Abstract
The Cymanus is a novel flex sensor glove for measuring hand kinematics in primates. It was used to monitor 9 joints of a rhesus macaque performing a grasping task with 25 objects. Over 6 days, the monkey tolerated the glove and showed no significant impairment in performance. The sensors linearly tracked joint angles, with joint trajectories preserved over days. Angular positions discriminated objects as accurately as electromyograms recorded simultaneously from 24 arm and hand muscles, and were maximally informative of object identity at the end of reach-to-grasp. In a further final validation of the glove, muscle activity controlling a joint was correlated with the joint’s angular acceleration 70 ms later.
Keywords: motor, hand, finger, kinematics, monkey, grasping, muscle
INTRODUCTION
While researchers have successfully tracked human hand movements, tests of nonhuman kinematics present unique constraints. 1) Subject adaptability requires that a sensor glove be tolerated by the animal and non-disruptive of behavior. For monkeys, 2) hand geometry is a concern given their small joints but large ranges of motion. Additional considerations in protracted neurophysiological experiments are 3) repeatability and 4) relevance to motor control. For behaviors like object manipulation, the likelihood of marker occlusion may preclude video-based manual labeling (Wing et al., 1986) or automated tracking (Mason et al., 2004; Roy et al., 2002). In experiments involving simultaneous recording of kinematics and variables such as cortical activity, interference between systems may complicate electromagnetic sensing (Fahn and Sun, 2005) or Hall-effect designs (Dipietro et al., 2003). Optical fibers (Wise et al., 1990) and flex sensors are alternative measurement technologies. Here a novel flex sensor glove, Cymanus, was evaluated in terms of these considerations using data from a monkey performing a natural grasping behavior.
METHODS
Subject
Kinematic and electromyographic (EMG) data were collected from a 6.5-kg, 4-year-old male rhesus macaque (Macaca mulatta). The Cymanus was placed on the monkey on each of twelve days spanning 2.5 weeks. During the final six consecutive days the animal also performed a grasping task while gloved. Procedures were approved by the MIT Committee on Animal Care.
Behavior
As described in Overduin et al. (2008), the subject used its left hand to press a button, then remove an object in one of two wells within 1 s, and carry it to the opposing well within another 1 s. A different object was pseudorandomly selected after every 10 successful trials. The 25 objects systematically sampled shape and size, including cube width (1.5–3.6 cm), sphere diameter (1.6–3.6 cm), and cylinder height (0.6–5.7 cm), diameter (1.3–3.8 cm), or concavity (with a diameter of 3.2 cm at the cylinder ends that declined smoothly to a midpoint diameter ranging 0.6–3.2 cm). The monkey completed 6.3 ± 3.0 trials per day for each object position, shape, and size combination, or 1903 in total over the six sessions (each lasting 90 ± 20 min).
Sensors
The flex sensors (each ≤US$7.50; Spectra Symbol, Salt Lake City, UT) were laminated and custom-sized at 25.0 × 6.0 × 0.4 mm (or 75.0 × 6.0 × 0.5 mm for wrist sensors). Solder tabs extending 5.0 mm from the base of the sensors afforded wire-wrap connections; these were reinforced with heat-shrink tubing that extended about 12.5 mm along the sensors. The flex sensors increased in resistance in one direction of bend, and decreased in the other. Their baseline resistance when straight was 14.6 ± 2.0 kΩ (36–51 kΩ for wrist sensors).
Conditioning
Each flex sensor was referenced to an inactive sensor of approximately matched resistance placed around the monkey. Each of these sensors served as a variable resistance element of a four-arm Wheatstone bridge, along with two fixed 22-kΩ resistors. The bridges were implemented in circuit breadboards powered by a DC voltage supply. When the monkey was gloved and at rest, mechanical 10 or 100-kΩ potentiometers (Bourns, Riverside, CA) tapped onto the middle of the bridge circuit were adjusted to nullify any voltage difference between the active and inactive sensors, thereby centering the conditioned sensor signal at 0 V. Data were sampled at 2 kHz, with a nominal resolution of 0.02 ± 0.10° across joints and days.
Glove
The Cymanus actually consisted of two gloves, each made of elastic spandex (Spandex House Inc., New York, NY) and continuous with a halter worn over the upper torso and anchoring the glove in place (Fig. 1A). The gloves were cut from the same 2D template, which was optimized through iterative fittings performed under anesthesia (0.01 mg/kg Atropine, 5 mg/kg Telazol and/or 10 mg/kg Ketaset IM). The sensors were placed in pockets stitched onto the inner glove. The outer glove protected these pockets as well as the wires extending slackly from the sensors to a connector mounted behind the monkey. The ends of the finger tubes were open so that the monkey could apply its fingertips directly to grasped objects. The weight of the Cymanus including sensors, wires, and connector was 56 g, of which 14 g bore down directly on the hand.
Figure 1.
Cymanus and task. A) The glove and halter (dark gray) are shown worn by a monkey while it carried a sphere. B) The flex sensors spanned nine joints, depicted here while grasping a short cylinder. C) Raw kinematic data are shown for a sample trial from the final session wherein the monkey grasped this cylinder (at t = 0.5 s) and transported it from right to left. D) Both before and after glove introduction (dashed line), the monkey performed the task with 70–85% daily accuracy (averaged over all 25×2 objects and start positions). E) Reach and transport times on successful trials were comparable before and after glove introduction.
Joints
Sensors measured flexion/extension (as a positive/negative voltage response) at the metacarpophalangeal joints of digits 5, 3, 2, and 1 (f5, f3, f2, and f1), carpometacarpal opposition/reposition of digit 5 (o5), trapeziometacarpal abduction/adduction and opposition/reposition of digit 1 (a1 and o1), and extension/flexion and ulnar/radial deviation of the wrist (eW and dW) (Fig. 1B). Abduction of digits 2–5 was not measured given concern that sensors placed between these digits might apply lateral pressure keeping the fingers abducted. Other joints, e.g. digit 4 metacarpophalangeal and digit 2–5 proximal interphalangeal flexion/extension, could have been sampled but for a limitation in the number of available input channels.
Fitting
Although the subject was a relatively aggressive monkey, it did not resist placement of the glove and in fact was placated by this exercise in a way not unlike its reaction to grooming. During fitting, the two slits of the inner halter were first pulled over the animal’s head. The monkey’s right and left arms were then conducted in turn through the lower slit and into the open and gloved ends of the halter. This process was then repeated with the outer halter. Most of the fitting was spent palpating the monkey’s digits within the glove and then pushing each through the appropriate finger tube. The first fitting required two hours, but most of this time was spent troubleshooting the fitting procedure. This duration declined to 35 min on the second day and thereafter plateaued at 17 ± 4 min by the final six sessions. The subject did not resist this procedure, and tolerated the Cymanus despite having access to the glove with its mouth and other hand. Glove removal required only ~2 min.
Muscles
Arm and hand muscles were implanted with 24 EMG electrodes. Proximal muscles acting on the shoulder and elbow included cleidodeltoideus, spinodeltoideus, pectoralis major, triceps brachii ulnar and radial short heads, biceps brachii longus, and brachioradialis. Wrist and extrinsic hand extensors included abductor pollicis longus (AbPL) and extensors carpi radialis brevis (ECRB), digitorum communis (EDC), digiti secundi and tertii proprius (ED23), digiti quarti and quinti proprius (ED45), and carpi ulnaris (ECU). Wrist and extrinsic hand flexors included palmaris longus (PL) and flexors carpi radialis (FCR), digitorum superficialis (FDS), digitorum profundus ulnar (FDPu) and radial (FDPr), and carpi ulnaris (FCU). Intrinsic hand muscles included abductor pollicis brevis (AbPB), adductor pollicis (AdP), opponens pollicis (OP), and flexors pollicis brevis superficialis (FPBS) and digiti quinti brevis manus (F5BM). The contributions of these muscles to flexion or extension of the joint sensors were estimated based on monkey anatomy. The expected positive or negative correlations between flex sensor and muscle were: o5 α {+F5BM}, f5 α {−EDC, −ED45, +FDS, +FDPu, +F5BM}, f3 α {−EDC, −ED23, +FDS, +FDPu, +FDPr }, f2 α {−EDC, −ED23, +FDS, +FDPr }, f1 α {−AbPL, +FDPr, −AbPB, +AdP, +OP, +FPBS}, a1 α {+AbPL, +AbPB, −AdP}, o1 α {−AbPL, +AdP, +OP}, eW α {+AbPL, +ECRB, +EDC, +ED23, +ED45, +ECU, −PL, −FCR, −FDS, −FDPu, −FDPr, −FCU}, and dW α { −ECRB, +ECU, −FCR, +FCU}. EMG electrode design, implantation, recording, and data quality verification procedures are described in Overduin et al. (2008).
Calibration
Sensor voltages were linearly transformed into angular values. The necessary equations were estimated using a videotaped calibration done at the end of the final session in which each of the joints was rotated in view of the camera. Each was held near its two extremes for ~0.5 s, and the sensor values at these two angles were used to normalize its range for that day. The slope and offset parameters were set for each of the earlier sessions to equate the width of each day’s distribution of sensor values from completed trials (at 5th and 95th percentiles). While this calibration allowed voltages at each joint’s terminal positions to be equated across days (i.e. day-to-day normalization), a more precise calibration was still required in order to ascribe joint angles to intermediate voltages. The relative linearity of the sensor-angle curves was determined by a final calibration performed under anesthesia. Each joint was rotated through its entire range of rotation, first in one direction and then in the other. Voltages were read and averaged at 10° increments as estimated with a goniometer.
Analysis
EMG and sensor signals from successful trials were averaged within 10-ms bins, and the latter were low-pass-filtered (50-Hz 5th-order Butterworth) to minimize high-frequency noise in the raw data (Fig. 1C). A sensor’s repeatability was evaluated for each session as in Dipietro et al. (2003), but using data recorded during active start-button press rather than a static posture. Also, these standardized calculations required 10 blocks of 10 trials each; here this requirement was satisfied by taking the first 100 successful trials completed with each object position, and dividing them into 10 consecutive 10-trial blocks (regardless of the object grasped following each button press). The repeatability range and S.D. were then averaged across positions and days. For other analyses, signals were time-aligned to the instant of object removal from the first well, and restricted to a fixed 1.1-s time window that encompassed 0.5 s of reaching and 0.6 s of transportation before and after object removal. Average correlation coefficients were computed by transforming a set of r values to Z scores, averaging these, and then back-transforming the mean into an r value. The significance of each such mean correlation coefficient was assessed with a two-tailed t-test done on the population of Z scores. Object position, shape, and size were decoded using instantaneous postural data, after these data were linearly recombined into a discriminant space maximizing between-to-within-condition variance (Johnson and Wichern, 1992). Also computed was the sensorimotor efficiency (SME; Santello and Soechting, 1998), a ratio of information the data transmitted about the 2×5×5 object conditions to the total amount of information distinguishing these conditions (log250 = 5.6 bits). For these information-theoretic analyses, an equal number of trials (the final 20 completed) were used for each condition. The baseline SME (≤0.50 ± 0.01 over 10 repetitions and 110 time-bins) was found by randomizing object conditions before recalculating SME. Data were analyzed in MATLAB (MathWorks, Natick, MA), and results were evaluated at a p < 0.01 significance threshold. Mean values are quoted ±S.D. and plotted ±S.E.
RESULTS
The monkey was introduced to the glove after it had already learned to use its left hand to grasp and transport a variety of objects between two wells. Subject performance was compared before (days −5:0) and after (days 1:6) it began executing the task while gloved. Skill was gauged both by accuracy (the fraction of trials in which the animal both grasped and deposited the object within 1-s limits) and movement times. Behavior was relatively stable over all 12 sessions, though with an initial 9% decrease in accuracy (Fig. 1D) and a 0.19-s increase in total movement time (Fig. 1E) following glove introduction. However, this handicap was brief and not significant even in comparisons of the final pre-glove day and the first per-glove day (all p > 0.01).
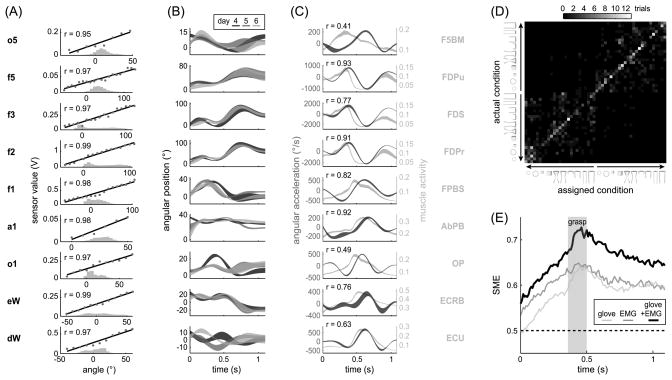
The sensors embedded in the Cymanus glove readily allowed joint angles to be estimated (Fig. 1C). They gave relatively linear voltage responses as a function of bend over the range of angles actually spanned during the task, as shown in Figure 2A (all linear fits r ≥ 0.95). The sensors were reliable over the six days of recording. Figure 2B depicts sensor traces averaged over trials in which the monkey handled a familiar object, for each of three consecutive days. While there were differences in these traces, attributable either to sensor or behavioral variation, for most sensors the profiles appeared to be conserved. This was confirmed by calculating linear correlations between the average sensor trajectory of one session and object condition with that of another session. Taken over all such pairings and then over joints, these coefficients averaged r = 0.8 ± 0.5 and were significant over the population of pairs (t[5426] = 74.58 p < 0.0001). Following previous investigators, within-day repeatability range and S.D. metrics were also computed; these were found to be 7.0° and 2.3°, averaged over sensors.
Figure 2.
Cymanus validations. A) Voltages recorded while joints were passively rotated followed relatively linear calibration curves. These relationships held over the range of behaviorally-related bend angles; histograms give the frequency of angles for all trials completed on a typical day. B) Joint trajectories were largely preserved over days, here illustrated by bend angles averaged over all leftward-transport trials completed with the cylinder of Figure 1BC on each of three days. C) Acceleration of a joint was maximally related to EMG activity recorded ~70 ms prior in those muscles acting on the joint, as indicated by sample linear correlations for muscles labeled at right. Data are averaged over all completed trials. D) A discriminant analysis assigned trials to object conditions based on grasp posture, sampled 50 ms before object removal from the first well. Each matrix element counts correct assignments; the diagonal clustering attests to sensors’ validity in distinguishing object conditions. E) Sensors were maximally informative about object properties during grasp, as measured by the SME index. These properties could be similarly discriminated based on EMG data, but best distinguished using a combination of these signals.
While flex sensors measured joint rotation, muscle signals provided an independent estimate of the force applied at each joint. Flex sensor measurements were further validated in a correlation of angular acceleration and EMG data, after the latter were shifted by a variable electromechanical delay. As in the examples with single muscles in Figure 2C, a delay of 70 ms maximized the significant average correlation between the subset of muscles acting on a given sensor and the resulting angular acceleration (r = 0.6 ± 0.6, t[42] = 5.36, p < 0.0001).
Joint angles had a complicated but often monotonic variation with object properties. Given this qualitative finding, kinematic data were used to decode the identity and position of a grasped object on a trial-by-trial basis. The confusion matrix resulting from this discriminant analysis, shown in Figure 2D, demonstrates that object conditions were frequently classified correctly or confused with similar conditions. The object information conveyed by the sensors is quantified by the SME index, which Figure 2E demonstrates rose from baseline at the beginning of the trial window to peak at 0.64 during grasp. A similar peak SME score (0.65) was observed when postures were discriminated using EMG data from 24 electrodes. However, these two kinds of information were not completely redundant, as the SME for kinematic and EMG data combined rose to 0.73 during grasp.
DISCUSSION
The Cymanus demonstrates that flex sensors can be adapted for kinematic measurement of nonhuman primate hands, as for humans (Santello and Soechting, 1998; Williams et al., 2000; Simone et al., 2007; Gentner and Classen, 2009). Validation tests showed that the sensor values were: 1) linearly correlated with joint angles (Fig. 2A); 2) consistent over days (Fig. 2B); 3) related to activation of muscular controllers after an appropriate electromechanical delay (Fig. 2C); 4) predictive of object properties (Fig. 2D); and 5) increasingly informative about target grasp posture as a reach evolved (Fig. 2E), consistent with earlier literature. As described below, the glove was also responsive to each of the unique constraints of nonhuman kinematic measurement raised in the Introduction.
In terms of subject adaptability, the glove was lightweight and flexible, and did not significantly impede the animal’s ability to grasp objects varying in location, shape, and size. The subject did not damage the glove—and regardless, replacement would have been straightforward given the simple design. At ~US$100, the glove was also 10–1000× less expensive than common commercial systems, and comparable to experimental human gloves (US$300–500; Simone et al., 2007; Gentner and Classen, 2009).
Regarding hand geometry, the sensors were small enough to isolate single joints of the monkey hand. Freedom in placing these sensors allowed us to measure movements such as digit 5 opposition (a combination of flexion, abduction, and rotation), and abduction and opposition involving the thumb’s trapeziometacarpal saddle joint. These degrees of freedom are rarely studied (Kessler et al., 1995; Dipietro et al., 2003) despite their specialized muscular controllers and involvement in grasping. For many joints, the sensor signals gave relatively linear responses over a range of motion larger than the human counterpart (e.g. ~150° for the finger metacarpophalangeal sensors). The moderate voltage-angle correlations (r ≥ 0.95, cf. ≥0.86 in Kessler et al., 1995, and ≥0.99 in Gentner and Classen, 2009) appear to reflect nonsystematic error, e.g. from imprecision in coregistering the goniometer with joint anatomy. The sensor responses, while monotonic, also demonstrated some nonlinearity in cases of hyperextension (e.g. dW). Sensor linearity could be improved with different signal conditioning (Gentner and Classen, 2009). But in neurophysiological studies such nonlinearities may not be as problematic as in other applications (e.g. clinical practice). Investigators with extensive access to their subjects can afford to take precise calibration measurements, rectifying these if necessary with nonlinear voltage-to-angle transformations (Williams et al., 2000; Simone et al., 2007).
The within-day repeatability of goniometer readings by expert clinicians is quoted as ±7° (Wise et al., 1990). Various tests with human flex sensors report ±5° error levels (Williams et al., 2000; Kessler et al., 1995). The repeatability range found here, 7.0° (S.D. 2.3°), was comparable to these values and also to standardized tests with human kinematic gloves worn during and between readings taken at a consistent grasp configuration (range 5.2°–7.5°, S.D. 1.6°–2.6°; Wise et al., 1990; Dipietro et al., 2003; Simone et al., 2007; Gentner and Classen, 2009). The present results were similar though based on kinematics measured during a movement (the start-button press) rather than a fixed hand posture, and though the object of the subsequent reach differed from trial to trial. Similarly, the estimate of across-day trial repeatability (inter-session r = 0.8) is a lower bound, based not on repeated static postures but on unconstrained trajectories.
Finally, the relevance of the Cymanus to motor control was demonstrated. The ~70-ms shift optimizing the correlation between muscles and the joints on which they act was consistent with previously-reported electromechanical delays (e.g. 80 ms in Inman et al., 1952). As in earlier studies with monkeys (Roy et al., 2002; Mason et al., 2004) and humans (Jeannerod, 1981; Wing et al., 1986), sensor data confirmed that grasp preshapes to an object during reach, in a process that may be mediated by a dedicated grasping synergy (Overduin et al., 2008). A lower peak SME score (0.64) was observed than reported in comparably-instrumented human subjects (0.86 in Santello and Soechting, 1998)—but with more object conditions to distinguish (50 vs. 15). Indeed, object properties could be discriminated with 9 sensors nearly as well as with 24 EMG electrodes. Such postural discrimination may be relevant to neuroprosthetics and other applications where it may be less important to find the precise mapping of cortical signals to joint movements than to use neural activity to decode motor goals.
Acknowledgments
The authors thank Allison Glinka, ShiLing Seow, Margo Cantor, and Steve Marchetti for technical assistance. The project was supported by NIH (NINDS) grant NS44393 to E.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dipietro L, Sabatini AM, Dario P. Evaluation of an instrumented glove for hand-movement acquisition. J Rehab Res Dev. 2003;40:179–90. [PubMed] [Google Scholar]
- 2.Fahn CS, Sun H. Development of data glove with reducing sensors based on magnetic induction. IEEE Trans Ind Electron. 2005;52:585–94. [Google Scholar]
- 3.Gentner R, Classen J. Development and evaluation of a low-cost sensor glove for assessment of human finger movements in neurophysiological settings. J Neurosci Methods. 2009;178:138–47. doi: 10.1016/j.jneumeth.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Inman VT, Ralston JH, Saunders CM, Feistein B, Wright EW. Relation of human electromyogram to muscular tension. Electroencephalogr Clin Neurophysiol. 1952;4:187–94. doi: 10.1016/0013-4694(52)90008-4. [DOI] [PubMed] [Google Scholar]
- 5.Jeannerod M. Intersegmental coordination during reaching at natural visual objects. In: Long J, Baddeley A, editors. Attention and Performance IX. Lawrence Erlbaum; Hillsdale, NJ: 1981. pp. 15–68. [Google Scholar]
- 6.Johnson RA, Wichern DW. Applied multivariate statistical analysis. Englewood Cliffs; Upper Saddle River, NJ: 1992. [Google Scholar]
- 7.Kessler GD, Hodges LH, Walker N. Evaluation of the CyberGlove as a whole-hand input device. ACM Trans Comput-Hum Interact. 1995;2:263–83. [Google Scholar]
- 8.Mason CR, Teverapperuma LS, Hendrix CM, Ebner TJ. Monkey hand postural synergies during reach-to-grasp in the absence of vision of the hand and object. J Neurophysiol. 2004;91:2826–37. doi: 10.1152/jn.00653.2003. [DOI] [PubMed] [Google Scholar]
- 9.Overduin SA, d’Avella A, Roh J, Bizzi E. Modulation of muscle synergy recruitment in primate grasping. J Neurosci. 2008;28:880–92. doi: 10.1523/JNEUROSCI.2869-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy AC, Paulignan Y, Meunier M, Boussaoud D. Prehension movements in the macaque monkey: Effects of object size and location. J Neurophys. 2002;88:1491–9. doi: 10.1152/jn.2002.88.3.1491. [DOI] [PubMed] [Google Scholar]
- 11.Santello M, Soechting JF. Gradual molding of the hand to object contours. J Neurophysiol. 1998;79:1307–20. doi: 10.1152/jn.1998.79.3.1307. [DOI] [PubMed] [Google Scholar]
- 12.Simone LK, Sundarrajan N, Luo X, Jia Y, Kamper DG. A low cost instrumented glove for extended monitoring and functional hand assessment. J Neurosci Meth. 2007;160:335–48. doi: 10.1016/j.jneumeth.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 13.Williams NW, Penrose JMT, Caddy CM, Barnes E, Hose DR, Harley P. A goniometric glove for clinical hand assessment. J Hand Surg [Br] 2000;25B:200–7. doi: 10.1054/jhsb.1999.0360. [DOI] [PubMed] [Google Scholar]
- 14.Wing AM, Turton A, Fraser C. Grasp size and accuracy of approach in reaching. J Mot Behav. 1986;18:245–60. doi: 10.1080/00222895.1986.10735380. [DOI] [PubMed] [Google Scholar]
- 15.Wise S, Gardner W, Sabelman E, Valainis E, Wong Y, Glass K, et al. Evaluation of a fiber optic glove for semi-automated goniometric measurements. J Rehabil Res Dev. 1990;27:411–24. doi: 10.1682/jrrd.1990.10.0411. [DOI] [PubMed] [Google Scholar]