Abstract
A fraction of cats exposed to feline leukemia virus (FeLV) effectively contain virus and resist persistent antigenemia/viremia. Using real-time PCR (qPCR) to quantitate circulating viral DNA levels, previously we detected persistent FeLV DNA in blood cells of non-antigenemic cats considered to have resisted FeLV challenge. In addition, previously we used RNA qPCR to quantitate circulating viral RNA levels and determined that the vast majority of viral DNA is transcriptionally active, even in the absence of antigenemia. A single comparison of all USDA-licensed commercially available FeLV vaccines using these modern sensitive methods has not been reported. To determine whether FeLV vaccination would prevent nucleic acid persistence, we assayed circulating viral DNA, RNA, antigen, infectious virus, and virus neutralizing (VN) antibody in vaccinated and unvaccinated cats challenged with infectious FeLV. We identified challenged vaccinates with undetectable antigenemia and viremia concomitant with persistent FeLV DNA and/or RNA. Moreover, these studies demonstrated that two whole inactivated virus (WIV) adjuvanted FeLV vaccines (Fort Dodge Animal Health’s Fel-O-Vax Lv-K® and Schering-Plough Animal Health’s FEVAXYN FeLV®) provided effective protection against FeLV challenge. In nearly every recipient of these vaccines, neither viral DNA, RNA, antigen, nor infectious virus could be detected in blood after FeLV challenge. Interestingly, this effective viral containment occurred despite a weak to undetectable VN antibody response. The above findings reinforce the precept of FeLV infection as a unique model of effective retroviral immunity elicited by WIV vaccination, and as such holds valuable insights into retroviral immunoprevention and therapy.
Keywords: FeLV, vaccine, whole inactivated virus, immunity, diagnosis, pathogenesis
1. Introduction
Feline leukemia virus (FeLV) was identified as a naturally occurring retroviral infection of cats over 40 years ago (Jarrett et al., 1964; Kawakami et al., 1967; Rickard et al., 1969). The primary route of transmission of this gammaretrovirus is horizontally through saliva (Francis et al., 1977; Hardy et al., 1976; Hardy et al., 1973; Hoover et al., 1977a). The pathogenic effects of FeLV infection are both cytoproliferative (e.g. lymphoma, myeloproliferative disorder) and cytosuppressive (e.g. immunodeficiency, myelosuppression) (Hoover and Mullins, 1991).
Historically, FeLV infection has represented a diametric paradigm of effective host response leading to regressive infection vs. ineffective host response leading to progressive infection and disease (Hoover et al., 1981). This model has been based on assays detecting either: (a) viremia by cell culture infectivity (VI) (de Noronha et al., 1977; Fischinger et al., 1974) or (b) intracellular antigenemia in leukocytes by immunofluorescent antibody (IFA) assay (Hardy et al., 1973; Hardy and Zuckerman, 1991a) or (c) extracellular antigenemia in plasma or serum by capture ELISA (Lutz et al., 1983a). Information obtained using these assays was used to estimate that in ~60% of young adult cats exposed to FeLV, neither p27 capsid antigen nor infectious virus were detectable in the blood after virus challenge (Hardy, 1980; Hardy et al., 1976; Hoover and Mullins, 1991; Rojko et al., 1979). In stark contrast, ~30% of exposed animals developed persistent antigenemia and viremia. However, subsequent widespread use of the p27 capture ELISA, in combination with the IFA and VI assays, prompted the identification of cats with discordant results (Hardy and Zuckerman, 1991b; Jarrett et al., 1982; Lutz et al., 1980b; Lutz et al., 1983b). In addition, several laboratories demonstrated that it is possible to reactivate FeLV from some cats with regressive infections (Madewell and Jarrett, 1983; Post and Warren, 1980; Rojko et al., 1982). These observations pointed to a more complex, less polar, view of FeLV:host relationships (Hoover and Mullins, 1991) and/or varying limits in assay sensitivity.
We have recently applied quantitative real-time PCR (qPCR) to examine vaccinated and unvaccinated cats challenged oronasally with FeLV-A/61E and found covert FeLV DNA, in both circulation and tissues, in the absence of detectable antigenemia (Torres et al., 2005). Investigators have shown that proviral integration occurs not only in cats with persistent antigenemia, but also in cats without detectable anitgenemia and with lower circulating proviral burdens (Cattori et al., 2006). Additionally, we have reported a near perfect agreement and strong linear correlation between FeLV DNA and RNA in the blood of FeLV-challenged cats, inferring that a substantial fraction of the detected FeLV DNA was indeed integrated into the host cell genome and initiated a transcriptionally active infection (Torres et al., 2008). Consequently, a spectrum of FeLV:host relationships have been identified, including cats with detectable nucleic acids and undetectable antigenemia (latent infections) and cats with both detectable nucleic acids and antigenemia (active infections). These findings, and those of colleagues (Cattori et al., 2006; Flynn et al., 2002; Gomes-Keller et al., 2006a; Gomes-Keller et al., 2006b; Hofmann-Lehmann et al., 2001; Hofmann-Lehmann et al., 2006; Tandon et al., 2005), demonstrated that DNA and RNA qPCR sensitivities are greater than p27 capsid antigen capture ELISA.
A singular feature of FeLV infection has been the development of effective vaccines providing protection against virulent virus challenge. At the time this study was initiated, four FeLV vaccines were commercially available in the USA, each with varying formulations and efficacy [reviewed by Loar (Loar, 1993) and Sparkes (Sparkes, 1997)]. Despite the accumulation of individual vaccine trials, the differences in experimental designs have made comparisons of vaccine efficacy virtually impossible. Moreover, most of these studies were performed before the advent of qPCR, thereby limiting the ability to detect evidence of viral infection. To our knowledge, a single comparison of every USDA-licensed FeLV vaccine that is commercially available in the USA has not been reported using modern viral nucleic acid detection methods. In addition, our previous work identified several protected vaccinates without any evidence of viral infection despite the use of DNA qPCR. Hofmann-Lehmann et. al. however, have not observed this unusual level of protection (Hofmann-Lehmann et al., 2008; Hofmann-Lehmann et al., 2007; Hofmann-Lehmann et al., 2006).
The present study, therefore, had two purposes: (1) to compare all USDA-licensed commercially available FeLV vaccines by determining whether they differed in ability to protect against both active and latent viral infection using contemporary sensitive methods and (2) to determine whether a neutralizing humoral immune response was associated with highly effective viral containment. Accordingly, we examined virulent FeLV challenge outcomes in cohorts of cats vaccinated with one of four commercially available vaccines and have assessed host:virus relationships by criteria of viral DNA, RNA, p27 capsid antigen, infectious virus, and neutralizing antibody.
2. Materials and Methods
2.1. Experimental animals
Forty specific-pathogen-free (SPF) cats were obtained from a commercial vendor (Harlan Sprague Dawley, Inc., Mt. Horeb, WI). The cats were randomly apportioned up to 5 cats per enclosure and housed at Harlan Sprague Dawley during the immunization phase of the experiment. Prior to virus challenge, they were transferred to Charmany Instructional Facility at the University of Wisconsin-Madison School of Veterinary Medicine (Madison, WI). For the remainder of the study, the animals were housed in identical groupings as before in accordance with the university animal care and use committee regulations.
2.2. Immunization
Four groups of n=8 cats each received one of four commercially available vaccines according to the manufacturer’s specifications and one group (n=8) served as the unvaccinated control. Group A received the adjuvanted whole inactivated virus (WIV) vaccine Fel-O-Vax Lv-K® (Fort Dodge Animal Health, Overland Park, KS). Group B received FEVAXYN FeLV® (Schering-Plough Animal Health Corporation, Summit, NJ), also an adjuvanted WIV vaccine. Group C received the adjuvanted, inactivated mixed subunit vaccine LEUKOCELL 2® (Pfizer Animal Health, New York, NY). Group D received PROTEX®-FeLV (Intervet, Millsboro, DE). It was a non-adjuvanted WIV vaccine which is no longer commercially available. The priming vaccination was administered when the cats were 15 – 16 weeks of age. The boosting vaccination was administered three weeks later when the cats were 18 – 19 weeks of age.
2.3. Challenge virus
Four months after receiving their boosting immunization, at 34 – 35 weeks of age, all cats were challenged intraperitoneally with 200 μL of 5 × 104 TCID50/mL FeLV-A/61E. This subgroup A virus strain is the highly replication competent, non-acutely pathogenic component of the FeLV-FAIDS complex (Donahue et al., 1988; Hoover et al., 1987; Mullins et al., 1986; Overbaugh et al., 1988). The cell-free infectious virus inoculum was prepared as supernatant from AH927 feline fibroblast cell cultures and determined to be equivalent to 1 CID100 (100% cat infective dose). Cats were observed twice daily for signs of illness after virus inoculation.
2.4. Sample collection and processing
Sample collections were performed on cats sedated with ketamine hydrochloride (11 mg/kg). Blood samples were collected immediately prior to challenge and every week thereafter through 8 weeks post-challenge (PC). Whole blood was shipped overnight on ice to Colorado State University (Ft. Collins, CO) where it was immediately processed upon arrival. Buffy coat cell pellets were stored at −70°C until analysis for FeLV DNA by qPCR. Plasma samples were separated into 1 mL aliquots and stored at −70°C until analysis for FeLV RNA by qPCR, FeLV p27 capsid antigen by capture ELISA, and infectious FeLV. Sera were stored at −70°C until analysis for FeLV neutralizing antibody.
2.5. Detection of FeLV DNA and RNA by qPCR assays
Weekly analysis of buffy coat cells for viral DNA and plasma for viral RNA was done by quantitative real-time PCR (qPCR) and reverse transcriptase qPCR, respectively. The DNA and RNA extractions, primer/probe set, and assay conditions were as described previously (Torres et al., 2005; Torres et al., 2008). The assays amplify exogenous and not endogenous FeLV sequences within the U3 region of FeLV-A/61E (Berry et al., 1988; Casey et al., 1981). The viral loads of the samples were determined by comparing the threshold cycle (CT) value of each nucleic acid sample with the standard curve of the co-amplified standard template. End-point dilutions of the standard templates yielded lower detection limits of 5 copies/DNA qPCR reaction and 10 copies/RNA qPCR reaction (1150 RNA copies/mL plasma).
2.6. Detection of FeLV by p27 capture ELISA
FeLV p27 capsid antigen was detected in plasma weekly as previously described (Torres et al., 2008). In addition, sera were assessed by the commercial SNAP® FeLV antigen diagnostic test (IDEXX Laboratories, Inc., Westbrook, ME). In rare instances of incongruous results, the perceived most sensitive finding of the two assays was chosen.
2.7. Virus stock
The FeLV-A/61E stock used for the virus infectivity and virus neutralization assays consisted of supernatant collected from Crandell feline kidney (CrFK) cells chronically infected with FeLV-A/61E. The virus stock was strongly positive by the p27 capture ELISA and contained 4.4 × 109 copies/mL by RNA qPCR. The 50% endpoint dilution of the virus stock was 2.5 × 103 TCID50/mL.
2.8. Detection of infectious FeLV
Plasma samples from cats inoculated with FeLV-A/61E 4 weeks previously were incubated with partially confluent AH927 cells and the presence of infectious FeLV (VI) was determined by p27 capture ELISA of the cell culture supernatant. Specifically, AH927 cells were seeded on a 24-well plate at a concentration of 1 × 105 cells/well. Cells were grown in 1 mL of minimal essential medium containing Earle’s salts and L-glutamine (Gibco Products, Invitrogen Corp., Carlsbad, CA) with the addition of 1% penicillin/streptomycin, 10% heat inactivated fetal bovine serum, and 4 μg/mL polybrene (Sigma-Aldrich Corp., St. Louis, MO). Following an overnight incubation at 37°C with 5% CO2, medium was removed from the subconfluent cells and 400 μL of freshly thawed sample plasma was added to each well. After a 2 hour incubation, the plasma was replaced with 1 mL of fresh medium without supplementation of polybrene and the plates were placed at 37°C with 5% CO2. This was day 1. The cells were examined daily for confluency and any cytopathic effects and were passaged (1:4 cell split) as necessary, approximately every 3 days. At each passage, the supernatants were assessed, in duplicate, for p27 by capture ELISA. The infectivity assay was stopped at day 21. The plasma sample was considered positive for infectious FeLV if a mean ELISA absorbance value of ≥ 0.05 was obtained (a threshold set above the negative control plus three times the standard deviation). Sham inoculated wells served as negative controls and an FeLV-A/61E virus stock as positive controls for the virus infectivity assay.
2.9. Detection of FeLV neutralizing antibodies
Virus neutralizing (VN) antibodies were measured at 2 time-points; post-vaccination just prior to receiving the virus challenge and at 8 weeks PC. Briefly, AH927 cells were seeded on a 96-well plate at a concentration of 1 × 104 cells/well and grown overnight in 100 μL of the same medium and under the same conditions as that used in the VI assay. The following day (day 1), sample sera were heat inactivated for 30 minutes at 56°C and 2-fold serial dilutions made (1:2 to 1:256). An equal volume (50 μL) of each serum dilution and 10 TCID50/mL FeLV-A/61E were incubated alone, in triplicate, for 1 hour at 37°C and 5% CO2 to allow antibody binding. Medium was removed from the subconfluent AH927 cells and the serum/virus mixtures were transferred onto the cells. After a 2 hour incubation at 37°C, the serum/virus mixtures were removed, the cells washed 3 times, 200 μL fresh medium without polybrene added, and the plates incubated at 37°C with 5% CO2. On day 4, approximately 50% of the medium was removed (100 μL) and the cells refed with 150 μL fresh medium. The assay was stopped on day 7 and cell culture supernatants assessed, singly, for p27 by capture ELISA. If the mean optical density value of each triplicate serum dilution was ≤ 0.05, the serum sample was considered positive for VN antibody. The titer of antibody was taken as the reciprocal of the serum dilution. Naïve serum served as the negative control and FeLV regressor serum served as the positive control for VN antibodies, respectively. In addition, sham inoculated wells served as negative controls and a back-titration of the virus stock as positive controls for virus infection, respectively.
2.10. Data analysis
In accord with USDA FeLV vaccine evaluation guidelines, a cat was considered FeLV-positive (actively infected) when 3 consecutive samples had positive antigen or virus results between weeks 3 to 8 PC (Shibley et al., 1991). The preventable fraction (PF) is used to express vaccine efficacy by accounting for the inherent resistance of some unvaccinated cats to development of persistent antigenemia/viremia after FeLV challenge (Pollack and Scarlett, 1990). The PF is calculated as
Undetectable results by both qPCR assays were corrected to a value of 1 and then all viral RNA and DNA levels were log transformed. Statistically significant differences in viral DNA and RNA levels between the FeLV:host categories and between the experimental groups were determined using repeated-measure analysis of variance (ANOVA) with the Tukey-Kramer post-hoc test. The chi-square test was used to determine if VI assay results and the FeLV:host categories were independent. A statistically significant difference between groups was considered to have occurred when a p value was < 0.05. Statistical tests were performed using StatView® version 5.0.1 for Macintosh, copyright 1999 (Abacus Concepts, Inc., Berkeley, CA).
3. Results
3.1. Host:virus relationships based on viral DNA and RNA levels, circulating p27, and infectious virus
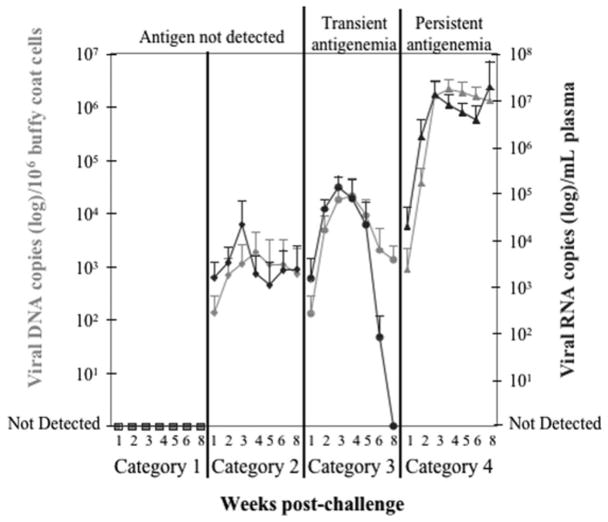
Virus challenge via the intraperitoneal route (vs. oronasal) produced animals representing the same 4 previously described response categories (Torres et al., 2005). However, in this manuscript we have reverted to numbered categories similar to those described by others in preceding reports (Hoover and Mullins, 1991; Lutz et al., 1980a; Lutz et al., 1983b), since terminology becomes to a degree imprecise or confusing. In 15 cats neither viral DNA, viral RNA, antigenemia, nor viremia were ever detected in blood at any time-point (Fig. 1). These animals previously described as having experienced abortive infection, were now simply expressed as a Category 1 response. Six cats were classified as having experienced regressive infection, here expressed as Category 2, on the basis of the continual absence of detectable circulating infectious virus and antigen but the presence of detectable viral RNA and/or DNA in blood albeit in a transient low or persistent low mode (DNA median: 176 DNA copies/106 buffy coat cells; DNA range: undetectable to 5,675 DNA copies/106 buffy coat cells; RNA median: undetectable; RNA range: undetectable to 120,267 RNA copies/mL plasma). Four cats classified as Category 3 (previously called latent infection) developed transient antigenemia and viral RNA burden (RNA median: 124 RNA copies/mL plasma; RNA range: undetectable to 255,238 RNA copies/mL plasma), yet retained a low to moderate circulating viral DNA load (DNA median: 2,487 DNA copies/106 buffy coat cells; DNA range: undetectable to 57,252 DNA copies/106 buffy coat cells). In addition, viremia was not detected even when viral DNA and RNA levels were moderate to low. Lastly, 15 animals that developed persistent antigenemia and persistent high circulating viral DNA and RNA levels (DNA median: 934,891 DNA copies/106 buffy coat cells; DNA range: 57 to 4,173,516 DNA copies/106 buffy coat cells; RNA median: 2,681,905 RNA copies/mL plasma; RNA range: undetectable to 161,904,762 RNA copies/mL plasma) were classified as Category 4 (aka progressive infection). Viremia was detected only in 12 of 15 cats classified into Category 4. In no sample was infectious virus detected without detectable viral DNA and RNA. In one animal, viremia was detected yet antigenemia was not detected at that timepoint.
Fig. 1.
Classification of host:virus relationships using circulating viral p27 antigen in conjunction with viral RNA and DNA levels. FeLV-A/61E-infected cats classified into Category 1 (squares) never had detectable p27, viral RNA (black symbols), or viral DNA (gray symbols) in blood (n = 15). Cats grouped into Category 2 (diamonds) did not have circulating p27 detected but viral RNA and/or DNA was detectable in blood, albeit at transient low or persistent low levels (n = 6). Cats considered to be in Category 3 (circles) developed a transient antigenemia and viral RNA burden, yet retained low to moderate viral DNA levels in blood (n = 4). Cats in Category 4 (triangles) were persistently antigenemic and had persistent high circulating viral RNA and DNA levels (n = 15). Statistically significant differences (p < 0.01) in viral DNA and RNA levels were present between all FeLV categories (with the exception of Category 2 vs. Category 3): Category 1 vs. Category 2, Category 1 vs. Category 3, Category 1 vs. Category 4, Category 2 vs. Category 4, and Category 3 vs. Category 4. Mean ± SD are plotted.
Statistically significant differences (p < 0.01) in viral DNA and RNA levels distinguished all FeLV categories (i.e. Categories 1 vs. 2; Categories 1 vs. 3; Categories 1 vs. 4; Categories 2 vs. 4; and Categories 3 vs. 4) with one exception--Categories 2 vs. 3. Infectious virus was easily detected in samples with high levels of viral DNA and RNA (≥105 DNA copies/106 buffy coat cells and ≥105 RNA copies/mL plasma) and this association was supported by chi square analysis (p < 0.0001). Low to moderate viral DNA and RNA levels (≥101 to <105 DNA copies/106 buffy coat cells and ≥101 to <105 RNA copies/mL plasma) were not demonstrably associated with plasma viral infectivity.
3.2. Vaccine efficacy
The assays above were applied to determine whether all USDA-licensed commercially available FeLV vaccines differed in ability to protect against both viral nucleic acid and antigenemia/viremia detection. The FeLV challenge induced adequate infection levels in the unvaccinated control group as seven of eight cats (88%) developed persistent antigenemia/viremia (Table 1). In addition, these seven unvaccinated cats developed persistent high viral DNA and RNA loads. However, neither viral DNA, RNA, antigenemia, nor viremia were detected at any time PC in seven of eight cats vaccinated with Vaccine A and in six of eight cats vaccinated with Vaccine B (Fig. 2 and Fig. 3). By contrast, viral DNA and RNA were detected in six of eight cats that received Vaccine C; three of which developed persistent antigenemia and detectable viremia. Lastly, all eight cats that received Vaccine D had detectable viral DNA and RNA; five of which developed persistent antigenemia and four of which had detectable viremia.
Table 1.
Efficacy of commercially available FeLV vaccines
| Experimental group | n | Persistent antigenemia/viremia | Preventable fraction (%) |
|---|---|---|---|
| Vaccine A | 8 | 0 | 100 |
| Vaccine B | 8 | 0 | 100 |
| Vaccine C | 8 | 3 | 57 |
| Vaccine D | 8 | 5 | 29 |
| Unvaccinated controls | 8 | 7 | not applicable |
Fig. 2.
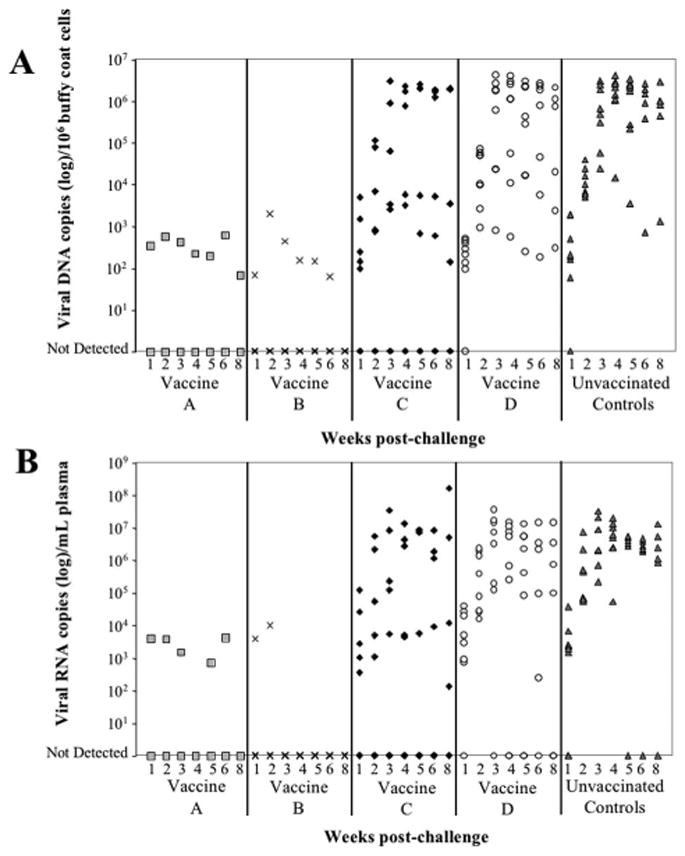
Vaccine induced immunity against FeLV challenge. Seven of 8 cats vaccinated with Vaccine A (light gray squares) and 6 of 8 cats vaccinated with Vaccine B (black Xs) had undetectable viral DNA (A) and RNA (B). By contrast, only 2 cats which received Vaccine C (black diamonds) had undetectable viral DNA and RNA. All 8 cats which received Vaccine D (open circles) had detectable viral DNA and RNA PC. Seven of 8 unvaccinated control cats (dark gray triangles) had persistent high viral DNA and RNA levels. Statistically significant differences (DNA and RNA p<0.01) were detected between Vaccine A vs. unvaccinated controls and between Vaccine B vs. unvaccinated controls. Results for Vaccine C and Vaccine D were not statistically different from the unvaccinated control group.
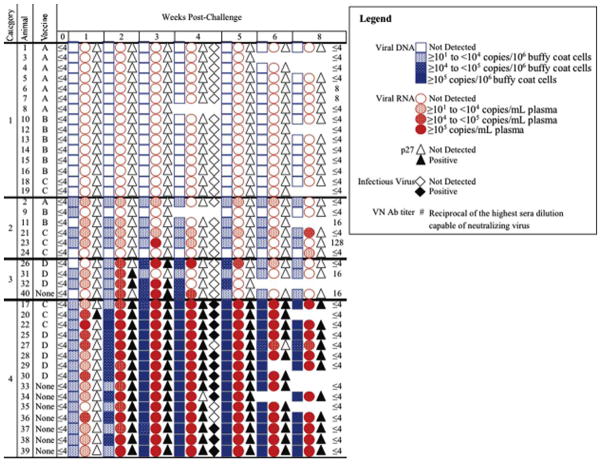
Fig. 3.
Outcome of FeLV challenge. Blood samples collected pre-challenge (0 weeks PC) and weekly thereafter were assessed for FeLV DNA (blue squares) and RNA (red circles) by qPCR, for p27 (triangles) by capture ELISA, and for infectious FeLV (diamonds) by VI at 4 weeks PC only. The VN antibody response was assessed post-vaccination pre-challenge and again at 8 weeks PC. The range of viral DNA and RNA levels is expressed by the intensity of the blue or red, respectively.
Statistically significant differences (DNA and RNA p < 0.01) were detected between Vaccine A vs. unvaccinated controls and between Vaccine B vs. unvaccinated controls. Results for Vaccine C and Vaccine D were not statistically different from the unvaccinated control group. For vaccines A and B the PF was100%.
3.3. Virus neutralizing antibody responses
To determine whether a neutralizing humoral immune response was associated with highly effective viral containment, virus neutralizing (VN) antibodies were measured post-vaccination just prior to receiving the virus challenge and at 8 weeks PC. FeLV vaccination was not distinguished by induction of substantial VN antibody titers (Fig. 3). Low VN antibody titers were observed in almost every vaccinated animal irrespective of the FeLV:host relationship that developed PC. Indeed, in all vaccinated cats in which neither viral DNA, RNA, antigen, nor infectious virus were ever detected, minimal VN immune responses were identified. In total, strong (1:16 to 1:128) VN antibody responses were detected in only four of 40 cats at 8 weeks PC. All of these animals had eliminated circulating viral RNA more than two weeks earlier, although in all four low viral DNA levels persisted in blood leukocytes. Thus, it appears that effective viral containment occurred despite a low to undetectable VN antibody response.
4. Discussion
Despite using a different route of challenge (intraperitoneal vs. oronasal), the present study reinforced previous data demonstrating that the first whole inactivated virus (WIV) FeLV vaccine (Vaccine A) provided substantial protection against FeLV challenge (Torres et al., 2005). A second WIV adjuvanted FeLV vaccine (Vaccine B) also provided effective protection against FeLV challenge. In nearly every recipient of the two WIV vaccines, neither viral DNA, RNA, antigen, nor infectious virus could be detected in blood. Thus, we found the preventable fraction for Vaccines A and B was 100%. These results are consistent with previously reported preventable fractions (PF) of 86 – 100% for Fel-O-Vax Lv-K® (Hoover et al., 1995, 1996; Legendre et al., 1991; Torres et al., 2005) and 90 – 100% for FEVAXYN FeLV® (Hines et al., 1991; Pedersen, 1993). In addition, the observed PF of 57% for Vaccine C was similar to previously reported PF of 35 – 88% (Haffer et al., 1990; Harbour et al., 2002; Legendre et al., 1991; Pollock and Haffer, 1991; Tizard and Bass, 1991). In the present study WIV Vaccines A and B appeared effective in preventing not only active but also latent FeLV infection following challenge. While our inability to detect evidence of viral infection by any assay employed at any point post exposure in these vaccinated cats is constrained by the limits of assay sensitivity and frequency of sampling, these results nevertheless lend support to the tenet that successful immunization against retroviral infection can be obtained by WIV immunoprophylaxis.
It should be mentioned that other studies using FeLV qPCR to evaluate vaccine efficacy have not identified this level of protection (Hofmann-Lehmann et al., 2008; Hofmann-Lehmann et al., 2007; Hofmann-Lehmann et al., 2006). Our viral DNA and RNA qPCR assays have slightly lower detection limits of 5 copies/DNA qPCR reaction and 10 copies/RNA qPCR reaction (1150 RNA copies/mL plasma), respectively, compared to that of Tandon et al. who reported 1 copy/DNA qPCR reaction detected 38% of the time and 180 copies/RNA qPCR reaction (2250 copies RNA/mL) (Tandon et al., 2005). The FeLV-61E subgroup A virus isolate used in the present study is a molecular clone of the highly replication competent, non-acutely pathogenic component of the FeLV-FAIDS complex (Donahue et al., 1988; Hoover et al., 1987; Mullins et al., 1986; Overbaugh et al., 1988) whereas Hofmann-Lehmann et al. (Hofmann-Lehmann et al., 2007; Hofmann-Lehmann et al., 2006) have used the moderately pathogenic FeLV-A/Glasgow-1. However, the two isolates are subgroup A homologous. The virus challenge dosage used here was satisfactory according to USDA guidelines (Shibley et al., 1991) in that at least 60% of the controls developed persistent viremia--88% in the present study and 70% in a previous study (Torres et al., 2005). In the studies of Hofmann-Lehmann (Hofmann-Lehmann et al., 2007; Hofmann-Lehmann et al., 2006) persistent viremia was induced in a comparable percentage (83 – 90%) of unvaccinated controls. However, the age (Hoover et al., 1976), outbred genetic background, and endogenous FeLV (enFeLV) repertoire (Tandon et al., 2008a; Tandon et al., 2008b; Tandon et al., 2007) of vaccinates at challenge could also explain this difference in any FeLV vaccine study.
We have shown in this study that the four putative FeLV:host response categories suggested previously by DNA qPCR were reinforced with the addition of circulating viral RNA levels (Torres et al., 2005). These FeLV:host relationships became established by 8 weeks PC as with our previous findings. Fifteen cats were classified into Category 1 because they did not have detectable infectious virus, antigen, viral DNA, or viral RNA in blood at any time-point. In this situation, these cats were indistinguishable from those never exposed to or never infected by FeLV on the basis of viral DNA and RNA, p27 capture ELISA, and viral infectivity assay results.
Since our inability to detect any indicator of virus infection did not reflect lack of exposure, it seems most plausible that vaccination primed the cats to resist infection. Statistically significant differences in the frequency of Category 1 animals were observed PC between cats receiving two of four vaccines vs. unvaccinated controls. Of the 15 cats in Category 1, seven received Vaccine A, six received Vaccine B, and two received Vaccine C. Consequently, it appears that Category 1 animals had effective adaptive immune responses which abrogated infection and eliminated FeLV-infected cells within one week after viral exposure and that two of the four vaccines used were able to effectively prime this successful response. It is probable, however, that virus could have been detected at time-points earlier than 1 week PC. Unfortunately, this study was not designed to include such sampling times. It is also possible that virus could have been harbored in some tissue site and not revealed by blood assay, although in an earlier study we found that in cats in which viral DNA was undetectable in blood cells, it was also not detected in bone marrow, spleen, mesenteric lymph node, thymus, tonsil, or retropharyngeal lymph node cells (Torres et al., 2005). In fact, we found circulating viral DNA was positively correlated with tissue viral DNA, in accord with other studies which quantified viral burdens using either the VI assay or semiquantitative PCR (Hoover et al., 1977a; Quackenbush et al., 1996a; Quackenbush et al., 1996b).
We previously demonstrated that viral DNA and RNA levels were highly correlated (Torres et al., 2008), thus in most instances, it appears that viral DNA initiates a transcriptionally active infection. It is assumed that measurement of extracellular (plasma) viral RNA indicates the presence of intact virions, however, here we report that in most blood samples with low to moderate viral DNA and RNA levels neither p27 nor infectious virus were detected. This finding is in agreement with other investigators (Flynn et al., 2002; Hofmann-Lehmann et al., 2006; Tandon et al., 2005) and thus it is possible that only a very small percentage of virions produced are actually infectious as has been observed in HIV infections (Layne et al., 1992; Piatak et al., 1993). In addition, multiple other factors could have contributed to our inability to detect infectious virus in animals with low nucleic acid levels, these include: sample deterioration, virus neutralizing antibody or some other inhibitory factor present in the assayed plasma, the use of cell-free plasma inocula, virus dilution through cell culture passage during the VI assay, and the sensitivity of p27 capture ELISA as the read-out (Dimitrov et al., 1993; Fischinger et al., 1976; Levy et al., 1975; Piatak et al., 1993; Sato et al., 1992; Wu et al., 2002). It seems most plausible to us that, given the high sensitivity of RNA qPCR, low levels of infectious virus and antigen were produced but were below the limits of detection. Consequently, the term ‘latent’ could be relative when describing animals which retain viral genome but do not produce detectable levels of virus particles.
Intermediate FeLV:host relationships such as Category 2 and 3 are potentially dynamic, making viral escape possible as was suggested by two cats with increased viral RNA levels at 8 weeks PC. While viral reactivation is also possible we, and others, have observed that these relationships can be maintained for years and a recurrence of antigenemia is a rare event (Hofmann-Lehmann et al., 2007; Torres et al., 2005). Although FeLV transmission from non-antigenemic and intermittently antigenemic cats has been reported (Hayes et al., 1989; Pacitti et al., 1986), we can now appreciate how such animals with ‘localized’ or ‘sequestered’ infections may have had sufficient virus, despite being undetectable, in milk or plasma to transmit to other cats. The possibility of transmission was not assessed in the discordant cats (detectable nucleic acids without detectable viremia or antigenemia) in the present study. Such issues are pertinent to use of FeLV antigen-negative cats for blood donation, tissue transplants, and adoptions, as well as to the use of therapeutic immunosuppressive drugs in antigen-negative cats (Coronado et al., 2000; Gregory et al., 1991; Nemzek et al., 1994, 1996). Until the clinical significance of nucleic acid-positive/antigen-negative, infectious virus-negative animals is understood, the diagnostic significance of applying FeLV qPCR in pet cats remains unclear and one should follow AAFP guidelines (Levy et al., 2008).
The virus neutralizing (VN) antibody titer produced following vaccination and PC was not a reliable correlate of vaccine protection. VN antibody was usually not detectable following vaccination and in only a few animals effecting successful viral containment were low level VN antibody titers elicited. In animals with the highest VN antibody responses, viral DNA remained detectable in circulating cells. Previous FeLV vaccine studies have observed that resistance to infection was attained either in the absence (Harbour et al., 2002; Hawks et al., 1991; Pedersen, 1993) or the occurrence of low and inconsistent (Haffer et al., 1990; Hardy et al., 1976; Hofmann-Lehmann et al., 2006; Hoover et al., 1976; Hoover et al., 1977b; Jarrett et al., 1977; Madewell and Jarrett, 1983; Pacitti et al., 1986; Schaller et al., 1977) VN antibody responses. It is possible, as reported by Hofmann-Lehmann et. al., that a VN antibody response is not detectable at the 8 week PC sampling time-point, but instead becomes detectable several weeks later (Hofmann-Lehmann et al., 2008; Hofmann-Lehmann et al., 2006). This lack of association between humoral responses and effective viral containment in protected vaccinates suggests a role for protective cell-mediated immune responses. Flynn et al. (Flynn et al., 2002; Flynn et al., 2000) have demonstrated an association between modest levels of FeLV-specific cytotoxic T lymphocyte activity in cats that resisted infection. Yet in summary, despite highly effective WIV vaccination, the determinants of effective FeLV immunity remain far from understood.
In conclusion, we have used contemporary methodologies with increased sensitivity to study FeLV host:virus relationships and vaccine-primed resistance. The results help explain instances of discordant results using traditional assays and offer opportunity for further insight into FeLV infection. In particular, further long-term studies are needed to determine the clinical relevance of persistent viral nucleic acids in non-antigenemic/non-viremic cats. Also merited is additional focus on the FeLV infection dynamics in very early infection, when virus containment or lack thereof transpires. Finally, we presented evidence for immunity against this retroviral infection elicited by WIV vaccination. It would seem that further examination of innate and cell-mediated immune responses is a requisite to unraveling the immune correlates of protection. The above findings reinforce the precept of FeLV infection as a model of the early immune responses that determine effective vs. ineffective retroviral containment, offering insights into immunoprevention and therapy.
Acknowledgments
The project described was supported by Grant K08AI054194 from the National Institute of Allergy and Infectious Disease. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. This work was also supported by Gift Funds to the Department of Pathobiological Sciences, School of Veterinary Medicine, University of Wisconsin-Madison. These studies were conducted by A. Torres as partial fulfillment for a PhD degree at Colorado State University.
Footnotes
Conflict of interest statement
None of the authors has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the paper entitled “Feline Leukemia Virus Immunity Induced by Whole Inactivated Virus Vaccination”.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berry BT, Ghosh AK, Kumar DV, Spodick DA, Roy-Burman P. Structure and function of endogenous feline leukemia virus long terminal repeats and adjoining regions. Journal of Virology. 1988;62:3631–3641. doi: 10.1128/jvi.62.10.3631-3641.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey JW, Roach A, Mullins JI, Burck KB, Nicholson MO, Gardner MB, Davidson N. The U3 portion of feline leukemia virus DNA identifies horizontally acquired proviruses in leukemic cats. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:7778–7782. doi: 10.1073/pnas.78.12.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattori V, Tandon R, Pepin A, Lutz H, Hofmann-Lehmann R. Rapid detection of feline leukemia virus provirus integration into feline genomic DNA. Molecular and Cellular Probes. 2006;20:172–181. doi: 10.1016/j.mcp.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Coronado GS, Swenson CL, Martinez SA, Burkhardt KS, Arnoczky SP. Effects of a 98% solution of glycerol or sterilization with ethylene oxide on FeLV in bone allografts and effects on bone incorporation of allografts in cats. American Journal of Veterinary Research. 2000;61:665–671. doi: 10.2460/ajvr.2000.61.665. [DOI] [PubMed] [Google Scholar]
- de Noronha F, Poco A, Post JE, Rickard CG. Virus isolation test for feline leukemia virus. Journal of the National Cancer Institute. 1977;58:129–131. doi: 10.1093/jnci/58.1.129. [DOI] [PubMed] [Google Scholar]
- Dimitrov DS, Willey RL, Sato H, Chang LJ, Blumenthal R, Martin MA. Quantitation of human immunodeficiency virus type 1 infection kinetics. Journal of Virology. 1993;67:2182–2190. doi: 10.1128/jvi.67.4.2182-2190.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue PR, Hoover EA, Beltz GA, Riedel N, Hirsch VM, Overbaugh J, Mullins JI. Strong sequence conservation among horizontally transmissible, minimally pathogenic feline leukemia viruses. Journal of Virology. 1988;62:722–731. doi: 10.1128/jvi.62.3.722-731.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger PJ, Blevins CS, Nomura S. Simple, quantitative assay for both xenotropic murine leukemia and ecotropic feline leukemia viruses. Journal of Virology. 1974;14:177–179. doi: 10.1128/jvi.14.1.177-179.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger PJ, Ihle JN, Bolognesi DP, Schafer W. Inactivation of murine xenotropic oncornavirus by normal mouse sera is not immunoglobulin-mediated. Virology. 1976;71:346–351. doi: 10.1016/0042-6822(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Flynn JN, Dunham SP, Watson V, Jarrett O. Longitudinal analysis of feline leukemia virus-specific cytotoxic T lymphocytes: correlation with recovery from infection. Journal of Virology. 2002;76:2306–2315. doi: 10.1128/jvi.76.5.2306-2315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JN, Hanlon L, Jarrett O. Feline leukemia virus: protective immunity is mediated by virus-specific cytotoxic T lymphocytes. Immunology. 2000;101:120–125. doi: 10.1046/j.1365-2567.2000.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DP, Essex M, Hardy WD., Jr Excretion of feline leukaemia virus by naturally infected pet cats. Nature. 1977;269:252–254. doi: 10.1038/269252a0. [DOI] [PubMed] [Google Scholar]
- Gomes-Keller MA, Gonczi E, Tandon R, Riondato F, Hofmann-Lehmann R, Meli ML, Lutz H. Detection of feline leukemia virus RNA in saliva from naturally infected cats and correlation of PCR results with those of current diagnostic methods. Journal of Clinical Microbiology. 2006a;44:916–922. doi: 10.1128/JCM.44.3.916-922.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes-Keller MA, Tandon R, Gonczi E, Meli ML, Hofmann-Lehmann R, Lutz H. Shedding of feline leukemia virus RNA in saliva is a consistent feature in viremic cats. Veterinary Microbiology. 2006b;112:11–21. doi: 10.1016/j.vetmic.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Gregory CR, Madewell BR, Griffey SM, Torten M. Feline leukemia virus-associated lymphosarcoma following renal transplantation in a cat. Transplantation. 1991;52:1097–1099. doi: 10.1097/00007890-199112000-00034. [DOI] [PubMed] [Google Scholar]
- Haffer KN, Koertje WD, Derr JT, Beckenhauer WH. Evaluation of immunosuppressive effect and efficacy of an improved-potency feline leukaemia vaccine. Vaccine. 1990;8:12–16. doi: 10.1016/0264-410x(90)90170-q. [DOI] [PubMed] [Google Scholar]
- Harbour DA, Gunn-Moore DA, Gruffydd-Jones TJ, Caney SM, Bradshaw J, Jarrett O, Wiseman A. Protection against oronasal challenge with virulent feline leukaemia virus lasts for at least 12 months following a primary course of immunisation with Leukocell 2 vaccine. Vaccine. 2002;20:2866–2872. doi: 10.1016/s0264-410x(02)00237-2. [DOI] [PubMed] [Google Scholar]
- Hardy WD., Jr . The virology, immunology and epidemiology of the feline leukemia virus. In: Hardy WD Jr, Essex M, McClelland AJ, editors. Feline Leukemia Virus. Elsevier North Holland, Inc; New York: 1980. pp. 33–78. [Google Scholar]
- Hardy WD, Jr, Hess PW, MacEwen EG, McClelland AJ, Zuckerman EE, Essex M, Cotter SM, Jarrett O. Biology of feline leukemia virus in the natural environment. Cancer Research. 1976;36:582–588. [PubMed] [Google Scholar]
- Hardy WD, Jr, Old LJ, Hess PW, Essex M, Cotter S. Horizontal transmission of feline leukaemia virus. Nature. 1973;244:266–269. doi: 10.1038/244266a0. [DOI] [PubMed] [Google Scholar]
- Hardy WD, Jr, Zuckerman EE. Development of the immunofluorescent antibody test for detection of feline leukemia virus infection in cats. Journal of the American Veterinary Medical Association. 1991a;199:1327–1335. [PubMed] [Google Scholar]
- Hardy WD, Jr, Zuckerman EE. Ten-year study comparing enzyme-linked immunosorbent assay with the immunofluorescent antibody test for detection of feline leukemia virus infection in cats. Journal of the American Veterinary Medical Association. 1991b;199:1365–1373. [PubMed] [Google Scholar]
- Hawks DM, Legendre AM, Rohrbach BW, Sebring R, Chavez L, Chu HJ, Acree WM. Antibody response of kittens after vaccination followed by exposure to feline leukemia virus-infected cats. Journal of the American Veterinary Medical Association. 1991;199:1463–1469. [PubMed] [Google Scholar]
- Hayes KA, Rojko JL, Tarr MJ, Polas PJ, Olsen RG, Mathes LE. Atypical localised viral expression in a cat with feline leukaemia. Veterinary Record. 1989;124:344–346. doi: 10.1136/vr.124.13.344. [DOI] [PubMed] [Google Scholar]
- Hines DL, Cutting JA, Dietrich DL, Walsh JA. Evaluation of efficacy and safety of an inactivated virus vaccine against feline leukemia virus infection. Journal of the American Veterinary Medical Association. 1991;199:1428–1430. [PubMed] [Google Scholar]
- Hofmann-Lehmann R, Cattori V, Tandon R, Boretti FS, Meli ML, Riond B, Lutz H. How molecular methods change our views of FeLV infection and vaccination. Veterinary Immunology and Immunopathology. 2008;123:119–123. doi: 10.1016/j.vetimm.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Hofmann-Lehmann R, Cattori V, Tandon R, Boretti FS, Meli ML, Riond B, Pepin AC, Willi B, Ossent P, Lutz H. Vaccination against the feline leukaemia virus: outcome and response categories and long-term follow-up. Vaccine. 2007;25:5531–5539. doi: 10.1016/j.vaccine.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Hofmann-Lehmann R, Huder JB, Gruber S, Boretti F, Sigrist B, Lutz H. Feline leukaemia provirus load during the course of experimental infection and in naturally infected cats. Journal of General Virology. 2001;82:1589–1596. doi: 10.1099/0022-1317-82-7-1589. [DOI] [PubMed] [Google Scholar]
- Hofmann-Lehmann R, Tandon R, Boretti FS, Meli ML, Willi B, Cattori V, Gomes-Keller MA, Ossent P, Golder MC, Flynn JN, Lutz H. Reassessment of feline leukaemia virus (FeLV) vaccines with novel sensitive molecular assays. Vaccine. 2006;24:1087–1094. doi: 10.1016/j.vaccine.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Hoover EA, Mullins JI. Feline leukemia virus infection and diseases. Journal of the American Veterinary Medical Association. 1991;199:1287–1297. [PubMed] [Google Scholar]
- Hoover EA, Mullins JI, Chu HJ, Wasmoen TL. Development and testing of an inactivated feline leukemia virus vaccine. Seminars in Veterinary Medicine & Surgery (Small Animal) 1995;10:238–243. [PubMed] [Google Scholar]
- Hoover EA, Mullins JI, Chu HJ, Wasmoen TL. Efficacy of an inactivated feline leukemia virus vaccine. AIDS Research & Human Retroviruses. 1996;12:379–383. doi: 10.1089/aid.1996.12.379. [DOI] [PubMed] [Google Scholar]
- Hoover EA, Mullins JI, Quackenbush SL, Gasper PW. Experimental transmission and pathogenesis of immunodeficiency syndrome in cats. Blood. 1987;70:1880–1892. [PubMed] [Google Scholar]
- Hoover EA, Olsen RG, Hardy WD, Jr, Schaller JP, Mathes LE. Feline leukemia virus infection: age-related variation in response of cats to experimental infection. Journal of the National Cancer Institute. 1976;57:365–369. doi: 10.1093/jnci/57.2.365. [DOI] [PubMed] [Google Scholar]
- Hoover EA, Olsen RG, Mathes LE, Schaller JP. Relationship between feline leukemia virus antigen expression and viral infectivity in blood, bone marrow, and saliva of cats. Cancer Research. 1977a;37:3707–3710. [PubMed] [Google Scholar]
- Hoover EA, Rojko JL, Olsen RG. Pathogenesis of feline leukemia virus infection. In: Olsen RG, editor. Feline Leukemia. CRC Press; Boca Raton, FL: 1981. pp. 31–51. [Google Scholar]
- Hoover EA, Schaller JP, Mathes LE, Olsen RG. Passive immunity to feline leukemia: evaluation of immunity from dams naturally infected and experimentally vaccinated. Infection and Immunity. 1977b;16:54–59. doi: 10.1128/iai.16.1.54-59.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett O, Golder MC, Weijer K. A comparison of three methods of feline leukaemia virus diagnosis. Veterinary Record. 1982;110:325–328. doi: 10.1136/vr.110.14.325. [DOI] [PubMed] [Google Scholar]
- Jarrett O, Russell PH, Stewart MF. Protection of kittens from feline leukaemia virus infection by maternally-derived antibody. Veterinary Record. 1977;101:304–305. doi: 10.1136/vr.101.15.304. [DOI] [PubMed] [Google Scholar]
- Jarrett WFH, Martin WB, Crighton GW, Dalton RG, Stewart MF. Leukemia in the cat. Transmission experiments with leukemia (lymphosarcoma) Nature. 1964;202:566–567. doi: 10.1038/202566a0. [DOI] [PubMed] [Google Scholar]
- Kawakami TG, Theilen GH, Dungworth DL, Munn RJ, Beall SG. C-type viral particles in plasma of cats with feline leukemia. Science. 1967;158:1049–1050. doi: 10.1126/science.158.3804.1049. [DOI] [PubMed] [Google Scholar]
- Layne SP, Merges MJ, Dembo M, Spouge JL, Conley SR, Moore JP, Raina JL, Renz H, Gelderblom HR, Nara PL. Factors underlying spontaneous inactivation and susceptibility to neutralization of human immunodeficiency virus. Virology. 1992;189:695–714. doi: 10.1016/0042-6822(92)90593-e. [DOI] [PubMed] [Google Scholar]
- Legendre AM, Hawks DM, Sebring R, Rohrbach B, Chavez L, Chu HJ, Acree WM. Comparison of the efficacy of three commercial feline leukemia virus vaccines in a natural challenge exposure. Journal of the American Veterinary Medical Association. 1991;199:1456–1462. [PubMed] [Google Scholar]
- Levy J, Crawford C, Hartmann K, Hofmann-Lehmann R, Little S, Sundahl E, Thayer V. 2008 American Association of Feline Practitioners’ feline retrovirus management guidelines. Journal of Feline Medicine and Surgery. 2008;10:300–316. doi: 10.1016/j.jfms.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JA, Ihle JN, Oleszko O, Barnes RD. Virus-specific neutralization by a soluble non-immunoglobulin factor found naturally in normal mouse sera. Proceedings of the National Academy of Sciences of the United States of America. 1975;72:5071–5075. doi: 10.1073/pnas.72.12.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loar AS. Feline Leukemia Virus: Immunization and Prevention. Veterinary Clinics of North America: Small Animal Practice. 1993;23:193–211. doi: 10.1016/s0195-5616(93)50012-8. [DOI] [PubMed] [Google Scholar]
- Lutz H, Pedersen NC, Durbin R, Theilen GH. Monoclonal antibodies to three epitopic regions of feline leukemia virus p27 and their use in enzyme-linked immunosorbent assay of p27. Journal of Immunological Methods. 1983a;56:209–220. doi: 10.1016/0022-1759(83)90413-1. [DOI] [PubMed] [Google Scholar]
- Lutz H, Pedersen NC, Harris CW, Higgins J, Theilen GH. Detection of feline leukemia virus infection. Feline Practice. 1980a;10:13–23. [Google Scholar]
- Lutz H, Pedersen NC, Higgins J, Harris CW, Theilen GH. Quantitation of p27 in the serum of cats during natural infection with feline leukemia virus. In: Hardy WD Jr, Essex M, McClelland AJ, editors. Feline Leukemia Virus. Elsevier North Holland, Inc; New York: 1980b. pp. 497–505. [Google Scholar]
- Lutz H, Pedersen NC, Theilen GH. Course of feline leukemia virus infection and its detection by enzyme-linked immunosorbent assay and monoclonal antibodies. American Journal of Veterinary Research. 1983b;44:2054–2059. [PubMed] [Google Scholar]
- Madewell BR, Jarrett O. Recovery of feline leukemia virus from non-viraemic cats. Veterinary Record. 1983;112:339–342. doi: 10.1136/vr.112.15.339. [DOI] [PubMed] [Google Scholar]
- Mullins JI, Chen CS, Hoover EA. Disease-specific and tissue-specific production of unintegrated feline leukaemia virus variant DNA in feline AIDS. Nature. 1986;319:333–336. doi: 10.1038/319333a0. [DOI] [PubMed] [Google Scholar]
- Nemzek JA, Arnoczky SP, Swenson CL. Retroviral transmission in connective tissue allotransplantation: an experimental study. Journal of Bone and Joint Surgery. 1994;76A:1036–1041. doi: 10.2106/00004623-199407000-00012. [DOI] [PubMed] [Google Scholar]
- Nemzek JA, Arnoczky SP, Swenson CL. Retroviral transmission in bone allotransplantation. Clinical Orthopaedics and Related Research. 1996;324:275–282. [PubMed] [Google Scholar]
- Overbaugh J, Donahue PR, Quackenbush SL, Hoover EA, Mullins JI. Molecular cloning of a feline leukemia virus that induces fatal immunodeficiency disease in cats. Science. 1988;239:906–910. doi: 10.1126/science.2893454. [DOI] [PubMed] [Google Scholar]
- Pacitti AM, Jarrett O, Hay D. Transmission of feline leukemia virus in the milk of a non-viraemic cat. Veterinary Record. 1986;118:381–384. doi: 10.1136/vr.118.14.381. [DOI] [PubMed] [Google Scholar]
- Pedersen NC. Immunogenicity and efficacy of a commercial feline leukemia virus vaccine. Journal of Veterinary Internal Medicine. 1993;7:34–39. doi: 10.1111/j.1939-1676.1993.tb03166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatak M, Jr, Saag MS, Yang LC, Clark SJ, Kappes JC, Luk KC, Hahn BH, Shaw GM, Lifson JD. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science. 1993;259:1749–1754. doi: 10.1126/science.8096089. [DOI] [PubMed] [Google Scholar]
- Pollack RVH, Scarlett JM. Randomized blind trial of a commercial FeLV vaccine. Journal of the American Veterinary Medical Association. 1990;196:611–616. [PubMed] [Google Scholar]
- Pollock VH, Haffer KN. Review of the first feline leukemia virus vaccine. Journal of the American Veterinary Medical Association. 1991;199:1406–1409. [PubMed] [Google Scholar]
- Post JE, Warren L. Reactivation of latent feline leukemia virus. In: Hardy WD Jr, Essex M, McClelland AJ, editors. Feline Leukemia Virus. Elsevier North Holland, Inc; New York: 1980. pp. 151–155. [Google Scholar]
- Quackenbush SL, Dean GA, Mullins JI, Hoover EA. Analysis of FeLV-FAIDS provirus burden and productive infection in lymphocyte subsets in vivo. Virology. 1996a;223:1–9. doi: 10.1006/viro.1996.0449. [DOI] [PubMed] [Google Scholar]
- Quackenbush SL, Mullins JI, Hoover EA. Replication kinetics and cell tropism of an immunosuppressive feline leukaemia virus. Journal of General Virology. 1996b;77 (Pt 7):1411–1420. doi: 10.1099/0022-1317-77-7-1411. [DOI] [PubMed] [Google Scholar]
- Rickard CG, Post JE, Noronha F, Barr LM. A transmissible virus-induced lymphocytic leukemia of the cat. Journal of the National Cancer Institute. 1969;42:987–1014. [PubMed] [Google Scholar]
- Rojko JL, Hoover EA, Mathes LE, Olsen RG, Schaller JP. Pathogenesis of experimental feline leukemia virus infection. Journal of the National Cancer Institute. 1979;63:759–768. doi: 10.1093/jnci/63.3.759. [DOI] [PubMed] [Google Scholar]
- Rojko JL, Hoover EA, Quackenbush SL, Olsen RG. Reactivation of latent feline leukaemia virus infection. Nature. 1982;298:385–388. doi: 10.1038/298385a0. [DOI] [PubMed] [Google Scholar]
- Sato H, Orenstein J, Dimitrov D, Martin M. Cell-to-cell spread of HIV-1 occurs within minutes and may not involve the participation of virus particles. Virology. 1992;186:712–724. doi: 10.1016/0042-6822(92)90038-q. [DOI] [PubMed] [Google Scholar]
- Schaller JP, Hoover EA, Olsen RG. Active and passive immunization of cats with inactivated feline oncornaviruses. Journal of the National Cancer Institute. 1977;59:1441–1450. doi: 10.1093/jnci/59.5.1441. [DOI] [PubMed] [Google Scholar]
- Shibley GP, Tanner JE, Hanna SA. United States Department of Agriculture licensing requirements for feline leukemia virus vaccines. Journal of the American Veterinary Medical Association. 1991;199:1402–1406. [PubMed] [Google Scholar]
- Sparkes AH. Feline leukemia virus: a review of immunity and vaccination. Journal of Small Animal Practice. 1997;38:187–194. doi: 10.1111/j.1748-5827.1997.tb03339.x. [DOI] [PubMed] [Google Scholar]
- Tandon R, Cattori V, Gomes-Keller MA, Meli ML, Golder MC, Lutz H, Hofmann-Lehmann R. Quantitation of feline leukaemia virus viral and proviral loads by TaqMan real-time polymerase chain reaction. Journal of Virological Methods. 2005;130:124–132. doi: 10.1016/j.jviromet.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Tandon R, Cattori V, Pepin AC, Riond B, Meli ML, McDonald M, Doherr MG, Lutz H, Hofmann-Lehmann R. Association between endogenous feline leukemia virus loads and exogenous feline leukemia virus infection in domestic cats. Virus Research. 2008a;135:136–143. doi: 10.1016/j.virusres.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Tandon R, Cattori V, Willi B, Lutz H, Hofmann-Lehmann R. Quantification of endogenous and exogenous feline leukemia virus sequences by real-time PCR assays. Veterinary Immunology and Immunopathology. 2008b;123:129–133. doi: 10.1016/j.vetimm.2008.01.027. [DOI] [PubMed] [Google Scholar]
- Tandon R, Cattori V, Willi B, Meli ML, Gomes-Keller MA, Lutz H, Hofmann-Lehmann R. Copy number polymorphism of endogenous feline leukemia virus-like sequences. Molecular and Cellular Probes. 2007;21:257–266. doi: 10.1016/j.mcp.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Tizard I, Bass EP. Evaluation of a killed, whole virion feline leukemia virus vaccine. Journal of the American Veterinary Medical Association. 1991;199:1410–1413. [PubMed] [Google Scholar]
- Torres AN, Mathiason CK, Hoover EA. Re-examination of feline leukemia virus: host relationships using real-time PCR. Virology. 2005;332:272–283. doi: 10.1016/j.virol.2004.10.050. [DOI] [PubMed] [Google Scholar]
- Torres AN, O’Halloran KP, Larson LJ, Schultz RD, Hoover EA. Development and application of a quantitative real-time PCR assay to detect feline leukemia virus RNA. Journal of Veterinary Immunology and Immunopathology. 2008;123:81–89. doi: 10.1016/j.vetimm.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Lee CG, Buckler-White A, Kozak CA. Genetic control of a mouse serum lipoprotein factor that inactivates murine leukemia viruses: evaluation of apolipoprotein F as a candidate. Journal of Virology. 2002;76:2279–2286. doi: 10.1128/jvi.76.5.2279-2286.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]