Abstract
Hexavalent chromium (Cr(VI)) compounds are well-established human lung carcinogens. Solubility plays an important role in their carcinogenicity with the particulate Cr(VI) compounds being the most carcinogenic. Epidemiology and animal studies suggest that zinc chromate is the most potent particulate Cr(VI) compound, however, there are few comparative data to support these observations. The purpose of this study was to compare the genotoxicity of zinc chromate with two other particulate Cr(VI) compounds, barium chromate and lead chromate, and one soluble Cr(VI) compound, sodium chromate. The clastogenic effects of barium chromate and zinc chromate were similar but lead chromate induced significantly less damage. The levels of DNA damage measured by gamma-H2A.X foci formation were similar for the three particulate chromium compounds. Corrected for chromium uptake differences, we found that zinc chromate and barium chromate were the most cytotoxic and lead chromate and sodium chromate were less cytotoxic. Zinc chromate was more clastogenic than all other chromium compounds and lead chromate was the least clastogenic. There was no significant difference between any of the compounds for the induction of DNA double strand breaks. All together, these data suggest that the difference in the carcinogenic potency of zinc chromate over the other chromium compounds is not due solely to a difference in chromium ion uptake and the zinc cation may in fact have an important role in its carcinogenicity.
Keywords: hexavalent chromium, zinc chromate, lead chromate, barium chromate, sodium chromate, cytotoxicity, chromosome damage, DNA damage
Introduction
Hexavalent chromium (Cr(VI)) compounds are well-established human lung carcinogens, however, Cr(VI) compounds appear to have different potencies. Solubility plays a key role in the potency of the various Cr(VI) compounds with the most potent compounds identified as the water-insoluble or `particulate' Cr(VI) compounds (1–4). Human autopsy studies of chromium workers have shown that chromium accumulates and persists at bronchial bifurcation sites where particles typically settle by impaction and these sites are where most Cr(VI)-induced tumors commonly form (5,6).
Epidemiology and whole animal studies suggest that zinc chromate may be the more potent particulate Cr(VI) compound (2,7–13). Langard and Vigander, 1983 found that all but one of their Cr(VI)-induced cancer cases were specifically exposed to zinc chromate (8). Another study reported increased cancer risk, particularly in workers only exposed to zinc chromate (14). Three out of twenty chromium compounds, one of which was zinc chromate, were found to significantly increase bronchial carcinomas in the rat lung in whole animal studies (15).
Cell culture studies also support a conclusion that zinc chromate is a potent carcinogen. For example, zinc chromate induced morphological transformation of Syrian hamster embryo cells (13) and chromosome aberrations and sister chromatid exchanges in CHO cells (16). More recently, data show that zinc chromate induces chromosome damage and DNA double strand breaks in human lung cells (17). However, while the epidemiology and whole animal studies suggest that zinc chromate is more potent than other Cr(VI) compounds, few studies have been performed to test this hypothesis directly. In fact, there are limited studies on Cr(VI) particulate compounds in general, as most investigators have focused on the less potent soluble compounds.
Of the available particulate Cr(VI) data, lead chromate has been studied the most. Lead chromate has been shown to induce tumors in experimental animals (1) and to induce genotoxicity (18–22), centrosome amplification (23), spindle assembly checkpoint bypass (24) and neoplastic transformation (25,26) in human lung cells. Barium chromate has been less studied and did not induce tumors in experimental animals (1,2). Undissolved barium chromate particles were mutagenic in Chinese hamster lung cells (27) and genotoxic to human lung cells (29), and pre-dissolved barium chromate particles induced neoplastic transformation of Syrian hamster embryo (SHE) cells (13). However, direct comparisons of these compounds with zinc chromate have not been done. Accordingly, this study compares the cytotoxicity and genotoxicity of three particulate Cr(VI) compounds, zinc chromate, barium chromate and lead chromate, and compares them with a soluble Cr(VI) compound, sodium chromate.
Methods
Chemicals and Reagents
A 50:50 mixture of Dulbecco's minimal essential medium and Ham's F-12 (DMEM/F-12) was purchased from Mediatech Inc. (Herndon, VA). Sodium pyruvate, penicillin/streptomycin, Gurr's buffer, trypsin/EDTA, and L-glutamine were purchased from Invitrogen Corporation (Grand Island, NY). Crystal violet and methanol were purchased from J.T. Baker (Phillipsburg, NJ) and cosmic calf serum (CCS) was purchased from Hyclone, (Logan, UT). Tissue culture dishes, flasks, and plastic-ware were purchased from Corning Inc. (Acton, MA). Lead chromate, sodium chromate, barium chromate, potassium chloride (KCl) and demecolchicine were purchased from Sigma/Aldrich. Zinc chromate was purchased from Alfa Aesar (Ward Hill, MA). Giemsa stain was purchased from Biomedical Specialties Inc. (Santa Monica, CA).
Cell Culture
WTHBF-6 cells, a clonal cell line derived from primary human bronchial fibroblasts (PHBF) with reconstituted telomerase activity and a genotoxic and cytotoxic response to metals which is the same as their parent cells (19), were routinely cultured in DMEM/F-12 supplemented with 15% CCS, 2 mM L-glutamine, 100 U/ml penicillin/100 μg/ml streptomycin, and 0.1 mM sodium pyruvate. Cells were maintained as adherent subconfluent monolayers by feeding at least twice weekly and subculturing at least once a week using 0.25% trypsin/1mM EDTA solution. Cells were tested routinely for mycoplasma contamination. All experiments were conducted on logarithmically growing cells.
Preparation of Cr(VI) Compounds
Lead chromate (CAS #7758-97-6, ACS reagent minimum 98% purity), barium chromate (CAS# 10294-40-3, ACS reagent minimum 98% purity) and zinc chromate (CAS#13530-65-9, ACS reagent minimum 98% purity) were used as particulate compounds. Lead chromate, zinc chromate and barium chromate were administered as suspensions as previously described (16,19). Sodium chromate (CAS #7775-11-3, ACS reagent minimum 98% purity) was used as a soluble compound and was administered as a solution in water as previously described (19).
Cytotoxicity
Cytotoxicity was determined by a clonogenic assay, which measures the reduction in plating efficiency in treatment groups relative to the controls as previously described (19). Briefly, ninety thousand cells were seeded into a 6 well tissue culture plate and allowed to grow for 48 h. The cultures were then treated for 24 h with chromate. After the exposure time, the treatment medium was collected (to include any loosely adherent mitotic cells); the cells were rinsed with PBS; and then removed from the dish with 0.25 % trypsin/1mM EDTA solution. Cells were centrifuged at 1000 rpm, 4°C for 5 min. The resulting pellet was re-suspended in 5 ml of medium, counted with a Coulter Multisizer III, and reseeded at colony forming density. Colonies were allowed to grow for 10 days, fixed with 100% methanol, stained with crystal violet, and the colonies counted. There were four dishes per treatment group and each experiment was repeated at least three times.
Clastogenicity
Clastogenicity was determined by measuring the production of chromosomal aberrations based on our published methods with minor modifications (19). Briefly, logarithmically growing cells were seeded in 100 mm dishes; allowed 48 h to plate and resume normal cycling; and then treated for 24 h with chromate. Shortly before the end of the treatment time, 0.1 g/ml demecolchicine was added to block the cells in metaphase. At the end of treatment, the culture medium and adherent cells were collected. Cells were then resuspended in 0.075M KCl hypotonic solution for 17 min to swell the cells and the nuclei. Next, cells were fixed in 3:1 methanol:acetic acid and the fixative was changed twice. Finally, cells were dropped onto clean wet slides and uniformly stained with 5% Giemsa stain in Gurr's buffer.
Chromosomal aberrations were scored blind using standard criteria by two different scorers (19). Because deletions can only be unequivocally distinguished from achromatic lesions if the distal acentric fragment is displaced, these aberrations were pooled. This approach also helps avoid artificial discrepancies between scorers due to different perceptions of the width of an achromatic lesion relative to the width of its chromatid. Furthermore, recent data show a much stronger correlation between DNA strand breaks and chromosomal aberrations if these achromatic lesions and deletions are pooled (30). Accordingly, chromatid deletions and achromatic lesions were pooled as chromatid lesions and isochromatid deletions and isochromatid achromatic lesions were pooled as isochromatid lesions. One hundred metaphases per data point were analyzed in each experiment. Each experiment was repeated at least three times.
Immunofluorescence for gamma-H2A.X foci formation
Gamma-H2A.X foci were used to detect the presence of chromate-induced DSBs. Cells were grown on chamber slides. After treatment with Cr(VI) for 24 h, the cells were fixed in 4% paraformaldehyde for 10 min, permeabilized with 0.2% Triton X-100 for 5 min and blocked with 1% BSA and 5% horse serum (Jackson Immunolaboratories, West Grove, PA) for 1 h. Cells were then incubated with anti-gamma-H2AX antibody (Cell Signaling, Beverly, MA) at 4 °C overnight and incubated with a FITC AlexaFluor 484-conjugated goat anti-rabbit IgG secondary antibody for 1 h. Nuclei were counterstained with propidium iodide. The slides were mounted and viewed with an Olympus laser scanning confocal microscope (LSCM) using a 60X objective. Images of the same antibody staining were obtained using the same LCSM parameters (brightness, contrast, pinhall, etc.).
Intracellular Chromium Ion Measurement
Intracellular chromium ion levels were determined according to previously published methods (31). Briefly, a monolayer of cells was treated for 0 or 24 h with varying concentrations of lead chromate, barium chromate, zinc chromate and sodium chromate. The cells were harvested and treated with a hypotonic solution followed by 2% SDS. This solution was sheered through a needle seven times and filtered into a vial. Chromium concentrations in the intracellular samples were then measured using a Perkin Elmer Optima 2000 inductively coupled plasma optical emission spectrometer (ICP-OES), equipped with a gem cone low flow nebulizer. Solutions were introduced to the nebulizer using a peristaltic pump operating at 2 mL/min. Samples of intracellular fluids were diluted 5× in 0.16 M aqueous HNO3 prior to analysis. Chromium was determined using emission wavelength at 267.716 with a minimum detection limit of 2 ppb. Yttrium (Y) was used as an internal standard for chromium determinations.
To account for the possibility that some undissolved particles may have passed through the filter or that some particles may have adhered to the outer membrane of the cells, a 0 h treatment was performed for each concentration. The final concentrations were corrected for this possible confounding factor by subtracting the 0 h values from the 24 h values. The corrected intracellular concentrations were converted from ug/L to uM by dividing by the volume of the sample, the atomic weight of the chemical, the number of cells in the sample and the average cell volume (determined to be 1.125 picoliters by a Beckman Coulter Multisizer 3).
Statistics
Linear regression analysis was employed to evaluate the relationship between administered dose and intracellular concentration. Comparisons among individual pairs of doses employed Student's t-test. Dose-response relationships for cytotoxicity, percent damage, total damage, and number of foci were fit using linear and polynomial regression analysis. Interactions between dose or concentration and type of chromate were evaluated by adding product terms to the regression models. Differences among the types of chromate were assessed using t-tests for linear combinations of parameters obtained from the regression analyses. Ninety-five percent confidence intervals were calculated based on the variance-covariance matrix. All analyses were conducted using SAS (32).
Results
Cytotoxicity of Hexavalent Chromium Compounds
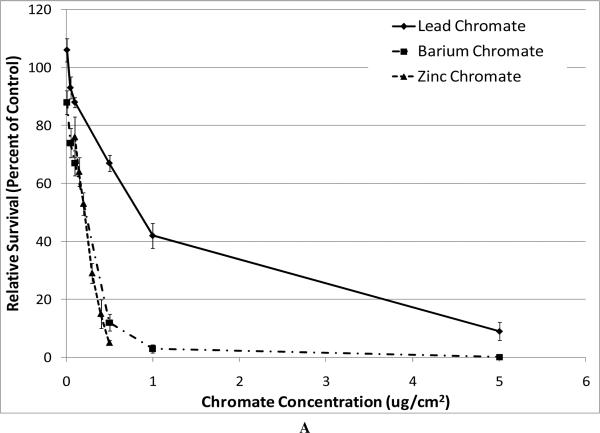
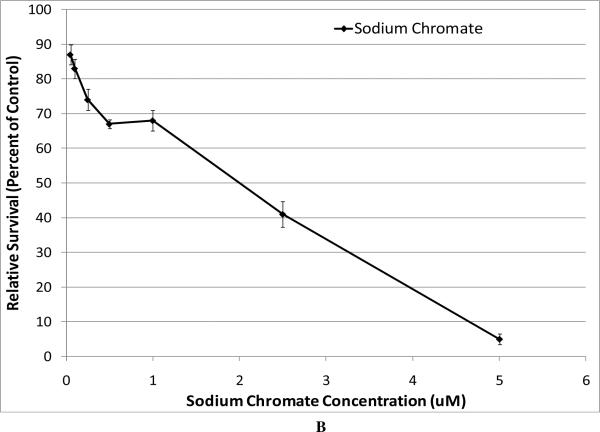
All compounds induced a concentration-dependent decrease in relative survival (Figure 1). When we compared the cytotoxicity of the particulate compounds based on administered concentration, we found that zinc chromate and barium chromate were significantly more cytotoxic than lead chromate. For example, at an administered concentration of 0.5 ug/cm2, lead chromate induced 67% survival compared to barium chromate which induced 12% survival and zinc chromate which induced 5% survival (Figure 1A). Sodium chromate was also cytotoxic with treatments of 0.05, 0.1, 0.25, 0.5, 1, 2.5, 5 and 10 uM sodium chromate inducing 87, 83, 74, 67, 68, 41, 5 and 0 percent relative survival, respectively (Figure 1B). Sodium chromate could not be compared directly to the particulates because of its water solubility.
Figure 1. Cytotoxicity of Four Cr(VI) Compounds.
This figure shows the cytotoxic effect of the chromium compounds. All compounds induced a concentration-dependent decrease in relative survival. Data represent an average of at least three experiments ± SEM for all four compounds. Panel A shows the cytotoxicity of the particulate compounds. Lead chromate concentrations of 0.01, 0.05, 0.1, 0.5, 1, 5 and 10 ug/cm2 induced 106, 93, 88, 67, 42, 9 and 1 percent relative survival, respectively; barium chromate concentrations of 0.01, 0.05, 0.1, 0.5, 1, 5 and 10 ug/cm2 induced 88, 74, 67, 12, 3, 0.1 and 0 percent relative survival, respectively; zinc chromate concentrations of 0.1, 0.15, 0.2, 0.3, 0.4 and 0.5 ug/cm2 induced 76, 64, 53, 29, 15, and 5 percent relative survival, respectively. For lead chromate, concentrations of 0.1, 0.5, 1, and 5 ug/cm2 are statistically different from control (p < 0.001); for barium chromate, concentrations of 0.05, 0.1, 0.5 and 1 ug/cm2 are statistically different from control (p < 0.05); for zinc chromate, 0.15, 0.2, 0.3, 0.4, and 0.5 ug/cm2 concentrations are statistically different from control (p < 0.005). Lead chromate was statistically different from barium chromate and zinc chromate at all concentrations (p < 0.05). Panel B shows the cytotoxicity of sodium chromate, administered concentrations of 0.05, 0.1, 0.25, 0.5, 1, 2.5, 5 and 10 uM sodium chromate induced 87, 83, 74, 67, 68, 41, 5 and 0 percent relative survival, respectively. All concentrations were statistically different from control (p < 0.05).
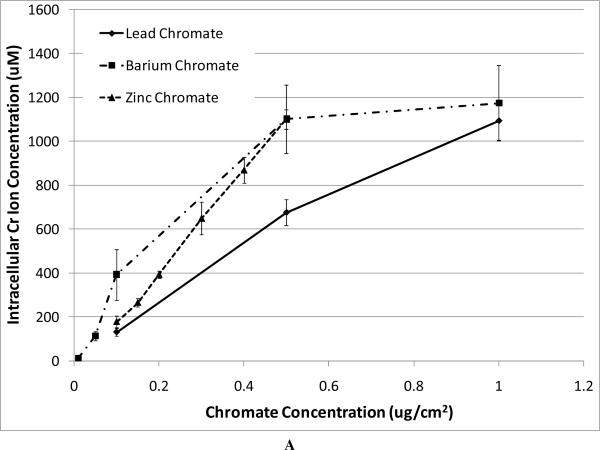
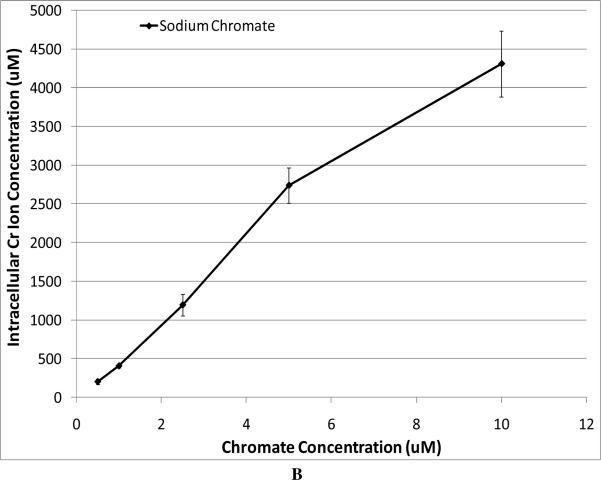
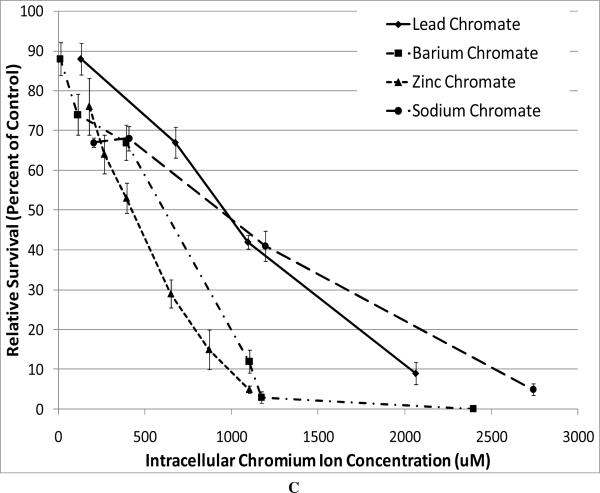
In order to compare the soluble compound with the particulate ones, we have to consider the intracellular Cr levels. Figure 2A shows that particulate chromate compounds induce similar levels of intracellular chromium, and Figure 2B shows the intracellular chromium levels achieved after exposure to sodium chromate. Thus, using these data we compared the cytotoxicity of all four compounds (Figure 2C). The data show that based on intracellular chromium concentration, there is less difference among zinc chromate, barium chromate, and lead chromate, but the trend remains with zinc chromate being the most cytotoxic. Sodium chromate induces a similar toxic effect as that of lead chromate. For example, at an intracellular Cr concentration of 1000 uM, lead chromate and sodium chromate induced 50% and 48% survival, respectively, compared to 20 % for barium chromate and only 9% for zinc chromate.
Figure 2. Intracellular Cr Ion Concentration after Exposure to Four Cr(VI) Compounds.
This figure shows the intracellular Cr concentrations achieved by the administered concentrations of the chromium compounds and compares the intracellular Cr concentration to the measured effects. All administered concentrations of the chromium compounds induced statistically significant increases in intracellular chromium ion concentrations (p < 0.05). Data represent an average of at least three experiments ± SEM for all four compounds. Panel A shows that exposure to particulate chromates (lead chromate, barium chromate and zinc chromate) induced concentration-dependent increases in intracellular Cr ion concentrations. All administered concentrations of lead chromate and zinc chromate induced a statistically significant increase in intracellular chromium ion concentrations (p < 0.05). For lead chromate, administered concentrations of 0.1, 0.5, 1 and 5 ug/cm2 yielded intracellular Cr concentrations of 131, 677, 1095 and 2065 uM respectively; for zinc chromate administered concentrations of 0.1, 0.15, 0.2, 0.3, 0.4 and 0.5 ug/cm2 yielded intracellular Cr concentrations of 179, 266, 394, 650, 870, and 1102 uM, respectively; for barium chromate administered concentrations of 0.01, 0.05, 0.1, 0.5, 1, and 5 ug/cm2 yielded intracellular Cr concentrations of 13, 113, 393, 1103, 1174, and 2396 uM, respectively. Concentrations of 0.05, 0.1, and 0.5 ug/cm2 barium chromate induced statistically significant increases in intracellular chromium ion concentrations (p < 0.05). Comparing the particulate compounds, only the concentration of 0.5 ug/cm2 induced a statistically significant difference in chromium ion concentration between lead chromate and zinc chromate (p < 0.001).
Panel B shows that exposure to soluble (sodium) chromate induced concentration-dependent increases in intracellular Cr ion concentrations. For soluble chromium, administered treatments of 0.5, 1, 2.5, 5, and 10 uM sodium chromate yielded intracellular Cr concentrations of 204, 407, 1195, 2740, and 4313 uM, respectively. Panel C shows the cytotoxic effect of all four compounds corrected for intracellular chromium ion concentration. Barium chromate and zinc chromate were significantly more cytotoxic than lead chromate and sodium chromate (p < 0.005); there was not a significant difference between barium chromate and zinc chromate or between lead chromate and sodium chromate.
Genotoxicity of Hexavalent Chromium Compounds
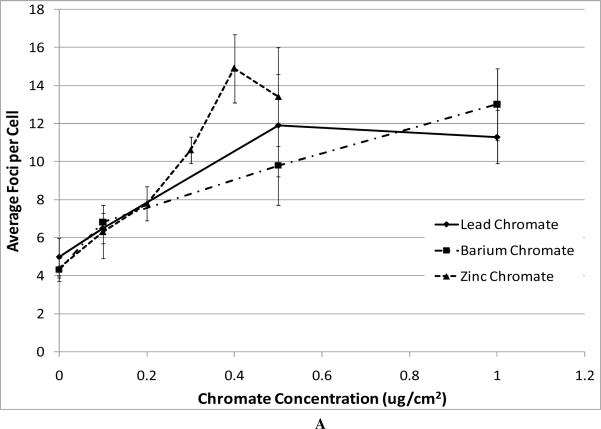
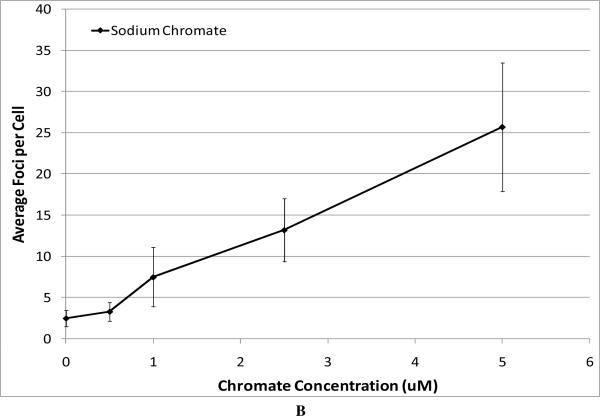
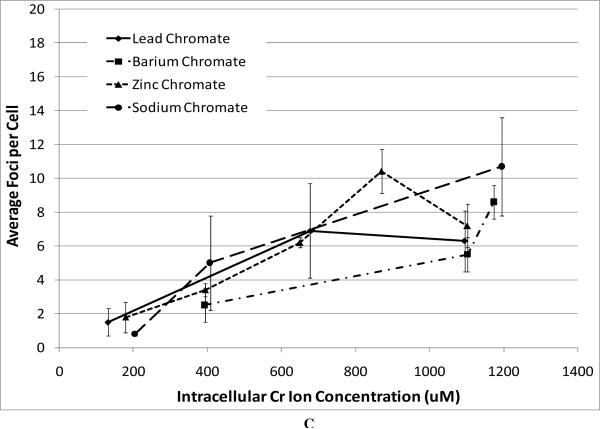
Next, we compared the genotoxicity of these four compounds. We considered chromosomal aberrations as a measure of large-scale DNA damage and DNA double strand breaks as a measure of damage on a finer scale. DNA double strand breaks were measured as the formation of gamma-H2A.X foci (Figure 3). All compounds induced a concentration-dependent increase in the amount of DNA double strand breaks (Figure 3). We found that the level of DNA double strand breaks was similar for all three particulate chromate compounds based on administered concentrations (Figure 3A). For example, concentrations of 0.5 ug/cm2 lead chromate, zinc chromate, and barium chromate induced an average of 11.9, 13.4, and 9.8 foci per cell, respectively. Sodium chromate also induced DNA double strand breaks (Figure 3B). Using the uptake data from Figure 2, we show that for all four compounds there was no difference in the DNA double strand breaks based on intracellular chromium concentration (Figure 3C).
Figure 3. Genotoxicity of Four Cr(VI) Compounds.
This figure shows the induction of DNA double strand breaks measured by gama-H2A.X foci formation. All of the particulate compounds induced a concentration-dependent increase in the average number of foci per cell. Data represent an average of three experiments ± SEM for all four compounds. Panel A shows that exposure to lead chromate, barium chromate and zinc chromate induced concentration-dependent increases in DNA double strand breaks. Lead chromate was statistically different from control at 1 ug/cm2 (p < 0.05); barium chromate was statistically different from control at 0.1 and 1 ug/cm2 (p < 0.05); zinc chromate was statistically different from control at 0.2, 0.3 and 0.4 ug/cm2 (p < 0.05). Panel B shows that exposure to sodium chromate induced concentration-dependent increases in DNA double strand breaks. Administered concentrations of 0, 0.5, 1, 2.5 and 5 uM sodium chromate induced an average of 2.5, 3.3, 7.5, 13.2 and 25.7 foci per cell, respectively. Panel C shows that all compounds induced similar levels of DNA double strand breaks at similar intracellular concentrations, for example 500 uM intracellular Cr induced 3, 4, 5 and 6 average foci per cell after exposure to barium chromate, zinc chromate, lead chromate and sodium chromate respectively.
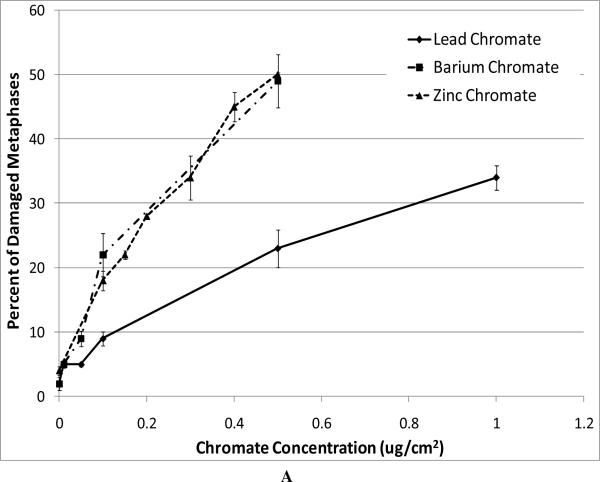
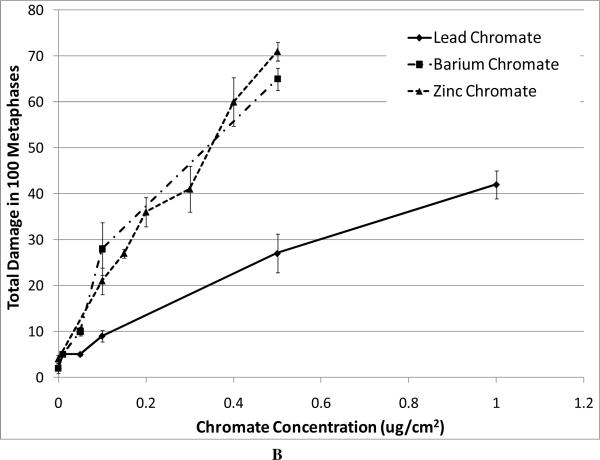
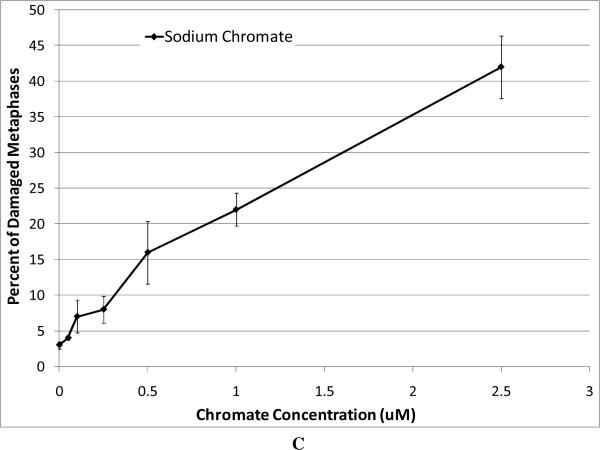
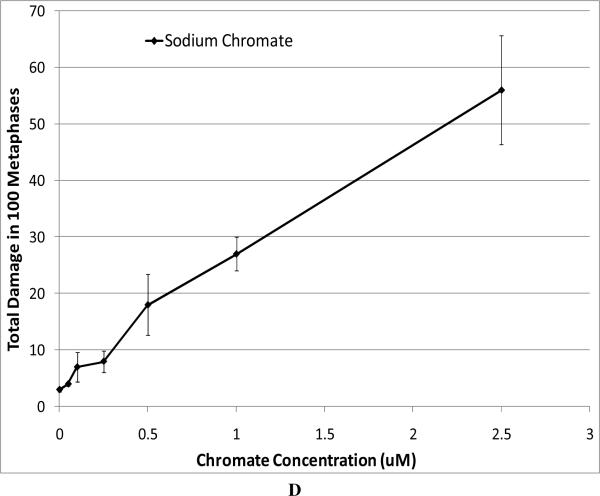
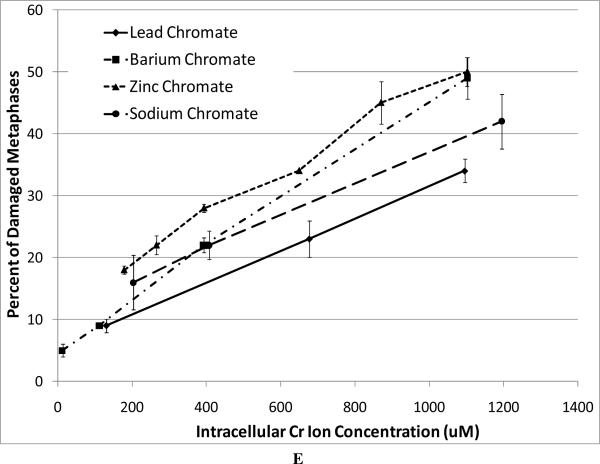
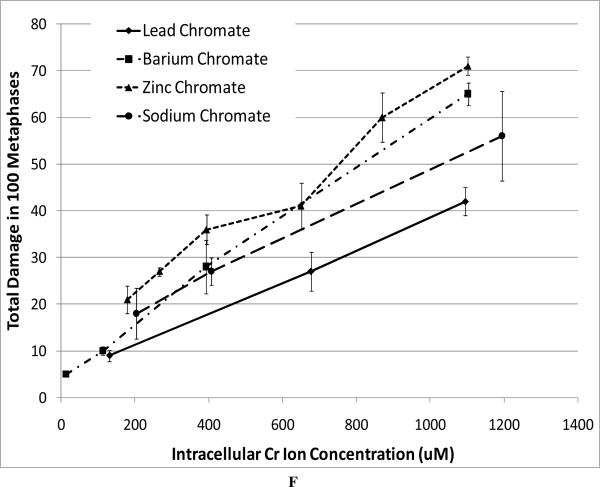
For our chromosome damage studies, we considered two measures of damage. We measured the percent of metaphases with damage, which determines the frequency of damaged cells. We also measured the total aberrations per 100 metaphases which measures the extent of damage within cells. Considered together, the difference in magnitude between the two genotoxicity measures indicates an increasing number of cells with multiple aberrations. All compounds induced a concentration-dependent increase in the percent of metaphases with damage and the total aberrations per 100 metaphases (Figure 4). Our data show that zinc chromate and barium chromate are the most clastogenic, while lead chromate is the least clastogenic. For example, concentrations of 0.5 ug/cm2 lead chromate, zinc chromate, and barium chromate induced 23, 50, and 49 percent of damaged metaphases, respectively (Figure 4A); and 27, 71, and 65 total chromosome aberrations per 100 cells, respectively (Figure 4B). Sodium chromate also induced a concentration-dependent increase in the percent of metaphases with damage and the total aberrations per 100 metaphases (Figure 4C). Using the uptake data from Figure 2, we show that when corrected based on intracellular chromium concentration, zinc chromate is the most clastogenic, followed by barium chromate and sodium chromate (Figure 4E). Lead chromate was the least clastogenic for both measurements and was statistically different from all other compounds (Figure 4E, F).
Figure 4. Clastogenicity of Four Cr(VI) Compounds.
This figure shows the clastogenic effect of the four chromium compounds. All four compounds induced a concentration-dependent increase in both the percent of damaged metaphases and in the total number of aberrations per 100 metaphases. Data represent an average of at least three experiments ± SEM for all four compounds. Panels A and B show the effect of lead chromate, barium chromate and zinc chromate based on administered concentration. For both percent of metaphases with damage and total damage, lead chromate was statistically different from control at all concentrations (p < 0.05); barium chromate was statistically different from control at concentrations of 0.5, 1, and 5 ug/cm2 (p < 0.05); zinc chromate was statistically different from control at all concentrations (p < 0.05). For the percent of metaphases with damage, lead chromate was statistically different from barium chromate and zinc chromate at concentrations of 0.1 and 0.5 ug/cm2 (p < 0.05). For total damage, lead chromate was statistically different from barium chromate at concentrations of 0.05 and 0.5 ug/cm2 and zinc chromate at concentrations of 0.1 and 0.5 ug/cm2 (p < 0.05). Panels C and D show the effect of sodium chromate based on administered dose. Concentrations of 1 and 2.5 uM are statistically different from control for both measurements (p < 0.05). Panels E and F show the clastogenic effect for all four compounds based on intracellular chromium ion concentrations. For the percent of damaged metaphases (Panel E), all the compounds were statistically different from one another (p < 0.01) except sodium chromate and barium chromate. Intracellular Cr ion concentrations of 500 uM induced approximately 18, 24, 26 and 30 percent damaged metaphases after exposure to lead chromate, zinc chromate, sodium chromate and barium chromate, respectively. For the total damage in 100 metaphases (Panel F), all the compounds were statistically different from one another (p < 0.01) except sodium chromate and barium chromate, and barium chromate and zinc chromate. Intracellular concentrations of 500 uM Cr induced approximately 21, 30, 33, and 38 damaged chromosomes after exposure to lead chromate, zinc chromate, sodium chromate and barium chromate, respectively.
Discussion
Particulate hexavalent chromium compounds are known human lung carcinogens. Zinc chromate appears to be a particularly potent Cr(VI) compound but only a few studies have considered it, reporting elevated carcinogenic risk in human workers, tumors in experimental animals and genotoxicity in human lung cells (8–12). However, the potency of zinc chromate has not been carefully compared to other Cr(VI) compounds. This study presents a direct comparison of the cytotoxic and genotoxic effects of zinc chromate with two other particulate compounds (lead chromate and barium chromate) and one soluble compound (sodium chromate). We find that consistent with the animal and epidemiological literature (8–12), zinc chromate is more cytotoxic and genotoxic than either sodium chromate or lead chromate with respect to cytotoxicity and chromosome damage. Consideration of the intracellular Cr ion levels indicates that this difference in potency is not simply due to differences in Cr ion uptake.
The explanation for these differences is uncertain. Previous studies with lead chromate have shown that the Cr ion is responsible for the cytotoxicity, genotoxicity, cell cycle and centrosome amplification effects of this compound and that it is similar to sodium chromate (33,34). The data in this report support those previous studies but indicate that a different mechanism may be occurring for zinc chromate that makes it more potent than lead chromate or sodium chromate. Most likely, the explanation for the increased cytotoxicity is a result of the increased genotoxicity observed. The underlying cause of the genotoxicity, however, is more uncertain.
One hypothesis, albeit untested, is that the zinc counterion may be playing a role. Zinc is an essential metal and is involved in hundreds of biological components such as DNA-binding proteins containing zinc fingers. There is extensive research on the effects of zinc deficiency and their role in cancer predisposition (35), but little is known about excessive zinc, because it is generally considered nontoxic. There is some work on zinc and cadmium interactions and generally zinc inhibits cadmium-induced carcinogenesis (36). However, there are some data showing that zinc facilitates cadmium-induced prostate cancer (36–37). The mechanism appears to be zinc blocking cadmium toxicity in the testes, leading to increased production of testicular hormones, which can then foster the development of prostate cancer. Some evidence suggests that zinc may have a role as a co-carcinogen by suppressing G2/M checkpoints (38) or by enhancing the genomic instability induced by other agents (35, 39); such a mechanism might fit if it is enhancing the genomic instability induced by Cr(VI). This possibility would suggest that zinc was active while lead ions were not. Such a possibility is consistent with the fact that lead ions are much bigger and bulkier than zinc ions and thus may be more able to interact with macromolecules. Further research will consider this possibility.
It is interesting to note that barium chromate and zinc chromate were similarly cytotoxic and genotoxic, but animal studies show that while zinc chromate induces tumors in experimental animals, barium chromate does not (2). The explanation for this difference is uncertain. It may reflect the fact that only a few animal studies have been done for barium chromate, and thus, it may not have been sufficiently studied (1,2). Alternatively, it may further suggest that the zinc is playing a co-carcinogen role that the larger barium ions cannot fulfill.
It is also interesting to note that zinc chromate and lead chromate were equipotent in their ability to induce DNA double strand breaks, but that there were significant differences in the amount of chromosome damage. This difference seems surprising given that double strand breaks are thought to lead to the breaks. However, it should be noted that the expression of the DNA double strand breaks occurs in G2 of the cell cycle (17), while the chromosome aberrations occur in M phase. Some breaks will be repaired, or cells with them may undergo apoptosis and thus, not all breaks will manifest themselves as chromosomal aberrations. One possible mechanism is that the zinc may be inhibiting the repair of these lesions, causing more of them to manifest as chromosome aberrations. Such a possibility is consistent with observations that excess zinc can increase chromosome instability (35, 39), though more study is needed to determine if this mechanism is indeed occurring. It could also be that the zinc ions are inhibiting apoptosis causing more breaks to manifest themselves as chromosomal aberrations, but such an explanation seems unlikely given the increased cytotoxicity we observed after zinc chromate exposure. Finally, it could be that some portion of the gamma-H2A.X foci do not represent breaks, but rather sites of chromatin remodeling. While it is well established that most gamma H2A.X do represent DNA double strand breaks (40), some are also involved in chromatin remodeling sites (41) and thus, it could be that lead chromate induced the same amount of gamma-H2A.X foci because it induced more chromatin remodeling sites than zinc chromate.
In sum, the data show that zinc chromate is indeed one of the more potent hexavalent chromium compounds in human lung cells. The explanation for its increased potency is uncertain but may involve effects of the zinc ion on DNA repair. Future research is aimed at understanding the co-carcinogenic effects of zinc and chromate.
Acknowledgements
We would like to thank Geron Corporation for the use of the hTERT materials. We would also like to thank Tolga Cavas, Julie Schuler, Jamie Young, Joe Keierleber, Wendy Harstock and Michael Ketterer for technical support; and Christy Gianios, Jr. for technology support. This work was supported by NIEHS grant ES016893 (J.P.W.), cooperative agreement #EP-08-01 through the Maine Space Grant Consortium (J.P.W.) and the Maine Center for Toxicology and Environmental Health.
References
- 1.IARC . Monographs on the Evaluation of Carcinogenic Risks to Humans: Chromium, Nickel and Welding. Vol. 49. Lyons; France: 1990. [PMC free article] [PubMed] [Google Scholar]
- 2.Levy LS, Vanitt S. Carcinogenicity and mutagenicity of chromium compounds: The association between bronchial metaplasia and neoplasia. Carcinogenesis. 1986;7:831–835. doi: 10.1093/carcin/7.5.831. [DOI] [PubMed] [Google Scholar]
- 3.Léonard A, Lauwerys RR. Carcinogenicity and mutagenicity of chromium. Mutat. Res. 1980;76:227–239. doi: 10.1016/0165-1110(80)90018-4. [DOI] [PubMed] [Google Scholar]
- 4.Patierno SR, Banh D, Landolph JR. Transformation of C3H/10T1/2 mouse embryo cells by insoluble lead chromate but not soluble calcium chromate: Relationship to mutagenesis and internalization of lead chromate particles. Cancer Res. 1988;47:3815–3823. [PubMed] [Google Scholar]
- 5.Ishikawa Y, Nakagawa K, Satoh Y, Kitagawa T, Sugano H, Hirano T, Tsuchiya E. Characteristics of chromate workers' cancers, chromium lung deposition and precancerous bronchial lesions: an autopsy study. Br. J. Cancer. 1994;70:160–166. doi: 10.1038/bjc.1994.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishikawa Y, Nakagawa K, Satoh Y, Kitagawa T, Sugano H, Hirano T, Tsuchiya E. “Hot spots” of chromium accumulation at bifurcations of chromate workers' bronchi. Cancer Res. 1994;54:2342–2346. [PubMed] [Google Scholar]
- 7.Kano K, Horikawa M, Utsunomiya T, Tati M, Satoh K, Yamaguchi S. Lung cancer mortality among a cohort of male chromate pigment workers. Japan Int. J. Epidemiol. 1993;22:16–22. doi: 10.1093/ije/22.1.16. [DOI] [PubMed] [Google Scholar]
- 8.Sheffet A, Thind I, Miller AM, Louria DB. Cancer mortality in a pigment plant utilizing lead and zinc chromates. Arch. Environ. Health. 1982;37:44–52. doi: 10.1080/00039896.1982.10667532. [DOI] [PubMed] [Google Scholar]
- 9.Dalager NA, Mason TJ, Fraumeni JF, Jr., Hoover R, Payne WW. Cancer mortality among workers exposed to zinc chromate paints. J. Occup. Med. 1980;22:25–29. [PubMed] [Google Scholar]
- 10.Davies JM. Lung cancer mortality among workers making lead chromate and zinc chromate pigments at three English factories. Br. J. Ind. Med. 1984;41:158–169. doi: 10.1136/oem.41.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langård S, Vigander T. Occurrence of lung cancer in workers producing chromium pigments. Br. J. Ind. Med. 1983;40:71–74. doi: 10.1136/oem.40.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langård S. One hundred years of chromium and cancer: a review of epidemiological evidence and selected case reports. Am. J. Ind. Med. 1990;17:189–215. doi: 10.1002/ajim.4700170205. [DOI] [PubMed] [Google Scholar]
- 13.Elias Z, Poirot O, Pezerat H, Suquet H, Schneider O, Danière MC, Terzetti F, Baruthio F, Fournier M, Cavelier C. Cytotoxic and neoplastic transforming effects of industrial hexavalent chromium pigments in Syrian hamster embryo cells. Carcinogenesis. 1989;10:2043–2052. doi: 10.1093/carcin/10.11.2043. [DOI] [PubMed] [Google Scholar]
- 14.Langard S, Norseth T. A cohort study of bronchial carcinoma in workers producing chromate pigments. Br. J. Ind. Med. 1975;32:62–953. doi: 10.1136/oem.32.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beaver LM, Stemmy EJ, Constant SL, Schwartz A, Little LG, Gigley JP, Chun G, Sugden KD, Ceryak SM, Patierno SR. Lung injury, inflammation and Akt signaling following inhalation of particulate hexavalent chromium. Toxicol. Appl. Pharmacol. 2009;235:47–56. doi: 10.1016/j.taap.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levis AG, Majone A. Cytotoxic and clastogenic effects of soluble and insoluble compounds containing hexavalent and trivalent chromium. Br. J. Cancer. 1981;44:219–235. doi: 10.1038/bjc.1981.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie H, Holmes AL, Young JL, Qin Q, Joyce K, Pelsue SC, Peng C, Wise SS, Jeevarjan AS, Wallace WT, Hammond D, Wise JP., Sr. Zinc chromate induces chromosome instability and DNA double strand breaks in human lung cells. Toxicol. Appl. Pharmacol. 2009;234:293–299. doi: 10.1016/j.taap.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh J, Pritchard DE, Carlisle DL, Mcean JA, Montaser A, Orenstein JM, Patierno SR. Internalization of carcinogenic lead chromate particles by cultured normal human lung epithelial cells: formation of intracellular lead-inclusion bodies and induction of apoptosis. Toxicol. Appl. Pharmacol. 1999;161:240–248. doi: 10.1006/taap.1999.8816. [DOI] [PubMed] [Google Scholar]
- 19.Wise JP, Wise SS, Little JE. The cytotoxicity and genotoxicity of particulate and soluble hexavalent chromium in human lung cells. Mutat. Res. 2002;517:221–229. doi: 10.1016/s1383-5718(02)00071-2. [DOI] [PubMed] [Google Scholar]
- 20.Xie H, Wise SS, Holmes AL, Xu B, Wakeman T, Pelsue SC, Singh NP, Wise JP., Sr. Carcinogenic lead chromate induces DNA double-strand breaks and activates ATM kinase in human lung cells. Mutat. Res. 2005;586:160–172. doi: 10.1016/j.mrgentox.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wise SS, Holmes AL, Wise JP., Sr. Particulate and soluble hexavalent chromium are cytotoxic and genotoxic to human lung epithelial cells. Mutat. Res. 2006;610:2–7. doi: 10.1016/j.mrgentox.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Holmes AL, Wise SS, Sandwick SJ, Wise JP., Sr. The clastogenic effects of chronic exposure to particulate and soluble Cr(VI) in human lung cells. Mutat. Res. 2006;610:8–13. doi: 10.1016/j.mrgentox.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Holmes AL, Wise SS, Sandwick SJ, Lingle WL, Negron VC, Thompson WD, Wise JP., Sr. Chronic exposure to lead chromate causes centrosome abnormalities and aneuploidy in human lung cells. Cancer Res. 2006;66:4041–4048. doi: 10.1158/0008-5472.CAN-05-3312. [DOI] [PubMed] [Google Scholar]
- 24.Wise SS, Holmes AL, Xie H, Thompson WD, Wise JP., Sr. Chronic exposure to particulate chromate induces spindle assembly checkpoint bypass in human lung cells. Chem. Res. Toxicol. 2006;19:1492–1498. doi: 10.1021/tx0601410. [DOI] [PubMed] [Google Scholar]
- 25.Xie H, Holmes AL, Wise SS, Huang S, Peng C, Wise JP., Sr. Neoplastic transformation of human bronchial cells by lead chromate particles. Am. J. Respir. Cell Mol. Biol. 2007;37:544–552. doi: 10.1165/rcmb.2007-0058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie H, Wise SS, Wise JP., Sr. Deficient repair of particulate chromate-induced DNA double strand breaks leads to neoplastic transformation. Mutat. Res. 2008;649:230–238. doi: 10.1016/j.mrgentox.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein CB, Lin S, Bowser D, Leszczynska J. Chromate-induced epimutations in mammalian cells. Environ. Health Perspect. 2002;110(5):739–743. doi: 10.1289/ehp.02110s5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wise SS, Schuler JHC, Katsifis SP, Wise JP., Sr. Barium chromate is cytotoxic and genotoxic to human lung cells. Environ. Mol. Mutagen. 2004;42:274–278. doi: 10.1002/em.10203. [DOI] [PubMed] [Google Scholar]
- 29.Wise SS, Schuler JHC, Holmes AL, Katsifis SP, Ketterer ME, Hartsock WJ, Zheng T, Wise JP., Sr. A comparison of two carcinogenic particulate hexavalent chromium compounds: Barium chromate is more potent than lead chromate in human lung cells. Environ. Mol. Mutagen. 2004;44:156–162. doi: 10.1002/em.20044. [DOI] [PubMed] [Google Scholar]
- 30.Paz-y-Miño C, Dávalos MV, Sánchez ME, Arévalo M, Leone PE. Should gaps be included in chromosomal aberration analysis? Evidence based on the comet assay. Mutat. Res. 2002;516(1–2):57–61. doi: 10.1016/s1383-5718(02)00021-9. [DOI] [PubMed] [Google Scholar]
- 31.Holmes AL, Wisem SS, Xie H, Gordon N, Thompson WD, Wise JP., Sr. Lead ions do not cause human lung cells to escape chromate-induced cytotoxicity. Toxicol. Appl. Pharmacol. 2005;203:167–176. doi: 10.1016/j.taap.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 32.SAS Institute, Inc. SAS/STAT® 9.1 user's guide. SAS Institute, Inc.; Cary, NC: 2004. [Google Scholar]
- 33.Wise SS, Holmes AL, Ketterer ME, Hartsock WJ, Fomchenko E, Katsfis SP, Thompson WD, Wise JP., Sr. Chromium is the proximate clastogenic species for lead chromate-induced clastogenicity in human bronchial cells. Mutat. Res. 2004;560:79–89. doi: 10.1016/j.mrgentox.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Wise SS, Holmes AL, Moreland JA, Xie H, Sandwich SJ, Stackpole MM, Fomchenko E, Teufack S, May A, Jr., Katsifas SP, Wise JP., Sr. Human lung cell growth is not stimulated by lead ions after lead chromate-induced genotoxicity. Mol. Cell. Biochem. 2005;279:75–84. doi: 10.1007/s11010-005-8217-0. [DOI] [PubMed] [Google Scholar]
- 35.Cheng W-H. Impact of inorganic nutrients on maintenance of genomic stability. Environ. Mol. Mutagen. 2009;50:349–360. doi: 10.1002/em.20489. [DOI] [PubMed] [Google Scholar]
- 36.Waalkes MP. Cadmium carcinogenesis in review. J. Inorganic Biochem. 2000;79:241–244. doi: 10.1016/s0162-0134(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 37.Filipic M, Fatur T, Vudrag M. Molecular mechanisms of cadmium induced mutagenicity. Hum. Exp. Toxicol. 2006;25:67–77. doi: 10.1191/0960327106ht590oa. [DOI] [PubMed] [Google Scholar]
- 38.Wong SH, Shih RS, Schoene NW, Lei KY. Zinc-induced G2/M blockage is p53 and p21 dependent in normal human bronchial epithelial cells. Am. J. Physiol. Cell Physiol. 2008;294:C1342–C1349. doi: 10.1152/ajpcell.00061.2008. [DOI] [PubMed] [Google Scholar]
- 39.Sliwinski T, Czechowska A, Kolodziejczak M, Jajte J, Wisniewska-Jarosinska M, Blasiak J. Zinc salts differentially modulate DNA damage in normal and cancer cells. Cell. Biol. Interact. 2009;33:542–547. doi: 10.1016/j.cellbi.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Watters GP, Smart DJ, Harvey JS, Austin CA. H2AX phosphorylation as a genotoxicity endpoint. Mutat. Res. 2009;679:50–58. doi: 10.1016/j.mrgentox.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Ashby J, Morrison JH, Nevan J, Greenblatt JF, Haber JE, Shen X. INO80 and gamma-H2AX interaction links ATP-dependent chromatin remodeling to DNA damage repair. Cell. 2004;119:767–775. doi: 10.1016/j.cell.2004.11.037. [DOI] [PubMed] [Google Scholar]