Summary
Regulation of epithelial tube size is critical for organ function. However, the mechanisms of tube-size control remain poorly understood. In the Drosophila trachea, tube dimensions are regulated by a luminal extracellular matrix (ECM) [1–4]. ECM organization requires apical (luminal) secretion of the protein Vermiform (Verm), which depends on the basolateral septate junction (SJ) [5, 6]. Here, we show that apical and basolateral epithelial polarity proteins interact to control tracheal tube-size independently of the Verm pathway. Mutations in yurt (yrt) and scribble (scrib), which encode SJ-associated polarity proteins [7, 8], cause an expansion of tracheal tubes, but do not disrupt Verm secretion. Reducing activity of the apical polarity protein Crumbs (Crb) suppresses the length defects in yrt but not scrib mutants, suggesting that Yrt acts by negatively regulating Crb. Conversely, Crb overexpression increases tracheal tube dimensions. Reducing crb dosage also rescues tracheal size defects caused by mutations in coracle (cora), which encodes a SJ-associated polarity protein [8, 9]. In addition, crb mutations suppress cora length defects without restoring Verm secretion. Together, these data indicate that Yrt, Cora, Crb and Scrib operate independently of the Verm pathway. Our data support a model in which Cora and Yrt act through Crb to regulate epithelial tube size.
Keywords: Tracheal morphogenesis, Tubulogenesis, epithelial polarity, Crumbs, Yurt, Coracle Scribble, Septate junction, Vermiform
Results and Discussion
Yrt is essential for tracheal tube size control, but is not required for Verm and Serpentine (Serp) secretion
The transmembrane protein Crb acts as an apical determinant during establishment of epithelial apical-basal polarity [10, 11]. During later stages of epithelial differentiation, Crb promotes apical membrane growth independently of its role in apical-basal polarity [12]. Crb activity is counteracted by different groups of basolateral polarity proteins including the Yrt/Cora group, which is composed of Yrt, Cora, Na+/K+-ATPase and Neurexin IV (Nrx-IV) [8]. Loss of Yrt results in Crb-dependent apical membrane growth during late stages of epithelial cell maturation in Drosophila [13]. Thus, the equilibrium between the activities of these polarity proteins is important to define the size of the apical domain. Precise control of the apical surface of tracheal cells is crucial to define epithelial tube size in the Drosophila respiratory system [14], suggesting a potential role for polarity proteins in tube morphogenesis. However, the contribution of polarity regulators to the regulation of epithelial tube shape and size is poorly understood. Controlling length and diameter of the lumen is important for organ function as illustrated by the deleterious tubule enlargements that occur in polycystic kidney disease [15].
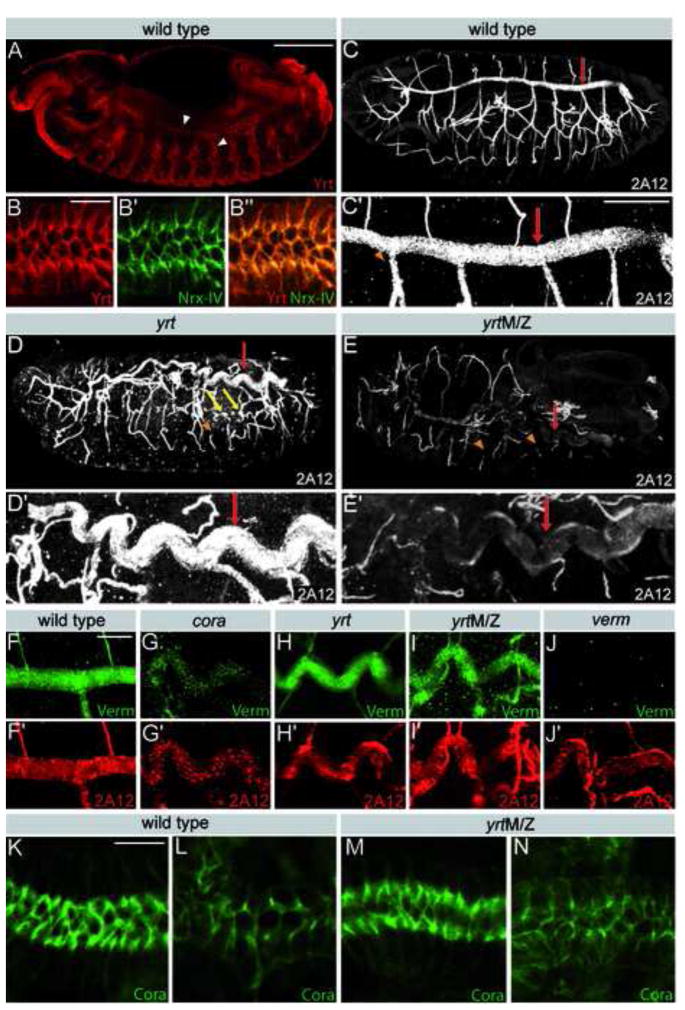
To better understand the role of polarity regulators and apical membrane growth in epithelial tube morphogenesis, we investigated the role of Yrt in the formation of the Drosophila respiratory system, a network of interconnected tubules that delivers oxygen throughout the body [16]. Yrt is mainly associated with the lateral membrane in tracheal cells and is enriched at SJs as shown by its co-localization with the SJ marker Nrx-IV (Fig. 1A,B) [17]. We characterized tracheal development in zygotic yrt mutants or yrt null embryos devoid of both maternal and zygotic yrt (yrtM/Z). Segmental tracheal placodes invaginated and established a normal branching pattern of tracheal tubes with intersegmental connections in both yrt and yrtM/Z mutants. yrtM/Z embryos display apical-basal polarity defects and irregularities in the tracheal epithelium at mid-embryogenesis. However, apical-basal polarity normalized during terminal differentiation [8, 13]. In contrast, zygotic yrt mutants have only minor, if any, polarity defects in tracheal cells (Fig. 4C) [13]. The most apparent defect in yrt mutant trachea was the excessively long and convoluted dorsal trunks compared to the straight dorsal trunks seen wild type (Fig. 1C–E). The average dorsal trunk length was 417 +/− 12 μm in wild type embryos, whereas it was 476 +/− 22 μm in yrt and 470 +/− 23 μm in yrtM/Z mutant embryos (Fig. S1A,B). Similar but milder tube-length defects were observed in other tracheal branches. In addition, the diameter of dorsal trunks in yrt and yrtM/Z mutants was uniform, but wider than in wild type embryos (Fig. 1C–E). The average dorsal trunk diameter was 9.1 +/− 0.6 μm in tracheal segment seven of wild type embryos compared to 12.2 +/− 0.9 μm in yrt and 12.7 +/− 0.6 μm in yrtM/Z mutant embryos (Fig. S1C,D). In smaller branches, some diameter expansions were also apparent (Fig. 1D). In addition, the smaller tracheal branches in yrtM/Z embryos showed frequent interruptions indicating either breaks or a failure in the luminal accumulation of the 2A12 antigen (Fig. 1E). These findings indicate that Yrt regulates the size of tracheal tubes and supports the integrity of segmental tracheal branches. Remarkably, despite the prominent differences in the apical-basal polarity defects between yrt and yrtM/Z mutant embryos [8, 13], both mutants exhibit very similar dorsal trunk elongation and diameter defects. This finding suggests that the transient loss of apical-basal polarity in yrtM/Z embryos is not the cause of dorsal trunk size defects, and that Yrt has therefore distinct functions during early and late stages of tracheal morphogenesis.
Figure 1. Yrt is required for epithelial tube size regulation, but not luminal secretion.
A) Stage 14 embryo stained for Yrt (red), which is expressed in the tracheal system (arrowheads). B) Yrt (red) is enriched in the upper region of the lateral membrane of tracheal cells and co-localizes with the SJ marker Nrx-IV (green). C–E) Stage 16 wild-type (C), yrt (D) or yrtM/Z (E) embryos stained with the tracheal luminal antigen 2A12 show the tracheal tree (upper panels) or a close up of a dorsal trunk of the same embryo (lower panels). Loss of Yrt causes an elongation of dorsal trunks (red arrows), results in diameter irregularities in small tracheal branches (yellow arrows) and is associated with discontinuous 2A12 staining (arrowheads). F–J) Portion of a dorsal trunk co-stained for Verm (green) and 2A12 (red) in stage 16 wild type embryo (F) or cora (G), yrt (H), yrtM/Z (I) and verm (J) mutant embryos. Note that loss of yrt does not interfere with Verm or 2A12 secretion. K,L) Staining of Cora in the salivary gland (K) and dorsal trunk (L) of a stage 16 wild-type embryo. M,N) Staining of Cora in the salivary gland (M) and dorsal trunk (N) of a stage 16 yrtM/Z mutant embryo. Cora distribution is normal in the absence of Yrt. Scale bars: A,C,D,E, 100 μm; C′-E′, 30 μm; B, F–N, 10 μm.
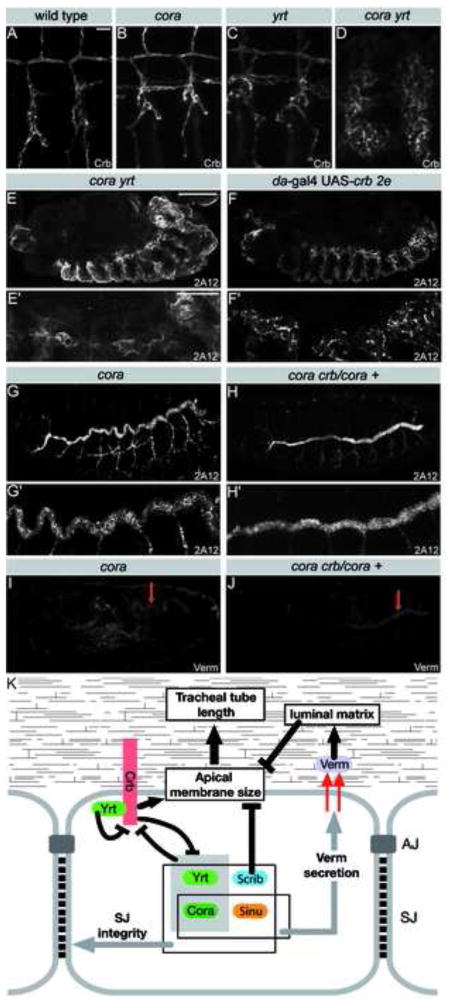
Figure 4. Cora and Yrt inhibit Crb to limit tracheal tube size.
A–D) Crb staining in tracheal cells of a stage 16 wild-type (A), cora (B), yrt (C) or cora yrt (D) embryo. E–H) Stage 16 embryos stained with the 2A12 luminal antigen showing the tracheal tree (upper panels) or a close up of a dorsal trunk (lower panels) of a cora yrt mutant embryo (E), an embryo overexpressing high levels of Crb (F), a cora mutant embryo (G) or a cora +/cora crb embryo (H). Simultaneous loss of Cora and Yrt or strong overexpression of Crb cause similar dramatic apicalization defects in the trachea. I,J) Verm staining in a cora mutant embryo (I) and a cora crb/cora + mutant embryo. K) Model of the pathways involved in the control of tracheal tube length by septate junction proteins. Scale bars: A–D, 10 μm; E–J, 100 μm; E′–J′, 30 μm.
The enlargement of the tracheal tube lumen observed in yrt mutants could be caused by an increase in cell number or an increase in the dimension of the apical surface of tracheal cells that surround the lumen. To address this question, we counted the number of dorsal trunk cells in yrt mutants and wild type embryos. No significant differences in cell numbers between wild type and yrt mutant embryos were found (Fig. S1E), indicating that the enlargement of tracheal tubes observed in yrt and yrtM/Z mutants must be accompanied by an increase in the dimension of the apical surface of tracheal cells.
Several other mutants display enlarged dorsal trunks similar to yrt. One group of genes required for limiting tube length encodes components of the SJ, including the Na+/K+ ATPase (α and β subunits), Cora, Nrx-IV, Scrib, Lachesin (Lac), Sinuous (Sinu), Megatrachea (Mega), and Varicose (Vari) [18–23]. Among these SJ proteins, Na+/K+ ATPase, Cora, Nrx-IV and Scrib also play a role as basolateral polarity proteins [7, 8]. In Drosophila and other invertebrates, SJs appear as a ladder-like group of septa basal to the cadherin-based adherens junctions. SJs have functions analogous to vertebrate tight junctions as they provide a transepithelial diffusion barrier [24]. Yrt is not required for normal septa formation [8] or localization of SJ components such as Cora (Fig. 1K–N), but is essential for the barrier function of SJs [8]. Zygotic yrt mutants show only minor defects in paracellular barrier function, whereas barrier function is fully compromised in yrtM/Z embryos [8]. This observation supports the notion that transepithelial barrier function and the regulation of tube dimension are independent functions of Yrt as yrt and yrtM/Z mutant embryos show similar tube size defects (Fig. 1D,E, S1A,C). This conclusion is consistent with previous findings suggesting that the regulation of tracheal tube elongation and of the paracellular diffusion barrier are distinct roles of SJ proteins [18, 25].
A second class of mutants showing abnormally long tracheal tubes are defective in the genes verm and serp, which encode enzymes that are predicted to modify the chitin-based luminal ECM, and mutants of which show structural defects in the luminal ECM [5, 6]. The chitin matrix filling the tracheal lumen is transiently present during lumen morphogenesis and is critical for determining lumen diameter and length [4]. Interestingly, all the mutations affecting SJ components tested so far are associated with a failure to secrete Verm into the tracheal lumen [5, 23]. This suggests that SJ proteins control tube size by regulating apical secretion and remodeling of the apical chitin matrix. Although Yrt is required for the barrier function of SJs [8], we found that luminal secretion of Verm (Fig. 1H,I) and Serp (Fig. S2A,B) was normal in yrt and yrtM/Z mutants. In contrast, low but detectable levels of Verm were observed in cora mutants (Fig. 1G). This finding suggests that Yrt regulates tracheal tube length through a pathway that is independent of Verm and Serp.
Antagonistic interactions between Yrt and Crb regulate tracheal tube length independent of Verm secretion
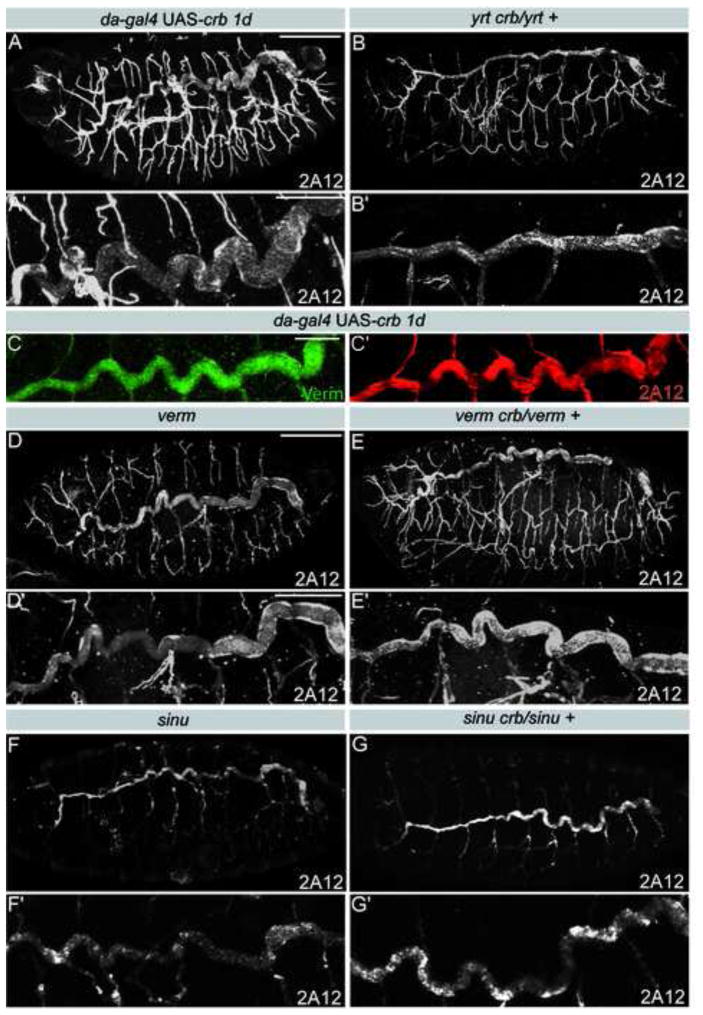
During late stages of epithelia maturation, Yrt is known to restrict apical membrane growth in epidermal and photoreceptor cells by limiting Crb activity. This interplay between Yrt and Crb governs apical membrane size in stage 14 and later embryos when tracheal tube size is defined [13]. This raises the possibility that Crb-dependent apical membrane growth is responsible for dorsal trunk expansion in yrt mutant embryos. To test this hypothesis, we reduced crb dosage in yrt mutant embryos by introducing one copy of a crb null allele into a yrt mutant background. Loss of one copy of crb suppressed the dorsal trunk elongation defects seen in yrt mutants, as the dorsal trunks appeared similar to wild type in yrt crb/yrt + mutants (Fig. 2B, S1A, S3F). In addition, moderate Crb overexpression increased dorsal trunk length and diameter without interfering with the integrity of the tracheal epithelium or the secretion of Verm or Serp, (Fig. 2A,C and Fig. S1A, S2C, S3C). These results show that Crb is required for promoting the expansion of tracheal tubes at late stages of embryogenesis. It was previously suggested that Crb also acts during early tracheal branch outgrowth [26], in addition to its role in apical-basal polarity. Therefore, Crb plays a critical role at several steps of tracheal development. Together, our findings indicate that the antagonistic interactions between Yrt and Crb determine tracheal tube size.
Figure 2. Epithelial tube size defects in yrt mutants results from Crb overactivation.
A,B, D–G) Stage 16 embryos stained for the 2A12 luminal antigen showing the tracheal tree (upper panels) or a close up of a dorsal trunk of the same embryo (lower panels). Moderate overexpression of Crb lengthens and widens dorsal trunks (A). Reducing crb dosage rescues tube size defects in yrt mutant embryos (B), but not in verm (D,E) or sinu (F,G) mutant embryos. C) Staining showing Verm (green) and 2A12 (red) in a dorsal trunk of an embryo overexpressing Crb. Overexpression of Crb does not interfere with Verm secretion. Scale bars: A,B,D–G, 100 μm; C, 30 μm; A′,B′,D′-G′, 30 μm.
Verm levels in yrt crb/yrt + and in yrt/yrt mutants were indistinguishable (not shown), indicating that the minor reduction in Verm levels sometime seen in yrt mutants were not the cause of the tracheal elongation defects. Accordingly, reduction of crb dosage in a verm or serp mutant background did not suppress tube size defects (Fig. 2D,E, S1A, S3G and not shown). Similarly, loss of one copy of crb in sinu mutant embryos, which fail to secrete Verm [5], had no impact on the length of dorsal trunks that remained enlarged as in sinu single mutants (Fig. 2F,G, S1A, S3G). These findings suggest that the apical secretion of matrix-modifying enzymes such as Verm and the control of Crb activity by Yrt are two independent and non-redundant modes of tracheal tube size regulation. Our data also establish that epithelial tube size control by SJ-associated proteins involves Verm-dependent and Verm-independent mechanisms.
The SJ-associated polarity proteins Yrt and Scrib define distinct Verm-independent mechanisms of tube size-control
To further characterize the function of SJ-associated polarity proteins in the regulation of tracheal tube size, we investigated tube elongation, the integrity of SJs and the secretion of Verm in scrib, lethal giant larvae (lgl) and discs large (dlg) mutant embryos [7, 21, 27–29]. Zygotic loss of scrib, lgl or dlg resulted in excessively long dorsal trunks, indicating that these genes are critical for tube size control (Fig. S4) [21]. Zygotic loss of lgl expression caused fully penetrant defects in SJ paracellular barrier function (trachea in 10/10 lgl4 embryos are dye permeable) (Fig. S4E), whereas zygotic scrib or dlg mutants did not have compromised transepithelial barriers (0/14 trachea in scrib2 embryos and 0/10 trachea in dlgm52 embryos were dye permeable; Fig. S4H,K). Luminal Verm deposition was not detected in lgl mutants but appeared near normal in dlg and normal in scrib mutants (Fig. S4D,G,J). Thus, Scrib and Dlg act like Yrt by controlling tracheal tube size through mechanisms distinct from Verm secretion.
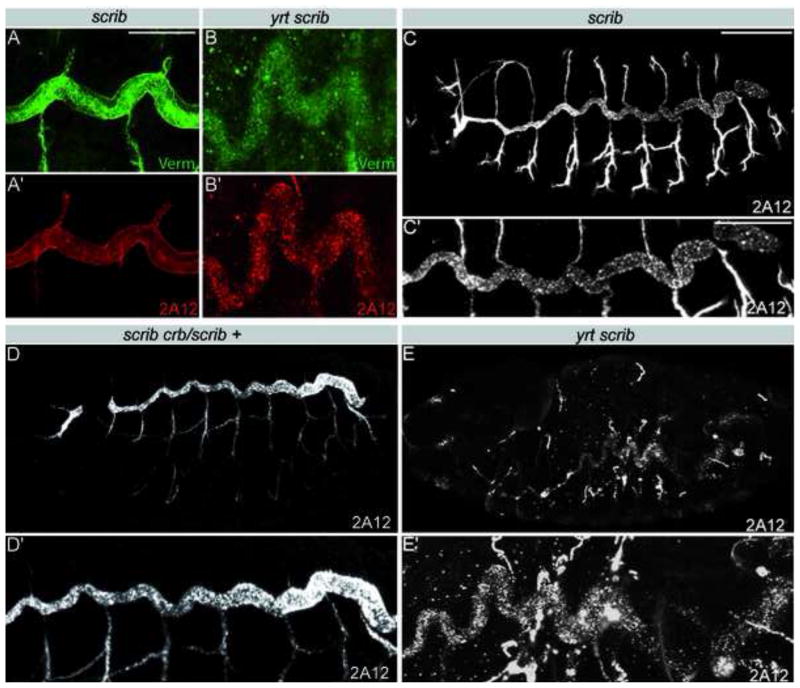
We concentrated our further analysis on Scrib and asked whether this protein, like Yrt, controls tracheal tube size by negatively regulating Crb activity. Scrib together with Lgl and Dlg show antagonistic interactions with Crb to regulate apical-basal epithelial polarity in early Drosophila embryos [29, 30]. However, the tracheal tube defects were not ameliorated in scrib crb/scrib + embryos compared to scrib single mutants (Fig. 3C,D). Thus, in contrast to Yrt, Scrib does not seem to limit tube length by restricting Crb activity. As Yrt and Scrib appear to control tracheal tube size through different mechanisms, we tested whether yrt scrib double homozygous mutant embryos had a more severe phenotype. The double mutants had Verm levels that were lower compared to yrtM/Z and scrib mutants (Fig. 1I, 3A,B). Moreover, the tracheal defects also appeared more severe in yrt scrib mutants than in yrt null mutant embryos, particularly in the smaller diameter branches. The defects in small diameter branches of the yrt scrib double mutants are not likely caused by the reduction in Verm secretion as the complete loss of Verm has only a mild effect on smaller branches (Fig. 2D) [5, 6]. The enhanced severity of the yrt scrib double mutant tracheal defects compared to the defects seen in yrt null embryos and the differences in the genetic interactions of scrib and yrt with crb suggest that Scrib and Yrt act in separate pathways to regulate the size of tracheal tubes, and that Scrib does not act by modulating Crb activity. It is possible that Scrib acts through other proteins, such as proteins of the Par complex [31], that promote apical domain formation. Therefore, SJ-associated polarity proteins use at least two Verm-independent mechanisms to restrict the dimension of tracheal tubes.
Figure 3. Yrt and Scrib control epithelial tube size through distinct Verm-independent pathways.
A,B) Portions of a dorsal trunk co-stained for Verm (green) and 2A12 (red) in scrib (A) and yrt scrib (B) mutant embryos. Loss of Scrib does not interfere with Verm accumulation in the tracheal lumen, whereas simultaneous loss of zygotic Yrt and Scrib reduces Verm secretion. C–E) 2A12 staining showing the tracheal system (upper panels) or a close up of a dorsal trunk (lower panels) in a scrib (C), scrib crb/scrib + (D) or yrt scrib (E) mutant embryos. Reducing the dosage of crb does not ameliorate the scrib mutant tracheal defects. yrt scrib double mutants show more severe tracheal defects than single mutants (B and E compare to A and C and Figure 1D and H). Scale bars: A,B 20 μm; C–E, 100 μm; C′–E′, 30 μm.
Cora limits Crb activity to restrict tracheal tube length
Cora is a SJ associated protein [9] required for optimal secretion of Verm (Fig. 1G, 4I). This suggests that Cora may control tracheal tube length through a Verm-luminal matrix pathway. However, Cora is also a basolateral polarity protein restricting the activity of Crb [8], which promotes Verm-independent expansion of the dorsal trunk (Fig. 2A,C). This led us to investigate the functional relationship between Cora and Crb in tracheal morphogenesis. In the epidermis, a striking redundancy between yrt and cora was observed in the regulation of apical-basal polarity [8]. Similarly, we found that tracheal cells in yrt cora double mutants show severe apicalization defects characterized by a broad expansion of the surface distribution of Crb (Fig. 4A–D). The antigen recognized by the monoclonal antibody 2A12 was found surrounding tracheal cells and not confined to the luminal cavity (Fig. 4E). In addition, the 2A12 antigen was not only associated with tracheal cells, but also with epidermal cells (Fig. 4E and S5A). These tracheal defects seen in cora yrt mutant embryos mimic defects that result from high levels of Crb overexpression (Fig. 4F, S5B). This observation argues that the tracheal defects observed in cora yrt double mutant embryos result from strong Crb over-activation, which is associated with a loss of basolateral polarity and an expansion of apical membrane character. As epidermal cells did not acquire expression of the tracheal cell marker Tango (Fig. S5C) [32], it is unlikely that epidermal cells adopt a tracheal cell fate in cora yrt mutants. The association of 2A12 with epidermal cells is therefore presumably due to the apicalization of tracheal cells (Fig. 4D), which would consequently secrete the 2A12 antigen not only on the luminal side but all around their cell surface allowing the 2A12 antigen to diffuse and bind to surrounding cells. Accordingly, cuticle deposition, taking place at the apical membrane, was seen at both luminal and abluminal sides of tracheal cells overexpressing Crb (Fig. S5D).
Our data indicate that Yrt and Cora cooperate to control apical-basal polarity of tracheal cells by limiting Crb to the apical cell pole, but they do not reveal whether Cora and Crb interact to control the length of tracheal tubes. To address this question, we examined cora +/cora crb embryos for a suppression of the tracheal size defects seen in cora single mutants. Reduction of crb dosage suppresses tube over-elongation defects resulting from the loss of Cora (Fig. 4G,H, S1A, S3G). This restriction of dorsal trunk elongation does not result from the restoration of Verm secretion as the level of Verm present in the dorsal trunk lumen was as low in cora +/cora crb embryos as in single cora mutants (Fig. 4I,J). Together, these data suggest that Crb overactivation is the primary cause of epithelial tube length defects observed in the absence of Cora. Thus, Cora and Yrt act independently from each other to counteract Crb activity and maintain the appropriate size of epithelial tubes. As the reduction of crb dosage does not rescue the verm mutant phenotype (Fig. 2D,E), we conclude that the residual amount of Verm found in cora mutants (Fig. 1G, 4I,J) is sufficient to maintain Verm pathway activity.
Conclusions
Our analysis suggests that basolateral proteins that are enriched at SJs have several critical functions in determining the size of epithelial tubes in the Drosophila tracheal system. We show that the increase in tube size is not caused by an increase in cell number, and, therefore, must be accompanied by an increase in the apical surface area of individual tracheal cells. Given that Crb is a well-known regulator of apical membrane size [9,10], our findings suggest that the interplay between Yrt, Cora and Crb modulates the dimensions of the apical surface of tracheal cells to control tracheal tube size. Moreover, this mechanism acts independently and in parallel to a previously proposed pathway depending on the apical secretion of the matrix modifying enzymes Verm and Serp, which requires several SJ-associated proteins [5, 23]. Yet another mechanism is revealed by our results that scrib mutants also have long trachea with normal Verm levels, but that in contrast to cora and yrt, tracheal defects in scrib mutants are not suppressed by loss of one copy of crb. Together, our findings suggest that basolateral proteins utilize at least three distinct mechanisms to regulate tube size in the Drosophila tracheal system (Fig. 4K). Unexpectedly, these mechanisms involve functional interactions between polarity proteins that appear to be different than those in establishing apical-basal polarity at earlier stages of development. For example, in promoting apical-basal polarity, Yrt and Cora act redundantly so that cora mutants show polarity defects only in a yrt mutant background and polarity defects in yrt mutants are strongly enhanced by removal of Cora [8]. In contrast, both cora and yrt single mutants show similar strong tracheal size defects. Furthermore, Scrib and Crb display antagonistic functional interactions during establishment of apical-basal polarity [29, 30], but not during tracheal elongation. An important challenge for future investigations will be to uncover the adaptations in the molecular pathways that allow polarity proteins to contribute to different aspects of epithelial development.
Experimental procedures
Drosophila genetics
The alleles used in this study were: yrt75a and yrt65a [13], cora1 [34], crb11A22 [10], scrib1 [7], scrib2 [35], vermKG07819 [6], serpe02821 [6], sinunwu7 [21], lgl4 [36] and dlgm52 [27]. Recombinant chromosomes generated: serp crb11A22, verm crb11A22. Overexpression of Crb was accomplished by crossing UAS-crbwt2e (strong overexpression) or UAS-crbwt1d (moderate overexpression) to the ubiquitous driver da-GAL4 [11] or the tracheal specific driver btl-GAL4 [37].
Immunofluorescence
Drosophila embryos were fixed as previously described [10]. Primary antibodies used: guinea pig anti-Yrt GP7 (1/500 dilution) [13], mouse anti-2A12 (1/5) and anti-Tango (1/50) (Developmental Studies Hybridoma Bank, DSHB), rabbit anti-Verm (1/500) [6], rabbit anti-Serp (1/500) [6], anti-Nrx-IV (1/500) [17] and rat anti-Crb (1/500) [12]. Secondary antibodies were conjugated to Cy3, Cy5 (Jackson Immunoresearch Laboratories) or Alexafluor 488 (Molecular Probes).
Measurements and statistical analysis
Dorsal trunks were analyzed in stage 16 embryos. Dorsal trunk length and diameter were determined using the Olympus Fluoview software. Dorsal trunk length was measured from the anterior in tracheal segment 1 to the junction of dorsal trunk and transverse connective in tracheal segment 10. Dorsal trunk diameter measurements were performed on maximal projections of Z-stacks. For each embryo, dorsal trunk diameter was determined by the average value of three independent measures taken in the central region of tracheal segment 7. Dorsal trunk length and diameter are expressed as average values +/− standard deviation. The Student two-sided unpaired t-test with equal variance was used to assess the statistical significance.
Electron microscopy
Electron microscopy was done following a modified version of the protocol described by Tepass and Hartenstein [38]. Embryos were dechorionated in a 50% bleach solution and pre-fixed in a 25% glutaraldehyde solution (diluted in 100 mM cacodylate buffer, pH 7.2) under a heptane phase. After removal of the vitelline membrane with a sharp needle, the embryos were incubated for 1 hour on ice in 2% glutaraldehyde solution (diluted in 100 mM cacodylate buffer pH 7.2). Following three washes in cacodylate buffer, embryos were fixed for 1 hour in a 1% OsO4, 0.8% w/v potassium ferricyanide solution (prepared in 100 mM cacodylate buffer pH 7.2) on ice (protected from light). The embryos were then washed three times in cacodylate buffer and twice with water and incubated overnight at 4°C in 2% uranylacetate. The embryos were finally dehydrated in an ethanol series (50%, 70%, 80%, 90% and 100%) and embedded in Spurr’s resin.
Septate junction permeability assay
Transepithelial permeability assays were carried out as described [18]. Stage 17 embryos were injected with 10 kDa Texan-Red dextran and dye diffusion across epithelia of the main trachea trunk was monitored by microscopy 10 minutes after injection.
Supplementary Material
Supplementary figure 1. Loss of Yrt increases dorsal trunk size. A,C) Table summarizing dorsal trunk (DT) length (A) and diameter (C) in the investigated genotypes. For each embryo (stage 16), dorsal trunk diameter was determined by the average value of three independent measures taken in the central region of tracheal segment 7. Std Dev. stands for standard deviation and n indicates the number of dorsal trunk measured. B,D) Histograms showing that the dorsal trunk length (B) and diameter (D) are significantly different in yrt and yrtM/Z mutants compared to wild type embryos. Of note, there is no proportional increase in embryo length. Therefore, the ratio dorsal trunk length/embryo length is significantly higher in yrt (p=0.00003) and yrtM/Z (p=0.00003) mutants compared to wild type embryos (F). E) Table summarizing the average number of cells per tracheal segment in wild type embryos or in yrt, yrtM/Z and cora mutant embryos in addition to embryos overexpressing Crb (moderate levels).
Supplementary figure 2. Loss of Yrt or moderate overexpression of Crb does not interfere with Serp secretion. A–C) Immunostaining showing Serp (green) and 2A12 (red) in a portion of a dorsal trunk of a wild-type embryo (A), a yrtM/Z embryo (B) and an embryo overexpressing Crb at moderate levels (C). Scale bar, 20 μm.
Supplementary figure 3. Crb overactivation increases dorsal trunk size. A,B) Armadillo (Arm) staining of a wild-type embryo (A) and of an embryo overexpressing Crb from the transgene UAS-crb 1d and the da-gal4 driver (B) showing that moderate Crb overexpression has low impact of embryonic morphology. This indicates that the convoluted aspect of dorsal trunks following Crb overexpression is not a consequence of a distortion of the embryo. C) Histogram showing that the increase in dorsal trunk length observed following Crb overexpression is statistically significant. D,E) Expression of Crb in tracheal cells using the btl-gal4 driver in a yrt sensitized background results in longer dorsal trunks (revealed by 2A12 staining (D) and statistical analysis (E)). F,G) Histograms showing that reducing crb dosage rescues tracheal elongation defects in yrt and cora mutants, but not in verm or sinu mutant embryos. Scale bar: A,B,D, 100 μm, D′, 30 μm.
Supplementary figure 4. Verm staining and septate junction permeability assay in mutants of the lgl group. (A,D,G,J) Staining of Verm in a dorsal trunk of a wild-type embryo (A) or lgl (D), dlg (G) and scrib (J) mutant embryos. In scrib and dlg mutant embryos, Verm is secreted into the lumen whereas no Verm is detected in the tracheal lumen of lgl mutants. (B,E,H,K) A 10kD fluorescent dye was injected in a wild-type embryo (B) or lgl (E), dlg (H) and scrib (K) mutant embryos and diffusion into the dorsal trunk was assessed. Note the presence of dye in the tracheal lumina of lgl but not wild-type, dlg or scrib mutant embryos. Dotted white lines outline the tracheal lumina in B, E, H and K. (C,F,I,L) GFP-fluorescence and DIC images corresponding to the images in (B,E,H,K) allow localization of the tracheal tubes in embryos in which dye does not penetrate to the tracheal lumen (trachea outlined in C,F,I and L with dotted lines; identical images without lines in C′,F′,I′, and L′). GFP fluorescence in C reveals the location of the tracheal tube in B, as well as the locations and sizes of the tracheal cell bodies that comprise the tube. Note that in the lgl mutant embryo, as is the case for strong septate junction mutants, the trachea is not visible by DIC at stage 17. For the lgl mutant, the location of the trachea in E,F and F′ is clearly revealed in E by the dye in the tracheal lumen. Scale bar, 10 μm.
Supplementary figure 5. 2A12 is associated with epidermal cells following strong Crb activation. A,B) cora yrt mutant embryo (A) and embryo strongly overexpressing Crb (B) stained with 2A12, which is associated with epidermal cells under these conditions. C) Embryo strongly overexpressing Crb stained with 2A12 and Tango. Tango is restricted to tracheal cells, suggesting that epidermal cell fate was unchanged. D) Electron microscope micrograph showing that apical membrane, revealed by the presence of cuticle (arrow), is inappropriately present on the abluminal (normally basal) side of tracheal cells following Crb overexpression. Scale bar, A,B, 10 μm; C, 100 μm, D, 200 nm.
Acknowledgments
We would like to thank Henry Hong for technical assistance. We are also grateful to E. Knust, M. Krasnow, H. Bellen, R. Fehon, the Developmental Studies Hybridoma Bank and the Bloomington Stock Center for reagents. Operating grants were provided by the Canadian Institutes of Health Research (CIHR) to P.L. and U.T. and the NIH (R01 GM069540 to G.J.B. and Lung Biology Training Grant 5 T32 HL076139-0 to S.M.P).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Araujo SJ, Aslam H, Tear G, Casanova J. mummy/cystic encodes an enzyme required for chitin and glycan synthesis, involved in trachea, embryonic cuticle and CNS development--analysis of its role in Drosophila tracheal morphogenesis. Dev Biol. 2005;288:179–193. doi: 10.1016/j.ydbio.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 2.Devine WP, Lubarsky B, Shaw K, Luschnig S, Messina L, Krasnow MA. Requirement for chitin biosynthesis in epithelial tube morphogenesis. Proc Natl Acad Sci U S A. 2005;102:17014–17019. doi: 10.1073/pnas.0506676102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moussian B, Tang E, Tonning A, Helms S, Schwarz H, Nusslein-Volhard C, Uv AE. Drosophila Knickkopf and Retroactive are needed for epithelial tube growth and cuticle differentiation through their specific requirement for chitin filament organization. Development. 2006;133:163–171. doi: 10.1242/dev.02177. [DOI] [PubMed] [Google Scholar]
- 4.Tonning A, Hemphala J, Tang E, Nannmark U, Samakovlis C, Uv A. A transient luminal chitinous matrix is required to model epithelial tube diameter in the Drosophila trachea. Dev Cell. 2005;9:423–430. doi: 10.1016/j.devcel.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Wang S, Jayaram SA, Hemphala J, Senti KA, Tsarouhas V, Jin H, Samakovlis C. Septate-junction-dependent luminal deposition of chitin deacetylases restricts tube elongation in the Drosophila trachea. Curr Biol. 2006;16:180–185. doi: 10.1016/j.cub.2005.11.074. [DOI] [PubMed] [Google Scholar]
- 6.Luschnig S, Batz T, Armbruster K, Krasnow MA. serpentine and vermiform encode matrix proteins with chitin binding and deacetylation domains that limit tracheal tube length in Drosophila. Curr Biol. 2006;16:186–194. doi: 10.1016/j.cub.2005.11.072. [DOI] [PubMed] [Google Scholar]
- 7.Bilder D, Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 2000;403:676–680. doi: 10.1038/35001108. [DOI] [PubMed] [Google Scholar]
- 8.Laprise P, Lau KM, Harris KP, Silva-Gagliardi NF, Paul SM, Beronja S, Beitel GJ, McGlade CJ, Tepass U. Yurt, Coracle, Neurexin IV and the Na(+), K(+)-ATPase form a novel group of epithelial polarity proteins. Nature. 2009;459:1141–1145. doi: 10.1038/nature08067. [DOI] [PubMed] [Google Scholar]
- 9.Lamb RS, Ward RE, Schweizer L, Fehon RG. Drosophila coracle, a member of the protein 4.1 superfamily, has essential structural functions in the septate junctions and developmental functions in embryonic and adult epithelial cells. Mol Biol Cell. 1998;9:3505–3519. doi: 10.1091/mbc.9.12.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tepass U, Theres C, Knust E. crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell. 1990;61:787–799. doi: 10.1016/0092-8674(90)90189-l. [DOI] [PubMed] [Google Scholar]
- 11.Wodarz A, Hinz U, Engelbert M, Knust E. Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell. 1995;82:67–76. doi: 10.1016/0092-8674(95)90053-5. [DOI] [PubMed] [Google Scholar]
- 12.Pellikka M, Tanentzapf G, Pinto M, Smith C, McGlade CJ, Ready DF, Tepass U. Crumbs, the Drosophila homologue of human CRB1/RP12, is essential for photoreceptor morphogenesis. Nature. 2002;416:143–149. doi: 10.1038/nature721. [DOI] [PubMed] [Google Scholar]
- 13.Laprise P, Beronja S, Silva-Gagliardi NF, Pellikka M, Jensen AM, McGlade CJ, Tepass U. The FERM protein Yurt is a negative regulatory component of the Crumbs complex that controls epithelial polarity and apical membrane size. Dev Cell. 2006;11:363–374. doi: 10.1016/j.devcel.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beitel GJ, Krasnow MA. Genetic control of epithelial tube size in the Drosophila tracheal system. Development. 2000;127:3271–3282. doi: 10.1242/dev.127.15.3271. [DOI] [PubMed] [Google Scholar]
- 15.Harris PC, Torres VE. Polycystic Kidney Disease. Annu Rev Med. 2008 doi: 10.1146/annurev.med.60.101707.125712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Affolter M, Caussinus E. Tracheal branching morphogenesis in Drosophila: new insights into cell behaviour and organ architecture. Development. 2008;135:2055–2064. doi: 10.1242/dev.014498. [DOI] [PubMed] [Google Scholar]
- 17.Baumgartner S, Littleton JT, Broadie K, Bhat MA, Harbecke R, Lengyel JA, Chiquet-Ehrismann R, Prokop A, Bellen HJ. A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell. 1996;87:1059–1068. doi: 10.1016/s0092-8674(00)81800-0. [DOI] [PubMed] [Google Scholar]
- 18.Paul SM, Ternet M, Salvaterra PM, Beitel GJ. The Na+/K+ ATPase is required for septate junction function and epithelial tube-size control in the Drosophila tracheal system. Development. 2003;130:4963–4974. doi: 10.1242/dev.00691. [DOI] [PubMed] [Google Scholar]
- 19.Genova JL, Fehon RG. Neuroglian, Gliotactin, and the Na+/K+ ATPase are essential for septate junction function in Drosophila. J Cell Biol. 2003;161:979–989. doi: 10.1083/jcb.200212054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Llimargas M, Strigini M, Katidou M, Karagogeos D, Casanova J. Lachesin is a component of a septate junction-based mechanism that controls tube size and epithelial integrity in the Drosophila tracheal system. Development. 2004;131:181–190. doi: 10.1242/dev.00917. [DOI] [PubMed] [Google Scholar]
- 21.Wu VM, Schulte J, Hirschi A, Tepass U, Beitel GJ. Sinuous is a Drosophila claudin required for septate junction organization and epithelial tube size control. J Cell Biol. 2004;164:313–323. doi: 10.1083/jcb.200309134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Behr M, Riedel D, Schuh R. The claudin-like megatrachea is essential in septate junctions for the epithelial barrier function in Drosophila. Dev Cell. 2003;5:611–620. doi: 10.1016/s1534-5807(03)00275-2. [DOI] [PubMed] [Google Scholar]
- 23.Wu VM, Yu MH, Paik R, Banerjee S, Liang Z, Paul SM, Bhat MA, Beitel GJ. Drosophila Varicose, a member of a new subgroup of basolateral MAGUKs, is required for septate junctions and tracheal morphogenesis. Development. 2007;134:999–1009. doi: 10.1242/dev.02785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banerjee S, Sousa AD, Bhat MA. Organization and function of septate junctions: an evolutionary perspective. Cell Biochem Biophys. 2006;46:65–77. doi: 10.1385/CBB:46:1:65. [DOI] [PubMed] [Google Scholar]
- 25.Wu VM, Beitel GJ. A junctional problem of apical proportions: epithelial tube-size control by septate junctions in the Drosophila tracheal system. Curr Opin Cell Biol. 2004;16:493–499. doi: 10.1016/j.ceb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Kerman BE, Cheshire AM, Myat MM, Andrew DJ. Ribbon modulates apical membrane during tube elongation through Crumbs and Moesin. Dev Biol. 2008;320:278–288. doi: 10.1016/j.ydbio.2008.05.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woods DF, Bryant PJ. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- 28.Strand D, Raska I, Mechler BM. The Drosophila lethal(2)giant larvae tumor suppressor protein is a component of the cytoskeleton. J Cell Biol. 1994;127:1345–1360. doi: 10.1083/jcb.127.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bilder D, Schober M, Perrimon N. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat Cell Biol. 2003;5:53–58. doi: 10.1038/ncb897. [DOI] [PubMed] [Google Scholar]
- 30.Tanentzapf G, Tepass U. Interactions between the crumbs, lethal giant larvae and bazooka pathways in epithelial polarization. Nat Cell Biol. 2003;5:46–52. doi: 10.1038/ncb896. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev Cell. 2007;13:609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonnenfeld M, Ward M, Nystrom G, Mosher J, Stahl S, Crews S. The Drosophila tango gene encodes a bHLH-PAS protein that is orthologous to mammalian Arnt and controls CNS midline and tracheal development. Development. 1997;124:4571–4582. doi: 10.1242/dev.124.22.4571. [DOI] [PubMed] [Google Scholar]
- 33.Hsu YC, Willoughby JJ, Christensen AK, Jensen AM. Mosaic Eyes is a novel component of the Crumbs complex and negatively regulates photoreceptor apical size. Development. 2006;133:4849–4859. doi: 10.1242/dev.02685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fehon RG, Dawson IA, Artavanis-Tsakonas S. A Drosophila homologue of membrane-skeleton protein 4.1 is associated with septate junctions and is encoded by the coracle gene. Development. 1994;120:545–557. doi: 10.1242/dev.120.3.545. [DOI] [PubMed] [Google Scholar]
- 35.Brumby AM, Richardson HE. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 2003;22:5769–5779. doi: 10.1093/emboj/cdg548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Timmons L, Hersperger E, Woodhouse E, Xu J, Liu LZ, Shearn A. The expression of the Drosophila awd gene during normal development and in neoplastic brain tumors caused by lgl mutations. Dev Biol. 1993;158:364–379. doi: 10.1006/dbio.1993.1195. [DOI] [PubMed] [Google Scholar]
- 37.Shiga Y, Tanaka-Matakatsu M, Hayashi S. A nuclear GFP/beta-galactosidase fusion protein as a marker for morphogenesis in the Drosophila tracheal system. Dev growth Diff. 1996;38:99–106. [Google Scholar]
- 38.Tepass U, Hartenstein V. The development of cellular junctions in the Drosophila embryo. Dev Biol. 1994;161:563–596. doi: 10.1006/dbio.1994.1054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1. Loss of Yrt increases dorsal trunk size. A,C) Table summarizing dorsal trunk (DT) length (A) and diameter (C) in the investigated genotypes. For each embryo (stage 16), dorsal trunk diameter was determined by the average value of three independent measures taken in the central region of tracheal segment 7. Std Dev. stands for standard deviation and n indicates the number of dorsal trunk measured. B,D) Histograms showing that the dorsal trunk length (B) and diameter (D) are significantly different in yrt and yrtM/Z mutants compared to wild type embryos. Of note, there is no proportional increase in embryo length. Therefore, the ratio dorsal trunk length/embryo length is significantly higher in yrt (p=0.00003) and yrtM/Z (p=0.00003) mutants compared to wild type embryos (F). E) Table summarizing the average number of cells per tracheal segment in wild type embryos or in yrt, yrtM/Z and cora mutant embryos in addition to embryos overexpressing Crb (moderate levels).
Supplementary figure 2. Loss of Yrt or moderate overexpression of Crb does not interfere with Serp secretion. A–C) Immunostaining showing Serp (green) and 2A12 (red) in a portion of a dorsal trunk of a wild-type embryo (A), a yrtM/Z embryo (B) and an embryo overexpressing Crb at moderate levels (C). Scale bar, 20 μm.
Supplementary figure 3. Crb overactivation increases dorsal trunk size. A,B) Armadillo (Arm) staining of a wild-type embryo (A) and of an embryo overexpressing Crb from the transgene UAS-crb 1d and the da-gal4 driver (B) showing that moderate Crb overexpression has low impact of embryonic morphology. This indicates that the convoluted aspect of dorsal trunks following Crb overexpression is not a consequence of a distortion of the embryo. C) Histogram showing that the increase in dorsal trunk length observed following Crb overexpression is statistically significant. D,E) Expression of Crb in tracheal cells using the btl-gal4 driver in a yrt sensitized background results in longer dorsal trunks (revealed by 2A12 staining (D) and statistical analysis (E)). F,G) Histograms showing that reducing crb dosage rescues tracheal elongation defects in yrt and cora mutants, but not in verm or sinu mutant embryos. Scale bar: A,B,D, 100 μm, D′, 30 μm.
Supplementary figure 4. Verm staining and septate junction permeability assay in mutants of the lgl group. (A,D,G,J) Staining of Verm in a dorsal trunk of a wild-type embryo (A) or lgl (D), dlg (G) and scrib (J) mutant embryos. In scrib and dlg mutant embryos, Verm is secreted into the lumen whereas no Verm is detected in the tracheal lumen of lgl mutants. (B,E,H,K) A 10kD fluorescent dye was injected in a wild-type embryo (B) or lgl (E), dlg (H) and scrib (K) mutant embryos and diffusion into the dorsal trunk was assessed. Note the presence of dye in the tracheal lumina of lgl but not wild-type, dlg or scrib mutant embryos. Dotted white lines outline the tracheal lumina in B, E, H and K. (C,F,I,L) GFP-fluorescence and DIC images corresponding to the images in (B,E,H,K) allow localization of the tracheal tubes in embryos in which dye does not penetrate to the tracheal lumen (trachea outlined in C,F,I and L with dotted lines; identical images without lines in C′,F′,I′, and L′). GFP fluorescence in C reveals the location of the tracheal tube in B, as well as the locations and sizes of the tracheal cell bodies that comprise the tube. Note that in the lgl mutant embryo, as is the case for strong septate junction mutants, the trachea is not visible by DIC at stage 17. For the lgl mutant, the location of the trachea in E,F and F′ is clearly revealed in E by the dye in the tracheal lumen. Scale bar, 10 μm.
Supplementary figure 5. 2A12 is associated with epidermal cells following strong Crb activation. A,B) cora yrt mutant embryo (A) and embryo strongly overexpressing Crb (B) stained with 2A12, which is associated with epidermal cells under these conditions. C) Embryo strongly overexpressing Crb stained with 2A12 and Tango. Tango is restricted to tracheal cells, suggesting that epidermal cell fate was unchanged. D) Electron microscope micrograph showing that apical membrane, revealed by the presence of cuticle (arrow), is inappropriately present on the abluminal (normally basal) side of tracheal cells following Crb overexpression. Scale bar, A,B, 10 μm; C, 100 μm, D, 200 nm.