Abstract
Almost every odor we encounter in daily life has the capacity to produce a trigeminal sensation. Surprisingly, few functional imaging studies exploring human neuronal correlates of intranasal trigeminal function exist, and results are to some degree inconsistent. We utilized activation likelihood estimation (ALE), a quantitative voxel-based meta-analysis tool, to analyze functional imaging data (fMRI/PET) following intranasal trigeminal stimulation with carbon dioxide (CO2), a stimulus known to exclusively activate the trigeminal system. Meta-analysis tools are able to identify activations common across studies, thereby enabling activation mapping with higher certainty. Activation foci of nine studies utilizing trigeminal stimulation were included in the meta-analysis. We found significant ALE scores, thus indicating consistent activation across studies, in the brainstem, ventrolateral posterior thalamic nucleus, anterior cingulate cortex, insula, precentral gyrus, as well as in primary and secondary somatosensory cortices – a network known for the processing of intranasal nociceptive stimuli. Significant ALE values were also observed in the piriform cortex, insula, and the orbitofrontal cortex, areas known to process chemosensory stimuli, and in association cortices. Additionally, the trigeminal ALE statistics were directly compared with ALE statistics originating from olfactory stimulation, demonstrating considerable overlap in activation. In conclusion, the results of this meta-analysis map the human neuronal correlates of intranasal trigeminal stimulation with high statistical certainty and demonstrate that the cortical areas recruited during the processing of intranasal CO2 stimuli include those outside traditional trigeminal areas. Moreover, through illustrations of the considerable overlap between brain areas that process trigeminal and olfactory information; these results demonstrate the interconnectivity of flavor processing.
Keywords: activation likelihood estimation, olfaction, nose, somatosensory, fMRI, PET
1. Introduction
Everyday chemosensory processing is based partly on the interaction between two systems, the olfactory and the trigeminal system. Whereas the olfactory system mediates the quality percept of an odor, the trigeminal system conveys sensations such as a burning, pungency, or stinging, as well as touch, pressure, and temperature. Although the processing of olfactory stimuli has received much attention, the neurological substrate of intranasal trigeminal function remains poorly understood. Further investigations of the intranasal trigeminal system are of great importance to an understanding of its role as a sentinel against potentially toxic substances and as the mediator of more animated percepts of odors and flavors.
Our current understanding of mechanisms underlying trigeminal stimulus processing is derived mostly from animal models (for review see Langley et al., 2008; Mogil, 2009). In both animals and humans, the nasal mucosa is innervated by the ophthalmic and maxillary branches of the trigeminal nerve, which transfers information about a painful stimulus to trigeminal nuclei in the spinal cord (Anton et al., 1991). From there, information is relayed via the lateral and the medial pain systems, two parallel organized systems with distinct projections (de Leeuw et al., 2005). The lateral pain system transmits information to lateral thalamic structures, which project to the primary (S I) and secondary (S II) somatosensory cortices. The medial pain system transfers information to medial thalamic nuclei and from there to prefrontal cortex, insula, cingulate gyrus, brain stem, and to the limbic system (Ingvar and Hsieh, 1999; Treede et al., 1999; Wiech et al., 2001). Significant genetic, neurochemical, and neuroanatomical differences distinguish non-human and human processing and experience of pain-related stimuli, as demonstrated by recent findings (Craig, 2009). Among other implications, these conclusions suggest that although animal models may provide an approximation of basic human trigeminal processing, there is no substitute for human subjects in the quest to reach a full understanding of how the human brain processes trigeminal stimuli.
Investigations of the human trigeminal system frequently rely on psychophysical or electrophysiological methods (Hari et al., 1997; Hummel and Kobal, 1999; Hummel and Livermore, 2002; Huttunen et al., 1986; Kobal and Hummel, 1988; Rombaux et al., 2006), which yield results that allow only indirect inferences of underlying cerebral processes due to methodological limitations. Psychophysical and electrophysiological tools lack direct links to functional processing and provide low spatial specificity. In contrast, non-invasive methods of functional brain imaging allow us to understand trigeminal processing with higher spatial resolution. In conjunction with these methods, the use of pure trigeminal stimuli, typically carbon dioxide (CO2), an odorless gas that stimulates the trigeminal system almost exclusively, enables isolation of an intranasal trigeminal sensation from an accompanying odor sensation (Fröhlich, 1851; Shusterman and Balmes, 1997; Stevens et al., 1982; Thürauf et al., 1991). Similarly, studies investigating olfactory processing often opt to use pure odorants that do not stimulate the trigeminal system, such as phenyl ethyl alcohol (PEA) or hydrogen sulfide (H2S) (Doty et al., 1978; Kobal et al., 1989).
Several comparisons of brain activation originating from stimulation with pure trigeminal stimuli to activation originating from stimulation with pure odorants have demonstrated considerable overlap in the structures mediating functional processing in each system (Boyle et al., 2007; Hummel et al., 2005; Hummel et al., 2009a; 2009b; Iannilli et al., 2008; Schoepf et al., 2009). Whereas pure trigeminal stimuli typically activate the brain stem, thalamus, caudate nucleus, anterior and dorsolateral orbitofrontal cortex, medial frontal gyrus, frontal operculum, superior temporal gyrus, cingulate, and the postcentral gyrus, stimulation with pure odors commonly induces activation in the medial orbitofrontal cortex, amygdala, parahippocampal gyrus, and cerebellum, exclusively. Functional overlaps between the trigeminal and olfactory networks were observed in the piriform cortex, the medial orbitofrontal cortex, peri-insular regions, as well as secondary somatosensory cortex (Boyle et al., 2007; Hummel et al., 2009b). Additional evidence for a close connection between the two chemosensory systems arises from comparisons of normosmic with anosmic subjects: trigeminally-mediated information is processed differently in the presence or absence of an intact sense of smell (Frasnelli and Hummel, 2007; Frasnelli et al., 2007; Hummel et al., 1996; Iannilli et al., 2007). Comparisons of trigeminal and olfactory imaging data have further revealed that trigeminal brain activations are often more pronounced than their olfactory counterparts (Bensafi et al., 2008; Boyle et al., 2007; Hummel et al., 2005), supporting evidence that the two systems differ with respect to intensity coding. Intensity coding in the olfactory system proceeds via a complex integrative system involving the cerebellum, entorhinal cortex, visual areas, and frontal regions, whereas the network involved in coding trigeminal stimulus intensity appears to be less complex and primarily recruits various subregions of the cingulate cortex (Bensafi et al., 2008).
Such functional imaging studies offer promising leads in the pursuit to understand intranasal trigeminal perception, however, only a limited number of these studies exist and their results are somewhat inconsistent. Due to the complex sensory and cognitive mechanisms involved in trigeminal processing, these studies often yield intricate and widespread neurological patterns rendering definitive conclusions difficult. And, though reviews of the functional imaging literature are well-suited to find activations common across studies based on a given variable of interest, much of the information about these activation patterns contained in the voxel-based data is lost in the transition from three- to two-dimensional space. A functional location meta-analysis can accomplish the three-dimensional comparison that a literature review cannot. This tool allows for a formal statistical integration of unbiased voxel-based data from multiple studies not only to determine common activations, but to provide a formal estimate of activation likelihood. Meta-analyses enable searches of emergent patterns undetectable in individual reports by providing objective methods for the post-hoc merging of data from several datasets. By utilizing a new meta-analysis tool, the activation likelihood estimation (ALE), we analyzed the datasets of functional imaging studies investigating stimulation of the nasal mucosa with CO2.
The aim of this meta-analysis was to map the human neuronal correlates of intranasal trigeminal stimulation with high statistical certainty. Cortical areas recruited during the processing of intranasal CO2 stimuli were expected to include both, those known for the processing of intranasal nociceptive stimuli and those known for the processing of common olfactory stimuli. A subsequent meta-analysis intended to further compare patterns of brain activation following trigeminal with that following olfactory stimulation.
2. Results
Results of literature search
Our literature search criteria identified a total of 15 original functional imaging studies. However, only nine of those fulfilled all stipulated inclusion criteria, as outlined in the method section, and were included in the meta-analyses. These nine studies, eight fMRI studies and one PET study (Table 1), rendered a total ten contrasts (nine fMRI contrasts, one PET contrast) and 207 activation foci.
Table 1.
Studies included in the meta-analysis about intranasal trigeminal stimulation
| Reference | Imaging modality | n | Age of the subjects (mean/range) | Side of stimulation | Concentration of CO2 stimuli | Contrast (tasks) | Correction for whole brain volume | Number of contrasts | Number of foci |
|---|---|---|---|---|---|---|---|---|---|
| a) published work | |||||||||
| Boyle et al. 2007 | fMRI (1.5 T) | 15 | 35.3 years/23-59 years | L/R | 60% | CO2L > BL/CO2R > BL | p < 0.001 uncorrected | 2 | 22 |
| Iannilli et al. 2007 | fMRI (1.5 T) | 12 | 61 years | R | 60% | CO2 > BL | p < 0.001 uncorrected | 1 | 20 |
| Bensafi et al. 2008 | fMRI (1.5 T) | 8 | 27.5 years | R | 37%/49% | CO2 > BL | p < 0.005 uncorrected | 1 | 10 |
| Iannilli et al. 2008 | fMRI (1.5 T) | 18 | 31 years | R | 60% | CO2 > BL | p < 0.002 uncorrected | 1 | 21 |
| Hummel et al. 2009 | 15O-H2O-PET | 12 | 36 years/30-58 years | L | 60% | CO2 > BL | p < 0.001 uncorrected/p < 0.05 SVC | 1 | 10 |
| Schöpf et al. 2009 | fMRI (3 T) | 22 | 29.0 years | L+R | 50-60% | CO2 > BL | p < 0.005 FDR corrected | 1 | 42 |
| b) unpublished work | |||||||||
| CO2 slightly painful | fMRI (1.5 T) | 26 | 28.4 years/22-38 years | L | 25-55% | CO2 > BL | p < 0.05 FWE corrected | 1 | 30 |
| CO2 severely painful | fMRI (1.5 T) | 30 | 27.6 years/21-38 years | L | 45-60% | CO2 > BL | p < 0.05 FWE corrected | 1 | 26 |
| CO2 3 concentrations | fMRI (1.5 T) | 27 | 28.9 years/22-42 years | L | 40-70% | CO2 > BL | p < 0.05 FWE corrected | 1 | 26 |
| 10 | 207 | ||||||||
An overview of the studies included in the olfactory ALE can be found in Table 2. Care was taken to balance the number of included contrasts and imaging modality as both can influence ALE analyses. Eight studies investigating mere olfactory stimulation-derived activation with a total of ten contrasts (nine fMRI contrasts, one PET contrast) and 119 foci were included in the olfactory ALE.
Table 2.
Olfactory functional imaging studies included in the comparison of significant ALE values between trigeminal and olfactory stimulation.
| Reference | Imaging modality | n | Age of the subjects (mean/range) | Number of contrasts | Number of foci |
|---|---|---|---|---|---|
| Gottfried and Dolan 2003 | fMRI (2 T) | 17 | 22-34 years | 1 | 11 |
| Gottfried et al. 2002 | fMRI (2 T) | 17 | 23 years / 18-31 years | 2 | 8 |
| Osterbauer et al. 2005 | fMRI (3 T) | 10 | 27 years / 22-35 years | 1 | 6 |
| Savic et al. 2000 | 15O-H2O-PET | 18 | 22-27 years | 1 | 5 |
| Sobel et al. 2000 | fMRI (1.5 T) | 8 | 25 years / 20-39 years | 1 | 32 |
| Wang et al. 2005 | fMRI (3 T) | 11 | 24 years / 21-26 years | 1 | 16 |
| Wicker et al. 2003 | fMRI (3 T) | 14 | 20-27 years | 2 | 26 |
| Wiesmann et al. 2006 | fMRI (1.5 T) | 22 | 27 years / 22-40 years | 1 | 15 |
| 10 | 119 | ||||
Significant ALE values for trigeminal stimulation
The ALE analysis revealed 29 significant clusters for intranasal trigeminal stimulation with CO2, the largest of which contained six local maxima (Figure 1, Table 3).
Figure 1.

Localization of significant FDR corrected (p < .05) ALE values due to intranasal trigeminal stimulation with CO2 (contrast “CO2 versus Baseline”). The ALE values are projected onto a standard template (colin1.1.nii) and are shown in Talairach space (z = - 49 to 66, L = left).
Table 3.
Localization of significant FDR corrected ALE values due to intranasal trigeminal stimulation with CO2 (contrast “CO2 versus Baseline”). Significant activation clusters are reported together with stereotaxic coordinates with regard to Talairach and Tournoux (1988), cluster volume, and activation likelihood estimation of the local activation maximum.
| Cluster number | Cluster volume (mm3) | ALE (*10-3) | Talairach coordinates | Brain area | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| 1 | 4640 | 18.13 | 44 | 16 | 10 | Frontal operculum |
| 12.34 | 46 | 10 | -2 | Frontal operculum | ||
| 12.13 | 38 | 20 | -8 | Basal operculum / Orbitofrontal cortex (OFC) | ||
| 11.85 | 36 | 6 | -16 | Dorsal temporopolar gyrus | ||
| 11.54 | 52 | 14 | 2 | Frontal operculum | ||
| 10.23 | 36 | 8 | 2 | Insula | ||
| 2 | 2408 | 15.11 | -38 | 36 | 28 | Middle frontal gyrus |
| 11.89 | -42 | 46 | 8 | Middle frontal gyrus | ||
| 3 | 1944 | 14.69 | -62 | -38 | 22 | Superior temporal gyrus |
| 13.49 | -62 | -40 | 14 | Superior temporal gyrus | ||
| 4 | 1896 | 15.99 | 0 | -58 | -26 | Cerebellum (declive) |
| 10.61 | 6 | -64 | -32 | Cerebellum (pyramis) | ||
| 5 | 1856 | 18.24 | 50 | -44 | 46 | Supramarginal gyrus |
| 6 | 1672 | 14.01 | -34 | -52 | -32 | Cerebellum (declive) |
| 11.33 | -24 | -64 | -32 | Cerebellum (declive) | ||
| 10.84 | -18 | -72 | -36 | Cerebellum (crus I) | ||
| 7 | 1560 | 16.96 | -32 | 26 | -8 | Posterior orbital gyrus / Orbitofrontal cortex (OFC) |
| 11.34 | -32 | 14 | 2 | Insula | ||
| 8 | 1336 | 13.11 | 44 | -14 | 26 | Postcentral gyrus / Primary somatosensory cortex (S I) |
| 9 | 1272 | 15.00 | 6 | 16 | 54 | Superior frontal gyrus |
| 10 | 1240 | 13.41 | 32 | -8 | 16 | Insula |
| 10.97 | 40 | -10 | 10 | Frontal operculum/insula | ||
| 11 | 696 | 12.71 | -46 | 14 | -2 | Frontal operculum |
| 12 | 688 | 12.41 | -4 | -6 | 62 | Superior frontal gyrus |
| 13 | 680 | 13.66 | 22 | -64 | -28 | Cerebellum (declive) |
| 14 | 616 | 12.94 | -58 | -24 | 32 | Parietal operculum / Secondary somatosensory cortex (S II) |
| 15 | 584 | 11.40 | -50 | -2 | 40 | Precentral gyrus |
| 16 | 552 | 11.10 | 4 | -28 | -20 | Mesencephalic trigeminal nucleus (brainstem) |
| 17 | 520 | 10.04 | -40 | -16 | -2 | Insula |
| 8.94 | -32 | -14 | -10 | Ventral putamen | ||
| 18 | 512 | 11.34 | -38 | -2 | -10 | Insula |
| 19 | 472 | 10.57 | 46 | 2 | 50 | Middle frontal gyrus |
| 20 | 448 | 10.24 | 20 | 42 | 8 | Superior frontal gyrus |
| 21 | 384 | 12.65 | 22 | -66 | -46 | Cerebellum (pyramis) |
| 22 | 368 | 12.07 | 20 | 8 | -10 | Piriform cortex |
| 23 | 288 | 10.53 | 6 | -76 | 42 | Superior parietal lobule |
| 24 | 240 | 10.21 | 2 | 32 | 22 | Anterior cingulate gyrus |
| 25 | 200 | 10.17 | 12 | -16 | 10 | Ventrolateral posterior thalamic nucleus |
| 26 | 152 | 9.47 | 8 | -14 | -6 | Mammillo-thalamic tract |
| 27 | 144 | 9.38 | 42 | 40 | 0 | Inferior frontal gyrus (orbital part) |
| 28 | 136 | 8.95 | 50 | -28 | 30 | Parietal operculum / Secondary somatosensory cortex (S II) |
| 29 | 104 | 8.52 | -48 | -54 | 46 | Supramarginal gyrus |
We found significant ALE scores in a network known for the processing of intranasal nociceptive stimuli – the brainstem (mesencephalic trigeminal nucleus), ventrolateral posterior thalamic nucleus, thalamic tract, anterior cingulate cortex, insula and adjoining frontal operculum, superior parietal lobule, precentral gyrus, as well as primary (S I) and secondary (S II) somatosensory cortices. Significant ALE values were also observed in the piriform cortex (Figure 4a), insula and adjoining orbitofrontal cortex (OFC) in area 13l, and the cerebellum, all areas known to process chemosensory stimuli. In addition, high ALE values were observed in association cortices, such as inferior, middle, and superior frontal gyri, as well as superior temporal gyrus and supramarginal gyrus.
Figure 4.
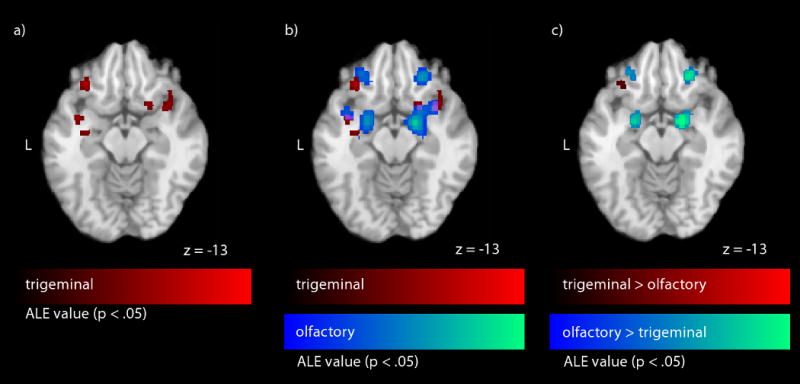
Significant FDR corrected (p < .05) ALE values in piriform cortex which a) are caused by intranasal stimulation with CO2 (contrast “CO2 versus Baseline”), b) overlap with regard to trigeminal and olfactory stimulation (10 contrasts each, olfactory + trigeminal), and c) differ with regard to trigeminal and olfactory stimulation (trigeminal > olfactory, olfactory > trigeminal). Significant ALE values (p < .05) are projected onto a standard template (colin1.1.nii) in Talairach space (L = left). The piriform cortex activation is shown on a selected axial slice (z = -13). Red colors represent trigeminal or trigeminal > olfactory ALE values, purple colors represent the overlap in ALE values (olfactory + trigeminal), blue colors represent olfactory or olfactory > trigeminal ALE values.
Comparison of trigeminal and olfactory ALE maps
We initially explored which areas are more likely to be activated by trigeminal stimulation than by olfactory stimulation by contrasting trigeminal to olfactory activations. Significant ALE values, related to higher likelihood for trigeminal stimulation were found in the brainstem, insula, frontal operculum, superior parietal lobule, precentral gyrus, and primary (S I) and secondary (S II) somatosensory cortices. In addition, high significant ALE values were found in parts of the orbitofrontal cortex (posterior parts in area 13l) and the cerebellum, areas known to process chemosensory stimuli, and in association cortices (inferior, middle, and superior frontal gyri, superior temporal gyrus, supramarginal gyrus) (Figure 2 & 4, Table 4).
Figure 2.

Localization of significant FDR corrected (p < .05) ALE values exclusively related to trigeminal stimulation (trigeminal > olfactory) and olfactory stimulation (olfactory > trigeminal) projected onto the standard template in Talairach space (z = - 49 to 66, L = left). Red colors represent trigeminal ALE results whereas blue colors represent olfactory ALE values.
Table 4.
Localization of significant FDR corrected ALE values: a) trigeminal stimulation (trigeminal > olfactory); b) olfactory stimulation (olfactory > trigeminal). Significant activation clusters are reported together with stereotaxic coordinates with regard to Talairach and Tournoux (1988), cluster volume, and activation likelihood estimation of the local activation maximum.
| Cluster number | Cluster volume (mm3) | ALE (*10-3) | Talairach coordinates | Brain area | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| a) Trigeminal > Olfactory | ||||||
| 1 | 2040 | 15.99 | 0 | -58 | -26 | Cerebellum (declive) |
| 10.61 | 6 | -64 | -32 | Cerebellum (pyramis) | ||
| 2 | 1856 | 16.79 | 44 | 16 | 10 | Frontal operculum |
| 11.40 | 52 | 14 | 2 | Frontal operculum | ||
| 10.99 | 46 | 12 | -4 | Frontal operculum | ||
| 3 | 1776 | 14.32 | -62 | -38 | 22 | Superior temporal gyrus |
| 12.96 | -62 | -40 | 14 | Superior temporal gyrus | ||
| 4 | 1744 | 14.00 | -34 | -52 | -32 | Cerebellum (declive) |
| 11.32 | -24 | -64 | -32 | Cerebellum (declive) | ||
| 10.84 | -18 | -72 | -36 | Cerebellum (crus I) | ||
| 5 | 1736 | 18.24 | 50 | -44 | 46 | Supramarginal gyrus |
| 6 | 1384 | 13.11 | 44 | -14 | 26 | Postcentral gyrus / Primary somatosensory cortex (S I) |
| 7 | 1248 | 15.00 | 6 | 16 | 54 | Superior frontal gyrus |
| 8 | 1160 | 14.54 | -38 | 36 | 28 | Middle frontal gyrus |
| 9 | 832 | 13.14 | 32 | -8 | 16 | Insula |
| 10.33 | 40 | -10 | 10 | Frontal operculum/Insula | ||
| 10 | 752 | 14.98 | -32 | 24 | -8 | Posterior orbital gyrus / Orbitofrontal cortex (OFC) |
| 9.08 | -32 | 16 | 2 | Insula | ||
| 11 | 664 | 13.66 | 22 | -64 | -28 | Cerebellum (declive) |
| 12 | 624 | 11.40 | -50 | -2 | 40 | Precentral gyrus |
| 13 | 480 | 10.72 | 4 | -28 | -20 | Mesencephalic trigeminal nucleus (brainstem) |
| 14 | 456 | 12.16 | -46 | 14 | -4 | Frontal operculum |
| 15 | 336 | 12.65 | 22 | -66 | -46 | Cerebellum (pyramis) |
| 16 | 296 | 10.64 | -4 | -8 | 62 | Superior frontal gyrus |
| 17 | 256 | 10.53 | 6 | -76 | 42 | Superior parietal lobule |
| 18 | 224 | 10.02 | -40 | -16 | -2 | Insula |
| 19 | 208 | 10.44 | -42 | 46 | 6 | Middle frontal gyrus |
| 20 | 152 | 8.52 | -48 | -54 | 46 | Supramarginal gyrus |
| 21 | 128 | 9.36 | 42 | 40 | 0 | Inferior frontal gyrus (orbital part) |
| 22 | 112 | 9.26 | -60 | -24 | 32 | Parietal operculum / Secondary somatosensory cortex (S II) |
| b) Olfactory > Trigeminal | ||||||
| 1 | 5712 | 18.05 | -6 | -2 | -2 | Thalamus |
| 28.21 | 18 | -6 | -8 | Amygdala | ||
| 2 | 1880 | 15.92 | -20 | -4 | -14 | Amygdala |
| 3 | 1864 | 18.33 | 24 | 32 | -10 | Posterior orbital gyrus |
| 4 | 1128 | 16.41 | -24 | 34 | -8 | Posterior orbital gyrus |
| 5 | 624 | 11.46 | 38 | 26 | 28 | Middle frontal gyrus |
| 6 | 456 | 12.33 | -36 | 18 | 14 | Frontal operculum |
| 7 | 384 | 12.49 | 30 | -72 | 48 | Parietal occipital transition area |
| 8 | 208 | 9.88 | -2 | 36 | 14 | Anterior cingulate gyrus |
For the reversed contrast, indicating which areas are more likely to be activated by olfactory stimuli than by trigeminal stimuli significant ALE scores were located in the posterior (temporal) portion of the piriform cortex, amygdala, thalamus, and anterior areas of the orbitofrontal cortex (area 13m), all of which are often reported in olfactory imaging studies. Additionally, high ALE scores were demonstrated in the frontal operculum, anterior cingulate gyrus, and parietal occipital transition area (Figure 2 & 4, Table 4).
Overlap in brain activation maps are most commonly explored using conjunction analyses (Nichols et al., 2005). Because conjunction analysis is not yet implemented in the ALE software, we tentatively explored significant areas commonly activated by olfactory and trigeminal stimulation by superimposing the olfactory and trigeminal ALE map on our anatomical template. The resulting images demonstrated clear overlaps of significant ALE values in piriform cortex, insula, and middle frontal gyrus, as well as a minor overlap located in orbitofrontal cortex areas 13l and 13m (Figure 3 & 4).
Figure 3.

Localization of significant FDR corrected (p < .05) ALE values that overlap with regard to intranasal trigeminal and olfactory stimulation (10 contrasts each) projected onto the standard template in Talairach space (z = - 49 to 66, L = left). Red colors represent trigeminal ALE results, purple colors represent the overlap in ALE values and blue colors represent olfactory ALE values.
3. Discussion
The use of a meta-analytical approach to any comparison of functional imaging datasets enables the mapping of cerebral activity with high statistical certainty because the ALE method excludes activations infrequently reported across studies. Using ALE meta-analysis, we provide a detailed in vivo mapping of human brain areas responsive to intranasal trigeminal stimulation.
Activation of the pain network by trigeminal stimuli
As hypothesized, results of the meta-analysis confirm that intranasal trigeminal stimulation activates areas of the human brain commonly associated with nociceptive stimuli. Significant ALE values were observed in a network encompassing the brainstem, the thalamus, the anterior cingulate cortex, the insula and adjoining operculum, the superior parietal lobule, the precentral gyrus, and the primary (S I) and secondary (S II) somatosensory cortices, areas known for the processing of intransal nociceptive stimuli (Bensafi et al., 2008; Boyle et al., 2007; Hari et al., 1997; Hummel et al., 2005; 2009b; Huttunen et al., 1986; Iannilli et al., 2007; 2008). Furthermore, published findings indicate that these areas process other painful stimuli applied to either the face (de Leeuw et al., 2005; de Leeuw et al., 2006; Ettlin et al., 2009; Iannilli et al., 2008) or hand (Bornhovd et al., 2002; Kwan et al., 2000; Peyron et al., 2000). In conjunction with these findings, our data suggest that the processing of intranasal CO2 stimulation does not utilize a unique network but rather accesses the general pain processing network, also known as the pain matrix. The functional significance of this network has been extensively reviewed elsewhere (for thoughtful reviews see May, 2007; Seifert and Maihofner, 2009; Tracey, 2008). The insula, however, appears to be activated by intranasal trigeminal stimulation uniquely, when compared to painful cutaneous electrical and irritating cutaneous mechanical stimulation of the right forehead (Iannilli et al., 2008), thereby possibly occupying a central position in the network that processes intranasal trigeminal stimuli.
The notion that peri-insular regions play an important role in the processing of intranasal trigeminal stimuli is supported by the finding that the largest cluster containing significant ALE scores was observed not in the somatosensory areas (SI or SII), as expected, but in peri-insular areas: the frontal operculum and the insular cortex. Pain perception is commonly divided into the sensory-discriminative and affective-motivational components (Treede et al., 1999). The sensory-discriminative component describes the localization of the painful stimulus as well as evaluation of intensity and quality. The emotional, arousal, and behavioral responses to a painful experience are described as the affective-motivational component of pain perception. There is evidence that the activation of the insula, particularly activation of the anterior insula, is related to the affective-motivational factor of pain perception (Craig, 2009; de Leeuw et al., 2005; Treede et al., 1999). Further support of the involvement of the insular cortex in processing pain-related emotions is provided by a set of studies investigating empathy or compassion for pain (Danziger et al., 2009; Immordino-Yang et al., 2009; Ochsner et al., 2008; Singer, 2007). We propose the hypothesis that the insula processes emotions linked to the perception of a chemosensory stimulus and consequently plays a major role in the overall processing of intranasal trigeminal stimuli. In light of this, the role of the insula in the processing of intranasal trigeminal stimuli may be of greater importance than the roles of the somatosensory cortices and the traditional pain network.
The existing literature contains evidence that insular activation is significantly reduced when subjects are distracted during pain perception (Brooks et al., 2002), and the heterogeneous insular cortex has been suggested to act as an integration area for chemosensory stimuli (Treede et al., 1999). In this context, the large cluster of significant ALE maxima in insular cortex may result from either an attentional shift directed towards the painful, and therefore salient, stimulus or from the proposed role of the insular cortex in sensory integration. Nevertheless, these findings confirm the central role of the insular cortex in trigeminal stimulus processing and in our understanding of the cerebral processes resulting from intranasal trigeminal stimulation.
Large clusters of significant ALE values were also observed in middle frontal and superior temporal gyri, two areas typically reported as association cortex for the processing of intransasal chemosensory stimuli (Cerf-Ducastel and Murphy, 2006; Kettenmann et al., 1997; Plailly et al., 2007; Porter et al., 2005). These authors suggested that chemosensory perception, localization, discrimination, and recognition memory lead to an activation in these areas. However, the function of these areas with respect to either trigeminal or chemosensory processing is not well explored.
Smaller clusters of significant ALE scores were localized to multiple sites within the cerebellum. Sobel and colleagues (1998b; 2000) have hypothesized that the cerebellum modulates sniff volume in relation to odor concentration, a theory supported by several reports of cerebellar activation resulting not only from odorant stimulation and sniffing of non-odorized air (Albrecht et al., 2009; Savic et al., 2002; Sobel et al., 1998b; 2000), but also from passive stimulus presentation (Yousem et al., 1997). This feedback mechanism might also be applicable to intranasal trigeminal perception. In light of the well-documented fact that intranasal trigeminal stimuli induce apnea (Alvaro et al., 1992; Boushey and Richardson, 1973; Yavari et al., 1996), a sentinel mechanism in place to limit the inhalation of harmful chemicals, and the hypothesis proposed by Sobel and colleagues, we suggest that the cerebellar activation commonly observed in trigeminal neuroimaging studies is related to a feedback mechanism regulating intranasal airflow.
Activation of the common olfactory network by trigeminal stimuli
Significant ALE values were observed in the piriform cortex and adjoining orbitofrontal cortex, which are cortical regions known traditionally as olfactory areas (Gottfried, 2006; Sobel et al., 1998b; Zald and Pardo, 2000; Zatorre et al., 1992).
The piriform cortex is a heterogeneous region spanning parts of two lobes, frontal (anterior piriform cortex) and temporal (posterior piriform cortex) lobe, and is most comonly associated with olfactory processing (Zatorre et al., 1992). However, this meta-analysis revealed that stimulation with a painful dose of CO2, as opposed to an odorous stimulus, also resulted in high activation likelihood estimate values in the anterior portion of the piriform cortex. That said, we would like to stress that the spatial resolution of an ALE analysis is restricted by the width of the kernel used for spatial smoothing (10 mm) of the data. Although, the piriform cortex is considerably larger than our smoothing diameter, as for many functional imaging studies, the exact peak location can not be accurately localized to subsections within the piriform cortex with an absolute certainty. Four potential hypotheses could be postulated in respect of this finding. First, activation of the piriform cortex may be due to a subset of human olfactory receptors that respond to odorous and CO2 stimulation alike. The possibility that CO2 is not a pure trigeminal stimulus provides a second, though unlikely, hypothesis for trigeminal stimulation-derived cortical olfactory activations. Third, the finding is spurious in that it is mediated by potential differences in sniff characteristics. Fourth, the piriform cortex has a role in the integration of chemosensations, plausible due to the known strong behavioral links between the chemical senses.
Evidence supporting the first hypothesis, the existence of olfactory receptors responsive to CO2, comes from a range of animal models, including bullfrogs (Coates and Ballam, 1990; Sakakibara, 1978), tegu lizards (Coates and Ballam, 1987), reptiles (Coates and Ballam, 1989), and mice (Hu et al., 2007). Furthermore it is possible that olfactory brain areas are activated during the perception of trigeminal CO2 stimuli via trigeminal ganglion cells with sensory endings in the nasal epithelium that also send branches into the olfactory bulb and the spinal trigeminal complex which were discovered by Schaefer and colleagues (2002). It is worth pointing out that unequivocal evidence that CO2 is not detected as an odor does not exist; this could be attributed to the fact that proving a negative result is a Sisyphus task. However, this gives rise to the second hypothesis. It is possible that activation in the piriform cortex is mediated by activity in the olfactory system, induced by CO2 that is either not perceived as an odor per se or is below perceptual awareness. In other words, it is possible that a weak odorous sensation, originating from CO2, produces the demonstrated activation in the piriform cortex. However, since it is challenging to map olfactory evoked piriform cortex activation due to the inherent weak neuronal signal in the olfactory system (due to adaptation and habituation) and known artifacts in functional imaging studies (for review see Gottfried, 2006; Sobel et al., 2003) such weak odorous stimuli tend not to be detected as activation in the piriform cortex (e.g. Bensafi et al., 2008; Boyle et al., 2007; Dade et al., 2001; Kettenmann et al., 1996; Kettenmann et al., 1997). In our view, the magnitudes of the activations elicited by CO2 are far too large to be elicited by a sub-threshold odor.
The third hypothesis, activation of the piriform cortex due to sniffing, originates from two studies of Sobel and colleagues (1998a; 2000) demonstrating that activation in piriform cortex is induced in the presence or absence of an odorant just by the simple act of sniffing. However, as stated above, trigeminal stimuli induce apnea which would effectively lead to a marked reduction, rather than an increase in sniff frequency. The apnea inducing nature of the stimulus merged with the fact that the included studies were conducted using velopharyngeal closure (personal communication), thus specifically prevented them to breathe through their noses, renders it unlikely that the demonstrated piriform cortex activation is mediated due to the secondary act of sniffing.
Support for the fourth hypothesis, a role for the piriform cortex in chemosensory integration, is derived from an array of studies showing that independent of odor detection, the piriform cortex seems to process stimuli from all three chemical senses. Though the piriform cortex is often labeled as primary olfactory cortex, it is a heterogeneous area proposed to respond in several higher order olfactory tasks, such as odor memory (Dade et al., 2002) and attention to odors (Zelano et al., 2005), as well as during odor imagery (Bensafi et al., 2007; Gonzalez et al., 2006). Efforts have recently been made to discriminate amongst primary olfactory processes mediated by the anterior and posterior piriform cortex. Gottfried and colleagues (2006; 2009) postulated that activation of the anterior piriform cortex corresponds to perception of an odor stimulus per se, whereas posterior piriform cortex activity is related to the quality of a chemosensory percept (but see also Gottfried et al., 2002). Additional evidence for activation of the anterior piriform cortex driven by odor detection has been published by Zelano and colleagues (2005). However, it is interesting to note that activations in the piriform cortex have also been reported in imaging studies investigating the cortical processing of taste stimuli (Small et al., 1997). In other words, the piriform cortex seems to process chemosensory stimuli from all the three chemical senses independent of whether or not an odor is detected. Based on this, we support the hypothesis that the piriform cortex is a chemosensory integration area. More evidence supporting the fourth hypothesis, an integrative role of the piriform cortex in chemosensory processing, comes from fMRI studies aimed at mapping the cerebral processing of odors with both trigeminal and olfactory percepts, commonly known as bimodal odors (Albrecht et al., 2007; Albrecht et al., 2009). Bimodal odorants typically evoke odor perceptions at low concentrations and burning or stinging sensations at higher concentrations, thus enabling the separation of trigeminal from olfactory processing. Stimulated with low concentrations of a bimodal odorant, subjects reported no trigeminal sensations despite clear activity in both the olfactory and the trigeminal neuronal systems were located, thus lending further support to the notion that the sensory systems within the nose might interact based on stimulus location rather than sensory system belonging. This integrational function could arise from the repetitive and complex paired association that our everyday percept of flavor is provoking, a complex interaction between odorous, trigeminal, and gustatory sensation.
Comparison of networks responsible for processing of olfactory and trigeminal stimuli
A comparison of the olfactory and trigeminal ALE maps displays a clear overlap of the two chemosensory networks, especially in the right piriform cortex, insula, and middle frontal gyrus, as well as in a minor portion of the orbitofrontal cortex. These areas are well-known members of the network processing trigeminal stimuli, mentioned above, and of the network processing olfactory stimuli (for review see Gottfried, 2006). However, the comparison also reveals areas that are specifically activated by either the trigeminal irritant (CO2) or by odorous stimuli. Although defining the peak ALE cluster scores to exact subdivisions of the various cortical regions is not an exact science due to the filters used and variation between subject and studies, one can see in Figure 4 that trigeminal and olfactory stimuli activate common areas but peak ALE scores are mostly separated within each gross anatomical division. If one studies the individual subdivisions of piriform cortex and orbitofrontal cortex one can find an interesting pattern. Trigeminal stimuli activate the anterior piriform cortex and the posterior orbitofrontal cortex (within area 131), whereas olfactory stimuli render the highest ALE values in the posterior piriform cortex and the anterior orbitofrontal cortex (within area 13m). Both piriform and orbitofrontal cortex are connected to the somatosensory system via afferent inputs from SII (Saleem et al., 2008). This demonstrates the large degree of interconnectivity within the flavor processing network and lends further support to the view that the three chemical senses are not isolated networks. Future studies seeking to distinguish olfactory from trigeminal functional networks should map and label activation within subregions rather than gross anatomical regions.
Overall conclusion
With the help of the meta-analytical tool ALE, we provide an in vivo detailed mapping of human brain areas responsive to intranasal trigeminal stimulation. In addition, our results demonstrate that both the trigeminal and olfactory systems are consistently activated with a high probabilistic certainty during perception of a pure trigeminal substance and illustrate significant activation overlaps within the piriform cortex. These system interactions demonstrate that only when we understand the integration of olfactory, gustatory, and trigeminal network processing can we start to fully understand how we process food and flavor.
4. Experimental procedure
Identification of publications
Different data sources were used for identification of publications using functional neuroimaging methods to investigate the neuronal processing of intranasal trigeminal stimulation. We searched for publications using the online citation index service (Medline) and the keywords “intranasal trigeminal fMRI” and “intranasal trigeminal PET” (including acronyms and synonyms like “intranasal pain”, “functional magnetic resonance imaging”, “positron emission tomography”). We utilized publications published before 1 March 2009 and analyzed the reference lists of these publications to find additional articles. Due to the limited number of published studies, we opted to include the datasets of one submitted (Schoepf et al., 2009) and three unpublished studies from our group. Regarding the unpublished studies, the first experiment was aimed at investigating cerebral activation in response to a low concentration of CO2, the second experiment was aimed at investigating cerebral activation in response to a high CO2 concentration, and the third experiment explored cerebral activation due to different concentrations of CO2 stimuli. The methods and results of the unpublished studies are described in detail in the Supplementary Materials.
Inclusion criteria
The following inclusion criteria were applied to the identified publications: (1) use of functional imaging methods (fMRI/PET) to investigate brain activation following mere intranasal stimulation with CO2; (2) results of a whole brain volume or small volume correction analysis; (3) 3D coordinate-based data (x, y, z) reported in standardized stereotactic space (Montreal Neurological Institute (MNI) or Talairach); (4) results for a group of healthy subjects; (5) results originating from a main CO2 contrast (CO2 versus Baseline). Contrasts meeting these criteria were included regardless of tasks during or after scanning. We opted to use a less stringent task-specific inclusion criterion since the ALE methodology identifies brain activations that are unrelated to the processing of intranasal CO2 stimulation due to their inconsistent activation pattern across studies.
Activation likelihood estimations (ALE)
The original ALE meta-analysis algorithm was developed by Turkeltaub et al. (2002) and was recently modified by Laird and colleagues (2005) to include the possibility of a correction for multiple comparisons, the comparison of two different ALE analyses, and an automatic meta-analysis in Talairach space. Detailed descriptions of the ALE algorithm can be found in Turkeltaub et al. (2002) and Laird et al. (2005), and these algorithms are implemented in BrainMap (BrainMap GingerALE 1.1, http://brainmap.org/ale). During standard analysis of neuroimaging data, functional images are spatially normalized to a stereotactic template that might differ between studies. These anatomical templates are not directly comparable due to minor anatomical deviances. Therefore, in order to facilitate comparisons, all data included in the statistical meta-analysis were transformed into Talairach space, the template supported by the ALE software. After transformation into Talairach space, all coordinates were imported into the Java-based version of the ALE software and analyzed using a fully automated procedure.
A whole-brain ALE map was created by dividing the Talairach space into 8 mm3 voxels and modelling a Gaussian probability distribution centered at each reported activation coordinate. A voxel-wise calculation of the probability that each focus was located within that particular voxel was then performed using a 3D Gaussian function with a 10 mm FWHM (full-width, half-maximum) filter. Subsequent non-parametric permutation tests (n = 7,000) were used to test the null hypothesis that activation foci are distributed uniformly across the brain. Resulting statistical maps were corrected for multiple comparisons utilizing the false discovery rates (FDR) (Genovese et al., 2002; Laird et al., 2005) at a level of p < 0.05 and a minimal cluster volume of 100 mm3.
In order to compare ALE values due to trigeminal and olfactory stimulation, we created a whole-brain ALE map for olfactory stimulation by using an automated randomization algorithm to select studies from the pool of studies used for an ALE meta-analysis of olfactory functional brain imaging data (2009). All studies included in the olfactory ALE meta-analysis utilized a main olfactory contrast (olfactory stimulation versus baseline). The algorithm matched the number of included contrasts (n = 10) and assured that these contrasts originated from the same ratio of fMRI and PET methodolgy (9 fMRI contrasts, 1 PET contrast). To keep the structure of the obtained data we used only whole experimental datasets instead of including random contrasts. The olfactory ALE map was created using the methods described above for the trigeminal ALE map.
The two ALE maps (trigeminal and olfactory) were compared by creating an additional ALE map of voxels in which there is a statistical difference in likelihood of activation by stimulation with the two modalities. Both lists of foci were loaded into the ALE software (BrainMap GingerALE 1.1, http://brainmap.org/ale), one as the main foci list (trigeminal stimulation) and one as the subtraction foci list (olfactory stimulation). These two datasets were then statistically compared for convergences as described in detail elsewhere (Laird et al., 2005). Briefly, the main difference from the procedure described above is that after the probability is calculated in each voxel for both groups, the obtained ALE values are subtracted between groups rendering a measure of inter-map convergence for each voxel. A statistical measure of the level of convergence is then obtained by the methods and settings described above. This was performed for each contrast [(trigeminal > olfactory), (olfactory > trigeminal)].
For visualization purposes, the anatomical template provided on the Ginger ALE website (colin1.1.nii, Kochunov et al. (2002), http://brainmap.org/ale) was overlaid with the different thresholded ALE maps using MRIcron (version beta 31, http://www.sph.sc.edu/comd/rorden/mricron/). To visualize overlaps between olfactory and trigeminal stimulation, the olfactory ALE map was superimposed on the trigeminal ALE map. To visualize differences in the ALE maps the trigeminal > olfactory ALE map was superimposed on the olfactory > trigeminal ALE map. Local maxima of activation clusters were labeled using an anatomical atlas (Mai et al., 2004) and cross-checked using MRIcron. Subdivisions of the OFC were labeled using architectonic maps from within Carmichael and Price (1994).
Supplementary Material
Acknowledgments
This work was supported by the National Institute on Deafness and other Communication Disorders (NIDCD R03DC009869) awarded to JNL and a fellowship within the postdoctoral program of the German Academic Exchange Service (DAAD) awarded to JA. We are grateful to Amy R. Gordon for proofreading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albrecht J, Kopietz R, Kleemann AM, Schöpf V, Fesl G, Anzinger A, Schreder T, Kobal G, Wiesmann M. Brain activation of olfactory and trigeminal cortical areas is independent from perceptual strength – a functional magnetic resonance imaging study using nicotine as chemosensory stimulus. Chem Senses. 2007;32:A124. [Google Scholar]
- Albrecht J, Kopietz R, Linn J, Sakar V, Anzinger A, Schreder T, Pollatos O, Bruckmann H, Kobal G, Wiesmann M. Activation of olfactory and trigeminal cortical areas following stimulation of the nasal mucosa with low concentrations of S(-)-nicotine vapor – an fMRI study on chemosensory perception. Hum Brain Mapp. 2009;30:699–710. doi: 10.1002/hbm.20535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvaro RE, Weintraub Z, Kwiatkowski K, Cates DB, Rigatto H. A respiratory sensory reflex in response to CO2 inhibits breathing in preterm infants. J Appl Physiol. 1992;73:1558–63. doi: 10.1152/jappl.1992.73.4.1558. [DOI] [PubMed] [Google Scholar]
- Anton F, Peppel P, Euchner I, Handwerker HO. Controlled noxious chemical stimulation: responses of rat trigeminal brainstem neurones to CO2 pulses applied to the nasal mucosa. Neurosci Lett. 1991;123:208–11. doi: 10.1016/0304-3940(91)90932-j. [DOI] [PubMed] [Google Scholar]
- Bensafi M, Sobel N, Khan RM. Hedonic-specific activity in piriform cortex during odor imagery mimics that during odor perception. J Neurophysiol. 2007;98:3254–62. doi: 10.1152/jn.00349.2007. [DOI] [PubMed] [Google Scholar]
- Bensafi M, Iannilli E, Gerber J, Hummel T. Neural coding of stimulus concentration in the human olfactory and intranasal trigeminal systems. Neuroscience. 2008;154:832–8. doi: 10.1016/j.neuroscience.2008.03.079. [DOI] [PubMed] [Google Scholar]
- Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C, Buchel C. Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: a single-trial fMRI study. Brain. 2002;125:1326–36. doi: 10.1093/brain/awf137. [DOI] [PubMed] [Google Scholar]
- Boushey HA, Richardson PS. The reflex effects of intralaryngeal carbon dioxide on the pattern of breathing. J Physiol. 1973;228:181–91. doi: 10.1113/jphysiol.1973.sp010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle JA, Heinke M, Gerber J, Frasnelli J, Hummel T. Cerebral activation to intranasal chemosensory trigeminal stimulation. Chem Senses. 2007;32:343–53. doi: 10.1093/chemse/bjm004. [DOI] [PubMed] [Google Scholar]
- Brooks JC, Nurmikko TJ, Bimson WE, Singh KD, Roberts N. fMRI of thermal pain: effects of stimulus laterality and attention. Neuroimage. 2002;15:293–301. doi: 10.1006/nimg.2001.0974. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. J Comp Neurol. 1994;346:366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]
- Cerf-Ducastel B, Murphy C. Neural substrates of cross-modal olfactory recognition memory: an fMRI study. Neuroimage. 2006;31:386–96. doi: 10.1016/j.neuroimage.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Coates EL, Ballam GO. Upper airway CO2 receptors in tegu lizards: localization and ventilatory sensitivity. J Comp Physiol [B] 1987;157:483–9. doi: 10.1007/BF00691833. [DOI] [PubMed] [Google Scholar]
- Coates EL, Ballam GO. Breathing and upper airway CO2 in reptiles: role of the nasal and vomeronasal systems. Am J Physiol. 1989;256:R91–7. doi: 10.1152/ajpregu.1989.256.1.R91. [DOI] [PubMed] [Google Scholar]
- Coates EL, Ballam GO. Olfactory receptor response to CO2 in bullfrogs. Am J Physiol. 1990;258:R1207–12. doi: 10.1152/ajpregu.1990.258.5.R1207. [DOI] [PubMed] [Google Scholar]
- Craig AD. A rat is not a monkey is not a human: comment on Mogil. Nature Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606-c1. [DOI] [PubMed] [Google Scholar]; Nat Rev Neurosci. 10:466. doi: 10.1038/nrn2606-c1. [DOI] [PubMed] [Google Scholar]
- Dade LA, Zatorre RJ, Evans AC, Jones-Gotman M. Working memory in another dimension: functional imaging of human olfactory working memory. Neuroimage. 2001;14:650–60. doi: 10.1006/nimg.2001.0868. [DOI] [PubMed] [Google Scholar]
- Dade LA, Zatorre RJ, Jones-Gotman M. Olfactory learning: convergent findings from lesion and brain imaging studies in humans. Brain. 2002;125:86–101. doi: 10.1093/brain/awf003. [DOI] [PubMed] [Google Scholar]
- Danziger N, Faillenot I, Peyron R. Can we share a pain we never felt? Neural correlates of empathy in patients with congenital insensitivity to pain. Neuron. 2009;61:203–12. doi: 10.1016/j.neuron.2008.11.023. [DOI] [PubMed] [Google Scholar]
- de Leeuw R, Albuquerque R, Okeson J, Carlson C. The contribution of neuroimaging techniques to the understanding of supraspinal pain circuits: implications for orofacial pain. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:308–14. doi: 10.1016/j.tripleo.2004.11.014. [DOI] [PubMed] [Google Scholar]
- de Leeuw R, Davis CE, Albuquerque R, Carlson CR, Andersen AH. Brain activity during stimulation of the trigeminal nerve with noxious heat. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:750–7. doi: 10.1016/j.tripleo.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Doty RL, Brugger WE, Jurs PC, Orndorff MA, Snyder PJ, Lowry LD. Intranasal trigeminal stimulation from odorous volatiles: psychometric responses from anosmic and normal humans. Physiol Behav. 1978;20:175–85. doi: 10.1016/0031-9384(78)90070-7. [DOI] [PubMed] [Google Scholar]
- Ettlin DA, Brugger M, Keller T, Luechinger R, Jancke L, Palla S, Barlow A, Gallo LM, Lutz K. Interindividual differences in the perception of dental stimulation and related brain activity. Eur J Oral Sci. 2009;117:27–33. doi: 10.1111/j.1600-0722.2008.00590.x. [DOI] [PubMed] [Google Scholar]
- Frasnelli J, Hummel T. Interactions between the chemical senses: Trigeminal function in patients with olfactory loss. Int J Psychophysiol. 2007;65:177–81. doi: 10.1016/j.ijpsycho.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Frasnelli J, Schuster B, Hummel T. Subjects with congenital anosmia have larger peripheral but similar central trigeminal responses. Cereb Cortex. 2007;17:370–7. doi: 10.1093/cercor/bhj154. [DOI] [PubMed] [Google Scholar]
- Fröhlich R. Ueber einige Modificationen des Geruchsinnes. Wiener Sitzungsberichte, Math-naturw Classe Bd. 1851;6:322–8. [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gonzalez J, Barros-Loscertales A, Pulvermuller F, Meseguer V, Sanjuan A, Belloch V, Avila C. Reading cinnamon activates olfactory brain regions. Neuroimage. 2006;32:906–12. doi: 10.1016/j.neuroimage.2006.03.037. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Deichmann R, Winston JS, Dolan RJ. Functional heterogeneity in human olfactory cortex: an event-related functional magnetic resonance imaging study. J Neurosci. 2002;22:10819–28. doi: 10.1523/JNEUROSCI.22-24-10819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, Dolan RJ. The nose smells what the eye sees: crossmodal visual facilitation of human olfactory perception. Neuron. 2003;39:375–86. doi: 10.1016/s0896-6273(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Gottfried JA. Smell: central nervous processing. Adv Oto-Rhino-Laryngol. 2006;63:44–69. doi: 10.1159/000093750. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Winston JS, Dolan RJ. Dissociable codes of odor quality and odorant structure in human piriform cortex. Neuron. 2006;49:467–79. doi: 10.1016/j.neuron.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Hari R, Portin K, Kettenmann B, Jousmaki V, Kobal G. Pain. Vol. 72. 1997. Right-hemisphere preponderance of responses to painful CO2 stimulation of the human nasal mucosa; pp. 145–51. [DOI] [PubMed] [Google Scholar]
- Howard JD, Plailly J, Grueschow M, Haynes JD, Gottfried JA. Odor quality coding and categorization in human posterior piriform cortex. Nat Neurosci. 2009;12:932–8. doi: 10.1038/nn.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Zhong C, Ding C, Chi Q, Walz A, Mombaerts P, Matsunami H, Luo M. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317:953–7. doi: 10.1126/science.1144233. [DOI] [PubMed] [Google Scholar]
- Hummel T, Barz S, Lotsch J, Roscher S, Kettenmann B, Kobal G. Loss of olfactory function leads to a decrease of trigeminal sensitivity. Chem Senses. 1996;21:75–9. doi: 10.1093/chemse/21.1.75. [DOI] [PubMed] [Google Scholar]
- Hummel T, Kobal G. Chemosensory event-related potentials to trigeminal stimuli change in relation to the interval between repetitive stimulation of the nasal mucosa. Eur Arch Otorhinolaryngol. 1999;256:16–21. doi: 10.1007/s004050050115. [DOI] [PubMed] [Google Scholar]
- Hummel T, Livermore A. Intranasal chemosensory function of the trigeminal nerve and aspects of its relation to olfaction. Int Arch Occup Environ Health. 2002;75:305–13. doi: 10.1007/s00420-002-0315-7. [DOI] [PubMed] [Google Scholar]
- Hummel T, Doty RL, Yousem DM. Functional MRI of intranasal chemosensory trigeminal activation. Chem Senses. 2005;30 1:i205–i206. doi: 10.1093/chemse/bjh186. [DOI] [PubMed] [Google Scholar]
- Hummel T, Iannilli E, Frasnelli J, Boyle J, Gerber J. Central processing of trigeminal activation in humans. Ann N Y Acad Sci. 2009a;1170:190–5. doi: 10.1111/j.1749-6632.2009.03910.x. [DOI] [PubMed] [Google Scholar]
- Hummel T, Oehme L, van den Hoff J, Gerber J, Heinke M, Boyle JA, Beuthien-Baumann B. PET-based investigation of cerebral activation following intranasal trigeminal stimulation. Hum Brain Mapp. 2009b;30:1100–4. doi: 10.1002/hbm.20573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen J, Kobal G, Kaukoranta E, Hari R. Cortical responses to painful CO2 stimulation of nasal mucosa; a magnetoencephalographic study in man. Electroencephalogr Clin Neurophysiol. 1986;64:347–9. doi: 10.1016/0013-4694(86)90159-8. [DOI] [PubMed] [Google Scholar]
- Iannilli E, Gerber J, Frasnelli J, Hummel T. Intranasal trigeminal function in subjects with and without an intact sense of smell. Brain Res. 2007;1139:235–44. doi: 10.1016/j.brainres.2006.12.082. [DOI] [PubMed] [Google Scholar]
- Iannilli E, Del Gratta C, Gerber JC, Romani GL, Hummel T. Trigeminal activation using chemical, electrical, and mechanical stimuli. Pain. 2008;139:376–88. doi: 10.1016/j.pain.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Immordino-Yang MH, McColl A, Damasio H, Damasio A. Neural correlates of admiration and compassion. Proc Natl Acad Sci U S A. 2009;106:8021–6. doi: 10.1073/pnas.0810363106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvar M, Hsieh JC. The image of pain. In: Wall PD, Melzack R, editors. Textbook of pain. Vol. Churchill Livingstone; London: 1999. pp. 215–32. [Google Scholar]
- Kettenmann B, Jousmaki V, Portin K, Salmelin R, Kobal G, Hari R. Odorants activate the human superior temporal sulcus. Neurosci Lett. 1996;203:143–5. doi: 10.1016/0304-3940(95)12280-x. [DOI] [PubMed] [Google Scholar]
- Kettenmann B, Hummel C, Stefan H, Kobal G. Multiple olfactory activity in the human neocortex identified by magnetic source imaging. Chem Senses. 1997;22:493–502. doi: 10.1093/chemse/22.5.493. [DOI] [PubMed] [Google Scholar]
- Kobal G, Hummel C. Cerebral chemosensory evoked potentials elicited by chemical stimulation of the human olfactory and respiratory nasal mucosa. Electroencephalogr Clin Neurophysiol. 1988;71:241–50. doi: 10.1016/0168-5597(88)90023-8. [DOI] [PubMed] [Google Scholar]
- Kobal G, Van Toller S, Hummel T. Is there directional smelling? Experientia. 1989;45:130–2. doi: 10.1007/BF01954845. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Lancaster J, Thompson P, Toga AW, Brewer P, Hardies J, Fox P. An optimized individual target brain in the Talairach coordinate system. Neuroimage. 2002;17:922–7. [PubMed] [Google Scholar]
- Kwan CL, Crawley AP, Mikulis DJ, Davis KD. An fMRI study of the anterior cingulate cortex and surrounding medial wall activations evoked by noxious cutaneous heat and cold stimuli. Pain. 2000;85:359–74. doi: 10.1016/S0304-3959(99)00287-0. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–64. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley CK, Aziz Q, Bountra C, Gordon N, Hawkins P, Jones A, Langley G, Nurmikko T, Tracey I. Volunteer studies in pain research--opportunities and challenges to replace animal experiments: the report and recommendations of a Focus on Alternatives workshop. Neuroimage. 2008;42:467–73. doi: 10.1016/j.neuroimage.2008.05.030. [DOI] [PubMed] [Google Scholar]
- Lundstrom JN, Djordjevic J. The localization of human olfactory cortex: An ALE meta-analysis of functional neuroimaging studies. 2009. submitted. [Google Scholar]
- Mai JK, Assheuer J, Paxinos G. Atlas of the human brain. Vol. Elsevier Academic Press; Amsterdam: 2004. [Google Scholar]
- May A. Neuroimaging: visualising the brain in pain. Neurol Sci. 2007;28 2:S101–7. doi: 10.1007/s10072-007-0760-x. [DOI] [PubMed] [Google Scholar]
- Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–94. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–60. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Zaki J, Hanelin J, Ludlow DH, Knierim K, Ramachandran T, Glover GH, Mackey SC. Your pain or mine? Common and distinct neural systems supporting the perception of pain in self and other. Soc Cogn Affect Neurosci. 2008;3:144–60. doi: 10.1093/scan/nsn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterbauer RA, Matthews PM, Jenkinson M, Beckmann CF, Hansen PC, Calvert GA. Color of scents: chromatic stimuli modulate odor responses in the human brain. J Neurophysiol. 2005;93:3434–41. doi: 10.1152/jn.00555.2004. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30:263–88. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Plailly J, Radnovich AJ, Sabri M, Royet JP, Kareken DA. Involvement of the left anterior insula and frontopolar gyrus in odor discrimination. Hum Brain Mapp. 2007;28:363–72. doi: 10.1002/hbm.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J, Anand T, Johnson B, Khan RM, Sobel N. Brain mechanisms for extracting spatial information from smell. Neuron. 2005;47:581–92. doi: 10.1016/j.neuron.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Rombaux P, Mouraux A, Bertrand B, Guerit JM, Hummel T. Assessment of olfactory and trigeminal function using chemosensory event-related potentials. Neurophysiol Clin. 2006;36:53–62. doi: 10.1016/j.neucli.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Sakakibara Y. Localization of CO2 sensor related to the inhibition of the bullfrog respiration. Jpn J Physiol. 1978;28:721–35. doi: 10.2170/jjphysiol.28.721. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Kondo H, Price JL. Complementary circuits connecting the orbital and medial prefrontal networks with the temporal, insular, and opercular cortex in the macaque monkey. The Journal of Comparative Neurology. 2008;506:659–693. doi: 10.1002/cne.21577. [DOI] [PubMed] [Google Scholar]
- Savic I, Gulyas B, Larsson M, Roland P. Olfactory functions are mediated by parallel and hierarchical processing. Neuron. 2000;26:735–45. doi: 10.1016/s0896-6273(00)81209-x. [DOI] [PubMed] [Google Scholar]
- Savic I, Gulyas B, Berglund H. Odorant differentiated pattern of cerebral activation: comparison of acetone and vanillin. Hum Brain Mapp. 2002;17:17–27. doi: 10.1002/hbm.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer ML, Bottger B, Silver WL, Finger TE. Trigeminal collaterals in the nasal epithelium and olfactory bulb: a potential route for direct modulation of olfactory information by trigeminal stimuli. J Comp Neurol. 2002;444:221–6. doi: 10.1002/cne.10143. [DOI] [PubMed] [Google Scholar]
- Schoepf V, Kopietz R, Albrecht J, Kleemann AM, Haegler K, Brueckmann H, Wiesmann M. Analyzing fMRI data using ICA: a validation study using intranasal chemosensory trigeminal stimulation. 2009. submitted. [Google Scholar]
- Seifert F, Maihofner C. Central mechanisms of experimental and chronic neuropathic pain: findings from functional imaging studies. Cell Mol Life Sci. 2009;66:375–90. doi: 10.1007/s00018-008-8428-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shusterman DJ, Balmes JR. A comparison of two methods for determining nasal irritant sensitivity. Am J Rhinol. 1997;11:371–8. doi: 10.2500/105065897781286007. [DOI] [PubMed] [Google Scholar]
- Singer T. The neuronal basis of empathy and fairness. Novartis Found Symp. 2007;278:20–30. discussion 30-40, 89-96, 216-21. [PubMed] [Google Scholar]
- Small DM, Jones-Gotman M, Zatorre RJ, Petrides M, Evans AC. A role for the right anterior temporal lobe in taste quality recognition. J Neurosci. 1997;17:5136–42. doi: 10.1523/JNEUROSCI.17-13-05136.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Desmond JE, Glover GH, Goode RL, Sullivan EV, Gabrieli JD. Sniffing and smelling: separate subsystems in the human olfactory cortex. Nature. 1998a;392:282–6. doi: 10.1038/32654. [DOI] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Hartley CA, Desmond JE, Zhao Z, Glover GH, Gabrieli JD, Sullivan EV. Odorant-induced and sniff-induced activation in the cerebellum of the human. J Neurosci. 1998b;18:8990–9001. doi: 10.1523/JNEUROSCI.18-21-08990.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Zhao Z, Desmond JE, Glover GH, Sullivan EV, Gabrieli JD. Time course of odorant-induced activation in the human primary olfactory cortex. J Neurophysiol. 2000;83:537–51. doi: 10.1152/jn.2000.83.1.537. [DOI] [PubMed] [Google Scholar]
- Sobel N, Johnson B, Mainland J. Functional neuroimaging of human olfaction. In: Doty R, editor. Handbook of olfaction and gustation. 2nd. Marcel Dekker; New York: 2003. pp. 251–73. [Google Scholar]
- Stevens JC, Plantinga A, Cain WS. Reduction of odor and nasal pungency associated with aging. Neurobiol Aging. 1982;3:125–32. doi: 10.1016/0197-4580(82)90008-2. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Vol. Thieme; New York: 1988. [Google Scholar]
- Thürauf N, Friedel I, Hummel C, Kobal G. The mucosal potential elicited by noxious chemical stimuli with CO2 in rats: is it a peripheral nociceptive event? Neurosci Lett. 1991;128:297–300. doi: 10.1016/0304-3940(91)90283-y. [DOI] [PubMed] [Google Scholar]
- Tracey I. Imaging pain. Br J Anaesth. 2008;101:32–9. doi: 10.1093/bja/aen102. [DOI] [PubMed] [Google Scholar]
- Treede RD, Kenshalo DR, Gracely RH, Jones AK. The cortical representation of pain. Pain. 1999;79:105–11. doi: 10.1016/s0304-3959(98)00184-5. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–80. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Wang J, Eslinger PJ, Smith MB, Yang QX. Functional magnetic resonance imaging study of human olfaction and normal aging. J Gerontol A Biol Sci Med Sci. 2005;60:510–4. doi: 10.1093/gerona/60.4.510. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–64. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Wiech K, Preissl H, Birbaumer N. Neural networks and pain processing. New insights from imaging techniques. Anaesthesist. 2001;50:2–12. doi: 10.1007/s001010050956. [DOI] [PubMed] [Google Scholar]
- Wiesmann M, Kopietz R, Albrecht J, Linn J, Reime U, Kara E, Pollatos O, Sakar V, Anzinger A, Fesl G, Bruckmann H, Kobal G, Stephan T. Eye closure in darkness animates olfactory and gustatory cortical areas. Neuroimage. 2006;32:293–300. doi: 10.1016/j.neuroimage.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Yavari P, McCulloch PF, Panneton WM. Trigeminally-mediated alteration of cardiorespiratory rhythms during nasal application of carbon dioxide in the rat. J Auton Nerv Syst. 1996;61:195–200. doi: 10.1016/s0165-1838(96)00072-0. [DOI] [PubMed] [Google Scholar]
- Yousem DM, Williams SC, Howard RO, Andrew C, Simmons A, Allin M, Geckle RJ, Suskind D, Bullmore ET, Brammer MJ, Doty RL. Functional MR imaging during odor stimulation: preliminary data. Radiology. 1997;204:833–8. doi: 10.1148/radiology.204.3.9280268. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. Functional neuroimaging of the olfactory system in humans. Int J Psychophysiol. 2000;36:165–81. doi: 10.1016/s0167-8760(99)00110-5. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Jones-Gotman M, Evans AC, Meyer E. Functional localization and lateralization of human olfactory cortex. Nature. 1992;360:339–40. doi: 10.1038/360339a0. [DOI] [PubMed] [Google Scholar]
- Zelano C, Bensafi M, Porter J, Mainland J, Johnson B, Bremner E, Telles C, Khan R, Sobel N. Attentional modulation in human primary olfactory cortex. Nat Neurosci. 2005;8:114–20. doi: 10.1038/nn1368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


