Abstract
There is no known antiviral drug treatment that routinely terminates persistent virus infections. A recent provocative report indicated that low dosage of the sphingosine analog FTY720 caused lymphopenia in mice persistently infected with lymphocytic choriomeningitis virus (LCMV) -clone 13 (−Cl 13) and induced viral clearance within 30 days post-treatment (Premenko-Lanier et al., 2008). However, we find that low dosage of FTY720 fails to purge LCMV-Cl 13 infection and does not induce lymphopenia in LCMV-Cl 13-infected mice. In fact, infection with non-persistent LCMV-Arm53b or with persistent LCMV-Cl 13 induces an equivalent lymphopenia, demonstrating that the quantity of circulating cells has little bearing on viral persistence. In addition, treatment with FTY720 or the sphingosine-1-phosphate receptor 1 (S1P1) -specific agonist, AUY954, does not alleviate T cell exhaustion and exacerbates disruption of the CD8+ T cells response following LCMV-Cl 13 infection. Therefore, treatment with a sphingosine analog does not ameliorate persistent LCMV-Cl 13 infection.
Keywords: viral persistence, FTY720, lymphopenia, lymphocytic choriomeningitis virus, sphingosine, influenza, sphingosine-1-phosphate receptor 1, AUY954
Introduction
Human pathogens such as human immunodeficiency virus, hepatitis B and hepatitis C viruses induce inactivation of T cells, which leads to persistent viral infection associated with the exhaustion (nonfunctional activity) of virus-specific antiviral T cells (reviewed by (Oldstone, 2009)). Such T cell exhaustion was first reported using the experimental model of persistent lymphocytic choriomeningitis virus (LCMV)-clone 13 (Cl 13) infection in its natural murine host (Moskophidis et al., 1993; Wherry et al., 2003; Zajac et al., 1998). This model compares LCMV-Cl 13, a virus that induces persistence, with the parental strain, LCMV-Armstrong 53b (Arm53b), that does not. These variants differ by 5 nucleotides which results in a coding change of 2 amino acids (aa) (Salvato et al., 1991; Salvato et al., 1988). The LCMV-Cl 13 aa changes include a phenylalanine to leucine substitution at aa 260 of the glycoprotein and a lysine to glutamine change at aa 1079 of the RNA polymerase protein (Salvato et al., 1991). The receptor for LCMV and other old world arenaviruses is α-dystroglycan (Cao et al., 1998) which among cell of the immune system is preferentially located on dendritic cells (DC) (Kunz et al., 2001; Sevilla et al., 2000). The glycoprotein (GP) mutation of LCMV-Cl 13 dramatically increases the affinity of the GP for the LCMV receptor (Kunz et al., 2001), α-dystroglycan, two and a half logs over the binding affinity of the LCMV-Arm53b GP, resulting in an enhanced dendritic cell tropism (Kunz et al., 2001; Sevilla et al., 2000). Due to these observations, LCMV-Cl 13 has been used extensively to study the molecular basis of immune suppression and subsequent viral persistence (Ahmed et al., 1984; Barber et al., 2006; Brooks et al., 2008; Brooks et al., 2006). Recently the Altman lab (Premenko-Lanier et al., 2008) reported that alteration of T cell trafficking by low dosage of the sphingosine analog, FTY720, was associated with the termination of persistent infection induced by LCMV-Cl 13. According to this report (Premenko-Lanier et al., 2008), the strong lymhopenia induced by the non-persistent LCMV-Arm53b strain was mimicked by FTY720 treatment of LCMV-Cl 13-infected mice and was the cause viral clearance, although the mechanism of how the drug achieved this was not uncovered.
Administration of sphingosine analogs results in the redistribution of lymphocytes into secondary lymphoid organs (Mandala et al., 2002), which can be mediated via activation of the S1P1 receptor (Sanna et al., 2004). Due to the profound effect of these compounds on lymphocyte localization, the drug has been tested as a treatment to alter trafficking of killer T lymphocytes in patients with solid graft rejection (Nikolova et al., 2001) and multiple sclerosis (O'Connor et al., 2009). FTY720 as well as other chemical modulators of the sphingosine-1-phosphate (S1P) immunoregulatory axis can impair the immune response following acute viral infections (Marsolais et al., 2008; Marsolais et al., 2009). In this instance, local delivery of FTY720 analogs into the airway inhibits migration and expansion of influenza-specific T cells into the lung, the release of cytokines and chemokines in the lung, and antigen presentation by DC in the mediastinal lymph node and lung (Marsolais et al., 2008; Marsolais et al., 2009). It is not fully known how systemic delivery of drug affects immunomodulation during viral infection, although sequestration of normally expanding T cells in secondary lymphoid organs occurs (Pinschewer et al., 2000).
In this report, we utilize the LCMV model to probe the effects of sphingosine analogs on the lymphocyte response in both acute and persistent infection. In addition, we test the relevance of sphingosine analogs as therapeutic agents to treat a persistent infection. Our results reveal that early or late treatment with a low dose of FTY720 (0.004 mg/kg) has no significant effect on the outcome of persistent virus infection. We find that both LCMV-Cl 13 and -Arm53b induce equivalent levels of lymphopenia during the acute phase of infection. Induction of lymphopenia with FTY720 or the S1P1-specific agonist, AUY954, does not alleviate T cell exhaustion or LCMV-Cl 13 persistence and reduces the number of virus-specific T cells. Lastly, using influenza virus, we observe that lymphopenia induced by systemic administration of AUY954 inhibits the generation of influenza virus-specific T cells. Thus, it is clear that modulation of the S1P immunoregulatory axis does not terminate persistent LCMV infection and instead can adversely alter the anti-viral immune T cell response.
Materials and Methods
Mice and viruses
C57Bl/6, Balb/c and C57Bl/6 Thy1.1+ DbGP33–41 TCR-tg male mice (P14) (6–8 weeks of age) were used. All mice were maintained in pathogen-free conditions and handling conforms to the requirements of the National Institutes of Health and The Scripps Research Institute animal research committee. Isolation of CD8+ T cells and T cell adoptive transfers were performed as described (Marsolais et al., 2009). LCMV-Cl 13 and -Arm53b strains were grown, stored and quantified according to published methods (Borrow, Evans, and Oldstone, 1995). Generation and growth of mutant influenza A/WSN/33 H1N1 virus containing T cell-specific GP33 and GP65 LCMV sequences in its neuraminidase stalk (FLU-LCMV) was described elsewhere (Marsolais et al., 2009).
Mononuclear cell isolation
Cells were obtained by mechanically disrupting the spleens, lungs, inguinal and mediastinal lymph nodes through a 100-µm filter. Before lungs were removed, mice were perfused with 10 ml of phosphate buffered saline (PBS; Invitrogen, Carlsbad, CA) via cardiac puncture. Red blood cells were lysed by treating splenocytes and lungs with red blood cell lysis buffer (Ammonium Chloride: Tris HCl 0.02M, NH4Cl 0.14M), washed, and resuspended in cell culture medium. For anlysis of lymphopenia, 350 µl of blood was harvested via cardiac puncture and placed in 10 ml of red blood cell lysis buffer for 1 hr on ice. Peripheral blood mononuclear cells (PBMCs) were centrifuged and then subjected to a second round of red blood cell lysis (3 min at rt). Cells were collected by centrifugation and resuspended in a known volume of PBS. The number of viable cells per tissue or volume of blood were obtained by the use of a hemocytometer and the trypan blue exclusion method.
Formulation and Administration of Compounds
All experiments concerning FTY720 were performed in accordance with the previous study by Premenko-Lanier et al. (Premenko-Lanier et al., 2008). In short, FTY720 (Cayman Chemical, Ann Arbor, MI) was dissolved in DMSO and stored at −20°C. On the day of the experiment, stock solution was diluted in water to obtain working concentrations in a final volume of 200 ul. In parallel experiments, PBS containing 0.1% BSA was used to dilute DMSO stock of FTY720. Results obtained with both formulations were identical (data not shown). Mice were administered i.v. with indicated doses of FTY720 once a day, for 3 days, starting 1h after infection. For FTY720 treatment of persistently-infected mice, mice were treated on days 30, 31 and 32 post-infection. AUY954 (gifted by Dr Nathanael S. Gray, Dana Farber Hospital, Boston, Massachusetts) was dissolved in a 1:1 mixture of PEG300 and 5% dextrose and administered by gavage at a dose of 3 mg/kg.
Plaque Assays
Blood was drawn from the retro-orbital sinus under isoflurane anesthesia. Serum was isolated by centrifugation to remove red and white blood cells. 10 or 100 ul of serum was used to perform 10-fold serial dilutions for plaque assays on VERO cells (Ahmed et al., 1984). Brains and kidneys were harvested from mice euthanized on day 62 post-infection and frozen at −80 °C. At the time of the assay, tissues were thawed, homogenized, centrifuged and 100 µL of the supernatant was used to perform 10-fold serial dilutions for plaque assays (Ahmed et al., 1984).
Cell surface staining
Mononuclear cells were stained with antibodies raised against murine CD4 Pacific Blue (L3T4; eBioscience, San Diego, CA), CD8 APC (53-6.7; BD Biosciences, San Jose, CA), CD19 FITC (1D3; BD Biosciences, San Jose, CA), CD11b PE-Cy7 (M1/70; eBioscience, San Diego, CA), CD45.2 Alexa Fluor 750 (104; ebioscience, San Diego, CA) and CD90.1 FITC (HIS51; eBioscience, San Diego, CA) as described (Walsh, Lanier, and Lane, 2008). For tetramer staining, mononuclear cells were incubated for 1 hr on ice with MHC class I tetramers for DbGP33–41 APC, DbNP396–404 APC, and KbNP205–212 APC. MHC II tetramer staining with I-AbGP67–77 APC was performed for 1 hr at room temperature. Biotinylated monomers were obtained from the NIH Tetramer Core Facility and tetramerized using streptavidin-APC (Invitrogen, Carlsbad, CA).
Intracellular cytokine staining
Splenocytes were stimulated for 5 h with 2 µg/ml of the MHC class I restricted LCMV-GP33–41, LCMV-NP396–404, or the MHC class II restricted peptide LCMV-GP61–80 peptide (>99% pure; The Scripps Research Institute, Center for Protein Science, La Jolla, CA) in the presence of 50 U/ml recombinant murine IL-2 (R&D Systems, Minneapolis, MN) and 4 µg/ml brefeldin A (Sigma, St Louis, MO). Cells were fixed, permeabilized, and stained intracellulary with antibodies to TNF-α FITC (MP6-XT22; BD Biosciences, San Jose, CA), IFN-γ PE (XMG1.2; BD Biosciences, San Jose, CA) and IL-2 APC (JES6-5H4; BD Biosciences, San Jose, CA). Flow cytometric analysis was performed using Digital LSR II (Becton Dickinson) and data was analyzed using FlowJo (Tree Star, Inc., Ashland, OR). Absolute number of cells was determined by multiplying the frequency of specific cell populations by the total number of viable cells.
Statistics
Data are presented as means ± SEM. ANOVA or unpaired two-tailed Student’s t-test was used, when appropriate, to determine significance, which was set at 5%.
Results
LCMV infection induces lymphopenia
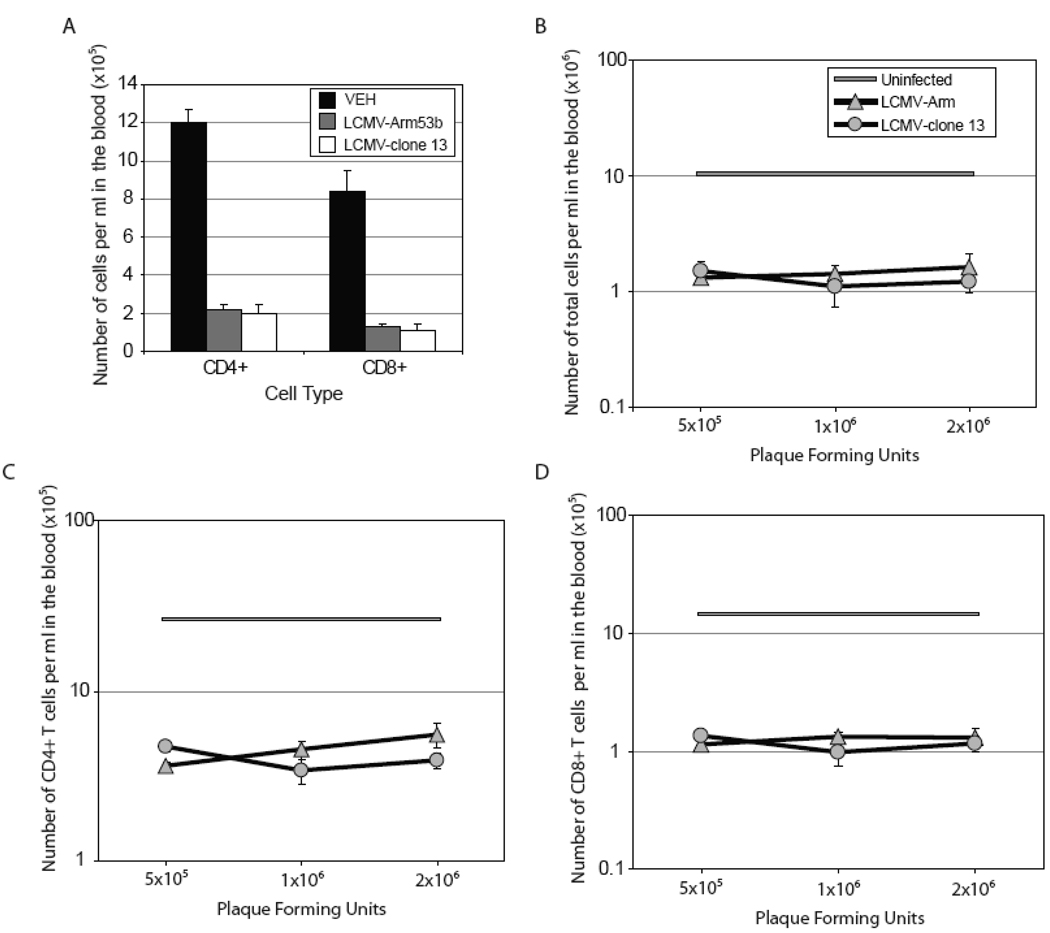
We compared the extent of acute lymphopenia induced by the LCMV variant -Cl 13 that causes a persistent infection with the parental LCMV strain, LCMV-Arm53b, that results in a vigorous antiviral cytotoxic T lymphocyte (CTL) response, clears virus and does not cause a persistent infection (Ahmed et al., 1984). Infection with LCMV-Arm53b or -Cl 13 induces similar levels of lymphopenia characterized by a reduced number of circulating CD4+ and CD8+ T cells in the blood, when compared to uninfected mice (Figure 1A). In order to determine if lymphopenia depends on the infectious dose, mice were infected with of 5×105, 1×106 and 2×106 PFU of either LCMV-Arm53b or -Cl 13. Infection with either virus at any of the three doses results in a profound reduction in the total number of cells within the blood (Figure 1B). Similarly, there is a decrease in numbers of CD4+ and CD8+ T cells in the blood with both strains of virus, at all infectious doses employed (Figure 1C and D). This data is contradictory to the previous study in which the extent of lymphopenia was enhanced in LCMV-Arm53b-infected mice when compared to mice infected with LCMV-Cl 13 (Premenko-Lanier et al., 2008). Thus, the degree of lymphopenia following LCMV-Cl 13 and -Arm53b infections is equivalent and does not differ due to the infectious dose administered.
Figure 1. Lymphopenia occurs at equivalent levels following either LCMV-Arm53b or LCMV-Cl 13 infection.
(A) Infection with 2×106 PFU i.v. of either LCMV-Arm53b or LCMV -Cl 13 causes a decrease in circulating CD4+ and CD8+ T cells on day 3 post-infection, when compared to uninfected mice. (B–D) Mice infected with varying doses of LCMV-Arm53b (shaded triangles) or -Cl 13 (shaded circles) exhibit an equivalent degree of lymphopenia regardless of virus strain or inoculation dose. Total number of cells (B), CD4+ (C) and CD8+ (D) T cells in the blood from infected mice are reduced on day 3 post-infection, compared to uninfected mice. (B–D) Uninfected mice are represented by the shaded boxed area, which represents the average number of cells ± SEM. 4 mice were used per group and data is represented as average ± SEM.
Treatment with FTY720 induces lymphopenia independently of virus infection
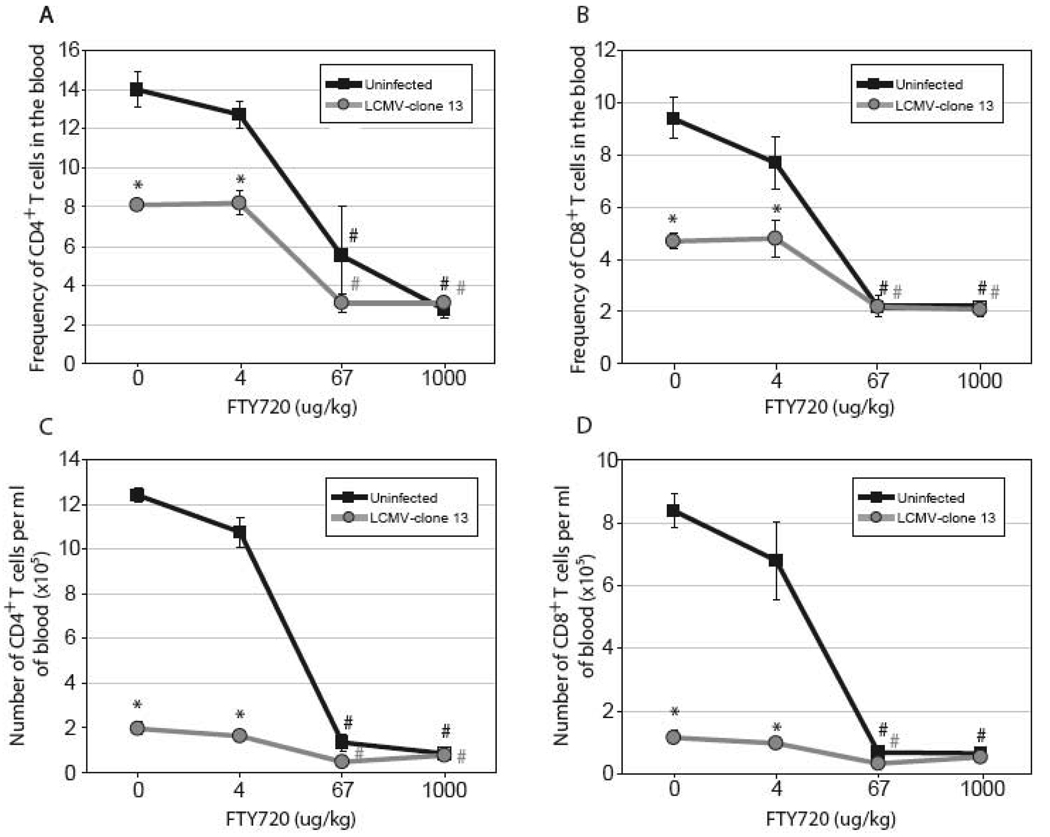
Sphingosine analogs are effective at inducing lymphopenia. We assessed if treatment with FTY720, a sphingosine analog, would further enhance lymphopenia following LCMV-Cl 13 infection. Infection with LCMV-Cl 13 results in a decrease in the frequencies (Figures 2A and B) and numbers (Figures 2C and D) of CD4+ and CD8+ T cells in the blood compared to uninfected mice on day 3 post-infection. An earlier report by Premenko-Lanier et al. (Premenko-Lanier et al., 2008) indicated that intravenous (i.v.) treatment with a low dose of FTY720 (4 µg/kg) was sufficient to enhance lymphopenia in LCMV-Cl 13-infected mice. However, we find that administration of FTY720 (4 µg/kg) does not significantly alter the frequencies or numbers of circulating T cells when compared to vehicle treatment (Figure 2A–D). As expected, i.v. treatment with a medium (67 µg/kg) or a high dose (1 mg/kg) of FTY720 enhances lymphopenia in both LCMV-Cl 13-infected and uninfected mice. The extent of lymphopenia observed between the 67 µg/kg and 1 mg/kg doses of FTY720 is equivalent between LCMV-Cl 13-infected and uninfected mice, demonstrating that maximum lymphopenia can be achieved with 67 µg/kg independently of virus infection. In summary, our results show that treatment with a low dose of FTY720 (4 µg/kg) does not enhance lymphopenia in uninfected or LCMV-Cl 13-infected mice and the extent of lymphopenia induced by medium (67 µg/kg) and high doses (1 mg/kg) of FTY720 is equivalent between infected and uninfected mice. Therefore, FTY720 does not synergize with virus-induced lymphopenia and acts independently of viral infection.
Figure 2. FTY720 induces lymphopenia in LCMV-Cl 13 infected and uninfected mice.
(A–D) Mice were mock-infected (black lines, black squares) or infected with 2×106 PFU i.v. of LCMV-Cl 13 (grey lines, grey circles) and treated with increasing doses of FTY720 at 1 hr, 1 and 2 days post infection. Mice were euthanized on day 3 post infection and blood was removed via cardiac puncture. Frequencies (A–B) and concentrations (C–D) of CD4+ (A and C) and CD8+ T cells in blood (B and D) are decreased by treatment with 67 and 1000 µg/kg of FTY720. 4 mice were used per group and data is represented as average ± SEM. * Significantly different from uninfected, p ≤ 0.05. # Significantly different from vehicle (dose 0), p ≤ 0.05.
FTY720 hinders the anti-viral T cell immune response and does not alter LCMV persistence
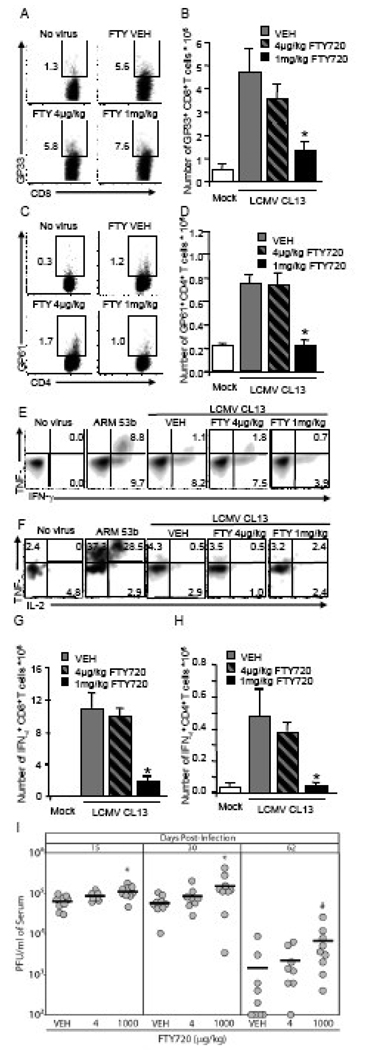
Premenko-Lanier and colleagues demonstrated that a low dose of FTY720 (4 µg/kg.) induced lymphopenia in LCMV-Cl 13-infected mice, which induced viral clearance by preventing T cell exhaustion (Premenko-Lanier et al., 2008). Since our study does not support induction of lymphopenia with 4 µg/kg of FTY720, we also addressed the effect of FTY720 treatment on T cell exhaustion. Intravenous injection of 4 µg/kg of FTY720 to LCMV-Cl 13-infected mice does not alter the frequency or number of virus-specific CD8+ (Figure 3A and B) or CD4+ (Figure 3C and D) T cells in the spleen on day 8 post-infection, when compared to mice treated with vehicle. However, treatment with 1 mg/kg of FTY720 results in a dramatic decrease in the number of splenocytes (data not shown) which results in a significant decrease in the numbers of LCMV-specific CD8+ (Figure 3B) and CD4+ (Figure 3D) T cells. In a control stain, streptavidin-APC by itself did not bind splenocytes from infected mice demonstrating that free streptavidin-APC does not artificially enhance the tetramer positive signal. Analysis of cytokine production by peptide stimulation reveals that CD8+ (Figure 3E) and CD4+ (Figure 3F) T cells from mice infected with LCMV-Cl 13, regardless of FTY720 treatment, had diminished cytokine profiles compared to mice infected with LCMV-Arm53b. Treatment with 4 µg/kg of FTY720 does not affect the number of IFN-γ producing CD8+ (Figure 3G) of CD4+ (Figure 3H) T cells when compared to vehicle, however treatment with 1 mg/kg significantly reduces IFN-γ producing LCMV-specific T cells which correlates with the reduction in the total number of LCMV-specific tetramer+ T cells (Figures 3B and D). These data demonstrate that treatment with 4 µg/kg FTY720 does not alleviate T cell exhaustion and administration of a dose of FTY720 effective at inducing lymphopenia (1 mg/kg) can adversely affect the antiviral immune response, as defects in the T cell response were detectable even 5 days after the last dose of FTY720. In correlation, treatment with 1 mg/kg of FTY720 results in a slight, but significant increase in viral burden within the serum compared to vehicle-treated mice, while treatment with 4 µg/kg does not alter viremia on days 15 and 30 post-infection (Figure 3I). All mice treated with FTY720 had detectable virus in the serum on day 62 post infection while virus was undetectable in 4 out of 9 mice (44%) treated with vehicle demonstrating that FTY720 treatment delays LCMV Cl 13 clearance from the serum. Similarly, administration of a medium dose of FTY720 (40µg/kg), does not affect viral burden within the serum of LCMV-Cl 13-infected mice (data not shown), demonstrating that a large dose of FTY720 (1 mg/kg) is required to increase viral burden in the serum. Experiments performed in Balb/c mice yielded similar results (data not shown). Thus, low dose FTY720 (4 µg/kg) does not alter viral persistence or T cell exhaustion while high dose of FTY720 (1 mg/kg) negatively affects the antiviral immune response.
Figure 3. FTY720 does not prevent T cell exhaustion or promote viral clearance, but impairs the T cell immunity in response to LCMV-CL 13 infection.
(A–H) Mice were mock infected (white bars), infected with 1×105 PFU LCMV-Arm53b i.p. (E–F only) or with 2×106 PFU LCMV-Cl 13 i.v. and treated i.v., once a day for 3 days, starting 1 h post-infection with water (VEH; grey bars), 4 µg/kg (dashed bars) or 1 mg/kg (black bars) FTY720, and spleens were harvested on day 8 post-infection. (A–D) Frequencies of CD8+ GP33–41 (A) and CD4+ GP65–77 (C) tetramer positive cells are not altered by FTY720 when compared to VEH. (B and D) Administration of 1mg/kg FTY720 significantly reduces the numbers of these two cell subsets, when compared to vehicle and treatment with 4 µg/kg. (E) GP33–41 peptide stimulation of CD8+ T cells from LCMV-Cl 13-infected mice results in reduced expression of TNF-α and IFN-γ compared to cells from LCMV-Arm53b infected mice. (F) Similarly, IL-2 and TNF-α are strongly produced in response to GP61–80 peptide stimulation from IFN-γ+ CD4+ T cells of mice infected with LCMV-ARM53b, but not with LCMV-CL 13. Treatment with FTY720, at any dose, fails to modify the frequencies of cytokine-producing CD8+ (E) or CD4+ (F) T cells. However, treating with FTY720 at 1mg/kg, but not 4µg/kg, decreases the number of IFN-γ-producing CD8+ (G) or CD4+ (H) T cells, in response to GP33–41 or GP61–80 peptide simulation, respectively, when compared to VEH. (I) Mice were infected with 2×106 PFU LCMV-Cl 13 i.v. and treated i.v., once a day for 3 days, starting 1 h post-infection. Administration of FTY720 at 1mg/kg, but not 4µg/kg, significantly increases LCMV-Cl 13 burden in serum when compared to VEH (n=8–9 mice per group) on days 15 and 30 post-infection. Also 1 mg/kg of FTY720 enhances viral burden and delays LCMV Cl 13 clearance from the serum on day 62 post infection when compared to vehicle-treated mice, but the difference is not statistically significant. (A, C, E, and F) Representative density plots are shown; n ≥ 4 mice per group; average ± SEM; *Significantly different from VEH, p < 0.05. #, p < 0.09.
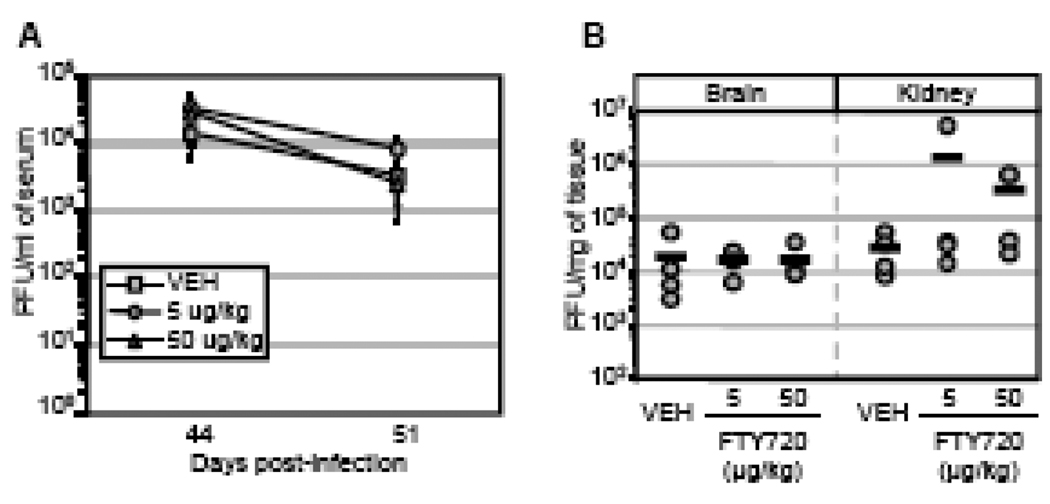
We next assessed the effects of FTY720 treatment during the persistent phase of LCMV-Cl 13 infection. Mice were treated with vehicle, 5 µg/kg or 50 µg/kg of FTY720 i.v. on days 30, 31, and 32 post-infection and monitored for serum viral burden. Viral load was similar between vehicle- and FTY720-treated groups on days 44 and 51 post-infection, demonstrating that FTY720 administration does not purge LCMV-Cl 13 from the blood (Figure 4A). The spatiotemporal course of LCMV-Cl 13 burden has previously been characterized (Ahmed et al., 1984; Brooks et al., 2006; Moskophidis et al., 1995; Wherry et al., 2003). In brief, LCMV-Cl 13 is detectable within the serum and most organs until 70 days post infection, but persists within the brain until 100–120 days post infection (Oldstone et al., 1986) and within the kidneys for up to two years due to the formation of LCMV-specific antibody immune complexes (reviewed by (Oldstone, 2006)). According to Premenko-Lanier and colleagues (Premenko-Lanier et al., 2008), virus was purged from brain and kidney 30 days post-FTY720 administration, i.e. 60 days post infection. In contrast to these results, we detect virus in both brain and kidney after treatment with 5 and 50 µg/kg of FTY720 on day 62 post-infection, i.e. day 32 post-treatment (Figure 4B). Viral titers were equivalent in tissues regardless of the treatment regimens. These results were also observed using a different vehicle for FTY720 (0.1% fatty acid-free bovine serum albumin in PBS) to increase drug solubility, and in another series of experiments using Balb/c mice (data not shown). These data indicate that FTY720 treatment does not alleviate LCMV-Cl 13 persistence nor does it significantly alter viral burden within sera and tissues. Our observations of virus persisting in the brain and kidneys following FTY720 treatment are not surprising since reconstitution with immune memory-specific antiviral LCMV T cells by adoptive transfer therapy, while purging virus from sera by 15 days, takes over 100 days to clear virus from the brain (Oldstone et al., 1986), and longer to clear virus from the kidney (Oldstone et al., 1986).
Figure 4. FTY720 does not reduce viremia or virus titer in tissues of mice persistently infected with LCMV-Cl 13.
(A and B) Mice were infected i.v. with 2×106 PFU LCMV-Cl 13. Mice were treated with vehicle (VEH, shaded squares), 5 (shaded circles) or 50 µg/kg (shaded triangles) of FTY720 on days 30, 31 and 32 post-infection. (A) Plaque assays of serum samples from all cohorts display no significant difference in viral titers. n=4 mice per group; data is represented as average ± SEM. (B) Tissues harvested on day 62 post infection were used for determination of viral titer by plaque assay. Differences between experimental groups are not statistically significant. Circles represent individual mice while bars indicate the group average. Experiments were replicated 3 times by two independent groups, which obtained similar results.
S1P1-induced lymphopenia inhibits the generation of virus-specific T cells
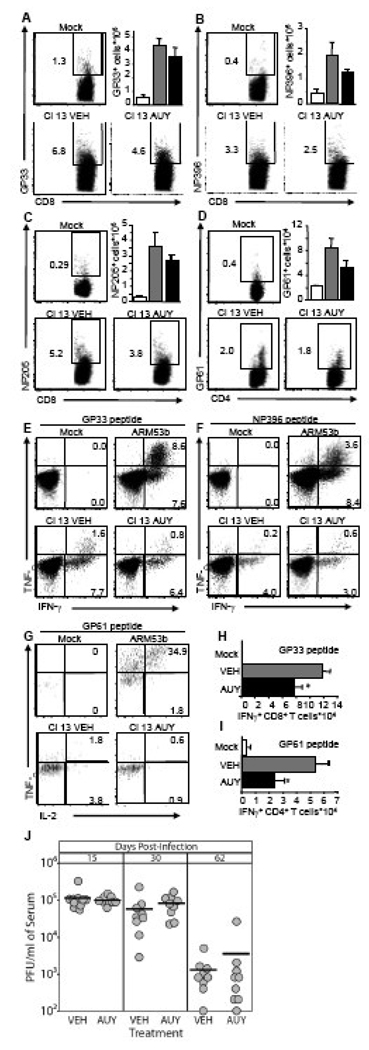
Phosphorylated FTY720 binds to four of the five S1P receptors (S1P1 and S1P3-5). These receptors have redundant and non-redundant functions and are differentially expressed on individual immune cell populations. We utilized AUY954, an S1P1-specific agonist previously shown to induce lymphopenia (Pan et al., 2006), to assess how S1P1 signaling would affect LCMV-Cl 13 infection and the development and functionality of virus-specific T cells. Mice were infected with 2×106 PFU of LCMV-Cl 13 and treated with vehicle or 3 mg/kg of AUY954 by gavage 1 hr, 1 and 2 days post-infection. Virus-specific T cells from the spleen were analyzed on day 8 post-infection using tetramer staining. AUY954 treatment does not significantly reduce the frequencies and numbers of virus-specific CD8+ (Figures 5A–C) and CD4+ (Figure 5D) T cells. Infection with LCMV-Cl 13 results in reduced frequencies of IFN-γ+/TNF-α+ CD8+ T cells, when compared to mice infected with LCMV-Arm53b (Figures 5E and F). Similarly, LCMV-specific CD4+ T cells from LCMV-Cl 13-infected mice treated with AUY954 or vehicle express less TNF-α and IL-2 compared to LCMV-Arm53b-infected mice (Figure 5G). Although frequencies of IFN-γ-producing cells are not drastically impaired (Figures 5E–F; data not shown), the absolute number of virus-specific CD8+ (Figure 5H) and CD4+ (Figure 5I) T cells expressing IFN-γ is significantly decreased by AUY954, when compared to mice treated with vehicle. Lastly, LCMV-Cl 13 titer within the serum on day 15, 30 and 62 post-infection reveals that AUY954 treatment does not affect viral replication when compared to mice treated with vehicle (Figure 5J). Thus, AUY954 treatment does not alleviate T cell exhaustion, but hampers the antiviral T cell response without significantly affecting LCMV-Cl 13 viral burden.
Figure 5. An S1P1-specific agonist does not alter T cell exhaustion, LCMV-CL 13 viral burden, but hampers the antiviral T cell response.
(A–J) Mice were infected i.v. with mock, 2×106 PFU LCMV-Cl 13 or 1×105 PFU LCMV-Arm53b i.p. (E–G) and treated by gavage, daily for 3 days, starting 1h post-infection, with vehicle (VEH) or 3 mg/kg AUY954. Spleens were harvested 8 days post-infection. Frequencies (representative dot plots) and numbers (bar graphs) of CD8+ GP33–41 (A), NP396–404 (B), NP205–212 (C), tetramer positive and CD4+ GP61–80 (D) tetramer positive cells are increased in LCMV-Cl 13 infected mice (grey and black bars), compared to mock infected mice (white bars). AUY954 treatment (black bars) does not significantly decrease frequencies or numbers of these T cell subsets, when compared to VEH (grey bars). In vitro stimulation with GP33–41 (E) or NP396–404 peptide (F) results in strong induction of TNF-α and IFN-γ production in CD8+ T cells isolated from mice infected with LCMV-Arm53b, when compared to CD8+ T cells from mock-infected mice and LCMV Cl 13-infected mice treated with VEH or AUY954. Similarly, in vitro simulation with GP61–80 (G) results in an increased in TNF-α and IL-2 expression in IFN-γ+ CD4+ T cells isolated from LCMV ARM53b-infected mice when compared to mock-infected and LCMV Cl 13-infected mice treated either with VEH or AUY954. Numbers of IFN-γ producing CD8+ and CD4+ T cells in response to in vitro GP33–41 (H) or GP61–80 (I) peptide stimulation, respectively, are significantly decreased by AUY954 treatment, when compared to VEH. (J) Mice treated with VEH or AUY954 display similar serum virus titer on days 15, 30, and 62 post-infection. Representative density plots are shown; n > 4 mice per group; average ± SEM; *Significantly different from VEH, p < 0.05.
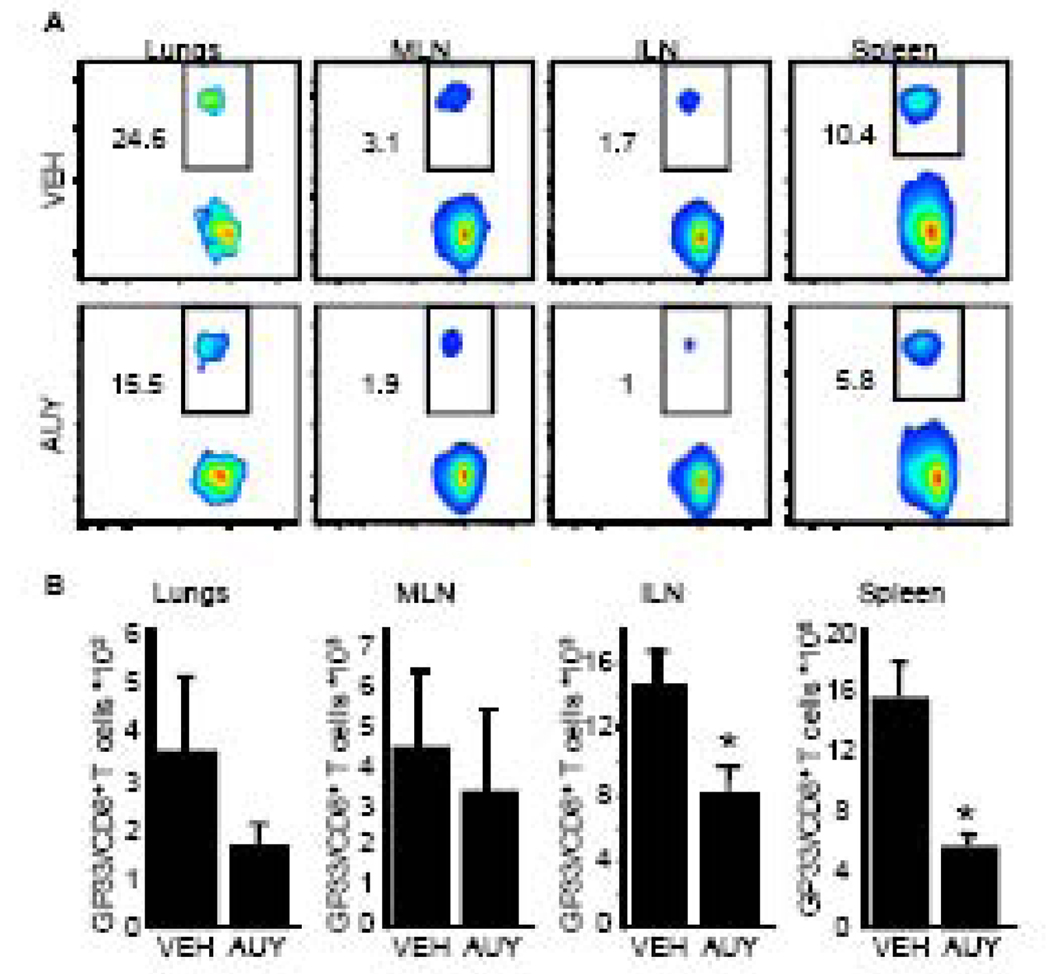
Because LCMV-Cl 13 induces T cell exhaustion, it is difficult to interpret how sphingosine analogs negatively affect the antiviral immune response. To establish how AUY954 treatment alters the virus-specific CD8+ T cell response, mice that received 1×104 Thy1.1+ DbGP33–41 TCR-tg cells one day prior were infected with 1×106 PFU i.v. with an engineered A/WSN/33 (H1N1) influenza virus bearing LCMV immunodominant epitopes H-2b CD8+ and I-Ab CD4+ into the neuraminidase stalk (FLU-LCMV) (Marsolais et al., 2009). AUY954 was administered by gavage on days 2 and 3 post infection, i.e. at the time of initial T cell-DC interactions in lymphoid organs. On day 6 post-infection, there is a reduction in the frequencies of virus-specific CD8+ T cells in the inguinal and mediastinal lymph nodes, lung and spleen (Figure 6A). This correlates with a significant reduction in the number of virus-specific CD8+ T cells within the inguinal lymph node and spleen (Figure 6B). Therefore, treatment with an S1P1-specific agonist, AUY954, negatively affects the generation of virus-specific CD8+ T cells during the acute phase of a systemic infection.
Figure 6. Treatment with the S1P1-specific agonist AUY954 interferes with the host’s response to virus infection.
(A and B) 5×104 GP33–41 TCR-transgenic Thy1.1+ CD8+ T cells were adoptively transferred into mice 1 day before infection. 1×106 PFU of FLU-LCMV was administered i.v. and 3mg/kg of AUY954 (AUY) or vehicle (VEH) was administered by gavage on days 2 and 3 post infection. (A) Reduced frequencies of virus-specific GP33–41 TCR-transgenic Thy1.1+ CD8+ T cells are detected by flow cytometric analysis in the lungs, mediastinal and inguinal lymph nodes as well as in the spleen of mice treated with AUY954, when compared to VEH. Representative density plots are shown. (B) Absolute numbers of virus-specific CD8+ cells are significantly decreased by AUY954 treatment in inguinal lymph nodes and spleen. n=4 mice per group; data is represented as average ± SEM. *Significantly different from VEH, p ≤ 0.05.
Discussion
Here we compare the lymphopenic effect induced by infection with a LCMV strain that generates an antiviral CTL response and clears an acute viral infection (Arm53b) with a LCMV variant (Cl 13) that fails to generate a vigorous CTL response to terminate an acute infection causing a resultant persistence of virus. We also examine the modulation of LCMV-Cl 13 infection by the sphingosine analog FTY720 and an S1P1 receptor-specific agonist, AUY954. Our studies make the following four points. First, transient treatment with a low dose of FTY720 (4 µg/kg) does not significantly modify viral burden following infection with LCMV-Cl 13. This was confirmed at 2 different onsets of treatment, i.e. at the time of infection and during the phase of persistence, as well as in 2 different MHC groups of mice, H-2b: C57Bl/6 and H-2d Balb/c and by two separate groups (Walsh, Marsolais, Rosen and Welch, Oldstone). Secondly, the extent of lymphopenia induced by LCMV-Cl 13 and Arm53b is equivalent over a dose range. Thirdly, LCMV-Cl 13-induded lymphopenia is weakly enhanced by sphingosine analogs.
Although the frequency of T cells in the blood are significantly reduced by FTY720 treatment during LCMV-Cl 13 infection, the impact on the total number of circulating T cells is minute since the virus induced a robust lymphopenia by itself. Fourthly, treatment with a high dose of FTY720 (1 mg/kg, i.v.) or the S1P1-specific receptor agonist, AUY954 (3 mg/kg, gavage), adversely affects the anti-LCMV T cell response and using influenza virus, impairs the generation of influenza virus-specific CD8+ T cells.
FTY720 does not significantly alter the course of persistent virus infection induced by LCMV-Cl 13. Our results agree with a recent study that administration of FTY720 was ineffective at curing macaques persistently infected with SIV:SHIVSF162P3 virus (Kersh et al., 2009) and our results contrast with the report of Premenko-Lanier and colleagues (Premenko-Lanier et al., 2008) that transient FTY720-induced lymphopenia successfully treated a persistent LCMV-Cl 13 infection and purged virus from serum, brain and kidneys within 30 days after treatment. The Premenko-Lanier and colleagues observations (Premenko-Lanier et al., 2008) are difficult to reconcile with the literature since LCMV persists within kidneys for up to 2 years due to the formation of virus-antibody immune complexes and in the brain for over 100 days after adoptive transfer of anti-LCMV-specific memory T cells that is able to clear virus from sera by 15 days (Garidou et al., 2009; Oldstone et al., 1986).
Previous studies support the spleen to be a primary site of T cell inactivation. During LCMV clone-13 infection, B cells and spleen DC were shown to up-regulate the production of the immunosuppressive molecule IL-10 (Brooks et al., 2008; Brooks et al., 2006). In addition, expression of the immunosuppressive receptor, programmed death-1 (PD-1), is enhanced on LCMV-specific CD8+ T cells located within the spleen following LCMV-Cl 13 infection (Barber et al., 2006). Since FTY720 eliminates naïve and activated T cells from spleen and peripheral organs (Hofmann, Brinkmann, and Zerwes, 2006; Sawicka et al., 2003) by sequestration into lymph nodes and Peyer’s patches (Mandala et al., 2002; Sanna et al., 2004), one could speculate that this alteration of cellular distribution might allow restoration of T cell function. In contradiction with this hypothesis, our results demonstrate that administration of FTY720 at doses sufficient to enhance LCMV-Cl 13-induced lymphopenia does not have an effect on T cell exhaustion or viral persistence, suggesting that soluble factors like IL-10 could maintain T cell exhaustion outside of the spleen and/or that the PD-1/PD-L1 pathway is effective in lymph nodes or Peyer’s patches. Furthermore, our results show that the extent of lymphopenia induced after LCMV-Cl 13 infection is not related to persistence of LCMV-Cl 13, since infection with the non-persisting strain of LCMV (Arm53b) induces the same degree of lymphopenia.
Acute lymphopenia is independent of infection by LCMV-Arm53b or -Cl 13 and can be enhanced by FTY720. In this study, we find that infection with LCMV-Arm53b and -Cl 13 induced profound lymphopenia (Figure 1 and Figure 2), and that the extent of lymphopenia induced by these two viruses are equivalent 3 days following infection. Early after viral infection, proinflammatory cytokines such as type I interferons (Kamphuis et al., 2006) and tumor necrosis factor-α (Young et al., 2000) induce the retention of T cells in draining lymphoid organs. Importantly, LCMV-Arm53b and -Cl 13 induce the release of type I interferon in similar amounts in serum early after infection (Zuniga et al., 2008), which could explain the similar levels of lymphopenia induced by both LCMV variants. Virus-induced lymphopenia is, at least partially, due to type-I interferon-induced CD69 up-regulation on the surface of T cells (Shiow et al., 2006). However, FTY720 can down-modulate the expression of CD69 on the surface of T cells (Rosen et al., 2003), arguing that enhancement of LCMV-induced lymphopenia by FTY720 may likely act through a different mechanism. Together, these results favor a model where enhancement of lymphopenia by FTY720 during viral infection is due to enforcement of the endothelial barrier in the lymph node, as previously observed in naïve mice (Mandala et al., 2002; Wei et al., 2005). This is further supported by our observation that the S1P1 receptor-specific agonist AUY954, which is sufficient in inducing lymphopenia and tighten the endothelial barrier (Marsolais and Rosen, 2009), disrupts the T cell response via S1P1 receptor activation.
We find that sphingosine analogs can alter the antiviral immune response. Low doses (4 and 40 µg/kg) of FTY720, a precursor of the S1P1 and S1P3-5 agonist, FTY720-phosphate, administered at the time of infection does not affect the antiviral T cell response or viral burden within the serum. However, treatment with 1 mg/kg of FTY720, a dose previously shown to induce lymphopenia in mice, results in increased serum viral burden on days 15, 30 and 62 post-infection which correlates with a significant reduction in the numbers of LCMV-specific T cells and the number of IFN-γ producing T cells following peptide stimulation. The reduction in LCMV-specific T cells following FTY720 treatment is at least partially due to S1P1 signaling since treatment with the S1P1-specific agonist, AUY954, significantly reduces the number of IFN-γ producing cells. However, the negative antiviral effect FTY720 exerts appears to be due to the multipotent activities of this drug as S1P1 receptor-specific signaling fails to replicate the effect of FTY720 on viral burden. This observation is not surprising as FTY720 was previously shown to interact with many targets including S1P lyase (Bandhuvula et al., 2005), lipid phosphate phosphatase (Mechtcheriakova et al., 2007) and sphingosine kinases (Billich et al., 2003). In agreement with a previous report (Pinschewer et al., 2000), our data reveals that sphingosine analogs alters the distribution of T cells during LCMV infection. In addition, we provide evidence that high doses of FTY720 as well as S1P1-induced lymphopenia during the acute phase of an infection hinders the expansion of virus-specific T cells.
Our data agrees with other studies demonstrating that immunosuppressive drugs result in reactivation of viruses, normally latent, due to suppression of viral replication by the host’s immune system. For instance, cyclosporine has resulted in the recrudescence of hepatitis B virus and Epstein-Barr virus in organ recipients (Ho et al., 1997; Seth et al., 2002) and anti-α4-integrin antibody, Natalizumab, has caused progressive multifocal leukoencephalopathy by reactivating JC virus (Langer-Gould et al., 2005). FTY720 does not appear to be an exception to this rule. Recently, a patient on FTY720 phase III trials for multiple sclerosis died from recrudescence of varicella zoster while another suffered herpes virus-mediated encephalitis (Garber, 2008). Sphingosine analogs have negative antiviral affects in rodent models as well. The S1P1 receptor-specific agonist, SEW2871, was shown to reduce cytokine secretion in CD4+ T cells during diabetes (Srinivasan et al., 2008). Also, CD4+ T cells from S1P1 transgenic mice expressed high levels of IL-4 suggesting S1P1 induces a Th2 cytokine profile that would be deleterious for an anti-viral response (Wang, Huang, and Goetzl, 2007). Differences in pharmacokinetics of the different drugs may also account for the slight differences seen between FTY720 and AUY954 treatments of LCMV-Cl 13-infected mice. Nonetheless, our data demonstrates that induction of lymphopenia via S1P1 receptor signaling negatively affects the immune response against two viral pathogens, ie. LCMV and influenza, by hampering the generation of virus-specific T cells.
Taken together, our work does not support acute lymphopenia as a predictive or therapeutic parameter for persistent viral infections. Instead, we show that FTY720, or S1P1-specific agonist AUY954, can negatively affect the host’s ability to respond to viral infection.
Acknowledgments
This work was supported in part by USPHS grants AI074564 (MBAO, HR, DM, KBW), AI009484 (MBAO), AI05509 (HR) and NIMH-074404 (HR). KBW is supported by the NIH postdoctoral fellow training grant NSO41219. DM is supported by Le Fonds de la Recherche en Santé du Québec, Canada. The authors acknowledge the technical assistance of Hanna Lewicki and thank the TSRI Flow Cytometry Core Facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160(2):521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandhuvula P, Tam YY, Oskouian B, Saba JD. The immune modulator FTY720 inhibits sphingosine-1-phosphate lyase activity. J Biol Chem. 2005;280(40):33697–33700. doi: 10.1074/jbc.C500294200. [DOI] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Billich A, Bornancin F, Devay P, Mechtcheriakova D, Urtz N, Baumruker T. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem. 2003;278(48):47408–47415. doi: 10.1074/jbc.M307687200. [DOI] [PubMed] [Google Scholar]
- Borrow P, Evans CF, Oldstone MB. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J Virol. 1995;69(2):1059–1070. doi: 10.1128/jvi.69.2.1059-1070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DG, Ha SJ, Elsaesser H, Sharpe AH, Freeman GJ, Oldstone MB. IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc Natl Acad Sci U S A. 2008;105(51):20428–20433. doi: 10.1073/pnas.0811139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12(11):1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Henry MD, Borrow P, Yamada H, Elder JH, Ravkov EV, Nichol ST, Compans RW, Campbell KP, Oldstone MB. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science. 1998;282(5396):2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- Garber K. Infections cast cloud over Novartis' MS therapy. Nat Biotechnol. 2008;26(8):844–845. doi: 10.1038/nbt0808-844. [DOI] [PubMed] [Google Scholar]
- Garidou L, Heydari S, Truong P, Brooks DG, McGavern DB. Therapeutic Memory T Cells Require Costimulation for Effective Clearance of a Persistent Viral Infection. J Virol. 2009 doi: 10.1128/JVI.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho BM, So SK, Esquivel CO, Keeffe EB. Liver transplantation in Asian patients with chronic hepatitis B. Hepatology. 1997;25(1):223–225. doi: 10.1002/hep.510250140. [DOI] [PubMed] [Google Scholar]
- Hofmann M, Brinkmann V, Zerwes HG. FTY720 preferentially depletes naive T cells from peripheral and lymphoid organs. Int Immunopharmacol. 2006;6(13–14):1902–1910. doi: 10.1016/j.intimp.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Kamphuis E, Junt T, Waibler Z, Forster R, Kalinke U. Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood. 2006;108(10):3253–3261. doi: 10.1182/blood-2006-06-027599. [DOI] [PubMed] [Google Scholar]
- Kersh EN, Luo W, Adams DR, Mitchell J, Garcia-Lerma JG, Butera S, Folks T, Otten R. Evaluation of the lymphocyte trafficking drug FTY720 in SHIVSF162P3-infected rhesus macaques. J Antimicrob Chemother. 2009 doi: 10.1093/jac/dkp008. [DOI] [PubMed] [Google Scholar]
- Kunz S, Sevilla N, McGavern DB, Campbell KP, Oldstone MB. Molecular analysis of the interaction of LCMV with its cellular receptor [alpha]-dystroglycan. J Cell Biol. 2001;155(2):301–310. doi: 10.1083/jcb.200104103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353(4):375–381. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296(5566):346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- Marsolais D, Hahm B, Edelmann KH, Walsh KB, Guerrero M, Hatta Y, Kawaoka Y, Roberts E, Oldstone MB, Rosen H. Local not systemic modulation of dendritic cell S1P receptors in lung blunts virus-specific immune responses to influenza. Mol Pharmacol. 2008;74(3):896–903. doi: 10.1124/mol.108.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsolais D, Hahm B, Walsh KB, Edelmann KH, McGavern D, Hatta Y, Kawaoka Y, Rosen H, Oldstone MB. A critical role for the sphingosine analog AAL-R in dampening the cytokine response during influenza virus infection. Proc Natl Acad Sci U S A. 2009;106(5):1560–1565. doi: 10.1073/pnas.0812689106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsolais D, Rosen H. Chemical modulators of sphingosine-1-phosphate receptors as barrier-oriented therapeutic molecules. Nat Rev Drug Discov. 2009;8(4):297–307. doi: 10.1038/nrd2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechtcheriakova D, Wlachos A, Sobanov J, Bornancin F, Zlabinger G, Baumruker T, Billich A. FTY720-phosphate is dephosphorylated by lipid phosphate phosphatase 3. FEBS Lett. 2007;581(16):3063–3068. doi: 10.1016/j.febslet.2007.05.069. [DOI] [PubMed] [Google Scholar]
- Moskophidis D, Battegay M, van den Broek M, Laine E, Hoffmann-Rohrer U, Zinkernagel RM. Role of virus and host variables in virus persistence or immunopathological disease caused by a non-cytolytic virus. J Gen Virol. 1995;76(Pt 2):381–391. doi: 10.1099/0022-1317-76-2-381. [DOI] [PubMed] [Google Scholar]
- Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362(6422):758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- Nikolova Z, Hof A, Baumlin Y, Hof RP. Combined FTY720/cyclosporine A treatment promotes graft survival and lowers the peripheral lymphocyte count in DA to lewis heart and skin transplantation models. Transpl Immunol. 2001;8(4):267–277. doi: 10.1016/s0966-3274(01)00031-4. [DOI] [PubMed] [Google Scholar]
- O'Connor P, Comi G, Montalban X, Antel J, Radue EW, de Vera A, Pohlmann H, Kappos L. Oral fingolimod (FTY720) in multiple sclerosis: two-year results of a phase II extension study. Neurology. 2009;72(1):73–79. doi: 10.1212/01.wnl.0000338569.32367.3d. [DOI] [PubMed] [Google Scholar]
- Oldstone MB. Viral persistence: parameters, mechanisms and future predictions. Virology. 2006;344(1):111–118. doi: 10.1016/j.virol.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Oldstone MB. Anatomy of Viral Persistence. PLoS Pathog. 2009 doi: 10.1371/journal.ppat.1000523. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone MB, Blount P, Southern PJ, Lampert PW. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature. 1986;321(6067):239–243. doi: 10.1038/321239a0. [DOI] [PubMed] [Google Scholar]
- Pan S, Mi Y, Pally C, Beerli C, Chen A, Guerini D, Hinterding K, Nuesslein-Hildesheim B, Tuntland T, Lefebvre S, Liu Y, Gao W, Chu A, Brinkmann V, Bruns C, Streiff M, Cannet C, Cooke N, Gray N. A monoselective sphingosine-1-phosphate receptor-1 agonist prevents allograft rejection in a stringent rat heart transplantation model. Chem Biol. 2006;13(11):1227–1234. doi: 10.1016/j.chembiol.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Pinschewer DD, Ochsenbein AF, Odermatt B, Brinkmann V, Hengartner H, Zinkernagel RM. FTY720 immunosuppression impairs effector T cell peripheral homing without affecting induction, expansion, and memory. J Immunol. 2000;164(11):5761–5770. doi: 10.4049/jimmunol.164.11.5761. [DOI] [PubMed] [Google Scholar]
- Premenko-Lanier M, Moseley NB, Pruett ST, Romagnoli PA, Altman JD. Transient FTY720 treatment promotes immune-mediated clearance of a chronic viral infection. Nature. 2008;454(7206):894–898. doi: 10.1038/nature07199. [DOI] [PubMed] [Google Scholar]
- Rosen H, Alfonso C, Surh CD, McHeyzer-Williams MG. Rapid induction of medullary thymocyte phenotypic maturation and egress inhibition by nanomolar sphingosine 1-phosphate receptor agonist. Proc Natl Acad Sci U S A. 2003;100(19):10907–10912. doi: 10.1073/pnas.1832725100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvato M, Borrow P, Shimomaye E, Oldstone MB. Molecular basis of viral persistence: a single amino acid change in the glycoprotein of lymphocytic choriomeningitis virus is associated with suppression of the antiviral cytotoxic T-lymphocyte response and establishment of persistence. J Virol. 1991;65(4):1863–1869. doi: 10.1128/jvi.65.4.1863-1869.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvato M, Shimomaye E, Southern P, Oldstone MB. Virus-lymphocyte interactions. IV. Molecular characterization of LCMV Armstrong (CTL+) small genomic segment and that of its variant, Clone 13 (CTL-) Virology. 1988;164(2):517–522. doi: 10.1016/0042-6822(88)90566-1. [DOI] [PubMed] [Google Scholar]
- Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, Webb B, Lefebvre S, Chun J, Gray N, Rosen H. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279(14):13839–13848. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- Sawicka E, Zuany-Amorim C, Manlius C, Trifilieff A, Brinkmann V, Kemeny DM, Walker C. Inhibition of Th1- and Th2-mediated airway inflammation by the sphingosine 1-phosphate receptor agonist FTY720. J Immunol. 2003;171(11):6206–6214. doi: 10.4049/jimmunol.171.11.6206. [DOI] [PubMed] [Google Scholar]
- Seth P, Alrajhi AA, Kagevi I, Chaudhary MA, Colcol E, Sahovic E, Aljurf M, Gyger M. Hepatitis B virus reactivation with clinical flare in allogeneic stem cell transplants with chronic graft-versus-host disease. Bone Marrow Transplant. 2002;30(3):189–194. doi: 10.1038/sj.bmt.1703614. [DOI] [PubMed] [Google Scholar]
- Sevilla N, Kunz S, Holz A, Lewicki H, Homann D, Yamada H, Campbell KP, de La Torre JC, Oldstone MB. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J Exp Med. 2000;192(9):1249–1260. doi: 10.1084/jem.192.9.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440(7083):540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Bolick DT, Lukashev D, Lappas C, Sitkovsky M, Lynch KR, Hedrick CC. Sphingosine-1-phosphate reduces CD4+ T-cell activation in type 1 diabetes through regulation of hypoxia-inducible factor short isoform I.1 and CD69. Diabetes. 2008;57(2):484–493. doi: 10.2337/db07-0855. [DOI] [PubMed] [Google Scholar]
- Walsh KB, Lanier LL, Lane TE. NKG2D receptor signaling enhances cytolytic activity by virus-specific CD8+ T cells: evidence for a protective role in virus-induced encephalitis. J Virol. 2008;82(6):3031–3044. doi: 10.1128/JVI.02033-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Huang MC, Goetzl EJ. Type 1 sphingosine 1-phosphate G protein-coupled receptor (S1P1) mediation of enhanced IL-4 generation by CD4 T cells from S1P1 transgenic mice. J Immunol. 2007;178(8):4885–4890. doi: 10.4049/jimmunol.178.8.4885. [DOI] [PubMed] [Google Scholar]
- Wei SH, Rosen H, Matheu MP, Sanna MG, Wang SK, Jo E, Wong CH, Parker I, Cahalan MD. Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nat Immunol. 2005;6(12):1228–1235. doi: 10.1038/ni1269. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77(8):4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AJ, Seabrook TJ, Marston WL, Dudler L, Hay JB. A role for lymphatic endothelium in the sequestration of recirculating gamma delta T cells in TNF-alpha-stimulated lymph nodes. Eur J Immunol. 2000;30(1):327–334. doi: 10.1002/1521-4141(200001)30:1<327::AID-IMMU327>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188(12):2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga EI, Liou LY, Mack L, Mendoza M, Oldstone MB. Persistent virus infection inhibits type I interferon production by plasmacytoid dendritic cells to facilitate opportunistic infections. Cell Host Microbe. 2008;4(4):374–386. doi: 10.1016/j.chom.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]