Abstract
Background:
Disruption of fibrinolytic homeostasis participates in the pathogenesis of severe lung diseases like acute respiratory distress syndrome (ARDS), idiopathic pulmonary fibrosis (IPF) and plastic bronchitis. We have developed a pulmonary formulation of tissue plasminogen activator (pf-tPA) that withstands nebulization and reaches the lower airways.
Objective:
Since treatment of ARDS, IPF and plastic bronchitis will require repeated administration of pf-tPA, the purpose of this study was to determine the safety of prolonged, repeated administration of pf-mouse tPA (pf-mtPA) to the lungs of healthy mice.
Methods:
Male and female B6C3F1 mice received one of two intratracheal (IT) doses of either nebulized pf-mtPA or sterile saline twice daily for 28 days. Weekly blood samples were collected to estimate hematocrit. Following the dosing period, animals were sacrificed for gross necropsy, the acquisition of bronchoalveolar lavage fluid (BALF), and histological assessment of the lungs and other major organs.
Results:
The low dose of pf-mtPA was well-tolerated by both female and male mice. However, female and male mice that received the high dose experienced a 16% and 8% incidence, respectively, of fatal pulmonary hemorrhage. Although male mice had a lower incidence of bleeding, these events occurred at lower mean (±S.E.) doses (1.06±0.02 mg/kg/d) of pf-mtPA compared with females (1.48±0.03 mg/kg/d, p < 0.001). In addition, male mice had higher BALF mtPA concentrations. Bleeding occurred six and 12 days in male and female mice, respectively, after the initiation of dosing suggesting that mtPA accumulated in the lungs.
Conclusion:
This study established a safe dose range and demonstrated the feasibility of prolonged, repeated dosing of pf-tPA. High doses (≥ 1mg/kg/d) were associated with pulmonary hemorrhage that may be due, in part, to accumulation of drug in the lungs.
Keywords: pulmonary drug delivery, therapeutic protein, plastic bronchitis, experimental lung injury, fibrinolysis, lung clearance, gender differences
Introduction
Severe, life-threatening lung diseases such as acute lung injury (ALI)/acute respiratory distress syndrome (ARDS), idiopathic pulmonary fibrosis (IPF) and plastic bronchitis, are categorized as rare but they represent significant hazards to human health because they are associated with significant morbidity and mortality [1-3]. Presently, no effective pharmacotherapy exists for these ailments and little progress has been made in identifying potential, viable therapeutics. The pathogeneses of these lung diseases are distinct and complex but inflammation and loss of fibrinolytic homeostasis appear to be common [4-8].
Tissue plasminogen activator (tPA) is a serine protease with a known fibrinolytic function that also possesses anti-inflammatory activity [9-11]. The amalgamation of these properties could make tPA advantageous in the treatment of lung illnesses in which inflammation and fibrosis are hallmark. However, the utility of tPA for these indications has limitations since higher systemic doses are required for its anti-inflammatory action compared with those used for fibrinolysis as in the treatment of myocardial infarction (MI) or stroke. In addition, unlike the treatment of MI or stroke, the use of tPA for pulmonary diseases will require repeated dosing because of the prolonged nature of these illnesses [6, 7, 12].
In hope of circumventing the systemic circulation and targeting drug therapy to the lungs, we developed a pulmonary formulation of human tPA (pf-tPA). We have previously demonstrated the feasibility of pf-tPA as evidenced by the retention of protein stability and activity following nebulization [13]. In addition, we have proved the concept of the delivery of active protein in a human lung replica by showing that the nebulized particle size distribution would permit diffuse airway distribution. We have also shown that a maximally feasible dose (3 mg/kg) of pf-tPA can be given repeatedly with increasing frequency over a short period of time without complications [14]. However, the anticipated clinical situation will require prolonged repeated administration of pf-tPA. Therefore, to test the feasibility and safety of this regimen, mice received intratracheal (IT) doses of either a nebulized pulmonary formulation of mouse tPA (pf-mtPA) or sterile saline twice daily for 28 consecutive days.
MATERIALS AND METHODS
Mouse tPA Formulation and Nebulization
Since mouse plasminogen is activated very slowly by human tPA, recombinant mouse tPA (mtPA; Molecular Innovations, Southfield, MI) was formulated to mimic a previously described pulmonary formulation of human tPA for use in this and our previous mouse studies [15, 16]. The stability and function of the mouse formulation (pf-mtPA) was assessed by measuring protein aggregation index (AI), an indicator of protein self-association, by ultraviolet (UV) spectroscopy and fibrinolytic activity as described in previous work [13, 14]. An AI of ≤ 10 was considered optimal.
In preparation for dosing, the liquid formulation of pf-mtPA was nebulized using a Micromist nebulizer (Hudson Respiratory Care, Temecula, CA) and a Sunrise compressor (Model 3655D, Somerset, PA). The aerosol condensate was collected and assayed for protein concentration and AI by UV spectroscopy. We have previously demonstrated that the aerosol consists of particles with a mass mean diameter and geometric standard deviation of 2.4 μm and 2.7 μm, respectively and the nebulized protein retains fibrinolytic activity [13, 14].
Aliquots (80 μL) of nebulized pf-mtPA were stored (4°C) until use at which time protein concentration and AI were re-assessed. Periodically, stored nebulized material was assayed for fibrinolytic activity by measuring its ability to cleave mouse plasminogen as previously described [13]. For dosing, the needed volume of pf-mtPA or sterile saline was drawn into a gavage needle (22G; Hallowell EMC, Pittsfield, MA) attached to a sterile syringe (1mL). The dose was prepared so that approximately 100μL of air followed the dose. We have previously shown that this approach results in diffuse distribution of the agent in the lungs including the lower airways [14, 17]. In addition, since mice are nose-breathers so inhalation of aerosols or liquids results in a substantial amount of deposition in the nasal passages and sinuses [18].
Animals
The animal protocol was approved by the University of Colorado Health Sciences Center Animal Care and Use Committee and the University of Michigan Committee on Use and Care of Animals. The research and the care and handling of the animals were in accordance with the National Institutes of Health Office of Laboratory Animal Welfare “Principles of Laboratory Animal Care” (http://grants.nih.gov/grants/olaw/references/phspol.htm).
Male and female mice (8-10 weeks old; B6C3F1; Taconic Farms, Hudson, NY) were studied in blocks of 16 (8 males, 8 females) in which an equal number of animals received either nebulized pf-mtPA or sterile saline. The pf-mtPA treated mice received one of two different doses of pf-mtPA were studied; a low dose (0.30 mg/kg/d) or a high dose (0.60 mg/kg/d). Mice received either saline or pf-mtPA every 12 h for 28 days by instilling the dose into the trachea via the oral route under gas anesthesia [14, 17]. This dosing regimen was derived from previous in vitro data based on protein concentrations achieved in a human lung replica [13]. Each mouse was anesthetized under isoflurane (IsoFlo, isoflurane, USP; Abbott Animal Health, North Chicago, IL) after which a rodent intubation table (Hallowell EMC) and a pediatric otoscope (Welch-Allyn Medical Products, Skaneateles Falls, NY) were used to visualize the vocal cords. The gavage needle was carefully moved past the vocal cords and into the trachea and the dose of pf-mtPA or saline was delivered by depression of the syringe's plunger. After IT administration, the mouse was held upright for ~5 s, after which it was returned to the cage and allowed to recover from anesthesia.
During the study, animals were weighed prior to dosing and were routinely assessed for changes in behavior that could be associated with morbidity (e.g., grooming, posturing). In addition, to monitor for occult bleeding, blood (50 μL) was acquired once a week from the retro-orbital sinus by carefully inserting a heparinized capillary tube into the medial canthus at a 30° angle to the nose while the animal was under gas (isoflurane) and local (proparacaine) anesthesia. Each capillary tube was sealed using Critoseal™ (LW Scientific, Atlanta, GA) and centrifuged in a micro-capillary centrifuge (International Equipment Company, Boston, MA) after which hematocrit was estimated as the percentage of packed red blood cell volume to the total sample volume.
Assay of Bronchoalveolar Fluid (BALF)
Upon the completion of the dosing period, mice were anesthetized with ketamine and xylazine and a tracheostomy was performed. The trachea was cannulated with a luer stub adapter (20 G; Becton Dickinson, Sparks, MD) and the lungs of some mice were lavaged twice with 400 μL of sterile phosphate-buffered saline (PBS). The BALF samples from each mouse were pooled and a cell count was performed using a hemacytometer. The BALF cells were pelleted by centrifugation (10,000 g, 4° C, 5 min) in preparation for fluorescence-activated cell sorting (FACS). The BALF supernatant was frozen (−80°C) until the time of assay.
Albumin concentrations in BALF samples were determined using a modified albumin assay kit (Bethyl Laboratories, Montgomery, TX). Purified mouse albumin (MP Biomedicals, Solon, OH) was used to create the standard curve (0-250 ng/mL). The concentration of mtPA was determined in BALF samples using a mtPA ELISA (Molecular Innovations) that measured functionally active mtPA. The absorbance data acquired from both assays were analyzed with Softmax PRO 4.1 on a ThermoMax microplate reader (Molecular Devices, Sunnyvale, CA).
The differential of the BALF cells from each sample was obtained by fluorescence-activated cell sorting as previously described [17]. Briefly, following staining with antibodies, cells were washed in staining buffer and fluorescence was detected by a Cytomics FC500 (Beckman Coulter, Fullterton, CA) at the University of Colorado Cancer Center Flow Cytometry Core Laboratory or a FACS Canto II (BD Biosciences) at the University of Michigan's Comprehensive Cancer Center.
Histology and Gross Necropsy
Following thoracotomy, the lungs of mice that were not lavaged were perfused blood-free with buffered formalin (10%) via catheterization of the right ventricle after which the lungs were inflated to 20 cm of water by instilling formalin into the trachea cannula. After 20 min, the lungs were removed en bloc and they, along with the carcasses, were submitted for gross necropsy by a veterinarian blinded to treatment group assignment. After gross necropsy, vital organs were paraffin-embedded and a single 5 μm section from each sample was acquired and stained with hematoxylin and eosin for microscopic histological evaluation. Paraffin embedding, sectioning and staining was performed by the University of Colorado Histology Core Laboratory and the University of Michigan's Laboratory Animal Medicine pathology service.
Measurement of mtPA Lung Clearance
To begin to investigate the ability of the lungs to degrade tPA, lungs of an untreated post-mortem male mouse were perfused blood-free with PBS and homogenized, in the absence of protease inhibitors, in tissue extraction reagent (Pierce, Rockford, IL). Residual red blood cells were lysed by the addition of red blood cell lysing buffer (1 mL; Sigma, St. Louis, MO) and the sample was homogenized (Tissuemiser, Fisher Scientific, Pittsburg, PA) on ice. Homogenate was transferred to microcentrifuge tubes, after which they were clarified by centrifugation (13,000 × g, 4° C) for 20 min. A known amount of mtPA (25 μg) was added to the homogenized mouse lung tissue (1 mL) and it was incubated (37°C). Periodically over time, an aliquot (50 μL) was removed and frozen (−80°C) until the time of assay. Upon completion of the experiment, an equal volume (30 μL) of each sample was subjected to 12.5% SDS-PAGE. Following electrophoresis, proteins were transferred to PVDF and the membrane was probed for mouse tPA (1:10,000 dilution of a rabbit anti-mouse tPA antibody; Molecular Innovations). Protein was detected using a secondary fluorescent antibody, the image of which was acquired using a Typhoon Variable Mode Imager (GE Healthcare). The amount of active mtPA was detected in the remaining lung homogenate (20 μL) using a mtPA ELISA as described above.
DATA ANALYSIS
Data were analyzed by a one-way analysis of variance (ANOVA) and if required a Fisher protected least significant difference post hoc analysis was performed. Some data, where appropriate, were compared by an unpaired two-tailed Student's t-test. Analyses were performed using StatPlus software (AnalystSoft, Vancouver, BC, Canada). In all cases a p value of ≤ 0.05 was considered statistically significant.
RESULTS
Mouse tPA Formulation and Dosing
As expected, nebulized pf-mtPA protein concentrations varied across the treatment blocks but aggregation indexes were similar and consistent with the feasibility criteria established for the human pulmonary formulation of tPA (Table 1). Freshly nebulized pf-mtPA was fibrinolytically active and remained so for up to four weeks of storage (4°C) (Fig. 1). The pf-mtPA (pre and post-nebulized) used primarily consisted of the double-chain form (~85%; MW ~34kDa).
Table 1.
Concentrations and Aggregation Indexes for Nebulized pf-mtPA
| Block | pf-mtPA (mg/mL, mean ± SE) |
Aggregation Index (AI, mean ± SE) |
|---|---|---|
| 1 | 1.151 ± 0.028 | 4.1 ± 1.5 |
| 2 | 1.179 ± 0.009 | 7.1 ± 0.9 |
| 3 | 1.182 ± 0.022 | 8.0 ± 0.8 |
| 4a | 0.833 ± 0.089 | 4.1 ± 1.5 |
| 5b | 1.411 ± 0.023 | 5.7 ± 0.8 |
| 6c | 1.471 | 4.4 |
| end materiald | 1.385 ± 0.034 | 6.5 ± 0.3 |
The mean mtPA concentrations in block 4 and 5 were lower and higher, respectively, compared with those of the other blocks (p ≤ 0.0017).
The mean mtPA concentrations in block 4 and 5 were lower and higher, respectively, compared with those of the other blocks (p ≤ 0.0017).
material was only nebulized once during this block
end material represents nebulized pf-mtPA that was nebulized and collected during block 1, stored (−4°C) and was assayed at the completion of block 6
Figure 1.
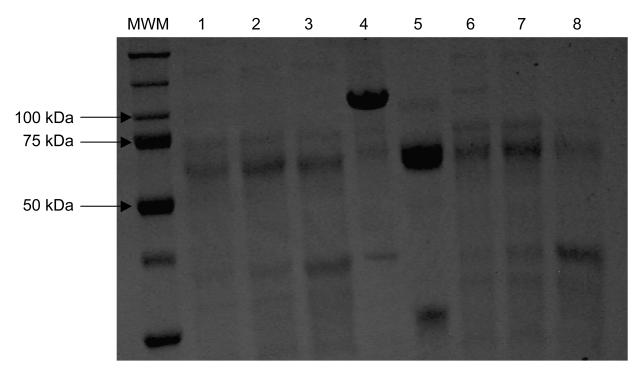
The fibrinolytic activity of nebulized pf-mtPA was maintained during storage. Samples were incubated (37°C) with fibrinogen (375 μg/mL) for 5 h after which they were subjected to SDS-PAGE (12.5%). Protein bands were visualized by staining the gel with Coomassie blue (freshly nebulized pf-mtPA + fibrinogen, lane 1: 50μg/mL; lane 2: 100μg/mL; lane 3: 250μg/mL and nebulized pf-mtPA after four weeks of storage at 4°C + fibrinogen, lane 6: 50 μg/mL; lane 7: 100 μg/mL; lane 8: 250 μg/mL). Mouse plasminogen (lane 4), plasmin (lane 5) and molecular weight markers (MWM) were used for protein identification and size determination.
The initial planned target doses of nebulized pf-mtPA were 0.15 mg/kg and 0.30 mg/kg in order to achieve measurable amounts of tPA in the lungs [13]. However, because female mice were much smaller than male mice, the weight-based volume of drug for the 0.15 mg/kg dose resulted in a volume of less than 5 μL. This volume was considered too small to accurately deliver to the lungs. Dilution of the formulation was avoided so that the consistency and integrity of the formulation was maintained. Therefore, the “low dose” drug and saline volume was administered as 5 μL. The “high dose” drug and saline volume was administered as 10 μL. Following the completion of the study the mean daily dose (mg/kg/d) for each dose range for male and female mice was calculated. Since male mice weighed more than female mice, they received lower mean doses than female mice in both the low and high doses (Table 2).
Table 2.
Nebulized pf-mtPA Doses (mg/kg/d) in Male and Female Mice
| Males range (mean ± SE) |
Females range (mean ± SE) |
|
|---|---|---|
| Low Dosea |
0.28 - 0.50 (0.41 ± 0.01) |
0.34 – 0.66 (0.50 ± 0.01) |
| High Doseb | 0.76 - 1.19 (1.01 ± 0.01) |
0.96 – 1.61 (1.37 ± 0.02) |
The male pf-mtPA doses were lower and higher, respectively, than those of female mice (p < 0.01).
The male pf-mtPA doses were lower and higher, respectively, than those of female mice (p < 0.01).
Animal Testing
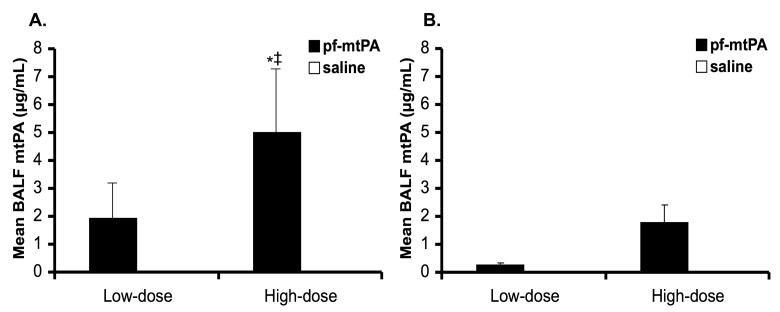
A total of six blocks of mice were studied (three low-dose and three high-dose) with an equal number of male and female mice in each. Prolonged, 28-day, IT twice daily low dose pf-mtPA was well tolerated in male and female B6C3F1 mice. However, in mice that received high dose pf-mtPA, there was an 8% (n = 1) incidence of fatal pulmonary hemorrhage in males and a 16% (n = 2) incidence in females. In addition to the cases of fatal lung hemorrhage, two male mice (one high dose pf-mtPA and one high dose saline) showed signs of hemorrhage in the lungs by histology. The most likely explanation for the higher incidence of fatal bleeding in the female mice is that they received, on average, higher doses (1.48±0.03 mg/kg/d) compared with male mice (1.06±0.02 mg/kg/d, p < 0.001 by unpaired Student's t-test). However, male mice may be at greater risk of tPA-induced pulmonary hemorrhage. This concept is illustrated by several observations. First, despite receiving a lower mean daily dose, male mice trended towards having higher BALF mtPA concentrations compared with those of females (Fig. 2). Second, pulmonary hemorrhage occurred 6 days after the initiation of pf-mtPA in male compared with 12 days in female mice, providing evidence that mtPA accumulated the lungs and that this may have been more pronounced in male mice.
Figure 2.
Male mice (A) that received high dose pf-mtPA had higher BALF mtPA levels compared with female mice (B). The difference between male and female high dose BALF mtPA approached significance (*p = 0.06) and there was a dose effect in male mice between the low and high doses (‡p = 0.05). The lack of significance between male and female groups is most likely due to the large variability associated with the BALF mtPA concentrations in male mice. In all saline treated mice, the BALF mtPA concentrations were below the limit of detection of the assay. Data are the mean (+S.E.) of 7-8 animals per group.
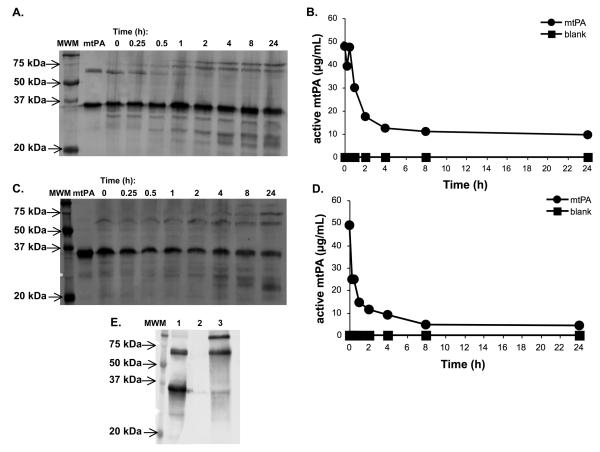
The slow elimination of mtPA in the lungs was exemplified by a mouse lung homogenate experiment in which single-chain mtPA was rapidly converted to its two-chain form (Fig. 3A) during which there appeared to be little to no reduction in the two-chain species (MW ~35 kDa) over time. Probable formation of a mtPA-plasminogen activator inhibitor (PAI; MW ~43 kDa) complex was evident by 1 h with the formation of a protein band at ~77 kDa. This conclusion is supported by the ELISA data which showed a rapid reduction in mtPA activity that coincided with the formation of the mtPA-PAI complex on Western blot (Fig. 3B). To investigate the fate of double-chain mtPA in lung homogenate, the experiment was repeated using two-chain mtPA. The results of this experiment were similar in that double-chain mtPA seemed to rapidly complex with PAI which was followed by slow degradation of the protein (Fig. 3C). This was mimicked by the ELISA data (Fig. 3D). In both experiments there was a detectable level of active mtPA at 24 h suggesting a slow terminal lung clearance. Using the ELISA data, the estimated initial clearance of the two-chain and single-chain species were 2.3 h (Fig. 3D) and 4.3 h (Fig. 3B), respectively. This difference may be attributable to the fact that two-chain mtPA is more catalytically active and has a greater affinity for PAI than one-chain mtPA [15]. Recombinant human tPA (alteplase, Activase™, Genentech, S. San Francisco, CA) is comprised of predominately the single-chain species (60-80%) [19]. In the presence of fibrin, the catalytic activity of human single- and double-chain are similar however, in its absence, like mtPA, double-chain human tPA is markedly more active than single-chain tPA.
Figure 3.
Western blot of mouse homogenate containing single- and double-chain mtPA (A). The single-chain species (~68 kDa) was rapidly converted to the double-chain species (~34 kDa) and by 1 h binding of the predominate double-chain species to plasminogen activator inhibitor (PAI) was apparent by the appearance of a protein band at ~77 kDa. The presumed binding of two-chain mtPA to PAI corresponded with a rapid decline in active mtPA concentration as measured by ELISA (B). By 4 h there was little change in the amount of double-chain mtPA by Western blot (A); this was mimicked by the ELISA data (B). When double-chain mtPA was added to lung homogenate, the Western blot showed a similar pattern (C) with PAI-binding occurring early followed by a prolonged elimination phase and some degradation of the protein by 4 h. The ELISA data followed a similar pattern (D). The pattern of single- and double-chain mtPA-PAI binding (A and C) were similar to that when mtPA was incubated in the presence of excess PAI (E; lane 1: mtPA, 5 μg; lane 2: CPAI, 15μg; lane 3: mtPA, 5μg, and CPAI, 15μg). This also showed the ability of the mtPA antibody to detect PAI-bound mtPA. Lane 1 (mtPA) on each blot (A and C) is a representative sample of the respective mtPA (0.75 μg) used in the experiment. mtPA was not detected in untreated lung homogenate by Western blot (data not shown) or by ELISA (blank, B and D).
In aggregate, these data support the concept that the elimination of mtPA from the lungs may be prolonged. This does not necessarily eliminate the need for twice daily dosing since the level of tPA needed to elicit a therapeutic response in the lungs is not yet known. In addition, other clearance mechanisms exist in the intact lungs (e.g., mucociliary action) that could contribute to the removal of mtPA. Investigation of these processes are beyond the scope of this work but future studies are warranted to more thoroughly study mechanisms of clearance and gender-related differences that may exist in these mechanisms as well as how the presence of disease may influence them.
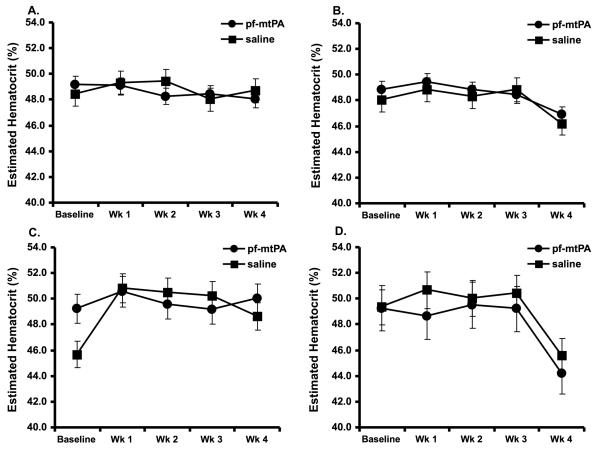
Notably, none of the mice, male or female, showed signs or symptoms of systemic hemorrhage. There was no evidence of systemic bleeding by gross necropsy or by micro-histological assessment of the other major organs and there were no differences in estimated hematocrit over time among the treatment groups (Fig. 4). There were however, instances of unanticipated euthanasia due to perforation of the trachea in five mice over the course of the study (n = 2 low dose pf-mtPA; n = 2 high dose saline; n = 1 high dose pf-mtPA). Other than these, there were no changes in animal behavior or moribund states that required euthanasia and there were no notable alterations in weight over the course of the study that could be attributed to the administration of pf-mtPA. Although we did not measure blood levels of mtPA, these data provide evidence that pf-mtPA most likely remained in the lungs and that the risk of systemic hemorrhage following its administration is low.
Figure 4.
Neither IT saline nor IT pf-mtPA administration in either low or high doses resulted in detectable changes in estimated hematocrit during the four week study period in male and female mice. There was also no evidence of systemic bleeding by gross necropsy or histology. Data are the mean (+S.E.) of 6-8 animals per group of females that received either a low (A) or high dose (B) pf-mtPA or saline or males that received either a low (C) or high dose (D) pf-mtPA or saline. The obvious declines in estimated hematocrit at week four in the high dose female and male groups (B and D) are most likely due to different operators.
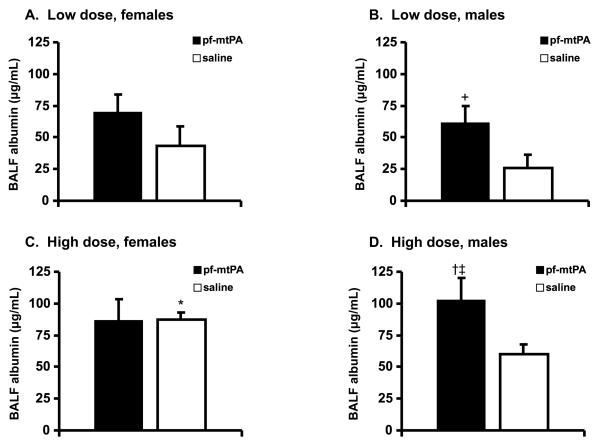
We measured BALF albumin as an indicator of lung injury. Extravasation of albumin into airways and the formation of non-cardiogenic pulmonary edema is an indicator of the loss of epithelial-endothelial barrier function [20, 21]. In male mice, administration of both doses of pf-mtPA were associated with increased BALF albumin concentration compared with saline controls (Fig. 5C & D). However, low-dose pf-mtPA-treated male mice had BALF albumin concentrations that were lower than those that are achieved (≥100 μg/mL) following the IT administration of substances known to cause lung injury (e.g., inflammatory cytokines) [17]. Collectively, these data show high dose pf-mtPA may lead to tPA-induced lung injury that could contribute to an increased risk of pulmonary hemorrhage. Although we were not able to measure BALF albumin levels in mice that succumbed to pulmonary bleeding, it is tempting to speculate that tPA-induced lung injury may have preceded these events. The mechanism by which this occurs will require further study but it is reasonable to suspect that enhanced proteolysis, either due to direct or indirect (e.g., tPA-induced activation of matrix metalloproteinases) actions of tPA, may lead to the degradation of the extracellular matrix and could have contributed to these adverse events [8].
Figure 5.
There was no difference in BALF albumin between the low dose male and female mice (A and B) or between pf-mtPA and saline treated female mice (A). However, the low dose pf-mtPA in male mice (B) resulted in higher BALF albumin compared with saline (+p = 0.05) but one that was lower than that which resulted from serious lung injury (see text). The high dose saline treated females had unexpectedly high BALF albumin concentrations (C) which resulted in the detection of a difference between low dose and high doses of saline-treated female mice (*p = 0.02). High dose pf-mtPA resulted in increased BALF albumin in male mice (D) compared with low dose pf-mtPA (†p = 0.05) and high dose saline (‡p = 0.04). Data are the mean (+S.E.) of 4-8 animals per group.
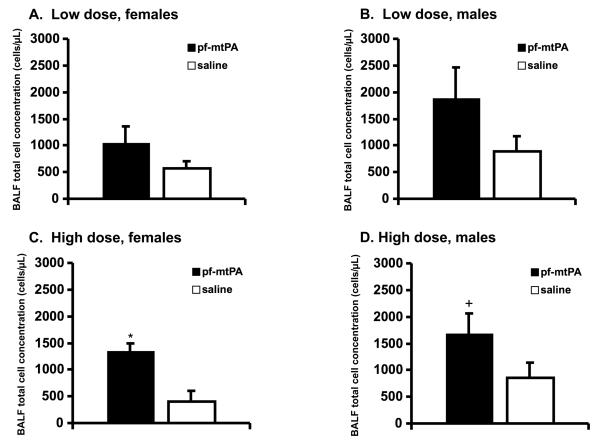
Finally, BALF cell concentrations were greater in high dose pf-mtPA-treated male and female mice compared with high dose saline controls (Fig. 6) but these counts are well below those that which resulted from the IT administration of inflammatory cytokines [17]. In all cases, most of the cells (≥ 75%) were macrophages and there were no differences in the differential between pf-mtPA-treated and saline-treated mice. The immunogenicity of the tPA protein in the lungs has not been studied. While the immunogenicity of single-dose systemically administered tPA is very low, additional studies are needed to investigate whether prolonged lung administration of pf-tPA would result in the production of antibodies that could negate the therapeutic function of the protein or result in immune-mediated adverse events [22].
Figure 6.
There was no difference in the BALF cell counts in female (A) or male (B) mice that received the low dose pf-mtPA or saline. The BALF cell counts were higher in female (C) and male (D) mice that received the high dose of pf-mtPA compared with their respective saline controls (*p = 0.03; +p = 0.03). Data are the mean (+ S.E.) of 5-12 animals per group.
Discussion
This study provides the first data to demonstrate the feasibility and safety of prolonged administration of pf-mtPA to the lungs in daily dose ranges of 0.34-0.66 and 0.28-0.50 mg/kg/d in healthy female and male B6C3F1 mice, respectively. This work is significant because lung delivered tPA could have utility in the treatment of a number of serious lung diseases that will require repeated administration. These data establish a safe range of doses that can be used for prolonged administration of pf-tPA and support the continued pursuit of this avenue of investigation. However, this work also identified doses associated with toxicity and uncovered the possibility of gender-related differences in the clearance of lung delivered tPA.
Doses of pf-mtPA ≥ 1 mg/kg/d were associated with an increased risk of acute, fatal pulmonary hemorrhage which may be due, in part, to the accumulation of tPA in the lungs and tPA-induced lung injury. This was evidenced by preliminary data that showed limited degradation of tPA protein in lung homogenate. In addition, high dose pf-mtPA-treated mice had increased BALF albumin concentrations compared with saline-treated mice that were consistent with those that are achieved following IT administration of inflammatory cytokines [17]. Importantly, while the safe dose ranges of pf-mtPA for female and male mice are similar, there appeared to be a gender difference in the associated risk of tPA-induced lung injury in the higher pf-mtPA dose ranges. This is illustrated by the fact that despite male mice having an overall lower incidence of fatal pulmonary bleeding, these events occurred at lower doses of pf-mtPA in males compared with those in females. Also, pulmonary hemorrhage occurred earlier in male mice compared with female mice. The gender effect was also evident by differences in BALF mtPA and albumin concentrations between male and female mice (Fig. 2 and 4). Taken together, these data suggest that male mice may be more susceptible to pf-tPA-induced lung injury. While gender in both mice and humans appears to influence the susceptibility to lung illnesses there are few data to suggest that the disposition of lung delivered drugs, in particular, therapeutic proteins, vary by gender [23, 24]. This makes our data the first to introduce this concept. One possibility for this difference includes distinct metabolic and/or clearance capabilities in the lungs of male and female mice that may be sex hormone mediated. Certainly, this avenue requires further investigation as well as whether there is application to the human situation.
Since lung delivered pf-mtPA appeared to accumulate in the lungs, we conducted a simple experiment to begin to investigate the lung clearance of tPA. This involved adding a known amount of mtPA to mouse lung homogenate. The results of this work provided important clues about the disposition of tPA in the lungs by showing the rapid conversion of single-chain mtPA to its double-chain form and subsequent probable binding to PAI which is known to be present in the lungs (Fig. 3) [25, 26]. While plasminogen probably contributed to the conversion of single-chain to double-chain mtPA, double-chain mtPA appeared only to undergo some degradation over time with a detectable amount of active mtPA in the homogenate for up to 24 h [27]. The changes observed in the Western blot were mimicked in the ELISA that showed a rapid reduction in active mtPA followed by a prolonged elimination phase with a half-life that exceeded 24 h. These data support the concept that twice daily dosing as used in the work described here, could lead to the accumulation of drug in the lungs.
Admittedly, these preliminary experiments only permitted us to address one (metabolism) of the many potential drug clearance mechanisms of the lungs albeit an important one. Certainly, in the live breathing mouse (and human) there are other routes of pulmonary clearance including non-absorptive mechanisms such as mucociliary clearance and alveolar macrophages that were not addressed by our preliminary lung homogenate experiment [28, 29]. In particular, there are processes in place for the maintenance of airway protein homeostasis. These may be especially important for lung delivered therapeutic proteins. For example, small, soluble proteins like insulin (5.8 kDa) typically dissolve quickly and/or are transported across the air-blood barrier to the systemic circulation [29]. Given the molecular weight (~68 kDa) of mtPA this is an unlikely mechanism. Also, given the absence of any evidence of systemic bleeding, it is unlikely that a substantial amount of tPA exited the lungs and entered the circulation. Alternatively, larger proteins are often removed from the airways as intact molecules via paracellular routes [30]. More recently however, the extracellular proteasome, present in the alveolar space of the human lungs, has been shown to play an important role in maintaining alveolar protein homeostasis [31]. Given these processes in the intact lungs, we expect that in a whole animal model, pf-tPA may be cleared from the lungs more quickly than it appears to be in the lung homogenate. Nevertheless, given the timing of pulmonary hemorrhage in our mice, it is apparent that the protein does remain in the lungs for some time. Exactly how long will need to be determined and confirmed in order to optimize a dosing regimen for the clinical use of pf-tPA. Work is ongoing to more fully characterize the pharmacokinetics of pf-tPA in the lungs.
Conclusions
In the present study we identified a safe dose range (mg/kg/d) of pf-mtPA for delivery to the lungs of healthy, normal mice. In the clinical situation of ALI/ARDS, IPF and plastic bronchitis, the presence of fibrin in the lungs would likely enhance the catalytic activity of tPA. In these environments, tPA-mediated plasmin generation would increase leading to the potential therapeutic benefit of fibrin degradation but also possibly augmentation of the risk of pulmonary hemorrhage. In fact, in the absence of fibrin, human tPA is generally considered a weak protease although the double-chain form is more active than the single-chain form [19]. In the presence of fibrin, single and double-chain tPA have similar proteolytic activity. Thus, it is reasonable to expect that the dose of tPA in the clinical situation of ALI/ARDS, IPF or plastic bronchitis will be lower than those identified as safe in the present work. Accordingly, the efficacy of pf-tPA in experimental models of disease still needs to be established. However, the safe dose ranges identified from the work described here provide support for the continued evaluation of pf-tPA for the treatment of severe lung diseases in which fibrin deposition is a component and for which there is presently no effective pharmacotherapy.
Acknowledgements
This work was supported by grant no. HL071439 from the National Heart, Lung and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung and Blood Institute or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 2.Taskar V, Coultas D. Exposures and idiopathic lung disease. Semin Respir Crit Care Med. 2008;29:670–9. doi: 10.1055/s-0028-1101277. [DOI] [PubMed] [Google Scholar]
- 3.Goo HW, Jhang WK, Kim YH, Ko JK, Park IS, Park JJ, et al. CT findings of plastic bronchitis in children after a Fontan operation. Pediatr Radiol. 2008;38:989–93. doi: 10.1007/s00247-008-0937-3. [DOI] [PubMed] [Google Scholar]
- 4.McClintock D, Zhuo H, Wickersham N, Matthay MA, Ware LB. Biomarkers of inflammation, coagulation and fibrinolysis predict mortality in acute lung injury. Crit Care. 2008;12:R41. doi: 10.1186/cc6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacLaren R, Stringer KA. Emerging role of anticoagulants and fibrinolytics in the treatment of acute respiratory distress syndrome. Pharmacotherapy. 2007;27:860–73. doi: 10.1592/phco.27.6.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Do TB, Chu JM, Berdjis F, Anas NG. Fontan patient with plastic bronchitis treated successfully using aerosolized tissue plasminogen activator: a case report and review of the literature. Pediatr Cardiol. 2009;30:352–5. doi: 10.1007/s00246-008-9312-2. [DOI] [PubMed] [Google Scholar]
- 7.Bringardner BD, Baran CP, Eubank TD, Marsh CB. The role of inflammation in the pathogenesis of idiopathic pulmonary fibrosis. Antioxid Redox Signal. 2008;10:287–301. doi: 10.1089/ars.2007.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sisson TH, Simon RH. The plasminogen activation system in lung disease. Curr Drug Targets. 2007;8:1016–29. doi: 10.2174/138945007781662319. [DOI] [PubMed] [Google Scholar]
- 9.Renckens R, Roelofs JJ, Florquin S, de Vos AF, Pater JM, Lijnen HR, et al. Endogenous tissue-type plasminogen activator is protective during Escherichia coli-induced abdominal sepsis in mice. J Immunol. 2006;177:1189–96. doi: 10.4049/jimmunol.177.2.1189. [DOI] [PubMed] [Google Scholar]
- 10.Renckens R, Roelofs JJ, Stegenga ME, Florquin S, Levi M, Carmeliet P, et al. Transgenic tissue-type plasminogen activator expression improves host defense during Klebsiella pneumonia. J Thromb Haemost. 2008;6:660–8. doi: 10.1111/j.1538-7836.2008.02892.x. [DOI] [PubMed] [Google Scholar]
- 11.Stringer KA, Dunn JS, Gustafson DL. Administration of exogenous tissue plasminogen activator reduces oedema in mice lacking the tissue plasminogen activator gene. Clin Exp Pharmacol Physiol. 2004;31:327–30. doi: 10.1111/j.1440-1681.2004.03999.x. [DOI] [PubMed] [Google Scholar]
- 12.Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27:337–49. doi: 10.1055/s-2006-948288. [DOI] [PubMed] [Google Scholar]
- 13.Dunn JS, Nayar R, Campos J, Hybertson BM, Zhou Y, Manning MC, et al. Feasibility of tissue plasminogen activator formulated for pulmonary delivery. Pharm Res. 2005;22:1700–7. doi: 10.1007/s11095-005-6335-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stringer KA, Tobias M, Dunn JS, Campos J, Van Rheen Z, Mosharraf M, et al. Accelerated dosing frequency of a pulmonary formulation of tissue plasminogen activator is well-tolerated in mice. Clin Exp Pharmacol Physiol. 2008;35:1454–60. doi: 10.1111/j.1440-1681.2008.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lijnen HR, van Hoef B, Beelen V, Collen D. Characterization of the murine plasma fibrinolytic system. Eur J Biochem. 1994;224:863–71. doi: 10.1111/j.1432-1033.1994.00863.x. [DOI] [PubMed] [Google Scholar]
- 16.Korninger C, Collen D. Studies on the specific fibrinolytic effect of human extrinsic (tissue-type) plasminogen activator in human blood and in various animal species in vitro. Thromb Haemost. 1981;46:561–5. [PubMed] [Google Scholar]
- 17.Serkova NJ, Van Rheen Z, Tobias M, Pitzer JE, Wilkinson JE, Stringer KA. Utility of magnetic resonance imaging and nuclear magnetic resonance-based metabolomics for quantification of inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L152–61. doi: 10.1152/ajplung.00515.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Southam DS, Dolovich M, O'Byrne PM, Inman MD. Distribution of intranasal instillations in mice: effects of volume, time, body position, and anesthesia. Am J Physiol Lung Cell Mol Physiol. 2002;282:L833–9. doi: 10.1152/ajplung.00173.2001. [DOI] [PubMed] [Google Scholar]
- 19.Loscalzo J, Braunwald E. Tissue plasminogen activator. N Engl J Med. 1988;319:925–31. doi: 10.1056/NEJM198810063191407. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Z, Kozlowski J, Schuster DP. Physiologic, biochemical, and imaging characterization of acute lung injury in mice. Am J Respir Crit Care Med. 2005;172:344–51. doi: 10.1164/rccm.200503-343OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker JC, Townsley MI. Evaluation of lung injury in rats and mice. Am J Physiol Lung Cell Mol Physiol. 2004;286:L231–46. doi: 10.1152/ajplung.00049.2003. [DOI] [PubMed] [Google Scholar]
- 22.Zwickl CM, Hughes BL, Piroozi KS, Smith HW, Wierda D. Immunogenicity of tissue plasminogen activators in rhesus monkeys: antibody formation and effects on blood level and enzymatic activity. Fundam Appl Toxicol. 1996;30:243–54. doi: 10.1006/faat.1996.0062. [DOI] [PubMed] [Google Scholar]
- 23.Card JW, Carey MA, Bradbury JA, DeGraff LM, Morgan DL, Moorman MP, et al. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. J Immunol. 2006;177:621–30. doi: 10.4049/jimmunol.177.1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carey MA, Card JW, Voltz JW, Germolec DR, Korach KS, Zeldin DC. The impact of sex and sex hormones on lung physiology and disease: lessons from animal studies. Am J Physiol Lung Cell Mol Physiol. 2007;293:L272–8. doi: 10.1152/ajplung.00174.2007. [DOI] [PubMed] [Google Scholar]
- 25.Shetty S, Padijnayayveetil J, Tucker T, Stankowska D, Idell S. The fibrinolytic system and the regulation of lung epithelial cell proteolysis, signaling, and cellular viability. Am J Physiol Lung Cell Mol Physiol. 2008;295:L967–75. doi: 10.1152/ajplung.90349.2008. [DOI] [PubMed] [Google Scholar]
- 26.Liu RM. Oxidative stress, plasminogen activator inhibitor 1, and lung fibrosis. Antioxid Redox Signal. 2008;10:303–19. doi: 10.1089/ars.2007.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishiuma T, Sisson TH, Subbotina N, Simon RH. Localization of plasminogen activator activity within normal and injured lungs by in situ zymography. Am J Respir Cell Mol Biol. 2004;31:552–8. doi: 10.1165/rcmb.2004-0162OC. [DOI] [PubMed] [Google Scholar]
- 28.Sakagami M. In vivo, in vitro and ex vivo models to assess pulmonary absorption and disposition of inhaled therapeutics for systemic delivery. Adv Drug Deliv Rev. 2006;58:1030–60. doi: 10.1016/j.addr.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Brain JD. Inhalation, deposition, and fate of insulin and other therapeutic proteins. Diabetes Technol Ther. 2007;9(Suppl 1):S4–S15. doi: 10.1089/dia.2007.0228. [DOI] [PubMed] [Google Scholar]
- 30.Folkesson HG, Matthay MA, Westrom BR, Kim KJ, Karlsson BW, Hastings RH. Alveolar epithelial clearance of protein. J Appl Physiol. 1996;80:1431–45. doi: 10.1152/jappl.1996.80.5.1431. [DOI] [PubMed] [Google Scholar]
- 31.Sixt SU, Beiderlinden M, Jennissen HP, Peters J. Extracellular proteasome in the human alveolar space: a new housekeeping enzyme? Am J Physiol Lung Cell Mol Physiol. 2007;292:L1280–8. doi: 10.1152/ajplung.00140.2006. [DOI] [PubMed] [Google Scholar]