receptors in chickens (Camacho-Arroyo, et al., 2007), quail and doves (Askew, et al., 1997; Belle, et al., 2005), but to our knowledge there is to date no report for the distribution of progestin receptors in songbirds (for progesterone autoradiography, see Lubischer and Arnold, 1990). Likewise, the binding characteristics for corticosteroid receptors have been described in the songbird brain (Breuner and Orchinik, 2009), but the neuroanatomical distribution of corticosteroid receptor types are unknown.
The avian ‘song system’: Traditionally, the ‘song system’ of passerine birds has been defined as the interconnected network of forebrain nuclei involved in the motor production of song, comprised of HVC (proper name), RA (robust nucleus of the arcopallium), and song learning, comprised of LMAN (lateral magnocellular nucleus of the anterior nidopallium), Area X, and DLM (dorsal lateral nucleus of the medial thalamus) (Farries, 2001; Leonardo and Fee, 2005). These nuclei regulate the activity of the hindbrain motor nucleus XIIts (tracheosyringeal region of the twelfth cranial nucleus), that in turn innervates the avian vocal organ, the syrinx, and patterns song. A parallel ascending auditory network in the forebrain is comprised of Field L, NCM (caudomedial nidopallium), CMM (caudomedial mesopallium) and NIf (nucleus interface of the nidopallium), and this network provides extensive auditory information to the motor pathway via HVC and RAshelf. Therefore, as a functional group, circuits within the auditory network (including HVC) are together characterized by their responsiveness to, and processing of song and other auditory stimuli. Because birdsong involves coordinated function of both motor and auditory circuits, this review will refer to the ‘song system’ as the interconnected network of forebrain nuclei involved in both auditory and motor processing of song.
Neurosteroids
The functional definition of a neurosteroid is a steroidal molecule that is synthesized and/or converted within the brain to achieve levels independent of the general circulation (after Baulieu, 1998). For the purposes of this review, we therefore refer to neurosteroids as steroidal compounds that are produced by the nervous system de novo from cholesterol or from precursors arriving from the periphery.
2. Neurosteroidogenic enzymes
2.1. Expression in the song system
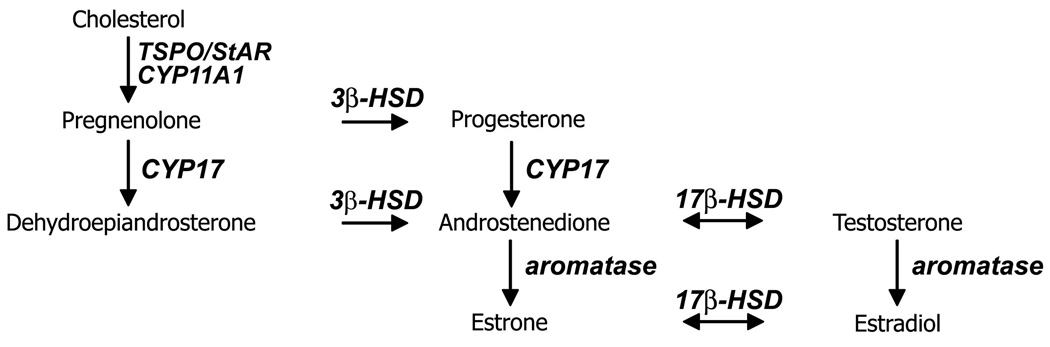
All steroids derive from cholesterol, which can be synthesized within the brain for de novo neurosteroidogenesis (Dietschy and Turley, 2001). To synthesize estrogen from cholesterol, four major steroidogenic enzymes and at least one carrier protein are necessary (Fig. 1). The initial step in steroid synthesis is the transport of cholesterol to the inner mitochondrial membrane, where the first steroidogenic enzyme resides. Two proteins have been implicated in cholesterol transport through the interstitial space of the mitochondria: the Steroidogenic Acute Regulatory Protein (StAR) and the mitochondrial translocator protein (TSPO; previously known as the peripheral-type benzodiazepine receptor). These proteins, potentially working in a complex (Papadopoulos, et al., 2007), facilitate the movement of cholesterol so it can be cleaved by cytochrome P450 side chain cleavage (CYP11A1) to produce pregnenolone, the first steroid in the steroidogenic pathway. Pregnenolone can be further metabolized by two enzymes: 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase (3 β -HSD) or cytochrome P450 17α-hydroxylase/17,20 lyase (CYP17). The activity of 3β-HSD converts pregnenolone to progesterone, and CYP17 converts pregnenlone to the androgen dehydroepiandrosterone (DHEA). 3β-HSD can also act on DHEA to synthesize another androgen, androstenedione (AE), and CYP17 converts progesterone to AE. Aromatase is the enzyme necessary to convert androgens such as AE and testosterone to the estrogens estrone and estradiol, respectively. AE and testosterone, and estrone and estradiol, can themselves be interconverted through the action of types of 17β-hydroxysteroid dehydrogenases (17β-HSD). The enzymes 5α- and 5β-reductase are also active in avian brain, and are involved in the reduction of androgens (e.g., to produce the potent androgen 5α-dihydrotestosterone (DHT)) and progestins (e.g., to produce the reduced pregnanes 5α- and 5β- allopregnanolone that allosterically modulate the GABA-A receptor, see below).
Figure 1.
The presence of aromatase in the zebra finch brain garnered particular attention because the sexually dimorphic zebra finch song circuit is steroid-sensitive but not dependent upon gonadally-synthesized steroids (Wade, et al., 1996). In particular, estradiol can functionally masculinize the song system in females, who ordinarily are not capable of singing (Adkins-Regan, et al., 1994). Other steroids have minimal or no masculinizing effects on the song system with no obvious behavioral impact (Schlinger and Arnold, 1991a; Grisham and Arnold, 1995; Jacobs, et al., 1995). It was therefore possible that the male brain could convert androgens from the periphery into masculinizing estradiol within the song system itself.
To date, aromatase remains the most studied steroidogenic enzyme in the zebra finch brain. Its mRNA expression (Shen, et al., 1994; Shen, et al., 1995; Jacobs, et al., 1999; Ramachandran, et al., 1999; Perlman and Arnold, 2003), protein localization (Balthazart, et al., 1990; Saldanha, et al., 2000) and enzymatic activity (Vockel, et al., 1990; Schlinger and Arnold, 1991b; Schlinger, et al., 1994; Wade, et al., 1995; Freking, et al., 1998; Rohmann, et al., 2007; Tam and Schlinger, 2007; Remage-Healey, et al., 2009) have all been measured. Generally, aromatase is found throughout the developing and adult male and female zebra finch brain when measured in a relatively “static” state (see comparisons below). Surprisingly, aromatase was essentially absent in the soma of neurons within song system nuclei themselves (Shen et al., 1995; Jacobs et al., 1999) (Saldanha et al., 2000) and with similar measures, no sex differences in aromatase were detected (but see below and Saldanha et al., 2000; Peterson, et al., 2005; Rohmann et al., 2007) For a long time, therefore, the potential role for neurally-produced estradiol within the song system was uncertain.
More recently, aromatase within synaptic terminals of song system neurons has been identified. Initially, aromatase was identified in neuronal fibers and punctuate terminals within the song system of zebra finches (Saldanha et al., 2000). When investigated using electron microscopy, it was discovered that within the song system, much or all aromatase is present within synaptic terminals rather than the somata (Peterson et al., 2005). In this study, no significant differences in aromatase levels were detected within each brain area, though there was an overall greater amount of aromatase-containing synaptic terminals in male brains compared to female brains (Peterson et al., 2005). This suggested that there was an alternative mechanism for estradiol provision within the male and female brain. A subsequent experiment reported higher levels of the biochemical activity of the enzyme aromatase (aromatase activity) within prepared synaptosomes as compared to other subcellular compartments where aromatase likely resides (Rohmann et al., 2007), confirming the potential for synaptic aromatase to have functional consequences. Further, this study demonstrated a regional difference in levels of synaptic aromatase activity, indicating the potential for higher estrogen concentrations in at least some components of the song system than in other, non-song brain regions (Rohmann et al., 2007). The potential for synaptic aromatase to be functionally relevant in this system has been recently illustrated by an increase in aromatase activity within the dorsal posterior telencephalon in adult males that have engaged in song (Remage-Healey et al., 2009). The implications of these observations are discussed further below.
Aromatase has been found in the auditory forebrain (Shen et al., 1995; Saldanha et al., 2000; Pinaud, et al., 2006), which has been shown to be required for auditory learning in developing and adult birds (Mello, et al., 1995; Vates, et al., 1996; Phan, et al., 2006; Gobes and Bolhuis, 2007; Dong and Clayton, 2008; London and Clayton, 2008). This region is comprised of field L, NCM, and CMM. There are few sex differences known in this area (Saldanha et al., 2000; Bolhuis and Gahr, 2006; Pinaud et al., 2006), but aromatase expression is high in at least one portion of this brain area in adult zebra finches, the caudal NCM (Saldanha et al., 2000; Pinaud et al., 2006). Functional implications of these findings are discussed in more detail below.
Aromatase is not, however, the only steroidogenic factor with neural relevance within the songbird brain. In fact, all of the steroidogenic factors required for de novo estrogen synthesis are present in developing and adult zebra finch brain, mostly identified by the presence of mRNA and enzymatic activity (Schlinger, et al., 1995; Wade et al., 1995; Vanson, et al., 1996; Cam and Schlinger, 1998; Freking et al., 1998; Schlinger, et al., 1999; London, et al., 2003; London, et al., 2006; Tam and Schlinger, 2007, London et al unpublished). This could have consequences for both the developmental organization and the mature function of the song system.
Steroidogenic factors have been detected in the zebra finch brain as early as the first day of posthatch life (London et al., 2003; Perlman and Arnold, 2003; London et al., 2006; London and Schlinger, 2007). Song system nuclei are not identifiable until approximately posthatch day 7–10 (Gahr and Metzdorf, 1999; Kim, et al., 2004). Thus, early neurosteroid production may impact the organization of the song system. The most likely place this would occur is in the cellular region surrounding the lateral ventricles.
The cellular zones along the lateral ventricles are the major sites of cell proliferation in adult and juvenile songbirds (Alvarez-Buylla, et al., 1990; DeWulf and Bottjer, 2002; DeWulf and Bottjer, 2005), and show high levels of expression of StAR, CYP11A1, 3β-HSD, CYP17, and 17β-HSD type 4 at posthatch days 1 and 5 (London et al., 2003; London and Schlinger, 2007, London et al unpublished). Interestingly, neither the distribution of highly mitotic regions nor the distribution of steroidogenic factor gene expression is uniform along the rostral-caudal and dorsal-ventral extents of the ventricle. In fact, the regions with the highest expression of steroidogenic genes (as measured by how far lateral the hybridization for steroidogenic genes extended) mimicked those of the highest proliferative activity (Alvarez-Buylla et al., 1990; DeWulf and Bottjer, 2002; DeWulf and Bottjer, 2005; London and Schlinger, 2007). The potential for steroid production in the same location as cell proliferation may have the most significance on the organization of song nuclei such as HVC. The newly-divided cells that populate HVC have been observed migrating from the zone along the lateral ventricles into HVC during development (Burek, et al., 1994). Further, the number of these cells was greater in males than in females even before they reached their destination within HVC (Burek et al., 1994). The androgens and estrogens that can be produced by the combination of these factors likely do not appear to directly impact cell proliferation in zebra finches as in other songbirds (Rasika et al., 1994; Williams et al., 1999;Hidalgo et al., 1995; Tamontin and Brenowitz, 1999;) although we note that cell proliferation along the adult lateral ventricle is regulated by corticosteroids (Katz, et al., 2008) and DHEA (unpublished observations). Therefore, it may be that neurosteroids impact the survival, differentiation, or migration of newly-divided cells that eventually become incorporated into song nuclei. A precedence for several of these mechanisms has been found in other songbirds (Rasika, et al., 1999; Williams, et al., 1999).
In adult zebra finches, the expression of at least one steroidogenic gene has been measured within each major song nucleus (Table 1). In HVC, there is a medial subregion with very few aromatase-containing cells (Shen et al., 1995; Saldanha et al., 2000), but more widespread expression of StAR, CYP11A1, 3β-HSD, CYP17, and 17β-HSD (London et al., 2003; London et al., 2006, London et al unpublished). In RA, the presence of StAR and CYP11A1 has been documented (London et al., 2006). Area X cells express CYP11A1, 3β-HSD, and CYP17, whereas LMAN cells contain StAR, CYP11A1, and 3β-HSD mRNAs (London et al., 2003; London et al., 2006). Notably, not all song nuclei express the same complement of steroidogenic factors, suggesting that different neurosteroids within particular regions act to modulate neural function. The specific action of neurosteroids within these regions is unknown. However, these steroids in other systems have been shown to, for example, affect NMDAR and GABAAR (Hosie, et al., 2007), the major excitatory and inhibitory receptors within the song system. In cultured zebra finch telencephalic neurons, 5α-and 5β-reduced pregnane steroids can allosterically modulate the GABAA receptor, whereas estradiol, testosterone, and corticosterone have no effects (Carlisle, et al., 1998). It is therefore possible that the steroids produced within the song nuclei exert similar effects on excitatory or inhibitory pathways with functional consequences for fine tuning the circuit for optimal song production. A summary of the current understanding of the expression of the major steroidogenic enzymes and receptors in the song system is presented in Table 1.
Table 1.
Expression of steroidogenic enzymes and classical steroid nuclear receptors in song system areas. Results are primarily from in situ hybridization studies in zebra finches (London et al., 2006; Saldanha et al., 2000; Schlinger and Brenowitz, 2002; Metzdorf et al., 1999; Jacobs et al., 1996). Hybridization intensity is shown as high (+++), medium (++), low (+), or undetected (−) for each region, from adult zebra finches of both sexes.
| Song system nucleus |
StAR | Cyp11A1 | 3βHSD | Cyp17 | Aromatase | Androgen Receptor |
Estrogen Receptor |
|---|---|---|---|---|---|---|---|
| HVC | + | ++ | + | + | +* | ++ | ++ |
| RA | + | ++ | + | − | − | ++ | − |
| Area X | − | + | + | + | − | + | − |
| LMAN | + | + | + | ++ | − | ++ | − |
| NCM | ++ | ++ | + | − | +++^ | ++ | − |
Expression is limited to synaptic terminals.
Expression is in both synaptic terminals and cell bodies.
2.2. Neurosteroidogenic enzymes: Regulation
For lipophilic hormones like sex-steroids that are unable to be stored, it is the regulation of their synthesis or their metabolism that ultimately governs their signal strength. Long term changes in steroid levels, such as those fluctuations seen across seasons, are best achieved by altering expression of genes coding for enzymes of steroidogenesis or metabolism. More rapid changes in steroid concentrations, across hours or even minutes, are best achieved by local and rapid modification of existent enzyme function. There is evidence for both short and long-term regulation of steroidogenic enzymes in the songbird brain.
2.2.1 Long term regulation
The best studied enzyme regulation involves long term changes in neural aromatase, which likely produce seasonal fluctuations in estrogen and androgen levels in some song related brain regions (aromatase simultaneously depletes local androgen levels as it converts them into increasing estrogen levels). The expression of the aromatase enzyme is conserved in several brain regions among vertebrates, including the hypothalamus and hippocampus (Forlano, et al., 2006; Roselli, 2007; Garcia-Segura, 2008). In many songbirds, the NCM, an auditory processing region akin to mammalian auditory cortex, is especially rich with aromatase. In seasonal breeding species, NCM aromatase is often elevated when singing and auditory song processing are maximal. In male song sparrows (Melospiza melodia morphna), aromatase activity in the NCM is highest in spring, when males are singing the most, as compared to winter or during molt when birds sing less (Soma, et al., 2003). Seasonal breeding male canaries show a similar pattern with aromatase expression in subregions of the NCM elevated in April compared to November (Fusani, et al., 2000). In wild caught male Lapland Longspurs (Calcarius lapponicus) aromatase activity in the caudal telencephalon that contains NCM is elevated when males sing as they display to females as well as when they mate-guard but is lower when the birds are incubating eggs and singing little (Soma, et al., 1999).
In each of these species, NCM aromatase is elevated when circulating testosterone levels are also high suggesting that gonadal testosterone upregulates aromatase expression in the NCM as it does in the avian hypothalamus (Ball, et al., 2002). Direct support for this idea came from studies of adult female canaries in which systemic testosterone stimulates and masculinizes song (Shoemaker, 1939). Treatment of female canaries with testosterone was found to increase aromatase expression and activity in the caudal NCM (Fusani, et al., 2001). This suggests that in at least some species, aromatase within the song system is sensitive to circulating testosterone so that long-term activation of the hypothalamic-pituitary-gonadal axis in males can increase estrogen concentrations in some neural circuits involved in song.
Patterns of aromatase expression in brain regions that impact song differ across species and region (Vockel et al., 1990; Foidart, et al., 1998; Soma et al., 1999; Silverin, et al., 2000; Soma et al., 2003). From an ultimate perpspective, these differences likely reflect variation in the behavioral ecology of song behavior in males of these species. Neurochemically, we must consider alternate regulatory mechanisms, including neurotransmitters, hormones or trophic factors as putative long term regulators of brain aromatase (zebra finch: Freking et al., 1998). Studies involving birds collected in the midst of complex social contexts must also consider how the rapid modulation of brain aromatase might be superimposed on or might replace long term regulation as a strategy to alter local estrogen levels (see below).
Aromatase is not the only enzyme potentially subject to long term regulation in and around song-related neural circuits. Song sparrows of the Pacific Northwest of North America are territorial and can sing year round despite having basal levels of circulating testosterone outside of the breeding season (Wingfield, 1994). The steroidogenic enzyme 3p-HSD is elevated in the song sparrow NCM of males during the non-breeding season as compared to the breeding season or when birds are molting (Soma and Schlinger, unpublished results). Presumably the androgen DHEA, which circulates at relatively high levels during the non-breeding season in song sparrows (Soma and Wingfield, 2001), is acted on by both 3β-HSD and aromatase to synthesize estrogens in NCM, which could then activate neural circuits that process conspecific song. Mechanisms regulating expression of neural 3β-HSD are unknown, but gonadal steroids may suppress 3β-HSD activity or expression within NCM during the breeding season.
We must also consider novel pathways for the regulation of neurosteroidogenesis in the songbird brain. One candidate signaling system involves nonapeptides such as vasotocin. Peptides in this family exert considerable influence on vertebrate social behavior (Goodson and Adkins-Regan, 1999; Young and Wang, 2004), with evidence in white-crowned sparrows for a stimulatory role in song production (Maney, et al., 1997) and for vasotocin binding sites in song related brain regions, including NCM (Leung, et al., 2009). Vasotocin can stimulate steroid synthesis in the frog brain (Do-Rego, et al., 2006) so it is possible that these peptides function, in part, to stimulate local synthesis of neuroactive steroids in the songbird brain. Additional studies are needed to assess long term changes in other steroidogenic enzymes in the brains of songbirds.
2.2.2 Acute regulation
In non-songbird avian models, the activity of steroid-producing enzymes has been shown to be regulated on the timescale of minutes in the brain. In male Japanese quail, copulatory behavior has been linked to acute (within minutes) changes in the activity of aromatase in the medial preoptic area (Balthazart, et al., 2003b; Cornil, et al., 2005; Cornil, et al., 2006). In addition, aromatase activity is regulated within seconds by Ca2+-dependent phosphorylation events that can be partially driven by glutamatergic activation (Balthazart, et al., 2003a; Balthazart, et al., 2006). In brooding males vs. non-breeding male quail the concentration of progesterone as well as the activity of the enzyme 3β-HSD within the diencephalon are both increased (Lea, et al., 2001), although whether this regulation is acute (minute-by-minute) is unknown. If similar mechanisms exist in the songbird forebrain, then this raises the possibility that acute changes in neurosteroid production and action can locally regulate the forebrain circuits involved in singing behavior (the song system).
In zebra finches, the activity of the 3β-HSD enzyme was shown to be rapidly responsive to stress in the forebrain (Soma, et al., 2004). Baseline neural 3β-HSD activity was lower in male vs. female zebra finches, especially within the forebrain. This relative sex difference was reversed following a brief period of stress (10 min), in that males showed higher 3β-HSD activity than females following acute stress. The relatively rapid time course of these changes indicates that local concentrations of steroids shift in a region-specific manner over the course of minutes in the songbird brain. More recently, it was observed that estradiol itself exerts rapid effects on the biochemical activity of steroidogenic enzymes, including 3β-HSD, in zebra finch telencephalic tissue (Pradhan, et al., 2008). However, whether acute regulation of neurosteroidogenic enzyme activity occurs specifically within the song system is currently unknown.
Synaptosomal aromatase enshrouds the song system in the zebra finch forebrain, and the biochemical activity of this aromatase in synaptic terminals is elevated in males vs. females (Rohmann et al., 2007). This observation is consistent with the hypothesis that forebrain synaptic aromatase activity regulates the male-biased production of song in this species. This hypothesis was tested in a recent set of biochemistry experiments (Remage-Healey et al., 2009). Males were individually exposed to females for 30 minutes and were divided into two groups based on their behavior. Males that sang at least one song bout during the 30-minute trial (‘singers’) had elevated aromatase activity in the posterior telencephalon as compared to males that did not sing during the 30 minute trial (‘nonsingers’). This experiment was repeated, and the elevated aromatase activity in singers was localized specifically to synaptic terminals in the posterior telencephalon (Remage-Healey et al., 2009). It therefore appears that courtship singing involves an up-regulation of aromatase activity specifically in synaptic terminals. This upregulation appears to be specific to motor aspects of song, since acoustic playback of conspecific song did not elicit significant changes in aromatase activity in synaptic terminals. It remains to be seen whether synaptosomal aromatase is rapidly upregulated during singing behavior, or is constitutively upregulated only in males that choose to sing (e.g., the ‘prior condition’ hypothesis, see (Schlinger and Callard, 1989). However, the localization of aromatase to pre-synaptic terminals suggests a dynamic mode of action for locally-produced estrogens that could be modulated by rapid events at the neuronal synapse.
3. Steroid receptors and coactivators in the song system
3.1. Nuclear receptor distribution
Steroids typically affect cellular function by binding to intracellular nuclear receptors. Once bound to steroid molecules in the cytoplasm, these receptors form dimers, enter the nucleus, and regulate gene expression by binding to specific sequences of DNA, or hormone response elements (HREs) on steroid-responsive genes (Norris, 1985). Nuclear receptors can be abundant within the song circuit (Gahr, 2001; Schlinger and Brenowitz, 2002), and represent the most well-understood mechanism by which neurosteroids act.
In zebra finches, it is androgen receptors (AR), but not estrogen receptors (ER), that delineate song nuclei. This has been shown mostly via autoradiographic binding (Arnold, et al., 1976; Arnold and Saltiel, 1979) (Nordeen, et al., 1987a; Nordeen, et al., 1987b) and in situ hybridization studies (Jacobs, et al., 1996; Gahr and Wild, 1997; Gahr and Metzdorf, 1999; Jacobs et al., 1999; Kim et al., 2004). In the zebra finch, one of the primary masculinizing features is the increase in AR expression within song nuclei. It is still unclear how estradiol is masculinizing, but AR within the song system seem to play a role (Grisham, et al., 2007). In other songbirds, song system nuclei express ER (Gahr, et al., 1993; Nastiuk and Clayton, 1995; Bernard, et al., 1999; Gahr and Metzdorf, 1999; Metzdorf, et al., 1999; Fusani et al., 2000; Grisham et al., 2007). Again, the functional significance of these differences is unknown. One hypothesis is that, in species that rely more on seasonal variation in singing may also depend more on peripherally-synthesized steroids, and it may be these species that show both AR and ER expression within the song system to more fully capture the changes in circulating levels of steroids.
The action of nuclear receptors is not quite as simple as depicted above; their ability to bind HREs can be modulated by other proteins called coactivators and corepressors. The investigation of coactivators is still young and no corepressors have yet been described within the brain, but a couple of steroid-receptor coactivators (SRCs) have now been identified in the songbird brain. The L7/SPA (also RPL7) is a SRC that interacts with ERα and progesterone receptors (Jackson, et al., 1997), possibly altering effectiveness of estradiol signaling. It has been detected in both developing and adult zebra finches (Duncan and Carruth, 2007). L7/SPA shows different patterns of expression across age and sex, and between mRNA and protein levels, illustrating the dynamic changes in steroid signaling that can occur within the brain. Interestingly, during early development L7/SPA is expressed along the lateral ventricles, and later in life is expressed within song nuclei (Duncan and Carruth, 2007). Therefore, it is likely that L7/SPA is integral to ERα-mediated signaling and impacts the developmental organization of the song system and possibly its function in adult males.
Another SRC has been investigated in birds: SRC-1. The SRC-1 coactivator can work in concert with several steroid receptors (Onate, et al., 1995) and has been demonstrated in vivo to alter neural sexual differentiation in rodents (Auger, et al., 2000). Its expression was described in the canary; SRC-1 mRNA was detected throughout the brain, and had high expression levels in all song nuclei except RA (Charlier, et al., 2002; Charlier, et al., 2003). This is intriguing; could it indicate a more important role for regulated steroid receptor-mediated signaling in the sensorimotor versus the motor component of the song circuit? The potential for SRC-1 to impact song comes indirectly from a study in the quail, a non-songbird. In quail, reduction of SRC-1 in reproductive brain areas caused a decrease in steroid-sensitive behaviors (Charlier, et al., 2005). It may be that similar deficits in song production would be caused by minimizing the availability of SRC-1 in the song system.
3.2. Potential non-classical sites for acute steroid actions
There are regions of the songbird brain that exhibit rich expression of steroidogenic enzymes, yet the expression of classical nuclear steroid receptors does not (in some cases) overlap with these local neurosteroid ‘sources’. One interesting example is the auditory region NCM, which has abundant expression of aromatase, but very limited expression of nuclear ERs (Jacobs et al., 1996; Metzdorf et al., 1999). Similarly, unlike in other songbirds, in zebra finches nucleus HVC exhibits relatively reduced ER expression levels, but there is a relative abundance of aromatase in HVC in presynaptic terminals (Jacobs et al., 1996; Metzdorf et al., 1999; Peterson et al., 2005). In seasonal-breeding songbirds, the estrogen-dependent annual growth of the song system (including HVC and RA) can precede gonadal recrudescence (Tramontin, et al., 2001; 2003; Caro, et al., 2005), and the relative dearth of classical nuclear receptors again suggests possible non-classical roles for the local production of neurosteroids. These observations, taken together, suggest that locally produced steroids (like estrogens) within the caudal forebrain could be acting via non-classical binding sites and/or receptors. To date, however, there have been no reports of acute (seconds-to-minutes) actions of any steroid in the avian song system.
4. Evidence for production of steroids by the song system
4.1. Systemic injections
The primary direct evidence that the male songbird brain is a major source of steroid production came from a series of studies with zebra finches. Radiolabeled aromatizable androgens were injected into both the jugular and carotid vasculature, and radiolabelled estrogen endproducts (resulting from aromatase activity) were collected and analyzed from cranial (jugular) vs. systemic (carotid) circulation. The predominant recovery of converted estrogens in males came from plasma that had been perfused through the brain via the jugular (Schlinger and Arnold 1992). Peripheral vasculature (infusion into the carotid) contributed significant estrogens only in females. Remarkably, the conversion of androgens into estrogens by the brain took place within only 5 min of infusion. These studies indicated that the central nervous system of zebra finches contains aromatase activity capable of acutely synthesizing estrogens from androgens. Given the abundant expression of aromatase in the zebra finch song system (see above) it is likely that a large amount of the estrogens generated in male brain is derived from the song system in these studies.
4.2. In vivo microdialysis
The recent advancement of neurosteroid microdialysis provides spatio-temporal precision in determining the local regulation of the song system in awake songbirds (Remage-Healey et al. 2008). Using microdialysis, it was observed that the NCM of zebra finches exhibits acute changes in local steroid concentrations in vivo. The presence of the neurosteroid estradiol in the brain of males was verified using highly-sensitive gas chromatography/mass spectrometry. The passive diffusion of steroids across the dialysis membrane was confirmed using in vitro methods with radiolabelled estradiol. When microdialyzed males were exposed to females in an adjacent cage for 30 minutes, local estradiol levels increased two-fold within the NCM region, while local testosterone levels remained unchanged. Local estradiol levels then returned to baseline immediately following the exposure to females. Futhermore, during brief periods of auditory activation, forebrain estradiol levels increased and testosterone levels decreased within NCM when males were exposed to other males’ song, but not when they were exposed to white noise or female chirps. These rapid fluctuations were specific to NCM, as they occurred independently from peripheral steroid changes. Fluctuations in forebrain steroids were also exceedingly localized, as acute neurosteroid changes that occurred within NCM were not observed in forebrain circuits that were adjacent to, but outside of, NCM (Remage-Healey et al., 2008). The measurement of acute changes using in vivo microdialysis therefore provides direct evidence that neurosteroids are produced and fluctuate within the forebrain. Locally-produced neurosteroids can change on a moment-by-moment basis, and these changes occur within a critical auditory circuit (NCM) that relays information to the song motor pathway in male zebra finches. These and other findings break down traditional categories for sex-steroids like estradiol as purely ‘masculinizing’ or ‘feminizing’ agents, since they have now been shown to be linked to momentary alterations in neural circuit function, much in the way of neuromodulators.
5. Evidence for the actions of brain-derived steroids on the song system
Circulating steroids, particularly testosterone, exert potent effects on the songbird brain, and lead to increases in singing behavior. In some species, experimental removal of the testes has been associated with decreases in singing and regression of song nuclei, and testosterone injection restores song and HVC size (Nottebohm, 1969; Prove, 1974; Arnold, 1975; Heid, et al., 1985). As circulating androgens reach the brain, they may be converted to highly-potent aromatized- or 5α-reduced-metabolites to exert effects on neural circuits. Because of the evidence for brain-derived steroid production, appreciation has grown recently for the local steroid ‘microenvironments’ that exist in the forebrain of songbirds (see below).
A modern understanding has emphasized that the effects of steroids on cells, circuits and behaviors occur in two separate time domains. The classical ‘long-term’ (hours-weeks) actions of steroids usually involve binding to intracellular receptors which then act as regulators of transcription to produce long lasting effects. The non-classical ‘acute’ (seconds-minutes) actions of steroids usually involve interactions with membrane receptors and/or second messenger pathways which can modulate cellular function within extremely fast time scales. These temporal criteria are not absolute, however, as there are genomic actions of steroids that can be manifest in as little as 30 min, while some membrane-initiated events can persist for hours or longer. Below, we consider both the long-term and acute actions of brain-derived steroids on the avian song system. Accordingly, long term steroid actions incorporate those that shape brain function and behavior over timescales ranging from days, to seasons, to the lifetime of the animal. Acute actions, by contrast, are those which affect ongoing behavior via candidate ‘neuromodulatory’ mechanisms. The current evidence is strongly biased toward long-term actions in the song system, in part because acute actions encompass very rapid changes in neurotransmission and ion channel function that shape ongoing behaviors, which have historically been very difficult to study.
5.1. Long term effects
The firing patterns of neurons in the avian song system can change over the course of the year, presumably to prepare males for the demands of courtship and territorial song in seasonal breeders. Seasonal rhythms in plasma sex steroids have been associated with season-dependent changes in firing patterns (Del Negro and Edeline, 2002) and nucleus volume (Brenowitz and Lent, 2002; Soma et al., 2003; Thompson, et al., 2007). In white-crowned sparrows, a seasonal plasticity in neuronal firing patterns has been observed in nucleus RA (Park, et al., 2005). Recent work has shown that the seasonality of RA firing patterns requires a transynaptic signal from nucleus HVC, and that one of the cues that can stimulate HVC to produce this transynaptic signal is increased circulating testosterone (Meitzen, et al., 2007). Using small implantable osmotic pumps, it was observed that the actions of testosterone in HVC also involved activation of either androgen- or estrogen-receptors to achieve its transynaptic effects on RA. Therefore, a long-term process of neurosteroid production (estrogen synthesis) could be linked to seasonal fluctuations in motor patterning in the song system.
In addition to seasonal changes in firing patterns, the size of nuclei in the song system can vary over the course of the year in some songbirds. It has generally been assumed that testosterone secretion from the gonads at the beginning of the breeding season provides a neuroendocrine cue for the song system to begin growing, although this has note always been observed. As described above, in some cases seasonal growth of the song nuclei HVC and RA can occur even before the recrudescence of the gonads and prior to any increases in circulating androgens (Tramontin et al., 2001; Caro et al., 2005). The contribution of brain-derived steroids to this ‘early’ seasonal growth could be significant, but this hypothesis has not yet been tested.
The immediate-early genes ZENK and Fos are responsive to auditory activation in songbirds, especially within the auditory regions NCM and CMM. Long term estrogen treatment can alter the immediate-early gene responses to male song in female white-throated sparrows (Maney, et al., 2006; Maney, et al., 2008), although it is currently unknown if a similar mechanism occurs in males, which would depend on local neurosteroidogenesis for estrogen production and action within the auditory forebrain.
Local estrogen production appears critical for the masculinization of the song circuit in zebra finches, as revealed by in vitro studies of Holloway and Clayton (Holloway and Clayton, 2001). Cultured slices that contained the forebrain song circuit were maintained for weeks under in vitro conditions and studied for the male-typical formation of synaptic connections between HVC and RA. Treatment of male slices with aromatase inhibitors and estrogen receptor antagonists actively suppressed the HVC-RA connection in males while co-incubating with male slices ‘masculinized’ these connections in slices from females. Lastly, treatment of female slices with estradiol also masculinized their HVC-RA circuit\ strongly suggesting that local steroidogenesis is part of the normal male-typical development of the forebrain in zebra finches (Holloway and Clayton, 2001).
Neurosteroids may also interact with neurogenetic mechanisms to shape the sex-specific development of forebrain circuits, as was revealed in an in-depth study of a gynandromorphic zebra finch (Agate, et al., 2003). The gynandromorph exhibited laterally-asymmetric expression of genes present on the female sex-chromosomes (ZW) on one half of it’s body (and brain) and male sex-chromosome genes (ZZ) on the other half. The lateralized development of the song system was consistent with that of the male neurogenetic complement on the ‘male’ side. However, it was also observed that the ‘female’ side of the gynandromorphic zebra finch brain had a more masculinized song system than the population distribution normally observed for female zebra finches. Since the bird had a mixture of testicular and ovarian tissue, and its circulating steroid levels were closer to those of normal females than males, the masculinization of the genetically female hemisphere suggested that some neurohormonal factors (perhaps neurosteroids?) produced on the ‘male’ side could have led to some degree of masculinization.
5.2. Acute effects
While there exists strong evidence for acute effects of steroids, particularly estrogens, on the activity of neurons and neural circuits in mammals and teleost fishes (Mermelstein, et al., 1996; Qiu, et al., 2003; Remage-Healey and Bass, 2007; Woolley, 2007), the evidence for acute (seconds-minutes) effects of steroids on the avian song system is scarce (for rapid estrogen effects on steroidogenic enzyme activity (see Pradhan et al., 2008). One recent study showed that removal of testosterone implants reduces the size of HVC to that of non-breeding condition after only 12 hours in white-crowned sparrows (Thompson et al., 2007). This indicates that elevated steroids help to maintain the seasonally-enlarged HVC volume via constitutively-active mechanisms that can be rapidly adjusted. It is possible that downstream metabolites of testosterone are responsible for these effects, which would implicate a role for neurosteroids (or their withdrawal) in acute mechanisms of apoptosis and/or cell volume shrinkage.
6. Neurosteroids and neurotransmitter interactions in the song system
We are only beginning to understand how neurosteroids may interact with neurotransmitters and neuropeptides within forebrain circuits in vertebrates. Several lines of evidence indicate that the predominant excitatory and inhibitory amino acids are co-expressed with neurosteroidogenic enzymes in the songbird brain, and that these systems are co-modulatory (see Table 2). Neuropeptides such as vasotocin and opioid peptides are also emerging as potential co-modulators of neurosteroid levels and actions in the songbird brain (Table 2).
Table 2.
Reports of interactions between neurosteroids and neurotransmitter/neuropeptide networks in the avian song system. Studies cited include evidence from neuroanatomical, neurophysiological, and neurochemical investigations.
| Neurotransmitter / neuropeptide |
Neurosteroid / enzyme |
Region | Evidence for interaction |
Reference(s) |
|---|---|---|---|---|
| GABA currents | 5-alpha- & 5-beta- reduced pregnanes |
Telencephalon | Electrophysiological | Carlisle et al., 1998 |
| NMDA currents | Testosterone/DHT | LMAN, RA | Electrophysiological | White et al., 1999 |
| NMDA-receptors | Aromatase | NCM | Anatomical co-localization | Saldanha et al., 2004 |
| Calbindin/GABA | Aromatase | NCM | Anatomical co-localization | Pinaud et al., 2006 |
| Glutamate/GABA | Estradiol / Testosterone | NCM | Neurochemical | Remage-Healey et al., 2008 |
| Catecholamines (tyrosine hydroxylase, DSP4) |
Androgen/ Estrogen Receptors |
HVC/RA/Hindbrain | Anatomical co-localization, Neurochemical |
Maney et al., 2001; Appeltants et al., 2003; Barclay et al. 1996; Vyas et al., 2008 |
| Glutamate | Aromatase | Hypothalamus / Preoptic area |
Biochemical | Balthazart et al., 2006# |
| Vasotocin (receptors) |
Aromatase* 3-beta-HSD* |
NCM | Anatomical co-localization | Leung et al., 2009; |
| Opioids (and receptors) |
Aromatase* | HVC/RA/ICo | Proposed anatomical co- localization |
Gulledge and Deviche, 1999; Deviche and Gϋntϋrkϋn, 1992 |
Results from songbirds, except where noted:
Japanese quail.
Proposed co-localization based on published aromatase distribution.
Using in vivo microdialysis, reverse-delivery of neurotransmitters into the songbird forebrain (retrodialysis) rapidly alters local steroid levels. Retrodialysis of the excitatory neurotransmitter glutamate acutely suppresses local estradiol levels, while retrodialysis of the inhibitory transmitter GABA causes acute increases in local testosterone levels (Remage-Healey, et al., 2008). These results indicate that steroid levels within forebrain circuits are linked to acute actions of conventional neurotransmitters. It remains to be seen how the rich diversity of neurochemicals in the songbird forebrain interact to regulate singing and audition.
7. Conclusions
The aim of this review has been to summarize the findings that demonstrate the remarkable ability of the songbird brain to locally synthesize its own suite of neurosteroids. The classical view of how steroids influence the songbird brain – via long term effects of hormones secreted from the gonads and adrenals – is not incorrect. However, in light of the most recent findings, this view must be updated to incorporate the understanding that forebrain circuits within and nearby the song system are local participants in their steroid-dependent regulatory and modulatory processes (see also London, et al., 2009). A revised understanding of the richness of steroid neurochemistry in songbirds stimulates new ideas and research directions. For example, as described above, the development and sex-dependent differentiation of the song system is clearly influenced by long-term actions of steroids (particularly estrogens). However, a modern understanding of neurosteroid biology makes it likely that as yet un-described changes in local levels of neurosteroids are involved in acute modulation of song learning, auditory encoding, and synaptic plasticity in juvenile birds. With this view in mind, the birdsong model system should continue to make us sit up and take notice of the rich possibilities for the neurochemical regulation of social behavior.
Citations in Table 2:
(Deviche and Gunturkun, 1992; Barclay, et al., 1996; Gulledge and Deviche, 1999; White, et al., 1999; Maney, et al., 2001; Appeltants, et al., 2003; Saldanha, et al., 2004; Vyas, et al., 2008)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkins-Regan E, Mansukhani V, Seiwert C, Thompson R. Sexual-Differentiation of Brain and Behavior in the Zebra Finch - Critical Periods for Effects of Early Estrogen-Treatment. J. Neurobiol. 1994;25(7):865–877. doi: 10.1002/neu.480250710. [DOI] [PubMed] [Google Scholar]
- Agate RJ, Grisham W, Wade J, Mann S, Wingfield J, Schanen C, Palotie A, Arnold AP. Neural, not gonadal, origin of brain sex differences in a gynandromorphic finch. Proc. Natl. Acad. Sci. U. S. A. 2003;100(8):4873–4878. doi: 10.1073/pnas.0636925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Kirn JR, Nottebohm F. Birth of projection neurons in adult avian brain may be related to perceptual or motor learning. Science. 1990 Sep 21;249(4975):1444–1446. doi: 10.1126/science.1698312. [DOI] [PubMed] [Google Scholar]
- Appeltants D, Ball GF, Balthazart J. Song activation by testosterone is associated with an increased catecholaminergic innervation of the song control system in female canaries. Neuroscience. 2003;121(3):801–814. doi: 10.1016/s0306-4522(03)00496-2. [DOI] [PubMed] [Google Scholar]
- Arnold AP. Effects of Castration and Androgen Replacement on Song, Courtship, and Aggression in Zebra Finches (Poephila-Guttata) Journal of Experimental Zoology. 1975;191(3):309–325. doi: 10.1002/jez.1401910302. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Nottebohm F, Pfaff DW. Hormone Concentrating Cells in Vocal Control and Other Areas of Brain of Zebra Finch (Poephila-Guttata) J. Comp. Neurol. 1976;165(4):487–511. doi: 10.1002/cne.901650406. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Saltiel A. Sexual Difference in Pattern of Hormone Accumulation in the Brain of a Songbird. Science. 1979;205(4407):702–705. doi: 10.1126/science.205.4407.702. [DOI] [PubMed] [Google Scholar]
- Askew JA, Georgiou GC, Sharp PJ, Lea RW. Localization of progesterone receptor in brain and pituitary of the ring dove: influence of breeding cycle and estrogen. Horm Behav. 1997;32(2):105–113. doi: 10.1006/hbeh.1997.1411. [DOI] [PubMed] [Google Scholar]
- Auger AP, Tetel MJ, McCarthy MM. Steroid receptor coactivator-1 (SRC-1) mediates the development of sex-specific brain morphology and behavior. Proc. Natl. Acad. Sci. U. S. A. 2000;97(13):7551–7555. doi: 10.1073/pnas.97.13.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball GF, Riters LV, Balthazart J. Neuroendocrinology of song behavior and avian brain plasticity: Multiple sites of action of sex steroid hormones. Frontiers in Neuroendocrinology. 2002;23(2):137–178. doi: 10.1006/frne.2002.0230. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF. Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology. 2006;147(1):359–366. doi: 10.1210/en.2005-0845. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Charlier TD, Ball GF. Calcium-dependent phosphorylation processes control brain aromatase in quail. Eur. J. Neurosci. 2003a;17(8):1591–1606. doi: 10.1046/j.1460-9568.2003.02598.x. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Charlier TD, Cornil CA, Ball GF. Multiple mechanisms control brain aromatase activity at the genomic and non-genomic level. J Steroid Biochem. 2003b;86(3–5):367–379. doi: 10.1016/s0960-0760(03)00346-7. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Foidart A, Surlemont C, Vockel A, Harada N. Distribution of Aromatase in the Brain of the Japanese-Quail, Ring Dove, and Zebra Finch - an Immunocytochemical Study. J. Comp. Neurol. 1990;301(2):276–288. doi: 10.1002/cne.903010210. [DOI] [PubMed] [Google Scholar]
- Barclay SR, Harding CF, Waterman SA. Central DSP-4 treatment decreases norepinephrine levels and courtship behavior in male zebra finches. Pharmacol Biochem Be. 1996;53(1):213–220. doi: 10.1016/0091-3057(95)00183-2. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Neurosteroids: A novel function of the brain. Psychoneuroendocrinology. 1998;23(8):963–987. doi: 10.1016/s0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- Belle MD, Sharp PJ, Lea RW. Aromatase inhibition abolishes courtship behaviours in the ring dove (Streptopelia risoria) and reduces androgen and progesterone receptors in the hypothalamus and anterior pituitary gland. Mol Cell Biochem. 2005;276(1–2):193–204. doi: 10.1007/s11010-005-4060-6. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Bentley GE, Balthazart J, Turek FW, Ball GF. Androgen receptor, estrogen receptor alpha, and estrogen receptor beta show distinct patterns of expression in forebrain song control nuclei of European starlings. Endocrinology. 1999;140(10):4633–4643. doi: 10.1210/endo.140.10.7024. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Gahr M. Neural mechanisms of birdsong memory. Nature Reviews Neuroscience. 2006;7(5):347–357. doi: 10.1038/nrn1904. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Johnson F. Circuits, hormones, and learning: Vocal behavior in songbirds. J. Neurobiol. 1997;33(5):602–618. doi: 10.1002/(sici)1097-4695(19971105)33:5<602::aid-neu8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA, Lent K. Act locally and think globally: Intracerebral testosterone implants induce seasonal-like growth of adult avian song control circuits. Proc. Natl. Acad. Sci. U. S. A. 2002;99(19):12421–12426. doi: 10.1073/pnas.192308799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuner CW, Orchinik M. Pharmacological characterization of intracellular, membrane, and plasma binding sites for corticosterone in house sparrows. Gen. Comp. Endocrinol. 2009 doi: 10.1016/j.ygcen.2009.01.027. In Press,Corrected Proof. [DOI] [PubMed] [Google Scholar]
- Burek MJ, Nordeen KW, Nordeen EJ. Ontogeny of Sex-Differences among Newly-Generated Neurons of the Juvenile Avian Brain. Dev. Brain Res. 1994;78(1):57–64. doi: 10.1016/0165-3806(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Cam V, Schlinger BA. Activities of aromatase and 3 beta-hydroxysteroid dehydrogenase Delta(4)-Delta(5) isomerase in whole organ cultures of tissues from developing zebra finches. Horm. Behav. 1998;33(1):31–39. doi: 10.1006/hbeh.1998.1434. [DOI] [PubMed] [Google Scholar]
- Camacho-Arroyo I, Hernandez-Molina VI, Rivas-Suarez M, Guerra-Araiza C, Gonzalez-Moran MG. Changes in progesterone receptor isoforms content in the brain of immature, mature and aged male and female chickens. Gen Comp Endocrinol. 2007;150(3):381–385. doi: 10.1016/j.ygcen.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Carlisle HJ, Hales TG, Schlinger BA. Characterization of neuronal zebra finch GABA(A) receptors: steroid effects. Journal of ComparativePhysiology a-Neuroethology Sensory Neural and Behavioral Physiology. 1998;182(4):531–538. [Google Scholar]
- Caro SP, Lambrechts MM, Balthazart J. Early seasonal development of brain song control nuclei in male blue tits. Neurosci Lett. 2005;386(3):139–144. doi: 10.1016/j.neulet.2005.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier TD, Ball GF, Balthazart J. Inhibition of steroid receptor coactivator-1 blocks estrogen and androgen action on male sex behavior and associated brain plasticity. J. Neurosci. 2005;25(4):906–913. doi: 10.1523/JNEUROSCI.3533-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier TD, Balthazart J, Ball GF. Sex differences in the distribution of the steroid receptor coactivator SRC-1 in the song control nuclei of male and female canaries. Brain Res. 2003;959(2):263–274. doi: 10.1016/s0006-8993(02)03758-7. [DOI] [PubMed] [Google Scholar]
- Charlier TD, Lakaye B, Ball GF, Balthazart J. Steroid receptor coactivator SRC-1 exhibits high expression in steroid-sensitive brain areas regulating reproductive behaviors in the quail brain. Neuroendocrinology. 2002;76(5):297–315. doi: 10.1159/000066624. [DOI] [PubMed] [Google Scholar]
- Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Dejace C, Ball GF, Balthazart J. Rapid decreases in preoptic aromatase activity and brain monoamine concentrations after engaging in male sexual behavior. Endocrinology. 2005;146(9):3809–3820. doi: 10.1210/en.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Taziaux M, Baillien M, Ball GF, Balthazart J. Rapid effects of aromatase inhibition on male reproductive behaviors in Japanese quail. Horm. Behav. 2006;49(1):45–67. doi: 10.1016/j.yhbeh.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro C, Edeline JM. Sex and season influence the proportion of thin spike cells in the canary HVc. Neuroreport. 2002;13(16):2005–2009. doi: 10.1097/00001756-200211150-00003. [DOI] [PubMed] [Google Scholar]
- Deviche P, Gunturkun O. Peptides for Calling - an Immunohistochemical Study of the Avian N-Intercollicularis. Brain Res. 1992;569(1):93–99. doi: 10.1016/0006-8993(92)90373-h. [DOI] [PubMed] [Google Scholar]
- DeWulf V, Bottjer SW. Age and sex differences in mitotic activity within the zebra finch telencephalon. J. Neurosci. 2002;22(10):4080–4094. doi: 10.1523/JNEUROSCI.22-10-04080.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWulf V, Bottjer SW. Neurogenesis within the juvenile zebra finch telencephalic ventricular zone: A map of proliferative activity. J. Comp. Neurol. 2005;481(1):70–83. doi: 10.1002/cne.20352. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr Opin Lipidol. 2001;12(2):105–112. doi: 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- Dong S, Clayton DF. Partial dissociation of molecular and behavioral measures of song habituation in adult zebra finches. Genes Brain Behav. 2008;7(7):802–809. doi: 10.1111/j.1601-183X.2008.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do-Rego JL, Acharjee S, Seong JY, Galas L, Alexandre D, Bizet P, Burlet A, Kwon HB, Luu-The V, Pelletier G, Vaudry H. Vasotocin and mesotocin stimulate the biosynthesis of neurosteroids in the frog brain. J. Neurosci. 2006;26(25):6749–6760. doi: 10.1523/JNEUROSCI.4469-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan KA, Carruth LL. The sexually dimorphic expression of L7/SPA, an estrogen receptor coactivator, in zebra finch telencephalon. Dev Neurobiol. 2007;67(14):1852–1866. doi: 10.1002/dneu.20539. [DOI] [PubMed] [Google Scholar]
- Farries MA. The oscience song system considered in the context of the avian brain: Lessons learned form comparative neurobiology. Brain Behav. Evol. 2001;58:80–100. doi: 10.1159/000047263. [DOI] [PubMed] [Google Scholar]
- Foidart A, Silverin B, Baillien M, Harada N, Balthazart J. Neuroanatomical distribution and variations across the reproductive cycle of aromatase activity and aromatase-immunoreactive cells in the pied flycatcher (Ficedula hypoleuca) Horm. Behav. 1998;33(3):180–196. doi: 10.1006/hbeh.1998.1448. [DOI] [PubMed] [Google Scholar]
- Forlano PM, Schlinger BA, Bass AH. Brain aromatase: New lessons from non-mammalian model systems. Frontiers in Neuroendocrinology. 2006;27(3):247–274. doi: 10.1016/j.yfrne.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Freking F, Ramachandran B, Schlinger BA. Regulation of aromatase, 5 alpha- and 5 beta-reductase in primary cell cultures of developing zebra finch telencephalon. J. Neurobiol. 1998;36(1):30–40. doi: 10.1002/(sici)1097-4695(199807)36:1<30::aid-neu3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Fusani L, Hutchison JB, Gahr M. Testosterone regulates the activity and expression of aromatase in the canary neostriaturn. J. Neurobiol. 2001;49(1):1–8. doi: 10.1002/neu.1061. [DOI] [PubMed] [Google Scholar]
- Fusani L, Van’tHof T, Hutchison JB, Gahr M. Seasonal expression of androgen receptors, estrogen receptors, and aromatase in the canary brain in relation to circulating androgens and estrogens. J. Neurobiol. 2000;43(3):254–268. [PubMed] [Google Scholar]
- Gahr M. Distribution of sex steroid hormone receptors in the avian brain: functional implications for neural sex differences and sexual behaviors. Microsc Res Tech. 2001;55(1):1–11. doi: 10.1002/jemt.1151. [DOI] [PubMed] [Google Scholar]
- Gahr M, Guttinger HR, Kroodsma DE. Estrogen-Receptors in the Avian Brain - Survey Reveals General Distribution and Forebrain Areas Unique to Songbirds. J. Comp. Neurol. 1993;327(1):112–122. doi: 10.1002/cne.903270109. [DOI] [PubMed] [Google Scholar]
- Gahr M, Metzdorf R. The sexually dimorphic expression of androgen receptors in the song nucleus hyperstriatalis ventrale pars caudale of the zebra finch develops independently of gonadal steroids. J. Neurosci. 1999;19(7):2628–2636. doi: 10.1523/JNEUROSCI.19-07-02628.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahr M, Wild JM. Localization of androgen receptor mRNA-containing cells in avian respiratory-vocal nuclei: An in situ hybridization study. J. Neurobiol. 1997;33(7):865–876. [PubMed] [Google Scholar]
- Garcia-Segura LM. Aromatase in the brain: Not just for reproduction anymore. J Neuroendocrinol. 2008;20(6):705–712. doi: 10.1111/j.1365-2826.2008.01713.x. [DOI] [PubMed] [Google Scholar]
- Gobes SMH, Bolhuis JJ. Birdsong memory: A neural dissociation between song recognition and production. Curr Biol. 2007;17(9):789–793. doi: 10.1016/j.cub.2007.03.059. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Adkins-Regan E. Effect of intraseptal vasotocin and vasoactive intestinal polypeptide infusions on courtship song and aggression in the male zebra finch (Taeniopygia guttata) J Neuroendocrinol. 1999;11(1):19–25. doi: 10.1046/j.1365-2826.1999.00284.x. [DOI] [PubMed] [Google Scholar]
- Grisham W, Arnold AP. A direct comparison of the masculinizing effects of testosterone, androstenedione, estrogen, and progesterone on the development of the zebra finch song system. J Neurobiol. 1995;26(2):163–170. doi: 10.1002/neu.480260202. [DOI] [PubMed] [Google Scholar]
- Grisham W, Park SH, Hsia JK, Kim C, Leung MC, Kim L, Arnold AP. Effects of long-term flutamide treatment during development in zebra finches. Neurosci Lett. 2007;418(1):92–96. doi: 10.1016/j.neulet.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge CC, Deviche P. Age- and sex-related differences in opioid receptor densities in the songbird vocal control system. J. Comp. Neurol. 1999;404(4):505–514. [PubMed] [Google Scholar]
- Heid P, Guttinger HR, Prove E. The Influence of Castration and Testosterone Replacement on the Song Architecture of Canaries (Serinus-Canaria) Zeitschrift Fur Tierpsychologie-Journal of Comparative Ethology. 1985;69(3):224–236. [Google Scholar]
- Holloway CC, Clayton DE. Estrogen synthesis in the male brain triggers development of the avian song control pathway in vitro. Nature Neuroscience. 2001;4(2):170–175. doi: 10.1038/84001. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, Smart TG. Neurosteroid binding sites on GABA(A) receptors. Pharmacology & Therapeutics. 2007;116(1):7–19. doi: 10.1016/j.pharmthera.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Jackson TA, Richer JK, Bain DL, Takimoto GS, Tung L, Horwitz KB. The partial agonist activity of antagonist-occupied steroid receptors is controlled by a novel hinge domain-binding coactivator L7/SPA and the corepressors N-CoR or SMRT. Mol. Endocrinol. 1997;11(6):693–705. doi: 10.1210/mend.11.6.0004. [DOI] [PubMed] [Google Scholar]
- Jacobs EC, Arnold AP, Campagnoni AT. Zebra finch estrogen receptor cDNA: Cloning and mRNA expression. J Steroid Biochem. 1996;59(2):135–145. doi: 10.1016/s0960-0760(96)00096-9. [DOI] [PubMed] [Google Scholar]
- Jacobs EC, Arnold AP, Campagnoni AT. Developmental regulation of the distribution of aromatase- and estrogen-receptor-mRNA-expressing cells in the zebra finch brain. Dev Neurosci-Basel. 1999;21(6):453–472. doi: 10.1159/000017413. [DOI] [PubMed] [Google Scholar]
- Jacobs EC, Grisham W, Arnold AP. Lack of a synergistic effect between estradiol and dihydrotestosterone in the masculinization of the zebra finch song system. J Neurobiol. 1995;27(4):513–519. doi: 10.1002/neu.480270406. [DOI] [PubMed] [Google Scholar]
- Katz A, Mirzatoni A, Zhen Y, Schlinger BA. Sex differences in cell proliferation and glucocorticoid responsiveness in the zebra finch brain. Eur J Neurosci. 2008;28(1):99–106. doi: 10.1111/j.1460-9568.2008.06303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Perlman WR, Arnold AP. Expression of androgen receptor mRNA in zebra finch song system: Developmental regulation by estrogen. J. Comp. Neurol. 2004;469(4):535–547. doi: 10.1002/cne.11033. [DOI] [PubMed] [Google Scholar]
- Lea RW, Clark JA, Tsutsui K. Changes in central steroid receptor expression, steroid synthesis, and dopaminergic activity related to the reproductive cycle of the ring dove. Microsc Res Techniq. 2001;55(1):12–26. doi: 10.1002/jemt.1152. [DOI] [PubMed] [Google Scholar]
- Leonardo A, Fee MS. Ensemble coding of vocal control in birdsong. J. Neurosci. 2005;25(3):652–661. doi: 10.1523/JNEUROSCI.3036-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CH, Goode CT, Young LJ, Maney DL. Neural distribution of nonapeptide binding sites in two species of songbird. J. Comp. Neurol. 2009;513(2):197–208. doi: 10.1002/cne.21947. [DOI] [PubMed] [Google Scholar]
- London SE, Boulter J, Schlinger BA. Cloning of the zebra finch androgen synthetic enzyme CYP17: A study of its neural expression throughout posthatch development. J. Comp. Neurol. 2003;467(4):496–508. doi: 10.1002/cne.10936. [DOI] [PubMed] [Google Scholar]
- London SE, Clayton DF. Functional identification of sensory mechanisms required for developmental song learning. Nature Neuroscience. 2008;11(5):579–586. doi: 10.1038/nn.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London SE, Monks DA, Wade J, Schlinger BA. Widespread capacity for steroid synthesis in the avian brain and song system. Endocrinology. 2006;147(12):5975–5987. doi: 10.1210/en.2006-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London SE, Remage-Healey L, Schlinger BA. Neurosteroid production in the songbird brain: A re-evaluation of core principles. Front Neuroendocrinol. 2009 doi: 10.1016/j.yfrne.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London SE, Schlinger BA. Steroidogenic enzymes along the ventricular proliferative zone in the developing songbird brain. J. Comp. Neurol. 2007;502(4):507–521. doi: 10.1002/cne.21335. [DOI] [PubMed] [Google Scholar]
- Lubischer JL, Arnold AP. Autoradiographic localization of progestin-concentrating cells in the brain of the zebra finch. J Comp Neurol. 1990;291(3):450–456. doi: 10.1002/cne.902910310. [DOI] [PubMed] [Google Scholar]
- Maney DL, Bernard DJ, Ball GF. Gonadal steroid receptor mRNA in catecholaminergic nuclei of the canary brainstem. Neurosci Lett. 2001;311(3):189–192. doi: 10.1016/s0304-3940(01)02157-7. [DOI] [PubMed] [Google Scholar]
- Maney DL, Cho E, Goode CT. Estrogen-dependent selectivity of genomic responses to birdsong. Eur. J. Neurosci. 2006;23(6):1523–1529. doi: 10.1111/j.1460-9568.2006.04673.x. [DOI] [PubMed] [Google Scholar]
- Maney DL, Goode CT, Lange HS, Sanford SE, Solomon BL. Estradiol Modulates Neural Responses to Song in a Seasonal Songbird. J. Comp. Neurol. 2008;511(2):173–186. doi: 10.1002/cne.21830. [DOI] [PubMed] [Google Scholar]
- Maney DL, Goode CT, Wingfield JC. Intraventricular infusion of arginine vasotocin induces singing in a female songbird. J Neuroendocrinol. 1997;9(7):487–491. doi: 10.1046/j.1365-2826.1997.00635.x. [DOI] [PubMed] [Google Scholar]
- Meitzen J, Moore IT, Lent K, Brenowitz EA, Perkel DJ. Steroid hormones act transsynaptically within the Forebrain to regulate neuronal phenotype and song stereotypy. J. Neurosci. 2007;27(44):12045–12057. doi: 10.1523/JNEUROSCI.3289-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C, Nottebohm F, Clayton D. Repeated Exposure to One Song Leads to a Rapid and Persistent Decline in an Immediate-Early Genes Response to That Song in Zebra Finch Telencephalon. J. Neurosci. 1995;15(10):6919–6925. doi: 10.1523/JNEUROSCI.15-10-06919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J. Neurosci. 1996;16(2):595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzdorf R, Gahr M, Fusani L. Distribution of aromatase, estrogen receptor, and androgen receptor mRNA in the forebrain of songbirds and nonsongbirds. J. Comp. Neurol. 1999;407(1):115–129. [PubMed] [Google Scholar]
- Nastiuk KL, Clayton DF. The Canary Androgen Receptor Messenger-Rna Is Localized in the Song Control Nuclei of the Brain and Is Rapidly Regulated by Testosterone. J. Neurobiol. 1995;26(2):213–224. doi: 10.1002/neu.480260206. [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW, Arnold AP. Sexual-Differentiation of Androgen Accumulation within the Zebra Finch Brain through Selective Cell Loss and Addition. J. Comp. Neurol. 1987a;259(3):393–399. doi: 10.1002/cne.902590307. [DOI] [PubMed] [Google Scholar]
- Nordeen KW, Nordeen EJ, Arnold AP. Estrogen Accumulation in Zebra Finch Song Control Nuclei - Implications for Sexual-Differentiation and Adult Activation of Song Behavior. J. Neurobiol. 1987b;18(6):569–582. doi: 10.1002/neu.480180607. [DOI] [PubMed] [Google Scholar]
- Norris DO. Vertebrate Endocrinology. In: Norris DO, editor. Vertebrate Endocrinology. Philadelphia: Lea&Fegiber; 1985. [Google Scholar]
- Nottebohm F. Critical Period for Song Learning. Ibis. 1969;111(3):386. [Google Scholar]
- Onate SA, Tsai SY, Tsai MJ, Omalley BW. Sequence and Characterization of a Coactivator for the Steroid-Hormone Receptor Superfamily. Science. 1995;270(5240):1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Liu J, Culty M. Is there a mitochondrial signaling complex facilitating cholesterol import? Mol.Cell. Endocrinol. 2007;265:59–64. doi: 10.1016/j.mce.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Park KHJ, Meitzen J, Moore IT, Brenowitz EA, Perkel DJ. Seasonal-like plasticity of spontaneous firing rate in a songbird pre-motor nucleus. J. Neurobiol. 2005;64(2):181–191. doi: 10.1002/neu.20145. [DOI] [PubMed] [Google Scholar]
- Perlman WR, Arnold AP. Expression of estrogen receptor and aromatase mRNAs in embryonic and posthatch zebra finch brain. J. Neurobiol. 2003;55(2):204–219. doi: 10.1002/neu.10190. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proceedings of the Royal Society B-Biological Sciences. 2005;272(1576):2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan ML, Pytte CL, Vicario DS. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proc. Natl. Acad. Sci. U. S. A. 2006;103(4):1088–1093. doi: 10.1073/pnas.0510136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaud R, Fortes AF, Lovell P, Mello CV. Calbindin-positive neurons reveal a sexual dimorphism within the songbird analogue of the mammalian auditory cortex. J. Neurobiol. 2006;66(2):182–195. doi: 10.1002/neu.20211. [DOI] [PubMed] [Google Scholar]
- Pradhan DS, Yu Y, Soma KK. Rapid estrogen regulation of DHEA metabolism in the male and female songbird brain. J Neurochem. 2008;104(1):244–253. doi: 10.1111/j.1471-4159.2007.04953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prove E. Der EinfluB von Kastration und Testoseronsubstitution auf das Sexualverhalten mannlicher Zebrafinken. J Orn. 1974;115:338–347. [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J. Neurosci. 2003;23(29):9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran B, Schlinger BA, Arnold AP, Campagnoni AT. Zebra finch aromatase gene expression is regulated in the brain through an alternate promoter. Gene. 1999;240(1):209–216. doi: 10.1016/s0378-1119(99)00399-6. [DOI] [PubMed] [Google Scholar]
- Rasika S, Alvarez-Buylla A, Nottebohm F. BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain. Neuron. 1999;22(1):53–62. doi: 10.1016/s0896-6273(00)80678-9. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Plasticity in brain sexuality is revealed by the rapid actions of steroid hormones. J. Neurosci. 2007;27(5):1114–1122. doi: 10.1523/JNEUROSCI.4282-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nature Neuroscience. 2008;11(11):1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Oyama RK, Schlinger BA. Elevated aromatase in forebrain synaptic terminals during song. J Neuroendocrinol. 2009 doi: 10.1111/j.1365-2826.2009.01820.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohmann KN, Schlinger BA, Saldanha CJ. Subcellular compartmentalization of aromatase is sexually dimorphic in the adult zebra finch brain. Dev Neurobiol. 2007;67(1):1–9. doi: 10.1002/dneu.20303. [DOI] [PubMed] [Google Scholar]
- Roselli CF. Brain aromatase: roles in reproduction and neuroprotection. J Steroid Biochem Mol Biol. 2007;106(1–5):143–150. doi: 10.1016/j.jsbmb.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha CJ, Schlinger BA, Micevych PE, Horvath TL. Presynaptic N-methyl-D-aspartate receptor expression is increased by estrogen in an aromatase-rich area of the songbird hippocampus. J. Comp. Neurol. 2004;469(4):522–534. doi: 10.1002/cne.11035. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J. Comp. Neurol. 2000;423(4):619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Schlinger B, Brenowitz EA. Neural and Hormonal Control of Birdsong. In: Pfaff DW, editor. Hormones, Brain and Behavior. Vol. 2. Elsevier; 2002. pp. 799–838. [Google Scholar]
- Schlinger BA. Sex steroids and their actions on the birdsong system. J. Neurobiol. 1997;33(5):619–631. [PubMed] [Google Scholar]
- Schlinger BA, Amurumarjee S, Campagnoni AT, Arnold AP. 5-Beta-Reductase and Other Androgen-Metabolizing Enzymes in Primary Cultures of Developing Zebra Finch Telencephalon. J Neuroendocrinol. 1995;7(3):187–192. doi: 10.1111/j.1365-2826.1995.tb00746.x. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Amurumarjee S, Shen P, Campagnoni AT, Arnold AP. Neuronal and Nonneuronal Aromatase in Primary Cultures of Developing Zebra Finch Telencephalon. J. Neurosci. 1994;14(12):7541–7552. doi: 10.1523/JNEUROSCI.14-12-07541.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA, Arnold AP. Androgen Effects on the Development of the Zebra Finch Song System. Brain Res. 1991a;561(1):99–105. doi: 10.1016/0006-8993(91)90754-j. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Arnold AP. Brain Is the Major Site of Estrogen Synthesis in a Male Songbird. Proc. Natl. Acad. Sci. U. S. A. 1991b;88(10):4191–4194. doi: 10.1073/pnas.88.10.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA, Callard GV. Aromatase-Activity in Quail Brain -Correlation with Aggressiveness. Endocrinology. 1989;124(1):437–443. doi: 10.1210/endo-124-1-437. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Lane NI, Grisham W, Thompson L. Androgen synthesis in a songbird: A study of Cyp17 (17 alpha-hydroxylase/C17,20-lyase) activity in the zebra finch. Gen. Comp. Endocrinol. 1999;113(1):46–58. doi: 10.1006/gcen.1998.7179. [DOI] [PubMed] [Google Scholar]
- Shen P, Campagnoni CW, Kampf K, Schlinger BA, Arnold AP, Campagnoni AT. Isolation and Characterization of a Zebra Finch Aromatase Cdna - in-Situ Hybridization Reveals High Aromatase Expression in Brain. Mol Brain Res. 1994;24(1–4):227–237. doi: 10.1016/0169-328x(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Shen P, Schlinger BA, Campagnoni AT, Arnold AP. An Atlas of Aromatase Messenger-Rna Expression in the Zebra Finch Brain. J. Comp. Neurol. 1995;360(1):172–184. doi: 10.1002/cne.903600113. [DOI] [PubMed] [Google Scholar]
- Shoemaker HH. Effect of testosterone propionate on Behavior of the female canary. Proceedings of the Society for Experimental Biology and Medicine. 1939;41(2):299–302. [Google Scholar]
- Silverin B, Baillien M, Foidart A, Balthazart J. Distribution of aromatase activity in the brain and peripheral tissues of passerine and nonpasserine avian species. Gen. Comp. Endocrinol. 2000;117(1):34–53. doi: 10.1006/gcen.1999.7383. [DOI] [PubMed] [Google Scholar]
- Soma KK, Alday NA, Hau M, Schlinger BA. Dehydroepiandrosterone metabolism by 3 beta-hydroxysteroid dehydrogenase/Delta 5-Delta 4 isomerase in adult zebra finch brain: Sex difference and rapid effect of stress. Endocrinology. 2004;145(4):1668–1677. doi: 10.1210/en.2003-0883. [DOI] [PubMed] [Google Scholar]
- Soma KK, Bindra RK, Gee J, Wingfield JC, Schlinger BA. Androgen-metabolizing enzymes show region-specific changes across the breeding season in the brain of a wild songbird. J. Neurobiol. 1999;41(2):176–188. [PubMed] [Google Scholar]
- Soma KK, Schlinger BA, Wingfield JC, Saldanha CJ. Brain aromatase, 5 alpha-reductase, and 5 beta-reductase change seasonally in wild male song sparrows: Relationship to aggressive and sexual behavior. J. Neurobiol. 2003;56(3):209–221. doi: 10.1002/neu.10225. [DOI] [PubMed] [Google Scholar]
- Soma KK, Wingfield JC. Dehydroepiandrosterone in songbird plasma: Seasonal regulation and relationship to territorial aggression. Gen. Comp. Endocrinol. 2001;123(2):144–155. doi: 10.1006/gcen.2001.7657. [DOI] [PubMed] [Google Scholar]
- Tam H, Schlinger BA. Activities of 3 beta-HSD and aromatase in slices of developing and adult zebra finch brain. Gen. Comp. Endocrinol. 2007;150(1):26–33. doi: 10.1016/j.ygcen.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Bentley GE, Brenowitz EA. Rapid seasonal-like regression of the adult avian song control system. Proc. Natl. Acad. Sci. U. S. A. 2007;104(39):15520–15525. doi: 10.1073/pnas.0707239104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontin AD, Perfito N, Wingfield JC, Brenowitz EA. Seasonal growth of song control nuclei precedes seasonal reproductive development in wild adult song sparrows. Gen. Comp. Endocrinol. 2001;122(1):1–9. doi: 10.1006/gcen.2000.7597. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Wingfield JC, Brenowitz EA. Androgens and estrogens induce seasonal-like growth of song nuclei in the adult songbird brain. J. Neurobiol. 2003;57(2):130–140. doi: 10.1002/neu.10263. [DOI] [PubMed] [Google Scholar]
- Vanson A, Arnold AP, Schlinger BA. 3 beta-hydroxysteroid dehydrogenase/isomerase and aromatase activity in primary cultures of developing zebra finch telencephalon: Dehydroepiandrosterone as substrate for synthesis of androstenedione and estrogens. Gen. Comp. Endocrinol. 1996;102(3):342–350. doi: 10.1006/gcen.1996.0077. [DOI] [PubMed] [Google Scholar]
- Vates GE, Broome BM, Mello CV, Nottebohm F. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches (Taenopygia guttata) J. Comp. Neurol. 1996;366(4):613–642. doi: 10.1002/(SICI)1096-9861(19960318)366:4<613::AID-CNE5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Vockel A, Prove E, Balthazart J. Effects of Castration and Testosterone Treatment on the Activity of Testosterone-Metabolizing Enzymes in the Brain of Male and Female Zebra Finches. J. Neurobiol. 1990;21(5):808–825. doi: 10.1002/neu.480210514. [DOI] [PubMed] [Google Scholar]
- Vyas A, Harding C, McGowan J, Snare R, Bogdan D. Noradrenergic Neurotoxin, N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride (DSP-4), Treatment Eliminates Estrogenic Effects on,Song Responsiveness in Female Zebra Finches (Taeniopygia guttata) Behav. Neurosci. 2008;122(5):1148–1157. doi: 10.1037/0735-7044.122.5.1148. [DOI] [PubMed] [Google Scholar]
- Wade J, Schlinger BA, Arnold AP. Aromatase and 5-Beta-Reductase Activity in Cultures of Developing Zebra Finch Brain - an Investigation of Sex and Regional Differences. J. Neurobiol. 1995;27(2):240–251. doi: 10.1002/neu.480270210. [DOI] [PubMed] [Google Scholar]
- Wade J, Springer ML, Wingfield JC, Arnold AP. Neither testicular androgens nor embryonic aromatase activity alters morphology of the neural song system in zebra finches. Biol Reprod. 1996;55(5):1126–1132. doi: 10.1095/biolreprod55.5.1126. [DOI] [PubMed] [Google Scholar]
- White SA, Livingston FS, Mooney R. Androgens modulate NMDA receptor-mediated EPSCs in the zebra finch song system. J. Neurophysiol. 1999;82(5):2221–2234. doi: 10.1152/jn.1999.82.5.2221. [DOI] [PubMed] [Google Scholar]
- Williams S, Leventhal C, Lemmon V, Nedergaard M, Goldman SA. Estrogen promotes the initial migration and inception of NgCAM-dependent calcium-signaling by new neurons of the adult songbird brain. Molecular and Cellular Neuroscience. 1999;13(1):41–55. doi: 10.1006/mcne.1998.0729. [DOI] [PubMed] [Google Scholar]
- Wingfield JC. Regulation of Territorial Behavior in the Sedentary Song Sparrow, Melospiza-Melodia Morphna. Horm. Behav. 1994;28(1):1–15. doi: 10.1006/hbeh.1994.1001. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang ZX. The neurobiology of pair bonding. Nature Neuroscience. 2004;7(10):1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]