Abstract
Structural restraints provided by solid-state NMR measurements of the metarhodopsin II intermediate are combined with molecular dynamics simulations to help visualize the structural changes in the light activation of rhodopsin. Since the time scale for the formation of the metarhodopsin II intermediate (> 1 ms) is beyond that readily accessible by molecular dynamics, we use NMR distance restraints derived from 13C dipolar recoupling measurements to guide the simulations. The simulations yield a working model for how photoisomerization of the 11-cis retinylidene chromophore bound within the interior of rhodopsin is coupled to transmembrane helix motion and receptor activation. The mechanism of activation that emerges is that multiple switches on the extracellular (or intradiscal) side of rhodopsin trigger structural changes that converge to disrupt the ionic lock between helices H3 and H6 on the intracellular side of the receptor.
Keywords: rhodopsin, G protein-coupled receptors, NMR, molecular dynamics, retinal
Introduction
G protein-coupled receptors (GPCRs) are a large superfamily of membrane receptors that have seven transmembrane (TM) helices and respond to a wide array of signaling ligands.1-3 The visual pigment rhodopsin is perhaps the best characterized GPCR. The crystal structure of the dark, inactive receptor was determined at 2.8 Å resolution by Palczewski and Okada4 and later improved by Okada5 and Schertler6 (Fig. 1). The crystal structures of several of the photointermediates in the rhodopsin photoreaction have been determined, including bathorhodopsin,7,8 lumirhodopsin9 and metarhodopsin I.10 However, these structures reveal only very slight differences with the dark rhodopsin structure. The transition to the active, metarhodopsin II (Meta II) intermediate is known to require a fluid, membrane environment,11 and involves an appreciable change in the conformation of the receptor.
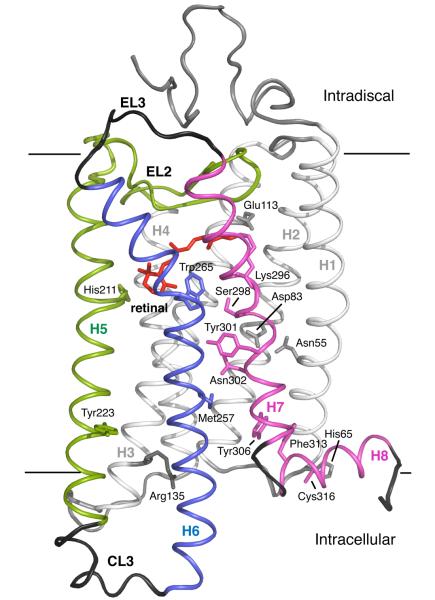
Fig. 1.
Crystal structure of rhodopsin. The photoreactive 11-cis retinal chromophore (red) is buried within the bundle of seven TM helices on the extracellular (or intradiscal) side of the receptor. Transmembrane helices H1-H4 (gray) form a rigid framework that is stabilized by tight packing mediated by group conserved amino acids and hydrogen bonding interactions.100 Guided MD simulations are used to characterize the motion of TM helices H5 (green), H6 (blue) and H7 (purple) upon isomerization of the retinal and deprotonation of the retinal – Lys296 Schiff base linkage.
Several important structures have been determined in the past few years that shed light on the activated state of rhodopsin. The most important of these are the crystal structures of opsin.12,13 Opsin is formed when the active Meta II intermediate decays and releases the “agonist” all-trans retinal SB from the retinal binding site. Opsin has a low, but detectable, basal activity. The 11-cis retinal serves as an ‘inverse agonist’; binding of this isomer of the retinal as a protonated Schiff base (PSB) lowers the basal activity of rhodopsin to undetectable levels. Nevertheless, opsin crystallized at low pH retains several features that reflect the active conformation14, including disruption of the ionic lock between the conserved E(D)RY sequence on TM helix H3 and Glu247 on TM helix H6. In fact, opsin has been co-crystallized with a peptide corresponding to the C-terminal 11 amino acids of the G subunit of transducin.13 The peptide adopts a conformation similar to that observed in NMR studies on the active Meta II intermediate.15,16
Molecular dynamics (MD) simulations complement the structural studies on rhodopsin and provide insights into the mechanism of light-induced activation. MD simulations of rhodopsin and the first photointermediate, bathorhodopsin, have been undertaken in lipid bilayers17-23 and in membrane mimetics.24 The simulations are based on the rhodopsin crystal structure and have addressed early events (ns-μs range) after isomerization, involving the protonation states of amino acids within the retinal binding site,24,25 the stability of the Glu113-retinal PSB salt bridge,18,26 the conformation of the retinal chain,27 and energy storage in the bathorhodopsin photoproduct.28,29
Several factors have limited the use of MD for probing the active Meta II conformation. First, the time scale for the formation of the Meta II intermediate (> 1 ms)30 is well beyond the time scale accessible by MD even with current state-of-the-art computational resources. Second, many simplifications must be introduced in the MD simulations both at the level of model construction and at the level of the molecular mechanics approach in order to make the computational problem tractable. For example, the inclusion of an explicit lipid bilayer in the calculations increases the demand on computational resources and makes it more difficult to extensively sample different receptor conformations. Recently, simulations have taken advantage of new computational methods to speed up the conformational searches of helix orientations25 and the inclusion of mass-weighted distance restraints to propose dynamical models of the Meta II state.31
To overcome some of the limitations of MD simulations in investigating the active Meta II structure, we take advantage of distance restraints obtained over the past several years using solid-state 13C and 15N NMR spectroscopy. The NMR distance restraints are generally within the bundle of TM helices and complement site directed EPR spin labeling studies of Hubbell and coworkers32,33 revealing that there is a large outward rotation of H6 on the cytoplasmic side of rhodopsin. Our NMR data have shown that retinal isomerization leads to a large rotation of the retinal C20 methyl group accompanied by movement of the β-ionone ring toward TM helix H5.34 In addition, hydrogen-bonding changes are observed within key conserved regions of the receptor.35,36 There are not a sufficient number of NMR measurements to define the three-dimensional structure of Meta II; rather the measurements that are used in the guided MD simulations are made in specific regions of the protein to address local changes in structure or environment. This approach provides a working model of the active state of rhodopsin in which our current experimental NMR restraints are satisfied. The resulting model for the active Meta II state can be compared with the crystal structures of opsin13 and Meta I.10
Results and Discussion
Retinal isomerization: Trajectory of the C20 methyl group
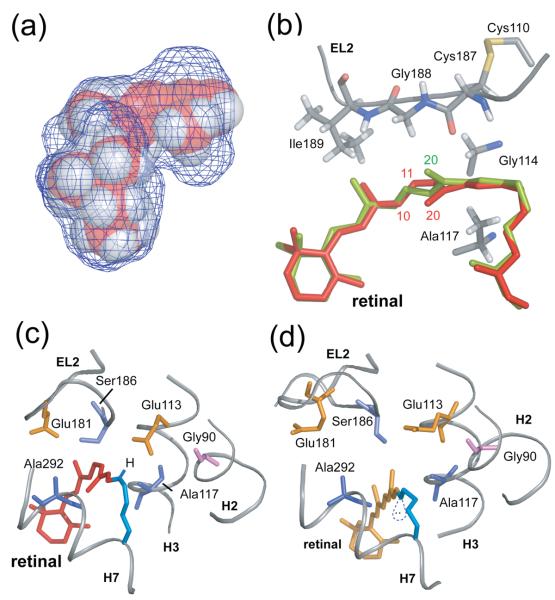
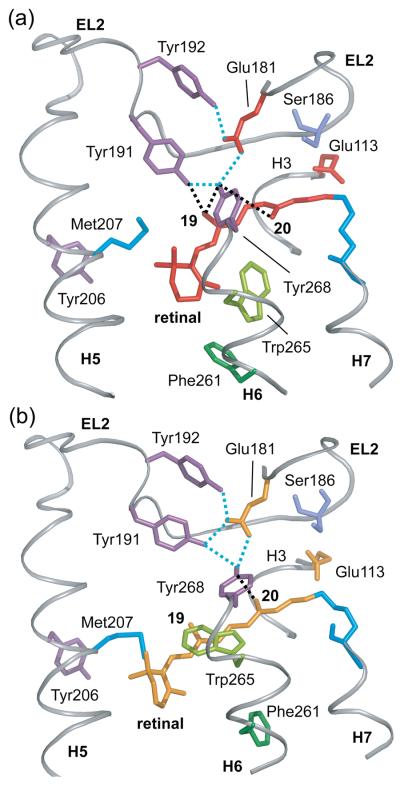
Fig. 2(a) presents a space-filling model of the retinal chromophore and amino acids forming the retinal binding site based on the crystal structure of rhodopsin (PDB ID: 1U195). The mesh surface illustrates the shape of the binding site and shows that the retinal C19 methyl group is tightly packed, while there is a cavity extending from the C20 methyl group toward the second extracellular loop (EL2). Part of that cavity is occupied by crystallographic water (Wat2a in the 1L9H rhodopsin structure37). In our simulations, isomerization is modeled by applying a strong torsional restraint to the C10-C11=C12-C13 dihedral angle, and the retinal isomerizes with motion of the C20 methyl group in the clockwise direction (viewed from the PSB end of the retinal) passing through the cavity seen in Fig. 2(a). There is a substantial body of data consistent with this trajectory and with the strong influence of the protein in determining the structure and photochemistry of the 11-cis retinal.7,8
Fig. 2.
Trajectory of the C20 methyl group and Schiff base proton upon retinal isomerization. (A) The packing of residues around the retinal chromophore in the crystal structure of rhodopsin is visualized by calculating a cavity surface for the binding site. The shape of this cavity likely determines the motion of the C19 and C20 methyl groups upon isomerization. The C19 methyl group is tightly packed against Ile189 and Tyr191, whereas a void exists above the retinal C20 methyl group in the direction of the EL2 loop. The view in this panel is from the β-ionone ring end of the retinal. (B) Structure of the retinal binding site in rhodopsin highlighting the position of the retinal in rhodopsin (red) and in a model from MD simulations immediately after isomerization (orange). (C) and (D) present views of the retinal binding site in rhodopsin viewed from the Schiff base end of the retinal highlighting the position, orientation and interactions of the retinal SB nitrogen. (C) In rhodopsin, the SB proton is oriented toward the extracellular side of the receptor and interacts with Glu113 (H3) and Ser186 (EL2). (D) In the guided MD simulations of Meta II, the electron pair on the SB nitrogen has rotated to a more hydrophobic environment and is pointing toward the cytoplasmic side of the protein.
The torsions about the C11=C12 and C12-C13 bonds are required for the extremely fast, selective photoreaction to the 11-trans isomer with a high quantum yield.38 There is both NMR39 and crystallographic5 data suggesting that the C20 methyl group contributes to “pre-twisting” the C11=C12 bond to favor the initial isomerization trajectory. A clockwise rotation about the C11=C12 bond moves the retinal C20 methyl group away from the C10H proton and the retinal C12H proton away from EL2. Liu et al.40 proposed that steric contact between the retinal C12H proton and the carbonyl of Cys187 helps drive the retinal C12H group away from EL2. Conversely, as we shall see below, this steric contact may also help to displace EL2 away from the retinal-binding site. The large rotation of the retinal C20 methyl group toward EL2 is in agreement with our recent solid-state NMR studies on Meta II (see below) showing that the retinal C20 methyl group gains a contact with Gly114 (H3).41,42 It is noteworthy that when the retinal C20 methyl group is removed (i.e. in 13-desmethyl-rhodopsin), the photoreaction is slowed43 and the quantum yield is reduced.44,45
Fig. 2(b) presents the structure of the retinal binding site and highlights several of the amino acids that influence the trajectory of the retinal C20 methyl group. Glu181 is also part of EL2 and may contribute to the retinal photochemistry by positioning a negative charge next to C12 and lowering the C11=C12 bond order (Fig. 2(c)).4,46-48 The conformation of the retinal in the dark state is shown in red, while the conformation immediately following isomerization (20 ps) is shown in green. In the simulations, the C10-C11=C12-C13 dihedral angle changes from −41° found in the rhodopsin crystal structure to approximately −169°. The conformation is distorted and almost identical to that modeled into the recent crystal structure of bathorhodopsin.7,8 Comparison of retinal binding site resulting from short (20 ps) isomerization simulations with the bathorhodopsin structure yields an RMS deviation for residues within 3 Å of the retinal of ~0.6 Å, and an RMS deviation of the retinal itself of ~0.3 Å. The retinal atoms in the region of isomerization (C11,C12,C19,C20) overlap with high accuracy in two structures.
The trajectory of the retinal C20 methyl group also defines the trajectory of the PSB N-H bond. The orientation of the N-H vector has implications for PSB deprotonation. One of the unusual findings in bacteriorhodopsin, the light-driven proton pump that has many similarities with rhodopsin, was that retinal isomerization displaces the PSB proton in a direction opposite to that of proton translocation.49 The displacement disrupts the hydrogen-bonding network of the PSB proton, lowers the PSB pKa and induces deprotonation.50,51 A similar mechanism may be operative in rhodopsin.
Fig. 2(c) shows the position of the PSB proton in rhodopsin. The NH proton is oriented toward Glu113 and the more hydrophilic residues on EL2 (Glu181 and Ser186). Fig. 2(d) shows the position of the unprotonated retinal Schiff base chromophore in Meta II determined from guided MD simulations. The orientation of the retinal C20 methyl group toward the extracellular surface of the receptor suggests that the free electron pair on the Schiff base nitrogen is oriented toward the receptor interior, a more hydrophobic environment. A comparison of the retinal chemical shifts in Meta II with those of retinal model compounds reveals that the C=N bond is highly polarized in a manner that would facilitate Schiff base hydrolysis.52
Displacement of EL2 upon activation
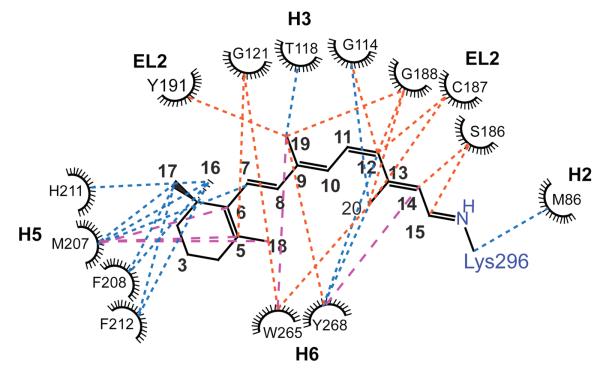
EL2 forms a lid over the retinal-binding cavity and the amino acids on the β4 strand of EL2 (Ser186, Cys187, Gly188 and Ile189) are interacting closely with the retinal polyene chain in rhodopsin. We have obtained distance restraints between amino acids on EL2 and the retinal (C9, C12, C14, C15 and C20) using solid-state 13C NMR spectroscopy.42 These new restraints are included in Table 1 and Fig. 3.
Table 1.
Distance restraints from solid-state MAS NMR measurements.
| # | Retinal β-Ionone Ring - H5 | Distance (Å) |
||
|---|---|---|---|---|
| Meta II | Rho (PDB ID:1U19) |
|||
| A1 | C6 | 207 Met CE41 | < 4 | 5.35 |
| A2 | C7 | 207 Met CE41 | < 4 | 4.9 |
| A3 | C16 | 207 Met CE41 | < 5 | 3.8 |
| A4 | C17 | 207 Met CE41 | < 5 | 4.5 |
| A5 | C16 | 207 Met C41 | < 5 | 4.9 |
| A6 | C16 | 211 His C41 | < 5 | 4.9 |
| A7 | C16 | 208,212 Phe C41*(ring) | < 5 | 4.7-6.2 |
| A8 | C17 | 208,212 Phe C41*(ring) | < 5 | 4.2-6.1 |
| A9 | C5 | 207 Met CE41 | < 5 | 6.7 |
| A10 | C18 | 207 Met CE41 | < 5 | 7.1 |
| A11 | C5 | 121 Gly CA41 | > 6 | 5.1 |
| A12 | C18 | 121 Gly CA41 | > 6 | 3.7 |
| A13 | C5 | 167 Cys CB41 | > 6 | 8.2 |
| A14 | C6 | 167 Cys CB41 | > 6 | 8.0 |
| A15 | C7 | 167 Cys CB41 | > 6 | 7.8 |
|
Retinal Polyene Chain – Protein | ||||
| B1 | C20 | 114 Gly CA41 | < 6 | 7.2 |
| B2 | C20 | 188 Gly CA41 | > 6 | 6.2 |
| B3 | C12 | 114 Gly CA41 | > 6 | 4.6 |
| B4 | C12 | 188 Gly CA41 | > 6 | 4.3 |
| B5 | C12 | 268 Tyr CZ41 | < 5 | 4.9 |
| B6 | C14 | 268 Tyr CZ41 | < 5 | 6.0 |
| B7 | C20 | 268 Tyr CZ41 | < 5 | 4.2 |
| B8 | C12 | 191 Tyr CZ41 | > 6 | 7.5 |
| B9 | C14 | 191 Tyr CZ41 | > 6 | 9.2 |
| B10 | C20 | 191 Tyr CZ41 | > 6 | 8.0 |
| B11 | C12 | 178 Tyr CZ41 | > 6 | 7.2 |
| B12 | C14 | 178 Tyr CZ41 | > 6 | 9.1 |
| B13 | C20 | 178 Tyr CZ41 | > 6 | 9.7 |
| B14 | C12 | 192 Tyr CZ41 | > 6 | 10.5 |
| B15 | C14 | 192 Tyr CZ41 | > 6 | 11.2 |
| B16 | C20 | 192 Tyr CZ41 | > 6 | 10.8 |
| B17 | C19 | 268 Tyr CZ34 | > 6 | 4.3 |
| B18 | C19 | 191 Tyr CZ34 | > 6 | 4.7 |
| B19 | C19 | 118 Thr C*41 | < 5 | 4.5-6.4 |
| B20 | C14 | 186 Ser CB34 | > 6 | 4.5 |
| B21 | C15 | 186 Ser CB34 | > 6 | 4.1 |
| B22 | C19 | 188 Gly CA34 | > 6 | 4.6 |
| B23 | C9 | 189 Ile C*42 | > 6 | 3.6-6.0 |
| B24 | C12 | 187 Cys C=O42 | > 6 | 4.2 |
| B25 | C20 | 187 Cys C=O42 | > 6 | 6.2 |
| B26 | C12 | 188 Gly C=O42 | > 6 | 5.6 |
| B27 | C20 | 188 Gly C=O42 | > 6 | 7.5 |
|
Retinal - Trp265 | ||||
| C1 | C19 | 265 Trp CE3,CZ3,CH236 | < 5 | 6.0-10.0 |
| C2 | 121 Gly CA | 265 Trp CE3,CZ3,CH236 | > 6 | 3.7-5.9 |
| C3 | C20 | 265 Trp Ring36 | > 6 | 3.9-8.3 |
| C4 | C18 | 265 Trp Ring41 | > 6 | 3.7-5.1 |
| C5 | C5 | 265 Trp Ring41 | > 6 | 3.7-5.3 |
|
Protein-Protein | ||||
| D1 | 167 Cys CB | 211 His CE41 | > 5 & < 6 | 3.9 |
| D2 | 167 Cys CB | 207 Met CE41 | < 5 | 7.8 |
| D3 | 167 Cys CB | 206 Tyr CZ41 | > 5 & < 6 | 5.3 |
| D4 | 207 Met CE | 211 His CE41 | < 5 | 9.7 |
| D5 | 163 Met CE | 211 His CE41 | < 5 | 4.0 |
| D6 | 211 His CE | 206 Tyr CZ41 | < 5 | 4.4 |
| D7 | 288 Met CE | 268 Tyr CZ42 | < 5 | 3.9 |
| D8 | 288 Met CE | 191 Tyr CZ42 | < 5 | 5.2 |
| D9 | 288 Met CE | 192 Tyr CZ42 | > 6 | 5.7 |
| D10 | 86 Met CE | 120 Gly CA36 | < 5 | 4.2 |
| D11 | 114 Gly CA | 178 Tyr CZ42 | > 6 | 4.5 |
| D12 | 296 Lys CE | 44 Met CE41 | > 5 & < 6 | 4.7 |
| D13 | 298 Ser CB | 86 Met CE42 | > 5 & < 6 | 4.8 |
| D14 | 298 Ser CB | 301 Tyr CZ42 | < 4 | 4.9 |
| D15 | 264 Cys CB | 301 Tyr CZ42 | < 5 | 4.8 |
| D16 | 316 Cys CB | 65 His CE142 | > 6 | 3.6 |
| D17 | 160Thr CA/CB | 126/161 Trp (ring) 42 | < 5 | (4.7-8.9)/(4.8-8.7) |
All carbons of the amino acid.
Fig. 3.
Two-dimensional residue map of close (< 6 Å) interactions near the retinal-binding site determined by 2D DARR NMR distance measurements in rhodopsin and Meta II. There are three main types interactions shown: 1) retinal-protein interactions observed in both rhodopsin and Meta II (blue dotted lines), 2) interactions that are observed in rhodopsin and are lost upon conversion to Meta II (orange dotted lines) and 3) interactions that are observed only in Meta II (broken magenta lines). See also Table 1.
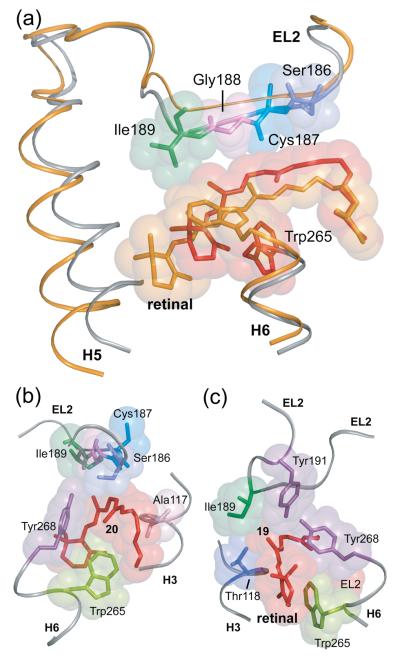
The guided MD simulations serve to integrate the NMR distance restraints into a working model for how retinal isomerization triggers local structural changes in the retinal binding site. First, the C20 methyl group undergoes a large rotation to place it near Gly114 on H3. The C13-C20 bond vector is oriented toward H3 at an angle of ~60° to the membrane normal. Independently, site-directed deuterium NMR studies on Meta I by Brown and coworkers39 have shown that the C13-C20 bond is oriented at an angle of 59±3°. The C9-C19 bond vector is also oriented toward H3 and the extracellular surface in the Meta II model, consistent with the observation that the retinal C19-Thr118 NMR contact is retained in Meta II. In order to satisfy the retinal – EL2 restraints, the retinal moves slightly toward the interior of the receptor and EL2 moves away from the retinal-binding site. The displacement of EL2 shown in Fig. 4(a) is consistent with studies showing that the retinal-binding site is accessible to water and hydroxylamine in Meta II.53-56
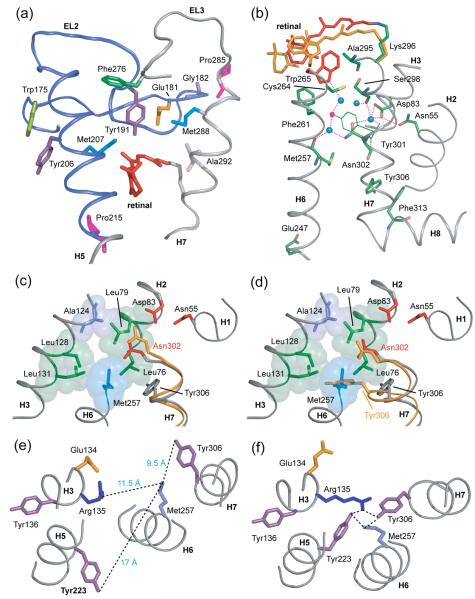
Fig. 4.
Displacement of EL2 upon rhodopsin activation. (A) The β4 strand of EL2 forms a lid of the retinal binding site and is connected to transmembrane helix H5. The rhodopsin crystal structure (gray) is superimposed on the Meta II model (orange) obtained from the guided MD simulations to illustrate the displacement of EL2 needed to satisfy the NMR restraints. (B) and (C) present space filling models of the retinal binding site highlighting the packing of the C20 and C19 methyl groups, respectively. (B) The retinal C20 methyl group is in contact with Trp265 and Tyr268 on H6. Clockwise rotation of the C20 methyl group upon isomerization changes its orientation toward EL2. (C) The C19 methyl group packs against Thr118 on H3, Tyr268 on H6 and Ile189 and Tyr191 on EL2. Counterclockwise rotation of the C19 methyl group would result in a steric clash with Tyr191 and Tyr268, and may be responsible for a shift in the hydrogen bonding interactions involving EL2.
The displacement of EL2 provides insights into the roles of the retinal C19 and C20 methyl groups in rhodopsin activation. The C20 methyl group in rhodopsin contacts Trp265 and Tyr268 on H6 (Fig. 4(b)). These residues are both important for receptor activation and likely contribute to establishing the pre-twist of the 11-cis retinal in rhodopsin. The large motion of the C20 methyl group is facilitated by the cavity shown in Fig. 2a. The retinal C19 methyl group in rhodopsin is tightly constrained in the retinal binding site and packs against Thr118 on H3, Ile189 and Tyr191 on EL2 and Tyr268 on H6 (Fig. 4(c)). NMR distance measurements show loss of contacts between the C19 methyl group and Tyr191 and Tyr268 in Meta II. The MD simulations suggest that the displacement of EL2 from the retinal binding site is responsible for the loss of these interactions. There is experimental support for the idea that the C19 methyl group facilitates the motion of EL2 in Meta II. First, removal of the C19 methyl group prevents receptor activation.57-59 Second, replacement of the retinal C19 methyl group with an ethyl or propyl group results in dark activity of rhodopsin, with the amount of activity being proportional to the size of the substituent at the C19 position.60 Functional studies on other GPCRs are consistent with a regulatory role of EL2 in activation. For example, in the melanocortin receptor a short EL2 facilitates receptor activation, presumably because it lacks inhibitory contacts with the TM helices. 61 In the C5a receptor, many substitutions in EL2 lead to constitutive activity, presumably by disrupting inhibitory contacts.62
Motion of the β-ionone ring: Stabilizing H5 in an active conformation
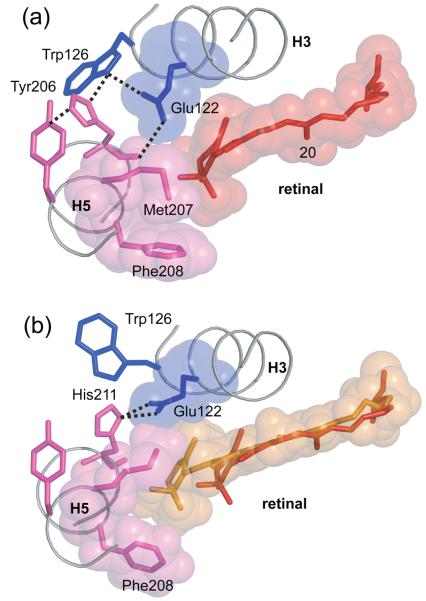
The β-ionone ring of the retinal is required for rhodopsin activation.63,64 Our recent solid-state NMR measurements have focused on the conformation of the ring and its position in the Meta II intermediate.41 Fig. 3 presents the experimental NMR contacts observed between the retinal and the protein that highlight the packing of the β-ionone ring with amino acids in the vicinity of His211 on H5 in Meta II. We observed strong crosspeaks between Cε-Met207 on H5 and the retinal C6 and C7 carbons in Meta II as compared to rhodopsin.41 The intensities of the Met207-retinal crosspeaks indicate the Cε methyl group of Met207 has moved into van der Waals contact with the retinal in Meta II. Additionally, NMR crosspeaks between the retinal C16 and C17 methyl groups and the backbone carbonyls of His211 and Met207 on H5 were found to increase in intensity in Meta II.41 These changes are consistent with a small movement of the β-ionone ring toward H5.
Fig. 5 shows the position of the β-ionone ring in Meta II based on guided MD simulations. The retinal straightens in Meta II and the ring translates ~2 Å toward H5. The flexible Met207 side chain changes position and lies along the face of the β-ionone ring that is closest to helices H3 and H4. There are several experimental observations that the hydrogen-bonding network involving Glu122 (H3), Trp126 (H3), Tyr206 (H5) and His211 (H5) rearranges in the transition from rhodopsin to Meta II. FTIR studies indicate that Glu122 is protonated in both rhodopsin and Meta II,65,66 while NMR studies show that His211 is neutral in both rhodopsin and Meta II.35 As a result, Glu122 and His211 do not form a salt bridge; rather, as seen in the crystal structure of rhodopsin, the Glu122 carboxyl group hydrogen bonds to the backbone carbonyl of His211. The His211 carbonyl is free to hydrogen bond due to the presence of a highly conserved Pro215 (73%) at the i+4 position of H5.
Fig. 5.
Hydrogen bonding of His211 in rhodopsin (A) and Meta II (B). Meta II formation is associated with a change in the hydrogen bonding network centered on His211. In rhodopsin, the backbone carbonyl of His211 forms a hydrogen bond with the side chain of Glu122, while the imidazole side chain of His211 interacts with Trp126. A shift in the position of the retinal β-ionone ring upon activation disrupts the interaction between the backbone carbonyl of His211 and Glu122, and a new hydrogen bond forms with the imidazole side chain δ-nitrogen.
The MD simulations suggest how the hydrogen-bonding interactions between H3 and H5 rearrange in Meta II, and how these changes may be related to Meta II stability and receptor activation. When viewed from the extracellular surface, the β-ionone ring of the retinal is packed against Glu122 in rhodopsin (Fig. 5(a)). The guided MD simulations suggest that when the retinal moves toward H5, the Glu122 side chain changes its hydrogen bonding interactions. This movement disrupts the hydrogen bond between the main chain carbonyl of His211 and the side chain of Glu122 as observed by NMR35 and a new hydrogen bond forms between Glu122 and the imidazole δ-nitrogen of His211. A direct side chain interaction would explain mutational data on His211 that point to a role of the His211 side chain in Meta II stability and activation.67 The changes in the orientation of His211 and Glu122 that lead to a direct interaction are observed in the crystal structure of opsin12,13 and provide a validation of the MD simulations assuming that the opsin structure in this region reflects the structure of the active Meta II intermediate.
Studies on the β2-adrenergic receptor first highlighted the importance of H5 in the activation mechanism.68 H5 connects EL2 and CL3. EL2 was briefly mentioned above in context to forming the retinal-binding site, and will be discussed more extensively below in terms of its role in activation. CL3 is the primary site for G protein interactions on the cytoplasmic surface of rhodopsin and other class A GPCRs.69-71 In contrast to the subfamily specific nature of the extracellular side of H5, the intracellular sequence of H5 contains sites that are conserved across the large family of class A GPCRs. For example, Tyr223 is highly conserved (93%). In the opsin crystal structure, the intracellular end of H5 has rotated to place the side chain of Tyr223 into close proximity of Arg135, which is part of the conserved ERY sequence on H3. The Tyr223-Arg135 interaction observed in opsin is responsible for breaking the ionic lock that exists between Arg135 (H3) and Glu247 (H6) in rhodopsin (see below). A similar change in hydrogen bonding interactions may occur in the adrenergic receptors.72 NMR studies on Meta II show that this feature of the opsin structure is reflective of the active state.42
The results described above allow us to trace the first connection between the structural changes on the extracellular side of rhodopsin and formation of the G protein binding site on the intracellular side of the receptor. Isomerization of the 11-cis retinal PSB in rhodopsin to the all-trans unprotonated SB results in displacement of EL2 due to SB deprotonation or steric interactions involving the C19 and C20 methyl groups, or both. Displacement of EL2 is coupled to the motion of H5 (see Fig. 4). The hydrogen-bonding network on the extracellular end of H5 holds the helix in an inactive orientation. An outward motion of the extracellular end of H5 with a slight rotation stabilized by a direct His211-Glu122 interaction leads to an inward rotation on the intracellular side of H5 (see Fig. 4 and 5). The motion of H5 places Tyr223 in a position to substitute for Glu247 and disrupt the ionic lock between H3 and H6. Interaction with Arg135 favors and stabilizes this new active state conformation.
Motion of the β-ionone ring: Trigger for motion of H6
Movement of TM helix H6 is one of the key elements in the activation of rhodopsin. The general model that has emerged following the pioneering EPR measurements of Hubbell and co-workers32 is that the relative motion of helices H3 and H6 couple retinal isomerization to the disruption of the “ionic lock” involving the ERY sequence and Glu247 at the cytoplasmic ends of H3 and H6, respectively. Crosslinking studies using cysteine disulfide formation32 and His-Zn coordination73 have shown that it is possible to block receptor activation by restraining the motion of the cytoplasmic ends of H3 and H6. In contrast, it is possible to produce a constitutively active receptor by mutating Glu134 to glutamine in the ERY sequence on H3.74,75
These studies raise the question as to how retinal isomerization on the extracellular side of H6 is coupled to the outward rotation of the cytoplasmic end of H6. On the extracellular side of H6 is a highly conserved aromatic cluster near Pro267 that is generally accepted to be essential for receptor activation. Pro267 is among the most highly conserved amino acids in the class A GPCRs. Computational and experimental studies suggest that Pro267 serves as a hinge to facilitate H6 motion.76-79
In rhodopsin, the aromatic cluster is formed by three residues (Phe261, Trp265 and Tyr268). Trp265 lies within the arc formed by the retinal polyene chain and the Lys296 side chain, and is packed between Gly121 on H3 and Ala295 on H7. The retinal appears to function as a clamp to prevent motion of Trp265 (and H6). We have previously proposed that motion of the β-ionone ring toward H5 is essential to release the steric restraints on Trp265.36 In the rhodopsin crystal structure, the C20 methyl group is closely packed near Trp265 and we observe the corresponding C20 – Trp265 NMR crosspeak. On conversion to Meta II, we lose the C20-Trp265 contact and gain a new contact between C19 and Trp265. The new C19 – Trp265 contact is one of the key restraints to emerge from the NMR studies on Meta II since the trajectory of the retinal is away from Trp26536 and the C19 methyl group is oriented toward EL2. That is, in order to gain a C19-Trp265 contact, there must be an appreciable change in the position of Trp265 towards the EL2 (see Fig. 4(a)).
Fig. 4(a) shows the results of MD simulations in the region of Trp265. The positive NMR restraint between the Trp265 side chain and the retinal C19 methyl group, along with the negative restraints between Trp265 and the C18 and C20 methyl groups, results in rotation of the Trp265 side chain toward the extracellular surface. Movement of the Trp265 sidechain is consistent with UV absorbance studies that show a motion of Trp265 into a more hydrophilic environment, perhaps due to accessibility of water at this site,80 and that the plane of the indole side chain of Trp265 changes from an orientation parallel to the bilayer normal to an orientation roughly perpendicular to the bilayer normal.81
The importance of the aromatic cluster was highlighted by studies on the dopamine receptor by Javitch and coworkers79 who proposed a rotamer toggle switch mechanism for triggering the motion of H6 and receptor activation. In these receptors, ligand binding is thought to induce a change in the side chain conformation of Phe6.52, which is coupled to changes in the side chain conformations of Cys6.47, Trp6.48 and Pro6.50 (using the Ballesteros and Weinstein numbering convention82). In the ligand-activated GPCRs, the ‚ligand-sensor’ is a phenylalanine.83,84 Ligand binding alters the packing of the Phe6.52 aromatic ring, which in turn allows the conserved tryptophan at position 6.48 to rotate.
Unlike other GPCRs, in rhodopsin, the residue at position 6.52 is an alanine. However, the small residue at this position in rhodopsin allows the retinal β-ionone ring to pack in the same position. Movement of the β-ionone ring due to light-induced retinal isomerization would have the same function as rotation of the Phe6.52 side chain due to ligand binding. The release of Trp265 allows the intracellular end of H6 to rotate outward and a new contact to form between Arg135 and Met257 (as observed in the opsin crystal structure12,13), effectively breaking the Arg135-Glu247 ionic lock between H3 and H6.
The guided MD simulations are also consistent with this mechanism in that rearrangement of the hydrogen bonding network involving EL2 and the rotation of the Trp265 side chain allow extracellular end of H6 to shift position in Meta II. Met288 contacts EL2 at the extracellular end of TM H7, while Tyr268 at the extracellular end of H6 forms hydrogen bonds to Glu181 and Tyr191 on EL2 (Figs. 6(a) and 7(a)). Glu181 is at the center of an extensive hydrogen-bonding network on EL2, which is connected through water-mediated hydrogen bonds to Ser186 and to Glu113 (H3). Glu113 is hydrogen-bonded to the backbone carbonyl of Cys187 (EL2) through a water molecule. A rearrangement in the hydrogen bonding network involving EL2 and H6 is shown in Figs. 6(a,b). In order to satisfy the NMR restraints, the MD simulations show that Tyr191 forms a hydrogen bond with Glu181 and Tyr268 shifts position toward Glu113. The unusual downfield 13Cζ-OH chemical shift of Tyr191 is consistent with an increase in hydrogen bonding character.42 Movement of Tyr268 toward Glu113 is consistent with the global toggle switch mechanism where metal ion coordination in the β2-adrenergic receptor between engineered cysteine or histidine residues at the equivalent positions of Tyr268 and Glu113 results in receptor activation.85
Fig. 6.
Hydrogen bonding changes involving EL2. (A) Crystal structure of rhodopsin highlighting the hydrogen bonding network centered on Glu181. Glu181 is hydrogen bonded (dotted blue lines) to Tyr192 and Tyr191 on EL2 and Tyr268 on H6. Tyr191 is also hydrogen bonded to Tyr268 on H6. Black dotted lines highlight the close interactions between Tyr268 and the retinal C19/C20 methyl groups, and between Tyr191 and the retinal C19 methyl group. (B) The guided MD simulations indicate that retinal isomerization and displacement of EL2 upon activation leads to a rearrangement of the hydrogen bonding network involving Glu181 on EL2. The Glu181 side chain remains hydrogen bonded to Tyr191, Tyr192 and Tyr268 in Meta II. However, Tyr191 is now hydrogen bonded to both Tyr192 and Tyr268. The Meta II model also shows that the retinal C19 methyl group is no longer packed against Tyr191 or Tyr268, although Tyr268 is still in close contact with the retinal C20 methyl group.
Fig. 7.
Coupling of retinal isomerization and displacement of EL2 to motion of H7. (A) The extracellular end of H7 contacts EL2 from Pro285 to Ala292. The H5-EL2 segment is presented in blue and EL3-H7 is presented in grey. In rhodopsin, Met288 on H7 is packed against Glu181 on EL2 and Pro285 on EL3 is packed against Gly182 on EL2. The displacement of EL2 upon retinal isomerization and deprotonation of the retinal SB linkage may allow EL3 and H7 to shift into their active conformations. (B) A view of the water-mediated hydrogen bonding network involving H7. The network extends from Trp265 (red; on H6) to Phe313 (green; on H8) through the conserved NPxxY (Asn302…Tyr306) on H7. Blue filled circles represent structural water. Positions of the all-trans retinal and Trp265 from guided MD simulations are shown in orange. In rhodopsin, Trp265 is packed between the side chain of Ala295 on H7 and the retinal β-ionone ring. The Meta II model shows a movement of the Trp265 side chain toward the extracellular side of the receptor away from the water cluster surrounding Asn302 (H7). (C) and (D) present a view of the protein pocket surrounding Asn302 on H7 in rhodopsin. One side of the pocket containing Asn302 is hydrophobic and composed of Leu76, Leu79, Ala124, Leu131 and Leu128. The other side of the pocket is hydrophilic containing Asn55 and Asp83. (C) In the Meta II model from guided MD simulations, Asn302 (orange) shifts toward and hydrogen bonds with Asp83. (D) An overlap of the rhodopsin (grey) and opsin (orange) structures shows a large movement of the side chain of Tyr306 on H7 from its position in rhodopsin to its new position facing H6 in opsin, which was previously occupied by the Met257 side chain on H6. The side chain of Tyr306 is now packed against the surrounding leucine residues on H3 and H2. Asn302 in opsin does not move significantly relative to its position in rhodopsin. (E) and (F) presents the interaction of amino acids around the conserved ERY motif on H3 in rhodopsin and opsin respectively.
Coupling of retinal isomerization to rotation of Tyr306
We have discussed above how retinal isomerization leads to rotation of the C20 methyl group, deprotonation of the SB nitrogen and displacement of EL2. These changes in turn are coupled to the motion of H5 and H6. Another key element that is required for rhodopsin activation involves the NPxxY motif on the intracellular end of H7. As with H5, TM helix H7 has two distinct ends that are both important in rhodopsin activation. The extracellular end of H7 contains residues that are specific for each class A GPCR subfamily.34,86 In rhodopsin, the retinal is covalently attached to Lys296, and the salt bridge between Glu113 and the retinal-lysine PSB must be broken for activation.87 In contrast, the cytoplasmic end of H7 is not subfamily specific, but rather contains the NPxxY sequence that is highly conserved across the class A GPCR family. The recent crystal structure of opsin shows that Tyr306 in the NPxxY motif is re-oriented toward the ionic lock.12 The lock thus has elements from H5 (Tyr223), H6 (Met257) and H7 (Tyr306).
How do retinal isomerization and Schiff base deprotonation lead to a change in the position of Tyr306 of the NPxxY sequence? In the previous two sections, we described how retinal isomerization leads to displacement of EL2 and rotation of Trp265 towards the extracellular side of rhodopsin in Meta II. In rhodopsin, Trp265 is connected to Asn302 of the conserved NPxxY sequence on the cytoplasmic side of H7 through water-mediated hydrogen bonds. The residues in the vicinity of Asn302 form a well-conserved cluster within GPCRs that appears to direct the outward rotation of H6. Figs. 7(b, c) show several of the unusual features of the Asn302 region. First, Asn302 is not directly hydrogen bonded to polar amino acids within the protein interior; rather a shell of water molecules surrounds the Asn302 side chain. Second, one side of the pocket containing Asn302 is hydrophobic and composed of Leu79 (H2), Ala124 (H3) and Leu128 (H3). At first glance, these residues are unremarkable until their sequence conservation is examined. Leu79 is very highly conserved (94%), Ala124 is group-conserved (78%), and Leu128 has a very high sequence identity (78%). The other side of the pocket containing Asn302 is hydrophilic, containing highly conserved Asn55 (99%) and Asp83 (89%). As a result, the Asn302 pocket is conserved across the class A GPCRs and highly amphipathic. Trp265 on H6 is on the extracellular side of Asn302 and part of the conserved aromatic cluster, while Met257 on H6 is on the intracellular side of Asn302 and packed against Leu76, Leu79, Leu128 and Leu131 (see Fig. 7(b)).
In the guided MD simulations, the outward rotation of H6 breaks the interaction with Asn302. Met257 moves away from the amphipathic binding pocket defined by Leu128, Leu131 and Asn302 (Fig. 7(c)). Asn302 forms a hydrogen bond with Asp83 on H2. A direct interaction of Asn302 with Asp83 would be consistent with FTIR studies showing an increase in hydrogen bonding of Asp83 in Meta II.65,88-90
We had previously found that mutation of Met257 alters the packing of the H6-H7 interface to allow opsin activation by addition of all-trans retinal as a diffusible ligand.91 The Met257 mutants with the highest constitutive activity were M257Y, M257N, and M257S, which are all capable of forming hydrogen bonds. The opsin crystal structure provides an explanation for the constitutive activity of these mutants. In opsin, Met257 has rotated into close proximity of Arg135. The more polar substitutions at position 257 would stabilize this active state structure. Nevertheless, the ability of the other Met257 mutants to more easily shift to an active conformation suggests that Met257 itself stabilizes the inactive conformation in rhodopsin rather than the active conformation in Meta II. For example, the mutation of Met257 to alanine or glycine would not be expected to generate stabilizing active state interactions, although they would disrupt the stabilizing interactions with Asn302. The same mechanism may explain constitutive activity associated with the mutation of Leu79 on H2 in rhodopsin86 and Leu128 on H3 in several other GPCRs.92-94
Finally, Figs. 7(e) and (f) show the structure of the ionic lock in rhodopsin and opsin. Tyr306 has shifted in the pocket vacated by Met257. This shift is most clearly seen in Fig. 7(d). The net result of the simulations is that Asn302 and Tyr306 switch orientations, but the backbone of H7 does not move appreciably. Further experimental studies are in progress to establish the structure and orientation of the intracellular end of H7.
Multiple switches in rhodopsin activation
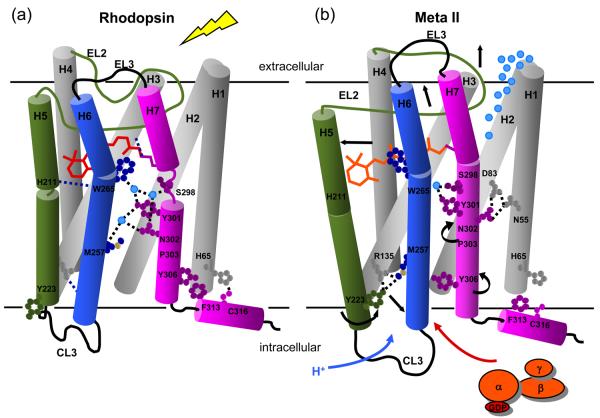
Fig. 8 presents our current working model of the activation mechanism. One of the unusual features of the rhodopsin crystal structure is that EL2 (residues 174-199) forms a lid over the retinal-binding site. EL2 connects H4 and H5, and folds deeply into the core of the receptor with van der Waals contacts with TM helices H6 and H7. As a result, EL2 is well positioned to regulate the motion of the three key helices (H5, H6 and H7) that change orientation upon receptor activation. Full activation of rhodopsin appears to involve multiple triggers or switches on the extracellular side of the receptor that converge to disrupt the ionic lock between helices H3 and H6 on the intracellular side of the receptor.
Fig. 8.
Working model of rhodopsin activation. Retinal isomerization leads to the strain in retinal binding site; all-trans retinal is not accommodated in the binding site in the dark. There are several studies probing the sequence of structural changes following isomerization. In detergent, Hofmann and coworkers118,119 have shown that SB deprotonation precedes the motion of H6 and proton uptake by Glu134. However, changes in the IR bands of Glu122 already accompany the formation of Meta I120 and the retinal SB in Meta I is accessible to hydroxylamine.121 Together these observations suggest that retinal isomerization first leads to displacement of EL2 and (at least limited) motion of H5 prior to deprotonation. Deprotonation of the SB then allows the retinal to shift into an active position that either guides or drives rotation of Trp265 and H6. This sequence of events is consistent with kinetic FTIR measurements122 indicating that there may be 15-20% deprotonation of the retinal Schiff base before changes in the IR bands between 1755 and 1740 cm−1 associated with Asp83 and Glu122. Rotation of Trp265 side chain towards EL2 disrupts the network of hydrogen bonding interactions involving the NPxxY motif on H7 and responsible for maintaining the inactive state of the receptor. The outward rotation of H6 is accompanied by the rotation of Tyr223 and Tyr306 into the H3-H6 interface.12 These residues face Met257 and hydrogen bond with Arg135, disrupting the ionic lock.
Light absorption by the retinal chromophore and subsequent isomerization to the all-trans isomer is the first switch in the activation of rhodopsin. Retinal isomerization, in turn, triggers the displacement of EL2 and deprotonation of the PSB. These three events are required for rhodopsin activation. We have recently shown that the displacement of EL2 and the outward rotation of the extracellular end of H5 are coupled.42 The coupled motion of EL2 and H5 constitutes a second switch in rhodopsin activation. EL2 is not a flexible linker between helices H4 and H5. Rather, a well-defined hydrogen-bonding network between the β3 and β4 strands of EL2 stabilizes its structure; EL2 functions as a rigid lever to shift the orientation of H5 when it is displaced from the retinal binding site. Coupling of EL2 displacement and H5 motion is reflected in the guided MD simulations (Fig. 4(a)), as well as in mutational studies in both rhodopsin42 and the ligand-activated GPCRs.95-98
A third switch involves the coupling of EL2 motion with the outward rotation of H6. This coupling is mediated by the conserved aromatic cluster on H6 as suggested by the rotamer toggle switch mechanism, which proposes that the rotamer configurations of Cys264 and the aromatic amino acids on H6 are tightly coupled and serve to modulate the proline kink in the middle of helix H6.79 The rotamer toggle switch mechanism is encompassed in the global toggle switch mechanism, which proposes that an inward movement of the extracellular end of H6 is needed to affect an outward motion of intracellular end of H6.85
The fourth switch in the activation mechanism couples motion of Asn302 and Tyr306. We discussed above how motion of Trp265 toward the extracellular surface disrupts the conserved hydrogen bonding interactions centered on Asn302. When this interaction is broken in the guided MD simulations, Asn302 forms a hydrogen bond with Asp83 on H2. Met257 moves away from the amphipathic binding pocket defined by Leu128, Leu131 and Asn302 (Fig. 7(c)), and Tyr306 shifts into the pocket and hydrogen bonds with Arg135. In a comprehensive mutational study of Tyr7.53 in the serotonin 5HT2C receptor, Prioleau et al.99 found a constitutively active “locked-on” mutant where the tyrosine of the conserved NPxxY motif was mutated to asparagine, an amino acid with a strong propensity to form interhelical hydrogen bonds.
The final switch involves the ionic lock between H3 and H6. The coordinated motions of H5-H7 upon rhodopsin activation place Tyr206, Met257 and Tyr306 between the intracellular ends of helices H3 and H6 and disrupt the ionic lock.12,13 Taken together, Figs. 4, 6 and 7 suggest how EL2 may play a pivotal role in controlling the motion of H5, H6 and H7. The confluence of several required structural changes is an example of coincidence counting often found in regulated biological processes.
Three levels of sequence conservation in class A GPCRs
The class A G protein-coupled receptors have presented a puzzle that may finally be close to solution. These receptors have a relatively simple architecture consisting of seven TM helices and just a handful of highly conserved amino acids. In the section above, we proposed a working model of the activation mechanism of rhodopsin. One test of the model is that it be able to explain the conservation of amino acids within the class A GPCR family.
There are at least three levels of sequence conservation in the class A GPCRs. The first level of conservation corresponds to those residues that have sequence identities of > 70% across the large GPCR family. There are 14 sites that fall into this category (Supplementary Material, Table 2), and we have discussed possible structural or functional roles of these sites in the context of the guided MD simulations.
The second level of conservation includes those residues that have low individual sequence identities, but are highly conserved (> 70%) when considered as a group. In the class A GPCRs, there are 14 positions that are group conserved as small and weakly polar (Gly, Ala, Ser, Thr and Cys) amino acids.100 These residues have high propensities for mediating helix-helix interactions.101 On this basis, we previously proposed that helices H1-H4, and possibly H7, form a tightly packed core that does not move upon activation.100 Additional support for the concept of the helix core comes from NMR and crystallography. For example, a close contact between Met86 (H2) and Gly120 (H3) does not change upon activation,36 while the opsin structure shows H5 and H6 move relative to H1-H4 and H7.12 In MD simulations by Karplus and coworkers,102 restraining the positions of each helix in the H1-H4 core individually did not influence the activation pathway, whereas restraining either H6 or H7 had an appreciable influence on helix interactions.
The third level of conservation involves residues that are highly conserved only within a subfamily of class A GPCRs. For example, Lys296 is the site of attachment of the retinal chromophore and is conserved in the rhodopsin subfamily of GPCRs. In general, EL2 and the extracellular ends of H5, H6 and H7 are specific for the receptor subfamilies. Each receptor subfamily will have different mechanisms for coupling motion of the H5-EL2 helix-loop segment with the H6-EL3-H7 HLH segment in order to change the positions of the intracellular ends of H5, H6 and H7. Understanding these subfamily specific interactions by combined structural and computational methods is the upcoming challenge for understanding the diversity of ligand recognition and activation in GPCRs.
Materials and Methods
Molecular dynamics simulations
Molecular dynamics (MD) simulations were undertaken to model possible rearrangements in the retinal binding site upon rhodopsin activation. Fig. 9 presents the system used for the simulations. The high-resolution (2.2 Å) crystal structure (chain A of 1U195) of rhodopsin was used as the initial model. Default protonation states were set for the solvent exposed amino acids. Asp832.50 and Glu1223.37 are set to be neutral.65,66 Glu1133.28 and Glu181 are assumed to be charged.24,53,54 Röhrig et al.24 found using a comparison of simulations with Glu181 in different protonation states that this residue is likely to be charged in the rhodopsin ground state. We have recently found that the unusual chemical shift at the retinal C12 carbon attributed to the interaction with Glu181 is lacking in the E181Q mutant of rhodopsin (unpublished results). All histidines are protonated in both the ε- and δ-positions (i.e. charged) except His2115.46 which is delta protonated.35 All heteroatoms but retinal are removed from the model. Internal water molecules are preserved; other crystallographic waters are deleted before the system is solvated by water to create a simulation box. For further reference, transmembrane helices are defined as follows: 34-69 (H1), 71-99 (H2), 107-140 (H3), 150-173 (H4), 200-229 (H5), 242-278 (H6), 288-309 (H7), and 311-322 (H8).
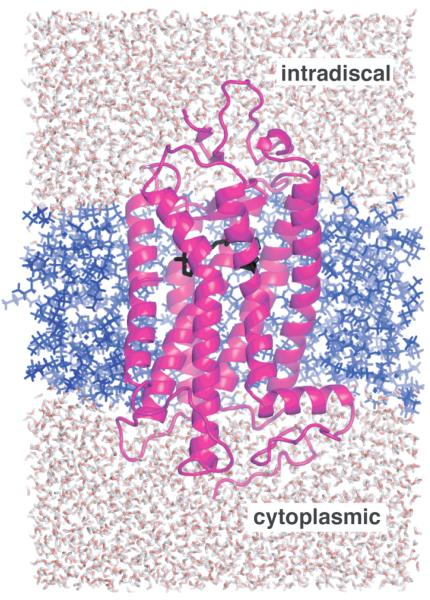
Fig. 9.
Computational model used in the MD simulations. Rhodopsin (magenta) is embedded in an octane bilayer (blue) and immersed in a box of water (red/white) which is ~32 Å on each side. The retinal chromophore (black) is close to extracellular side of the receptor (top).
All simulations were performed by the SANDER module from the molecular modeling package AMBER8 (D.A. Case, T.A. Darden, T.E. Cheatham, III, C.L. Simmerling, J. Wang, R.E. Duke, R. Luo, K.M. Merz, B. Wang, D.A. Pearlman, M. Crowley, S. Brozell, V. Tsui, H. Gohlke, J. Mongan, V. Hornak, G. Cui, P. Beroza, C. Schafmeister, J.W. Caldwell, W.S. Ross, and P.A. Kollman (2004), AMBER 8, University of California, San Francisco). Constant simulation temperature (300 K) and pressure (1 bar) were maintained by Berendsen temperature and pressure coupling. Periodic boundary conditions were applied, electrostatic interactions were treated by the Particle Mesh Ewald method, and integration time step was 2 fs. The overall setup procedure is similar to one previously described.24 The simulation system consisted of rhodopsin embedded in a membrane mimetic environment, which was modeled by a layer of octane molecules103 and with the cytoplasmic and extracellular domains solvated by water. The box containing the octane slab immersed in water was pre-equilibrated by constant volume molecular dynamics. The rhodopsin molecule was then inserted into the octane slab (approximately 30 Å thick) such that the principal axes of the slab and rhodopsin transmembrane region were aligned. The resulting system has approximately 400 octane molecules and 9000 molecules of water. The whole system was equilibrated in a 40 ps constant pressure MD simulation followed by a 40 ps constant volume MD simulation with rhodopsin restrained to its crystal structure coordinates in both equilibration steps. An additional 100 ps constant volume equilibration was performed before the system was switched to constant pressure for all production simulations.
The use of octane to model the membrane bilayer is a clear simplification, but due to its lower viscosity octane provides an environment that responds more readily to the applied distance restraints and thus facilitates receptor structural changes. Therefore, even though the octane mimetic is used because of its simplicity, it may better parallel the use of DDM detergent for trapping a homogeneous Meta II state in our NMR experiments. Nevertheless, it is important to point out that unsaturation in the acyl chains and the type of lipid headgroup can influence the Meta I to Meta II transition. For example, NMR 104,105 and computational 23 studies provide evidence that ω-3 polyunsaturated chains, such as docosahexaenoic acid, preferentially solvate rhodopsin with the unsaturated chains oriented toward the TM helices. Brown and co-workers 106,107 suggested that the conformational change associated with Meta II increases the hydrophobic thickness of the receptor and that the phosphotidylethanolamine headgroup, which favors hexagonal phase bilayers, will change the local curvature near the receptor to compensate for the hydrophobic mismatch between the receptor and membrane. The recent crystal structures of opsin12,13, which shows a lengthening of TM helices H5 and H6, support this idea.
All standard amino acids use Amber ff99 forcefield parameters.108,109 OPLS parameters are used for the octane layer.110 Retinal parameters were obtained from the antechamber module111 in AMBER8. Charges were adopted from Rohrig et al.24 and were derived using the RESP fitting procedure.112 The charge fitting procedure incorporates both the cis and trans configurations of the retinal. Torsional parameters were taken from quantum-mechanical calculations.113
Retinal in the equilibrated model was ‘isomerized’ from 11-cis to all-trans by applying a torsional restraint (50 kcal/mol/Å) on the C10-C11-C12-C13 dihedral. No sense of rotation is imposed by the restraint. The transition of the dihedral angle to a trans configuration requires MD runs of ~20 ps. Isomerization trials were repeated several times and resulted in very similar structures for the highly distorted all-trans retinal characteristic of the bathorhodopsin intermediate.
Two sets of restraints were used during MD. First, weak positional restraints (1.0 kcal/mol/Å) on the CA carbons were applied for helices H1-H4 in accord with the assumption that these helices form a stable rhodopsin core.100 Second, distance restraints from NMR measurements were applied to drive the structure to the Meta II state. One nanosecond long simulations were carried out employing these restraints and starting from a structure obtained after the isomerization step. Throughout the course of the simulation, initial distances were gradually changed toward the experimental distances characterizing Meta II. The distances start from those observed in the crystal structure of rhodopsin and slowly change toward the NMR-derived distances (Table 1). The goal is to introduce the changes to the crystal structure of rhodopsin gradually in order to avoid unrealistic structural distortions that would result from switching on experimental restraints in a single step. We then ran an additional 1 ns MD simulation during which the target restraints were held constant. The final model was obtained from this 1 ns MD run with the experimental restraints in place and during which the temperature is slowly annealed from 300 K to 50 K. Note, the actual restraints were well-shaped with a flat bottom and parabolic sides out to a defined distance followed by linear sides beyond (as implemented in Amber and described in the Amber manual). The energy penalty increases from zero as the distance deviates from the flat bottom of the well. The magnitude of that penalty is given by the force constant determining the “steepness” of the parabola (20 kcal/molA2 in our case). A typical simulation would have an average distance deviation from NMR restraints of 0.2 Å and energy penalty somewhere between 5-15 kcal/mol for the final model.
Control simulations of the dark (inactive) rhodopsin with 11-cis retinal were run for 8 ns to verify the stability of the system under our simulation protocol. No significant changes in the conformation of the retinal or position of the TM helices were observed, and the water-octane interface remained stable. We also ran simulations (up to 16 ns) without employing experimental distance restraints. These simulations were started from the same structures (after isomerization) as the restrained simulations, but provided rather different trajectories with a different location of the retinal in the binding site.
Solid-state NMR spectroscopy
Distance restraints that are used in the simulations were obtained by solid-state NMR spectroscopy. In this section, we briefly describe the nature of the NMR experiments and the nature of the restraints derived from these experiments. The NMR experiments on rhodopsin were all undertaken using recombinant protein that was affinity column purified from HEK293S cells adapted for suspension growth. The rhodopsin was regenerated with 13C-labeled 11-cis retinal and concentrated in DDM detergent. The procedures for sample preparation are described in several experimental papers36,114-117.
Internuclear 13C-13C distances were obtained using two-dimensional pulse sequences that re-introduce the dipolar couplings between 13C sites under conditions of magic angle spinning (MAS). MAS is used to obtain high resolution spectra, but otherwise has the disadvantage of averaging the dipolar interaction. The experiments were all performed at low temperature (−80°C) in order to trap the activated Meta II intermediate and to eliminate residual motion that would interfere with recoupling of the dipolar interaction. We typically used the two dimensional dipolar assisted rotational resonance (DARR) experiment.
Dipolar couplings between 13C sites in the DARR experiment result in cross peaks in the two-dimensional NMR spectrum. The intensity of the cross peaks is related to the strength of the dipolar coupling, which in turn is related to the internuclear distance. We typically obtain spectra with a mixing time of 600 msec and categorize the intensity of the cross peaks as strong, moderate or weak. Prior to measurements on Meta II, we obtain DARR NMR spectra of rhodopsin and compare the cross peak intensities with the 13C-13C distances obtained from the crystal structure. Based on many such comparisons, we categorize strong cross peaks as corresponding to internuclear distances of ~4 Å or less, moderate cross peaks as corresponding to distances between ~4 Å and 5 Å, and weak cross peaks corresponding to distances between ~5 Å and 6 Å. The longest internuclear contacts we have observed in rhodopsin are on the order of 6-6.5 Å.
Supplementary Material
Acknowledgements
This work was supported by NIH-NSF instrumentation grants (S10 RR13889 and DBI-9977553), and a grant from the NIH to S.O.S (GM-41412). MS acknowledges support from Kimmelman center for Biomolecular Structure and Assembly, and the United States-Israel Binational Science Foundation (BSF).
Abbreviations
- CL3
third cytoplasmic loop
- DARR
dipolar assisted rotational resonance
- DDM
n-dodecylmaltoside
- EL2
second extracellular loop
- GPCR
G protein-coupled receptor
- MAS
magic angle spinning
- MD
molecular dynamics
- Meta I
metarhodopsin I
- Meta II
metarhodopsin II
- PSB
protonated Schiff base
- TM
transmembrane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The nomenclature in superscript follows the convention established by Ballesteros and Weinstein82 where the helix number is followed by the sequence position relative to the most conserved residue in the helix designated as 50.
References
- 1.Menon ST, Han M, Sakmar TP. Rhodopsin: Structural basis of molecular physiology. Physiol. Rev. 2001;81:1659–1688. doi: 10.1152/physrev.2001.81.4.1659. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz TW, Frimurer TM, Holst B, Rosenkilde MM, Elling CE. Molecular mechanism of 7TM receptor activation - A global toggle switch model. Annu. Rev. Pharmacol. Toxicol. 2006;46:481–519. doi: 10.1146/annurev.pharmtox.46.120604.141218. [DOI] [PubMed] [Google Scholar]
- 3.Kobilka B, Schertler GFX. New G-protein-coupled receptor crystal structures: insights and limitations. Trends Pharmacol. Sci. 2008;29:79–83. doi: 10.1016/j.tips.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 5.Okada T, Sugihara M, Bondar AN, Elstner M, Entel P, Buss V. The retinal conformation and its environment in rhodopsin in light of a new 2.2 Å crystal structure. J. Mol. Biol. 2004;342:571–583. doi: 10.1016/j.jmb.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Edwards PC, Burghammer M, Villa C, Schertler GFX. Structure of bovine rhodopsin in a trigonal crystal form. J. Mol. Biol. 2004;343:1409–38. doi: 10.1016/j.jmb.2004.08.090. [DOI] [PubMed] [Google Scholar]
- 7.Nakamichi H, Okada T. Crystallographic analysis of primary visual photochemistry. Angew. Chem. Int. Ed. Engl. 2006;45:4270–4273. doi: 10.1002/anie.200600595. [DOI] [PubMed] [Google Scholar]
- 8.Schreiber M, Sugihara M, Okada T, Buss V. Quantum mechanical studies on the crystallographic model of bathorhodopsin. Angew. Chem. Int. Ed. Engl. 2006;45:4274–4277. doi: 10.1002/anie.200600585. [DOI] [PubMed] [Google Scholar]
- 9.Nakamichi H, Okada T. Local peptide movement in the photoreaction intermediate of rhodopsin. Proc. Natl. Acad. Sci. U.S.A. 2006;103:12729–12734. doi: 10.1073/pnas.0601765103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruprecht JJ, Mielke T, Vogel R, Villa C, Schertler GFX. Electron crystallography reveals the structure of metarhodopsin I. EMBO J. 2004;23:3609–3620. doi: 10.1038/sj.emboj.7600374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramon E, Marron J, del Valle L, Bosch L, Andres A, Manyosa J, et al. Effect of dodecyl maltoside detergent on rhodopsin stability and function. Vision Res. 2003;43:3055–3061. doi: 10.1016/j.visres.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature. 2008;454:183–187. doi: 10.1038/nature07063. [DOI] [PubMed] [Google Scholar]
- 13.Scheerer P, Park JH, Hildebrand PW, Kim YJ, Krauss N, Choe HW, et al. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008;455:497–502. doi: 10.1038/nature07330. [DOI] [PubMed] [Google Scholar]
- 14.Vogel R, Siebert F. Conformations of the active and inactive states of opsin. J. Biol. Chem. 2001;276:38487–38493. doi: 10.1074/jbc.M105423200. [DOI] [PubMed] [Google Scholar]
- 15.Kisselev OG, Kao J, Ponder JW, Fann YC, Gautam N, Marshall GR. Light-activated rhodopsin induces a structural binding motif in G protein α subunit. Proc. Natl. Acad. Sci. U.S.A. 1998;95:4270–4275. doi: 10.1073/pnas.95.8.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koenig BW, Kontaxis G, Mitchell DC, Louis JM, Litman BJ, Bax A. Structure and orientation of a G protein fragment in the receptor bound state from residual dipolar couplings. J. Mol. Biol. 2002;322:441–461. doi: 10.1016/s0022-2836(02)00745-3. [DOI] [PubMed] [Google Scholar]
- 17.Saam J, Tajkhorshid E, Hayashi S, Schulten K. Molecular dynamics investigation of primary photoinduced events in the activation of rhodopsin. Biophys. J. 2002;83:3097–3112. doi: 10.1016/S0006-3495(02)75314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crozier PS, Stevens MJ, Forrest LR, Woolf TB. Molecular dynamics simulation of dark-adapted rhodopsin in an explicit membrane bilayer: Coupling between local retinal and larger scale conformational change. J. Mol. Biol. 2003;333:493–514. doi: 10.1016/j.jmb.2003.08.045. [DOI] [PubMed] [Google Scholar]
- 19.Feller SE, Gawrisch K, Woolf TB. Rhodopsin exhibits a preference for solvation by polyunsaturated docosohexaenoic acid. J. Am. Chem. Soc. 2003;125:4434–4435. doi: 10.1021/ja0345874. [DOI] [PubMed] [Google Scholar]
- 20.Huber T, Botelho AV, Beyer K, Brown MF. Membrane model for the G-protein-coupled receptor rhodopsin: Hydrophobic interface and dynamical structure. Biophys. J. 2004;86:2078–2100. doi: 10.1016/S0006-3495(04)74268-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemaître V, Yeagle P, Watts A. Molecular dynamics simulations of retinal in rhodopsin: From the dark-adapted state towards lumirhodopsin. Biochemistry. 2005;44:12667–12680. doi: 10.1021/bi0506019. [DOI] [PubMed] [Google Scholar]
- 22.Pitman MC, Grossfield A, Suits F, Feller SE. Role of cholesterol and polyunsaturated chains in lipid-protein interactions: Molecular dynamics simulation of rhodopsin in a realistic membrane environment. J. Am. Chem. Soc. 2005;127:4576–4577. doi: 10.1021/ja042715y. [DOI] [PubMed] [Google Scholar]
- 23.Grossfield A, Feller SE, Pitman MC. A role for direct interactions in the modulation of rhodopsin by ω-3 polyunsaturated lipids. Proc. Natl. Acad. Sci. U.S.A. 2006;103:4888–4893. doi: 10.1073/pnas.0508352103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Röhrig UF, Guidoni L, Rothlisberger U. Early steps of the intramolecular signal transduction in rhodopsin explored by molecular dynamics simulations. Biochemistry. 2002;41:10799–10809. doi: 10.1021/bi026011h. [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharya S, Hall SE, Vaidehi N. Agonist-induced conformational changes in bovine rhodopsin: Insight into activation of G-protein-coupled receptors. J. Mol. Biol. 2008;382:539–555. doi: 10.1016/j.jmb.2008.06.084. [DOI] [PubMed] [Google Scholar]
- 26.Buss V, Sugihara M, Entel P, Hafner J. Thr94 and Wat2b effect protonation of the retinal chromophore in rhodopsin. Angew. Chem. Int. Ed. Engl. 2003;42:3245–3247. doi: 10.1002/anie.200351034. [DOI] [PubMed] [Google Scholar]
- 27.Sugihara M, Hufen J, Buss V. Origin and consequences of steric strain in the rhodopsin binding pocket. Biochemistry. 2006;45:801–810. doi: 10.1021/bi0515624. [DOI] [PubMed] [Google Scholar]
- 28.Gascon JA, Batista VS. QM/MM study of energy storage and molecular rearrangements due to the primary event in vision. Biophys. J. 2004;87:2931–2941. doi: 10.1529/biophysj.104.048264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gascon JA, Sproviero EM, Batista VS. Computational studies of the primary phototransduction event in visual rhodopsin. Acc. Chem. Res. 2006;39:184–193. doi: 10.1021/ar050027t. [DOI] [PubMed] [Google Scholar]
- 30.Thorgeirsson TE, Lewis JW, Wallace-Williams SE, Kliger DS. Effects of temperature on rhodopsin photointermediates from lumirhodopsin to metarhodopsin-Ii. Biochemistry. 1993;32:13861–13872. doi: 10.1021/bi00213a015. [DOI] [PubMed] [Google Scholar]
- 31.Tikhonova IG, Best RB, Engel S, Gershengorn MC, Hummer G, Costanzi S. Atomistic insights into rhodopsin activation from a dynamic model. J. Am. Chem. Soc. 2008;130:10141–10149. doi: 10.1021/ja0765520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farrens DL, Altenbach C, Yang K, Hubbell WL, Khorana HG. Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science. 1996;274:768–770. doi: 10.1126/science.274.5288.768. [DOI] [PubMed] [Google Scholar]
- 33.Altenbach C, Kusnetzow AK, Ernst OP, Hofmann KP, Hubbell WL. High-resolution distance mapping in rhodopsin reveals the pattern of helix movement due to activation. Proc. Natl. Acad. Sci. U.S.A. 2008;105:7439–7444. doi: 10.1073/pnas.0802515105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel AB, Crocker E, Eilers M, Hirshfeld A, Sheves M, Smith SO. Coupling of retinal isomerization to the activation of rhodopsin. Proc. Natl. Acad. Sci. U.S.A. 2004;101:10048–10053. doi: 10.1073/pnas.0402848101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel AB, Crocker E, Reeves PJ, Getmanova EV, Eilers M, Khorana HG, et al. Changes in interhelical hydrogen bonding upon rhodopsin activation. J. Mol. Biol. 2005;347:803–812. doi: 10.1016/j.jmb.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 36.Crocker E, Eilers M, Ahuja S, Hornak V, Hirshfeld A, Sheves M, et al. Location of Trp265 in metarhodopsin II: Implications for the activation mechanism of the visual receptor rhodopsin. J. Mol. Biol. 2006;357:163–172. doi: 10.1016/j.jmb.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 37.Okada T, Fujiyoshi Y, Silow M, Navarro J, Landau EM, Shichida Y. Functional role of internal water molecules in rhodopsin revealed by x-ray crystallography. Proc. Natl. Acad. Sci. U.S.A. 2002;99:5982–5987. doi: 10.1073/pnas.082666399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schoenlein RW, Peteanu LA, Wang Q, Mathies RA, Shank CV. Femtosecond dynamics of cis-trans isomerization in a visual pigment analog - isorhodopsin. J. Phys. Chem. 1993;97:12087–12092. [Google Scholar]
- 39.Struts AV, Salgad GFJ, Tanaka K, Krane S, Nakanishi K, Brown MF. Structural analysis and dynamics of retinal chromophore in dark and meta l states of rhodopsin from 2H NMR of aligned membranes. J. Mol. Biol. 2007;372:50–66. doi: 10.1016/j.jmb.2007.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu RSH, Hammond GS. Reflection on medium effects on photochemical reactivity. Acc. Chem. Res. 2005;38:396–403. doi: 10.1021/ar040246z. [DOI] [PubMed] [Google Scholar]
- 41.Ahuja S, Crocker E, Eilers M, Hornak V, Hirshfeld A, Ziliox M, et al. Location of the retinal chromophore in the activated state of rhodopsin. J. Biol. Chem. 2009;284:10190–10201. doi: 10.1074/jbc.M805725200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahuja S, Hornak V, Yan ECY, Syrett N, Goncalves JA, Hirshfeld A, et al. Helix movement is coupled to displacement of the second extracellular loop in rhodopsin activation. Nat. Struct. Mol. Biol. 2009;16:168–175. doi: 10.1038/nsmb.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Q, Kochendoerfer GG, Schoenlein RW, Verdegem PJE, Lugtenburg J, Mathies RA, et al. Femtosecond spectroscopy of a 13-demethylrhodopsin visual pigment analogue: The role of nonbonded interactions in the isomerization process. J. Phys. Chem. 1996;100:17388–17394. [Google Scholar]
- 44.Kochendoerfer GG, Verdegem PJE, van der Hoef I, Lugtenburg J, Mathies RA. Retinal analog study of the role of steric interactions in the excited state isomerisation dynamics of rhodopsin. Biochemistry. 1996;35:16230–16240. doi: 10.1021/bi961951l. [DOI] [PubMed] [Google Scholar]
- 45.Gärtner W, Ternieden S. Influence of a steric hindrance in the chromophore of rhodopsin on the quantum yield of the primary photochemistry. J. Photochem. Photobiol. B. 1996;33:83–86. [Google Scholar]
- 46.Han M, DeDecker BS, Smith SO. Localization of the retinal protonated Schiff base counterion in rhodopsin. Biophys. J. 1993;65:899–906. doi: 10.1016/S0006-3495(93)81117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han M, Smith SO. High-resolution structural studies of the retinal--Glu113 interaction in rhodopsin. Biophys. Chem. 1995;56:23–29. doi: 10.1016/0301-4622(95)00011-l. [DOI] [PubMed] [Google Scholar]
- 48.Lewis JW, Szundi I, Kazmi MA, Sakmar TP, Kliger DS. Time-resolved photointermediate changes in rhodopsin glutamic acid 181 mutants. Biochemistry. 2004;43:12614–12621. doi: 10.1021/bi049581l. [DOI] [PubMed] [Google Scholar]
- 49.Lin SW, Mathies RA. Orientation of the protonated retinal Schiff base group in bacteriorhodopsin from absorption linear dichroism. Biophys. J. 1989;56:653–660. doi: 10.1016/S0006-3495(89)82712-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gat Y, Sheves M. A mechanism for controlling the pKa of the retinal protonated schiff-base in retinal proteins - a study with model compounds. J. Am. Chem. Soc. 1993;115:3772–3773. [Google Scholar]
- 51.Rousso I, Friedman N, Sheves M, Ottolenghi M. pKa of the protonated Schiff-base and aspartic-85 in the bacteriorhodopsin binding-site is controlled by a specific geometry between the 2 residues. Biochemistry. 1995;34:12059–12065. doi: 10.1021/bi00037a049. [DOI] [PubMed] [Google Scholar]
- 52.Ahuja S, Eilers M, Hirshfeld A, Yan ECY, Ziliox M, Sakmar TP, et al. 6-s-cis conformation and polar binding pocket of the retinal chromophore in the photoactivated state of rhodopsin. J. Am. Chem. Soc. 2009 doi: 10.1021/ja9034768. in press, doi: 10.1021/ja9034768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakmar TP, Franke RR, Khorana HG. Glutamic acid-113 serves as the retinylidene Schiff base counterion in bovine rhodopsin. Proc. Natl. Acad. Sci. U.S.A. 1989;86:8309–8313. doi: 10.1073/pnas.86.21.8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhukovsky EA, Oprian DD. Effect of carboxylic acid side chains on the absorption maximum of visual pigments. Science. 1989;246:928–930. doi: 10.1126/science.2573154. [DOI] [PubMed] [Google Scholar]
- 55.Renk G, Crouch RK. Analog pigment studies of chromophore protein interactions in metarhodopsins. Biochemistry. 1989;28:907–912. doi: 10.1021/bi00428a075. [DOI] [PubMed] [Google Scholar]
- 56.Ridge KD, Lu Z, Liu X, Khorana HG. Structure and function in rhodopsin. Separation and characterization of the correctly folded and misfolded opsins produced on expression of an opsin mutant gene containing only the native intradiscal cysteine codons. Biochemistry. 1995;34:3261–3267. doi: 10.1021/bi00010a016. [DOI] [PubMed] [Google Scholar]
- 57.Corson DW, Cornwall MC, MacNichol EF, Tsang S, Derguini F, Crouch RK, et al. Relief of opsin desensitization and prolonged excitation of rod photoreceptors by 9-desmethylretinal. Proc. Natl. Acad. Sci. U.S.A. 1994;91:6958–6962. doi: 10.1073/pnas.91.15.6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vogel R, Fan GB, Sheves M, Siebert F. The molecular origin of the inhibition of transducin activation in rhodopsin lacking the 9-methyl group of the retinal chromophore: A UV-Vis and FTIR spectroscopic study. Biochemistry. 2000;39:8895–8908. doi: 10.1021/bi000852b. [DOI] [PubMed] [Google Scholar]
- 59.Meyer CK, Böhme M, Ockenfels A, Gärtner W, Hofmann KP, Ernst OP. Signaling states of rhodopsin: Retinal provides a scaffold for activating proton transfer switches. J. Biol. Chem. 2000;275:19713–19718. doi: 10.1074/jbc.M000603200. [DOI] [PubMed] [Google Scholar]
- 60.Han M, Groesbeek M, Sakmar TP, Smith SO. The C9 methyl group of retinal interacts with glycine-121 in rhodopsin. Proc. Natl. Acad. Sci. U.S.A. 1997;94:13442–13447. doi: 10.1073/pnas.94.25.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holst B, Schwartz TW. Molecular mechanism of agonism and inverse agonism in the melanocortin receptors - Zn2+ as a structural and functional probe. Ann. N. Y. Acad. Sci. 2003;994:1–11. doi: 10.1111/j.1749-6632.2003.tb03156.x. [DOI] [PubMed] [Google Scholar]
- 62.Klco JM, Wiegand CB, Narzinski K, Baranski TJ. Essential role for the second extracellular loop in C5a receptor activation. Nat. Struct. Mol. Biol. 2005;12:320–326. doi: 10.1038/nsmb913. [DOI] [PubMed] [Google Scholar]
- 63.Jäger F, Jäger S, Kräutle O, Friedman N, Sheves M, Hofmann KP, et al. Interactions of the β-ionone ring with the protein in the visual pigment rhodopsin control the activation mechanism. An FTIR and fluorescence study on artificial vertebrate rhodopsins. Biochemistry. 1994;33:7389–7397. doi: 10.1021/bi00189a045. [DOI] [PubMed] [Google Scholar]
- 64.Vogel R, Siebert F, Lüdeke S, Hirshfeld A, Sheves M. Agonists and partial agonists of rhodopsin: Retinals with ring modifications. Biochemistry. 2005;44:11684–11699. doi: 10.1021/bi0508587. [DOI] [PubMed] [Google Scholar]
- 65.Fahmy K, Jäger F, Beck M, Zvyaga TA, Sakmar TP, Siebert F. Protonation states of membrane-embedded carboxylic acid groups in rhodopsin and metarhodopsin II: A Fourier-transform infrared spectroscopy study of site-directed mutants. Proc. Natl. Acad. Sci. U.S.A. 1993;90:10206–10210. doi: 10.1073/pnas.90.21.10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fahmy K, Sakmar TP, Siebert F. Structural determinants of active state conformation of rhodopsin: Molecular biophysics approaches. Meth. Enzymol. 2000;315:178–196. doi: 10.1016/s0076-6879(00)15843-4. [DOI] [PubMed] [Google Scholar]
- 67.Lewis JW, Szundi I, Kazmi MA, Sakmar TP, Kliger DS. Proton movement and photointermediate kinetics in rhodopsin mutants. Biochemistry. 2006;45:5430–5439. doi: 10.1021/bi0525775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Strader CD, Candelore MR, Hill WS, Sigal IS, Dixon RAF. Identification of two serine residues involved in agonist activation of the β-adrenergic receptor. J. Biol. Chem. 1989;264:13572–13578. [PubMed] [Google Scholar]
- 69.O'Dowd BF, Hnatowich M, Regan JW, Leader WM, Caron MG, Lefkowitz RJ. Site-directed mutagenesis of the cytoplasmic domains of the human β2-adrenergic receptor. Localization of regions involved in G protein-receptor coupling. J. Biol. Chem. 1988;263:15985–15992. [PubMed] [Google Scholar]
- 70.Acharya S, Saad Y, Karnik SS. Transducin-α C-terminal peptide binding site consists of C-D and E-F loops of rhodopsin. J. Biol. Chem. 1997;272:6519–6524. doi: 10.1074/jbc.272.10.6519. [DOI] [PubMed] [Google Scholar]
- 71.Yamashita T, Terakita A, Shichida Y. Distinct roles of the second and third cytoplasmic loops of bovine rhodopsin in G protein activation. J. Biol. Chem. 2000;275:34272–34279. doi: 10.1074/jbc.M002954200. [DOI] [PubMed] [Google Scholar]
- 72.Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SGF, Thian FS, Kobilka TS, et al. High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sheikh SP, Zvyaga TA, Lichtarge O, Sakmar TP, Bourne HR. Rhodopsin activation blocked by metal-ion-binding sites linking transmembrane helices C and F. Nature. 1996;383:347–350. doi: 10.1038/383347a0. [DOI] [PubMed] [Google Scholar]
- 74.Cohen GB, Yang T, Robinson PR, Oprian DD. Constitutive activation of opsin: Influence of charge at position 134 and size at position 296. Biochemistry. 1993;32:6111–6115. doi: 10.1021/bi00074a024. [DOI] [PubMed] [Google Scholar]
- 75.Kim JM, Altenbach C, Thurmond RL, Khorana HG, Hubbell WL. Structure and function in rhodopsin: Rhodopsin mutants with a neutral amino acid at E134 have a partially activated conformation in the dark state. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14273–14278. doi: 10.1073/pnas.94.26.14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luo X, Zhang D, Weinstein H. Ligand-induced domain motion in the activation mechanism of a G-protein-coupled receptor. Protein Eng. 1994;7:1441–1448. doi: 10.1093/protein/7.12.1441. [DOI] [PubMed] [Google Scholar]
- 77.Sansom MSP, Weinstein H. Hinges, swivels and switches: The role of prolines in signalling via transmembrane α-helices. Trends Pharmacol. Sci. 2000;21:445–451. doi: 10.1016/s0165-6147(00)01553-4. [DOI] [PubMed] [Google Scholar]
- 78.Gether U, Lin S, Ghanouni P, Ballesteros JA, Weinstein H, Kobilka BK. Agonists induce conformational changes in transmembrane domains III and VI of the β2 adrenoceptor. EMBO J. 1997;16:6737–6747. doi: 10.1093/emboj/16.22.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shi L, Liapakis G, Xu R, Guarnieri F, Ballesteros JA, Javitch JA. β2 adrenergic receptor activation - Modulation of the proline kink in transmembrane 6 by a rotamer toggle switch. J. Biol. Chem. 2002;277:40989–40996. doi: 10.1074/jbc.M206801200. [DOI] [PubMed] [Google Scholar]
- 80.Lin SW, Sakmar TP. Specific tryptophan UV-absorbance changes are probes of the transition of rhodopsin to its active state. Biochemistry. 1996;35:11149–11159. doi: 10.1021/bi960858u. [DOI] [PubMed] [Google Scholar]
- 81.Chabre M, Breton J. Orientation of aromatic residues in rhodopsin. Rotation of one tryptophan upon the meta I to meta II transition after illumination. Photochem. Photobiol. 1979;30:295–299. doi: 10.1111/j.1751-1097.1979.tb07150.x. [DOI] [PubMed] [Google Scholar]
- 82.Ballesteros JA, Weinstein H. Integrated methods for the construction of three dimensional models and computational probing of structure-function relations in G-protein coupled receptors. Methods Neurosci. 1995;25:366–428. [Google Scholar]
- 83.Visiers I, Ballesteros JA, Weinstein H. Three-dimensional representations of G protein-coupled receptor structures and mechanisms. Meth. Enzymol. 2002;343:329–371. doi: 10.1016/s0076-6879(02)43145-x. [DOI] [PubMed] [Google Scholar]
- 84.Weinstein H. Hallucinogen actions on 5-HT receptors reveal distinct mechanisms of activation and signaling by G protein-coupled receptors. AAPS J. 2005;7:E871–E884. doi: 10.1208/aapsj070485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Elling CE, Frimurer TM, Gerlach LO, Jorgensen R, Holst B, Schwartz TW. Metal ion site engineering indicates a global toggle switch model for seven-transmembrane receptor activation. J. Biol. Chem. 2006;281:17337–17346. doi: 10.1074/jbc.M512510200. [DOI] [PubMed] [Google Scholar]
- 86.Madabushi S, Gross AK, Philippi A, Meng EC, Wensel TG, Lichtarge O. Evolutionary trace of G protein-coupled receptors reveals clusters of residues that determine global and class-specific functions. J. Biol. Chem. 2004;279:8126–8132. doi: 10.1074/jbc.M312671200. [DOI] [PubMed] [Google Scholar]
- 87.Cohen GB, Oprian DD, Robinson PR. Mechanism of activation and inactivation of opsin: Role of Glu113 and Lys296. Biochemistry. 1992;31:12592–12601. doi: 10.1021/bi00165a008. [DOI] [PubMed] [Google Scholar]
- 88.Rath P, DeCaluwe LLJ, Bovee-Geurts PHM, Degrip WJ, Rothschild KJ. Fourier transform infrared difference spectroscopy of rhodopsin mutants: light activation of rhodopsin causes hydrogen-bonding change in residue aspartic acid-83 during meta II formation. Biochemistry. 1993;32:10277–10282. doi: 10.1021/bi00090a001. [DOI] [PubMed] [Google Scholar]
- 89.Lüdeke S, Beck R, Yan ECY, Sakmar TP, Siebert F, Vogel R. The role of Glu181 in the photoactivation of rhodopsin. J. Mol. Biol. 2005;353:345–356. doi: 10.1016/j.jmb.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 90.Vogel R, Lüdeke S, Siebert F, Sakmar TP, Hirshfeld A, Sheves M. Agonists and partial agonists of rhodopsin: Retinal polyene methylation affects receptor activation. Biochemistry. 2006;45:1640–1652. doi: 10.1021/bi052196r. [DOI] [PubMed] [Google Scholar]
- 91.Han M, Smith SO, Sakmar TP. Constitutive activation of opsin by mutation of methionine 257 on transmembrane helix 6. Biochemistry. 1998;37:8253–8261. doi: 10.1021/bi980147r. [DOI] [PubMed] [Google Scholar]
- 92.Baranski TJ, Herzmark P, Lichtarge O, Gerber BO, Trueheart J, Meng EC, et al. C5a receptor activation - Genetic identification of critical residues in four transmembrane helices. J. Biol. Chem. 1999;274:15757–15765. doi: 10.1074/jbc.274.22.15757. [DOI] [PubMed] [Google Scholar]
- 93.Lu ZL, Hulme EC. The functional topography of transmembrane domain 3 of the M1 muscarinic acetylcholine receptor, revealed by scanning mutagenesis. J. Biol. Chem. 1999;274:7309–7315. doi: 10.1074/jbc.274.11.7309. [DOI] [PubMed] [Google Scholar]
- 94.Tao YX, Abell AN, Liu XB, Nakamura K, Segaloff DL. Constitutive activation of G protein-coupled receptors as a result of selective substitution of a conserved leucine residue in transmembrane helix III. Mol. Endocrinol. 2000;14:1272–1282. doi: 10.1210/mend.14.8.0503. [DOI] [PubMed] [Google Scholar]
- 95.Olah ME, Jacobson KA, Stiles GL. Role of the 2nd extracellular loop of adenosine receptors in agonist and antagonist binding - Analysis of chimeric A1/A3-adenosine receptors. J. Biol. Chem. 1994;269:24692–24698. [PMC free article] [PubMed] [Google Scholar]
- 96.Wurch T, Colpaert FC, Pauwels PJ. Chimeric receptor analysis of the ketanserin binding site in the human 5-hydroxytryptamine1D receptor: Importance of the second extracellular loop and fifth transmembrane domain in antagonist binding. Mol. Pharmacol. 1998;54:1088–1096. doi: 10.1124/mol.54.6.1088. [DOI] [PubMed] [Google Scholar]
- 97.Conner M, Hawtin SR, Simms J, Wootten D, Lawson Z, Conner AC, et al. Systematic analysis of the entire second extracellular loop of the V1a vasopressin receptor - Key residues, conserved throughout a G-protein-coupled receptor family, identified. J. Biol. Chem. 2007;282:17405–17412. doi: 10.1074/jbc.M702151200. [DOI] [PubMed] [Google Scholar]
- 98.Pfleger KDG, Pawson AJ, Millar RP. Changes to gonadotropin-releasing hormone (GnRH) receptor extracellular loops differentially affect GnRH analog binding and activation: Evidence for distinct ligand-stabilized receptor conformations. Endocrinology. 2008;149:3118–3129. doi: 10.1210/en.2008-0002. [DOI] [PubMed] [Google Scholar]
- 99.Prioleau C, Visiers I, Ebersole BJ, Weinstein H, Sealfon SC. Conserved helix 7 tyrosine acts as a multistate conformational switch in the 5HT2C receptor - Identification of a novel “locked-on” phenotype and double revertant mutations. J. Biol. Chem. 2002;277:36577–36584. doi: 10.1074/jbc.M206223200. [DOI] [PubMed] [Google Scholar]
- 100.Liu W, Eilers M, Patel AB, Smith SO. Helix packing moments reveal diversity and conservation in membrane protein structure. J. Mol. Biol. 2004;337:713–729. doi: 10.1016/j.jmb.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 101.Eilers M, Patel AB, Liu W, Smith SO. Comparison of helix interactions in membrane and soluble α-bundle proteins. Biophys. J. 2002;82:2720–2736. doi: 10.1016/S0006-3495(02)75613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kong YF, Karplus M. The signaling pathway of rhodopsin. Structure. 2007;15:611–623. doi: 10.1016/j.str.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 103.Moore PB, Zhong QF, Husslein T, Klein ML. Simulation of the HIV-1 Vpu transmembrane domain as a pentameric bundle. FEBS Lett. 1998;431:143–148. doi: 10.1016/s0014-5793(98)00714-5. [DOI] [PubMed] [Google Scholar]
- 104.Soubias O, Gawrisch K. Probing specific lipid-protein interaction by saturation transfer difference NMR spectroscopy. J. Am. Chem. Soc. 2005;127:13110–13111. doi: 10.1021/ja0538942. [DOI] [PubMed] [Google Scholar]
- 105.Soubias O, Teague WE, Gawrisch K. Evidence for specificity in lipidrhodopsin interactions. J. Biol. Chem. 2006;281:33233–33241. doi: 10.1074/jbc.M603059200. [DOI] [PubMed] [Google Scholar]
- 106.Brown MF. Modulation of rhodopsin function by properties of the membrane bilayer. Chem. Phys. Lipids. 1994;73:159–180. doi: 10.1016/0009-3084(94)90180-5. [DOI] [PubMed] [Google Scholar]
- 107.Wiedmann TS, Pates RD, Beach JM, Salmon A, Brown MF. Lipid-protein interactions mediate the photochemical function of rhodopsin. Biochemistry. 1988;27:6469–6474. doi: 10.1021/bi00417a041. [DOI] [PubMed] [Google Scholar]
- 108.Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz KM, Ferguson DM, et al. A 2nd Generation Force-Field for the Simulation of Proteins, Nucleic-Acids, and Organic-Molecules. J. Am. Chem. Soc. 1995;117:5179–5197. [Google Scholar]
- 109.Wang JM, Cieplak P, Kollman PA. How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules? J. Comput. Chem. 2000;21:1049–1074. [Google Scholar]
- 110.Kaminski G, Duffy EM, Matsui T, Jorgensen WL. Free-energies of hydration and pure liquid properties of hydrocarbons from the OPLS all-atom model. J. Phys. Chem. 1994;98:13077–13082. [Google Scholar]
- 111.Wang JM, Wolf RM, Caldwell JW, Kollman PA, Case DA. Development and testing of a general amber force field. J. Comput. Chem. 2004;25:1157–1174. doi: 10.1002/jcc.20035. [DOI] [PubMed] [Google Scholar]
- 112.Bayly CI, Cieplak P, Cornell WD, Kollman PA. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: The RESP model. J. Phys. Chem. 1993;97:10269–10280. [Google Scholar]
- 113.Tajkhorshid E, Baudry J, Schulten K, Suhai S. Molecular dynamics study of the nature and origin of retinal's twisted structure in bacteriorhodopsin. Biophys. J. 2000;78:683–693. doi: 10.1016/S0006-3495(00)76626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Reeves PJ, Thurmond RL, Khorana HG. Structure and function in rhodopsin: High level expression of a synthetic bovine opsin gene and its mutants in stable mammalian cell lines. Proc. Natl. Acad. Sci. U.S.A. 1996;93:11487–11492. doi: 10.1073/pnas.93.21.11487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Eilers M, Reeves PJ, Ying WW, Khorana HG, Smith SO. Magic angle spinning NMR of the protonated retinylidene schiff base nitrogen in rhodopsin: Expression of 15N-lysine and 13C-glycine labeled opsin in a stable cell line. Proc. Natl. Acad. Sci. U.S.A. 1999;96:487–492. doi: 10.1073/pnas.96.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Eilers M, Ying WW, Reeves PJ, Khorana HG, Smith SO. Magic angle spinning nuclear magnetic resonance of isotopically labeled rhodopsin. Meth. Enzymol. 2002;343:212–222. doi: 10.1016/s0076-6879(02)43137-0. [DOI] [PubMed] [Google Scholar]
- 117.Reeves PJ, Kim JM, Khorana HG. Structure and function in rhodopsin: A tetracycline-inducible system in stable mammalian cell lines for high-level expression of opsin mutants. Proc. Natl. Acad. Sci. U.S.A. 2002;99:13413–13418. doi: 10.1073/pnas.212519199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Arnis S, Hofmann KP. Two different forms of metarhodopsin II: Schiff base deprotonation precedes proton uptake and signaling state. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7849–7853. doi: 10.1073/pnas.90.16.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Knierim B, Hofmann KP, Ernst OP, Hubbell WL. Sequence of late molecular events in the activation of rhodopsin. Proc. Natl. Acad. Sci. U.S.A. 2007;104:20290–20295. doi: 10.1073/pnas.0710393104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Beck M, Sakmar TP, Siebert F. Spectroscopic evidence for interaction between transmembrane helices 3 and 5 in rhodopsin. Biochemistry. 1998;37:7630–7639. doi: 10.1021/bi9801560. [DOI] [PubMed] [Google Scholar]
- 121.Ratner VL, Bagirov IG, Fesenko EE. Metarhodopsin-I can react with hydroxylamine. Vision Res. 1981;21:251–253. doi: 10.1016/0042-6989(81)90118-8. [DOI] [PubMed] [Google Scholar]
- 122.Lüdeke S, Fonfría VAL, Siebert F, Vogel R. Time-resolved rapid-scan Fourier transform infrared difference spectroscopy on a noncyclic photosystem: Rhodopsin photointermediates from Lumi to Meta II. Biopolymers. 2006;83:159–169. doi: 10.1002/bip.20540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.