Abstract
HLA-mismatched grafts are a viable alternative source for patients without HLA-matched donors receiving ablative hematopoietic cell transplantation (HCT), though their use in reduced intensity or nonmyeloablative conditioning HCT has been not well established. Here we extended HCT to recipients of HLA-class I mismatched grafts to test whether nonmyeloablative conditioning can establish stable donor engraftment. Fifty-nine patients were conditioned with fludarabine 90 mg/m2 and 2 Gy total body irradiation (TBI) followed by immunosuppression with cyclosporine 5.0 mg/kg twice and mycophenolate mofetil 15 mg/kg three times daily for transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood cells from related (n=5) or unrelated donors (n=54) with one antigen ± one allele HLA-class I mismatch or two HLA-class I allele mismatches. Sustained donor engraftment was observed in 95% of evaluable patients. The incidences of grades II to IV acute and extensive chronic graft-versus-host disease were 69% and 41%, respectively. The cumulative probability of non-relapse mortality was 47% at 2 years. Two-year overall and progression-free survivals were 29% and 28%, respectively. Nonmyeloablative conditioning with fludarabine and low-dose TBI followed by HCT using HLA-class I mismatched donors leads to successful engraftment and long-term survival; however, the high incidence of acute GVHD and NRM needs to be addressed by alternate GVHD prophylaxis regimens.
Keywords: nonmyeloablative allogeneic hematopoietic stem cell transplantation, HLA-class I mismatched donors, low-dose total body irradiation, fludarabine
INTRODUCTION
Allogeneic hematopoietic cell transplantation (HCT) from HLA-matched donors is a well established curative strategy for patients with hematopoietic malignancies; however, only 20–30% of patients in need of HCT have genotypically matched sibling donors. Although unrelated donor registries have grown enormously, potential donors cannot be identified for 20% of Caucasians and more than 60% of African-American patients. In addition, patients with rare haplotypes are unlikely to find HLA-matched unrelated donors in a timely fashion. There is a need for a suitable transplantation procedure that can extend the application of HCT with reduced intensity or nonmyeloablative conditioning to patients who have no readily available HLA-matched donor. Alternative sources including HLA-mismatched unrelated donor for HCT have been explored to extend the pool of donors [1,2].
The use of HLA-class I mismatched unrelated donors is associated with an increased risk of graft rejection in the myeloablative setting [3–5]. In addition, the intensity of conditioning for HCT is also associated with successful engraftment. Thus, the risk of graft rejection may increase in the HLA-mismatched nonmyeloablative setting compared to the myeloablative setting due to less intensive conditioning. It is not known what degree of mismatch would lead to unacceptable levels of rejection for patients receiving nonmyeloablative HCT.
Based on preclinical studies [6], we have successfully applied a nonmyeloablative conditioning regimen involving fludarabine 90 mg/m2, 2 Gy total body irradiation (TBI), and post-grafting immunosuppression with mycophenolate mofetil (MMF) and cyclosporine (CSP) as conditioning for grafts from either HLA-matched related or unrelated donors in more than 1200 patients who were ineligible for high-dose HCT because of advanced age or co-morbidities [7–12]. Here, in this multi-center phase I/II trial, we extended nonmyeloablative HCT to include recipients of related or unrelated G-CSF mobilized peripheral blood stem cell (PBSC) grafts from donors who were mismatched for one HLA-class I antigen with or without one allele level HLA-class I mismatches, or donors who were mismatched for two HLA-class I alleles. In addition, we evaluated immune reconstitution after HCT in a subset of the unrelated recipients.
PATIENTS, MATERIALS, AND METHODS
This phase I/II multi-center trial included seven transplant centers: Fred Hutchinson Cancer Research Center (FHCRC), University of Utah, University of Torino, Italy, Medical College of Wisconsin, LDS Hospital, Rocky Mountain Cancer Center and Veterans Affairs Puget Sound Health Care System, Seattle, with the FHCRC acting as the coordinating center. The protocol was approved by the Institutional Review Boards of the FHCRC and each of the collaborating performance sites. Written informed consent was obtained from all patients.
Study endpoints
The primary objective of this trial was to determine whether stable allogeneic engraftment from related and unrelated HLA-mismatched PBSC donors could be safely established using the nonmyeloablative fludarabine/2Gy TBI conditioning regimen with or without escalating doses of the anti-CD52 monoclonal antibody, alemtuzumab, in patients with hematological malignancies. Secondary objectives included evaluation of: 1) acute and chronic GVHD, 2) infections, 3) disease progression and relapse, and 4) immunological reconstitution.
Eligibility criteria
Patients enrolled on this study were ineligible for conventional HCT and had disease expected to be stable for at least 100 days without further chemotherapy. Forty-one patients were more than 50 years old. Eighteen patients 50 years old or less met the eligibility criteria either because of previous HCT (autologous, n=14; allogeneic, n=1), neurological toxicity (n=1), fungal infection and a history of major toxicity to chemotherapy (n=1), or pancytopenia for 3 months (n=1).
Histocompatibility testing for donor selection was performed for all patients and donors, using previously described methods [13–15]. HLA-A, B, C, DRB1 and DQB1 alleles were typed prospectively by oligonucleotide hybridization or by DNA sequencing methods [16]. Compatibility of the donor with the recipient was tested further by lymphocyte cross-match (patient serum vs. donor T and B cells) before HCT [17]. Related or unrelated donors were allowed if matched for HLA-DRB1 and DQB1 alleles, and mismatched for a single antigen at HLA-A, -B, or -C, with or without an additional single allele mismatch at HLA-A, -B, or –C. Donors were excluded if the recipient was homozygous at the mismatched locus, or if both mismatches were at the same locus (HLA-A, -B, or -C).
Patients were ineligible for the present study if they were pregnant or breast-feeding, or had rapidly progressive intermediate or high-grade non-Hodgkin’s lymphoma (NHL), circulating leukemic blasts in the peripheral blood detected by standard pathology, central nervous system involvement refractory to intrathecal chemotherapy, infection with the human immunodeficiency virus, active bacterial or fungal infection unresponsive to therapy, decompensated liver disease, corrected pulmonary diffusion capacity less than 35%, cardiac ejection fraction of less than 35%, poorly controlled hypertension, or a Karnofsky performance status less than 50%.
Patient characteristics
Fifty-nine patients were enrolled between February 2002 and October 2008 (Table 1). When classified for risk of relapse as previously described [18], 16, 27 and 16 patients had high, standard or low risk diseases, respectively. Disease status at the time of HCT included complete remission (CR) (n=29), partial remission (PR) (n=14), stable disease (n=4), and refractory or relapsed disease (n=12). Patients with acute myeloid leukemia (AML), secondary acute myeloid leukemia from myelodysplastic syndrome (MDS/AML), and acute lymphocytic leukemia were all in CR at the time of HCT. Four patients received planned tandem autologous transplant followed by nonmyeloablative HCT (auto-allo). The HCT specific comorbidity index (HCT-CI) [19] was available in 57 of 59 patients. Twelve patients had an HCT-CI score of 0, 21 had scores of 1 or 2, and 24 had scores of 3 or higher.
Table 1.
Patient and disease characteristics
| No. (range) | |
|---|---|
| Patient No. | 59 |
| Median Age (yrs) | 56 (13–72) |
| Male/female | 44/15 |
| Unrelated/related donor | 54/5 |
| Prior chemotherapy (courses) | 6 (2–19) |
| Prior transplant (autologous/allogeneic) | 26/1 |
| Median time to HCT (mo) | 36 (5–205) |
| The median doses of CD34+ cells of G-PBSC | 7.0 (2.1–37.7) ×106 cells/kg |
| The median doses of CD3+ cells of G-PBSC | 2.6 (0.3–6.6) ×108 cells/kg |
| Diagnosis | No. (disease status at pre-transplant) |
| NHL | 18 (CR2: 2, CR3: 2, PR: 9, stable: 1, refractory: 4) |
| HL | 5 (CR1: 1, PR: 2, refractory: 2) |
| ATL | 1 (CR1: 1) |
| AML | 16 (CR1: 6, CR2: 5, CR3: 4, CR4: 1) |
| ALL | 5 (CR1: 1, CR2: 1, CR3: 3) |
| CLL | 6 (stable 1, refractory 5) |
| MM | 7 (CR2: 1, PR: 3, stable: 2, refractory: 1) |
| MDS/AML | 1 (CR1: 1) |
| HLA mismatch | Total (related donor) |
| Both HVG and GVHD vectors | 51 (4) |
| GVHD vector | 8 (1) |
| 1-HLA antigen alone | 48 (5) |
| HLA-A/-B/-C | 20 (5)/5/23 |
| 1-HLA antigen+1-allele | 10 (0) |
| HLA-A/-B/-C (additional allele mismatch) | 2/4/4 |
| 2 allele-level | 1 |
ALL: acute lymphocytic leukemia; AML: acute myeloid leukemia; ATL: adult T cell leukemia/lymphoma, CLL: chronic lymphocytic leukemia; CML: chronic myeloid leukemia; CR: complete remission; G-PBSC: granulocyte stimulating factor-mobilized peripheral blood stem cell; GVHD: graft-versus-host disease;
HL: Hodgkin’s lymphoma; HVG: host-versus-graft; MDS/AML: secondary acute myeloid leukemia from myelodysplastic syndrome; MM: multiple myeloma; NHL: non-Hodgkin’s lymphoma; PR: partial remission.
HLA typing and matching
Final selection of unrelated donors was based on the results of high resolution HLA typing for HLA-A, -B, -C, -DRB1, -DQB1 alleles (Table 1). Seven unrelated pairs and one related pair were mismatched only in the GVHD vector and were not considered evaluable for the rejection endpoint. Fifty-four unrelated pairs were mismatched for at least one antigen (19 at HLA-A, 8 at HLA-B, 26 at HLA-C) except one patient who had two allele mismatches (HLA-A and HLA-B). All five related pairs were mismatched for one HLA-A antigen. Ten patients had, in addition to the class I antigen mismatch, one allelic mismatch (two at HLA-A, four at HLA-B, four at HLA-C).
Conditioning regimen and GVHD prophylaxis
Patients received fludarabine (30 mg/m2/day) on days −4, −3, and −2 before HCT and 2 Gy TBI at the rate of 0.07 Gy/minute from a linear accelerator on day 0. Postgrafting immunosuppression with oral CSP, starting at 5 mg/kg twice a day (BID), on day −3 and continued to day 180, then tapered to day 365. MMF was given orally at 15 mg/kg, based on adjusted body weight, (rounded to nearest 250 mg increment), every 8 hours from the evening of day 0 (4 to 6 hours after HCT infusion) and to day 100, and then tapered at approximately 11% weekly for 8 weeks. The taper was completed by day 156 unless GVHD occurred. When significant nausea or vomiting occurred during MMF treatment, MMF was administrated intravenously at the same dose. CSP levels were measured by immunoassay and doses were targeted to achieve trough levels of 500 ng/ml for the first 28 days and thereafter 150–450 ng/ml. CSP was also administrated intravenously in patients who were not able to take CSP orally.
Escalation of alemtuzumab
The initial study design incorporated a provision for the addition of low-dose alemtuzumab if more than one rejection was seen in either of the first two cohorts of 7 evaluable patients treated without alemtuzumab. Eleven patients were enrolled in the first cohort, of whom 7 were evaluable for the chimerism endpoint and all engrafted. In the second cohort, only 1 of 7 patients rejected the graft. Therefore, the protocol was amended to allow continued accrual without alemtuzumab. A total 59 patients were transplanted without alemtuzumab.
Collection of hematopoietic cells
All patients received granulocyte colony-stimulating factor (G-CSF)-mobilized PBSC grafts. All related donors received 16 μg/kg/day G-CSF by subcutaneous injection for 5 consecutive days before collection on days −1 and 0.
Collection of unrelated donor PBSC was arranged through the National Marrow Donor Program (NDMP) or international donor centers. G-CSF, 10 μg/kg, was administered by subcutaneous injection starting 5 days before day 0 according to the NDMP protocol.
Supportive care
All patients received standard prophylactic antibiotics with a third-generation cephalosporin or quinolone when absolute neutrophil counts declined to less than 500 μL. All patients received fluconazole (400 mg/day) from the start of conditioning to at least day 75 as prophylaxis for yeast infection. Trimethoprim-sulfamethoxazole was used as first-line prophylaxis against Pneumocystis jiroveci and dapsone (50 mg BID) as second line until day 180 or until discontinuation of immunosuppressive therapy. The varicella zoster virus prophylaxis (acyclovir 250 mg/m2 intravenously followed by 800 mg orally or valacyclovir 500 mg orally, BID) was performed until 1 year after HCT or 6 months after discontinuation of all immunosuppression. Preemptive treatment with ganciclovir was started during the first 100 days after HCT when CMV polymerase chain reaction or pp65 antigenemia for weekly CMV surveillance became positive. After day 100, surveillance and preemptive therapy were recommended for CMV intermediate and high-risk patients on a weekly or biweekly basis.
GVHD grading and treatment
Diagnosis and clinical grading of acute and chronic GVHD were done by local investigators according to established criteria [20,21]. In most cases, biopsies confirmed clinical diagnosis. Treatment of acute GVHD was typically by administering prednisone (1–2 mg/kg/day) and reinitiating CSP at full doses if it had been previously tapered or discontinued. Prednisone (1 mg/kg/day) and CSP (5.0 mg/kg orally BID) were used for the primary treatment of extensive chronic GVHD.
Treatment of persistent/progressive or relapsed malignancies
Substantial persistent disease at day 84 or disease progression at any time was an indication for therapeutic intervention. If there was no GVHD, MMF was stopped and CSP was tapered over 2 weeks or per attending physician discretion. If there was no response to stopping immunosuppression, chemotherapy or radiation therapy could be considered. Donor lymphocyte infusions were not offered on this trial.
Chimerism analyses
Chimerism analysis of peripheral blood T cell (CD3+), granulocyte (CD33+) and whole marrow were performed on days +28, +56 +84, + 180, +365 and then yearly after HCT as previously described [7]. For the purposes of this study, full donor T-cell chimerism was defined as more than 95% donor CD3+ T cells, and graft rejection was defined as the inability to detect at least 5% donor CD3+ T cells, as a proportion of the total T cell population in the peripheral blood after HCT. Mixed or full donor chimerism was considered as evidence of donor engraftment. Sustained engraftment was defined as continued evidence of donor engraftment up to day 84 evaluation without subsequent loss at later evaluations.
Immune reconstitution
Immune reconstitution was studied in nine FHCRC recipients of HLA-class I mismatched unrelated grafts. Peripheral blood samples were obtained before conditioning, immediately before HCT on day 0, and at 1, 3, 6, and 12 months after HCT. Mononuclear cells (MNCs) were separated from blood specimens, stained with fluorochrome-conjugated monoclonal antibodies, and analyzed by using 3-color flow cytometry as described [22,23].
Naive B cells were represented by IgD+ B cells, since most IgD+ B cells lack somatic mutations [24–26]. Naive CD4+ T cells were defined as CD45RAhigh CD4+ T cells since this subset contains thymic emigrants [27–29]. Naive CD8+ T cells were defined as CD11alow CD8+ T cells because virtually all cord blood CD8+ T cells are CD11alow and become CD11ahigh after activation [30,31]. CD28+ T cells represent cells that can receive both the signal mediated by the T-cell receptor and the CD28-mediated costimulatory signal. Monocytes were defined as CD14+ MNCs. Natural killer (NK) cells were defined as MNCs expressing CD16 or CD56 and not expressing CD3 or CD14. Dendritic cells (DC) were defined as HLA-DRhigh MNCs not expressing CD3, CD14, CD16, CD20, CD34, or CD56.
Statistical analysis
Survival curves were estimated by the Kaplan-Meier method. Cumulative incidence curves for acute and chronic GVHD and relapse treated death as a competing risk. Cumulative incidence curves for non-relapse mortality treated relapse as a competing risk. Comparative analyses of mortality and competing risk endpoints were performed via Cox regression; all p-values reflect likelihood ratio statistics from these models and are 2-sided.
RESULTS
Engraftment, chimerism and graft rejection
The median numbers of CD34+ and CD3+ cells in the grafts were 7.0 (range, 2.1–37.7) ×106 and 2.6 (range, 0.3–6.6) ×108 cells/kg recipient body weight, respectively. The median neutrophil nadir was 100 cells/μL (range, 0–860). The median duration of neutropenia (absolute neutrophil counts <0.5 ×103/μL) was 9 days (range 0–33), and 9% of patients did not experience neutropenia. The median platelet nadir was 23 ×103/μL (range, 4–110 ×103). The median duration of thrombocytopenia (platelet counts <20 ×103/μL) was 0 days (range 0–23), and 62% of patients did not develop thrombocytopenia.
The rates of full donor T-cell chimerism were 76% at day +28, 84% at day +56 and 81% at day +84 in evaluable patients. Sixteen patients were excluded for evaluation of the graft rejection outcome: four died early (less than 30 days after HCT), eight were transplanted from donors mismatched only in the GVHD vector, and four had received planned tandem auto-allo transplants. Four patients died at day 8, 16, 23 and 29 after HCT. The causes of death were non-relapse mortality (NRM) in two patients and disease progression in the other two patients. In one patient who died at day 8 after HCT, hematopoietic recovery was not observed.
Among the 43 patients eligible for evaluation of engraftment outcome, 77% and 92% of patients achieved full donor T-cell chimerism at days +28 and +56, respectively. Sustained engraftment was observed in 95% of evaluable patients
Overall, two patients (one with follicular lymphoma and the other with AML) experienced early graft rejection (day 56 and 84, respectively), and there were no late graft rejections. Both patients with early graft rejection were successfully transplanted again, one from the same donor with conditioning consisting of fludarabine and 4 Gy TBI, and the other patient from an alternative HLA-class I mismatched donor with fludarabine, 4 Gy TBI and alemtuzumab (total dose of 10 mg), respectively.
GVHD
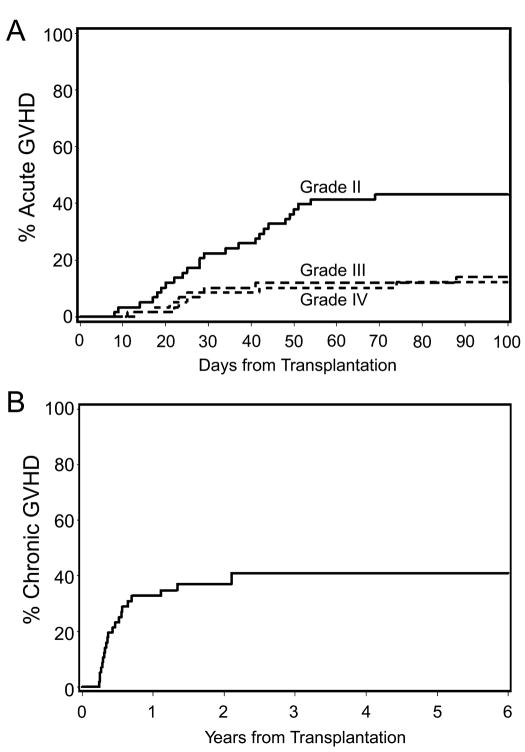
Forty-four of 57 patients (70%), excluding the two rejection patients, developed acute GVHD. Three of the 44 patients had grade I, 25 grade II, 8 grade III and 8 grade IV GVHD. The cumulative incidences of grades II to IV and grades III to IV acute GVHD were 69% and 26%, respectively (Figure 1A). Twenty one patients developed chronic GVHD, with a 3-year cumulative incidence of extensive chronic GVHD of 41% (Figure 1B).
Figure 1. Cumulative incidence of graft-versus-host disease (GVHD).
The cumulative incidence of grades II, III and IV acute GVHD (A) and clinical chronic extensive GVHD (B) are presented.
Toxicity and non-relapse mortality
Thirty-five patients (59%) experienced at least one non-hematopoietic grade III toxicity, and 20 (34%) had at least one non-hematopoietic grade IV toxicity. The most common grade III and IV non-hematopoietic toxicities were cardiovascular and pulmonary complications (Table 2). Grade IV cardiac toxicities included atrial fibrillation/flutter (n=3), elevated cardiac enzymes suggestive of ischemic events (n=3), pulmonary embolism (n=1), septic shock with kidney failure requiring dialysis (n=1), acute myocardial infarction (n=1), severe congestive heart failure (n=1), and acute vascular leak syndrome (n=1). Grade IV pulmonary toxicities consisted of hypoxia/apnea requiring intubation or pressure support (n=7) and acute respiratory distress syndrome (ARDS) (n=2). Grade IV gastrointestinal toxicity consisted of diarrhea or colitis associated with GVHD (n=6) or small bowel perforation (unknown etiology) (n=1).
Table 2.
Incidence of grade III and IV toxicities (n=59)
| No. episodes/No. patients (% of patients) |
||
|---|---|---|
| Grade III | Grade IV | |
| Cardiovascular | 16/16 (27) | 11/7 (12) |
| Pulmonary | 12/10 (17) | 9/8 (14) |
| Hepatic | 10/10 (17) | 5/5 (8) |
| Renal/Genitourinary | 6/6 (10) | 3/2 (3) |
| Gastrointestinal | 2/2 (3) | 7/7 (12) |
| Neurologic | 2/2 (3) | 2/2 (3) |
| Hemorrhage | 3/3 (5) | 0/0 (0) |
Infection rates were calculated based on infections within the first 100 days post-transplant or until death before day 100. The rates of documented viral (including cytomegalovirus reactivation), fungal and bacterial infection were 1.5, 0.3, and 2.1 per 100 patient days, respectively, with an overall infection rate of 3.9 per 100 patient days.
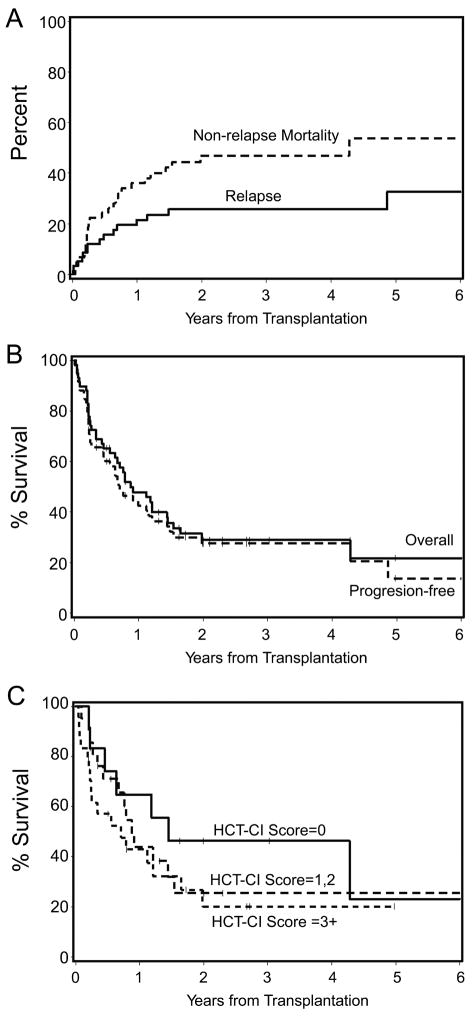
Overall, 26 of 59 patients died from non-relapse causes. The cumulative probabilities of NRM were 22% at day 100, 36% at 1 year and 47% at 2 years (Figure 2A). The cumulative incidences of NRM at 2 years for patients in CR and those not in CR at the time of transplant were 55% and 37%, respectively. The cumulative incidence of NRM for the patients with AML and MDS/AML was 56% at 2 years. Twenty patients died of non-relapse causes within 1 year after HCT. Causes of NRM within 1 year after HCT included infections associated with GVHD (n=6), multiorgan failure (n=3), infections without GVHD (n=2), GVHD (n=2), secondary AML (n=1), ARDS (n=1), bronchiolitis obliterans (n=1), diffuse alveolar damage (n=1), myocardial infarction (n=1), leukoencephalopathy (n=1), and congestive heart failure (n=1). Six patients died of NRM greater than 1 year after HCT. Causes of late NRM included infections associated with GVHD (n=2), infections without GVHD (n=2), cardiac and respiratory failure (n=1), and bronchiolitis obliterans organizing pneumonia (n=1). In 3 of the 6 cases, chronic GVHD contributed to the late death.
Figure 2.
Kaplan-Meier product estimates of non-relapse mortality and relapse (A), overall survival and progression-free survival (B), and overall survival as stratified by the HCT-CI (C).
Relapse and progression free survival
The cumulative probability of relapse/progression was 26% at 2 years (Figure 2A). Progression-free survival was 28% at 2 years (Figure 2B). The cumulative incidences of relapse/progression for patients in CR and patients not in CR at the time of HCT were 14% and 38% at 2 years, respectively. The cumulative incidence of relapse for the patients with AML and MDS/AML (who were all transplanted in CR) was 18% at 2 years. The 2-year probabilities of progression-free survival in high and standard/low risk disease were 26% and 28% (P=0.54), respectively.
Survival
Nineteen of 59 patients were alive a median of 24 (range, 2–79) months after transplant. Of the 19, 14 were in CR, 2 were in PR, 2 had stable disease, and 1 had relapsed. The 2-year overall survivals were 29% (Figure 2B). Grade III to IV acute GVHD adversely affected overall survival as time-dependent covariates in a Cox regression model (HR 8.35, 95%CI: 3.4–21, P< 0.0001).
The 2-year Kaplan-Meier probabilities of overall survival among patients receiving grafts from one HLA-class I antigen mismatched donors (n=48) and those with donors who were mismatched for 2 loci (n=11) were 26% and 42%, respectively (P=0.35). The 2-year probabilities of overall survival in high and standard/low-risk disease were 33% and 28%, respectively (P=0.92). Additionally, the 2-year survivals in the groups with an HCT-CI score of 0, scores of 1 or 2, and scores of 3 or higher were 46%, 26%, and 20%, respectively (P=0.46) (Figure 2C).
Immune reconstitution
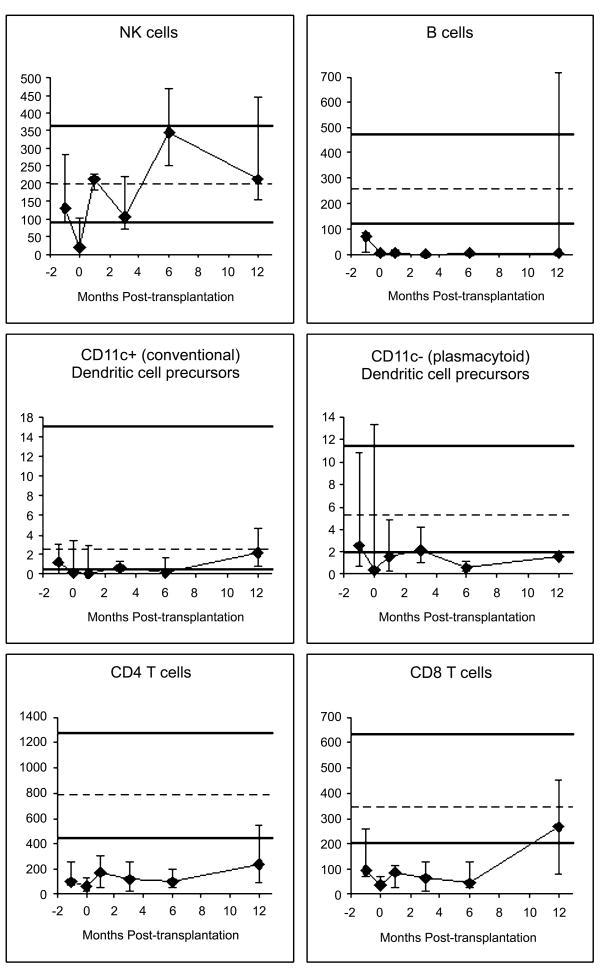
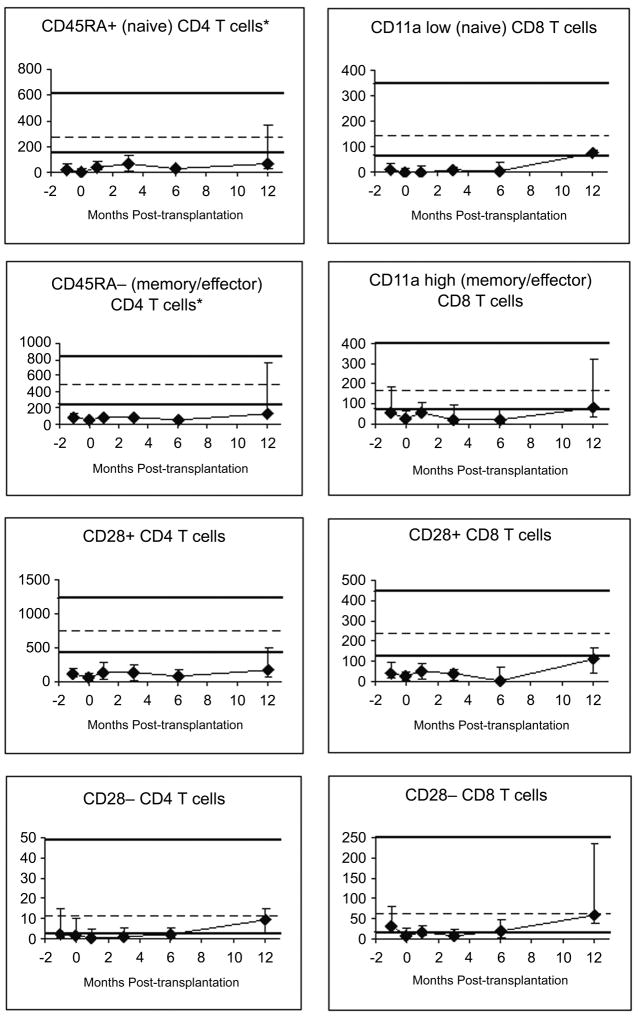
Innate immune cell (NK cells, neutrophils and monocytes) counts recovered within 1 month posttransplant (Figure 3A, data on neutrophils and monocytes not shown). DC recovery was slower than that of NK cells, neutrophils and monocytes, and more so for the plasmacytoid subset than for the conventional (“myeloid”) subset. T cells recovered slowly over several months. B-cell counts were typically still low at 12 months; this was true for both naive (surface IgD+) and memory (surface IgD−) B cells (data not shown). CD8+ T cell counts reached the normal range between 6 and 12 months, whereas CD4+T-cell counts were still low at 12 months. Both naive and memory/effector T cells recovered slowly (Figure 3B). CD28− T cells recovered faster than CD28+ T cells (Figure 3B).
Figure 3A and B. Recovery of lymphocyte subsets.
All y-axes show the number of cells per microliter of blood. Patient medians (diamonds) and 25th–75th percentiles (error bars) are displayed. Normal medians (of 104 normal adults) are indicated by the dashed horizontal lines; the thick horizontal lines denote the normal 10th–90th percentiles. Pre-transplant studies are arbitrarily shown as performed using blood collected at 1-month pre-transplant, despite the blood was drawn at any time between 1 month pre-transplant and the morning of the day of starting conditioning. Day 0 studies were performed using blood drawn immediately before graft infusion. The following numbers of patient blood samples were analyzed: nine samples pre-transplant, seven on day 0, six at 1 month, eight at 3 months, six at 6 months and five samples at 12 months post-transplant.
DISCUSSION
In studies of patients who receive myeloablative conditioning and unrelated marrow grafts, the incidence of graft rejection was higher among patients given grafts mismatched for one or more class I HLA antigens [5]. We sought to determine whether graft rejection was increased when class I HLA-mismatched grafts were transplanted after nonmyeloablative conditioning and found that sustained donor engraftment was observed in 95% of recipients. Although initially designed as a dose escalation trial of alemtuzumab according to the number of patients experiencing graft rejection, the dose-escalation rule was never activated. Thus the combination of fludarabine and 2 Gy TBI, followed by a post-transplant combination of MMF given three times a day (TID) and CSP, was sufficient to ensure a high rate of sustained full donor T-cell chimerism and engraftment even with HLA-class I mismatched grafts.
We previously had used 15 mg/kg MMF BID administrated from day 0 to day 40 with taper through day 96 in combination with CSP as GVHD prophylaxis after nonmyeloablative HLA-matched unrelated donor HCT. However, a high graft rejection rate of 21% was observed. By increasing the dosing of MMF to 15 mg/kg TID (because of the short half-life (t½) of only 3 hours of the active metabolite of MMF) [7] and using only G-PBSC as a stem cell source, we successfully reduced the rejection rate to 5 % in nonmyeloablative HCT from HLA-matched unrelated donors [32]. Therefore, in the current study we used MMF 15 mg/kg TID and extended the duration of MMF given until day 100 for the HLA-mismatched setting. As a result, sustained engraftment was observed in 95% of evaluable patients without the use of alemtuzumab.
We were unable to detect any enhancement of graft-versus-tumor effects with the use of HLA-mismatched unrelated grafts. Observed cumulative probabilities of relapse/progression of 22% at 1 year and 36% at 2 years were very similar to our previous HLA-matched unrelated HCT data (26% at 1 year and 31% at 2 years) [32]. If limited to patients not in CR at HCT, the CR rate in this study [8 of 30 (27%)] was lower rather than 48% of the previous our study.
An association between HLA-class I mismatches and a high incidence of GVHD has been described in recipients of ablative conditioning regimens followed by unrelated donor HCT [33–35]. Previous studies reported that the cumulative incidences of grades II to IV acute GVHD varied from 43% to 74% after myeloablative HCT with HLA-matched unrelated marrow and 63% to 80% after myeloablative HCT with HLA-one antigen mismatched unrelated marrow [36–39]. Chronic GVHD was observed in more than 55% of patients after myeloablative HCT with HLA-matched unrelated marrow and in 70% of patients after myeloablative HCT with HLA-one antigen mismatched unrelated marrow [21,36–39]. In a previous study using the same nonmyeloablative conditioning regimen (fludarabine and 2 Gy TBI), 103 patients received either 10/10 HLA-matched (n=93) or single HLA class I allele-level mismatched (single each; n=10) unrelated donor HCT. In that study, we reported cumulative incidences of grades II to IV acute and chronic extensive GVHD were 53% and 56%, respectively [32]. Interestingly, the observed 41% incidence of chronic extensive GVHD in the current study is comparable to or less than that observed in the HLA-matched unrelated setting. The prolonged CSP and MMF administration in this study may have contributed to the similar incidence of chronic GVHD. On the other hand, the observed 69% rate of grades II to IV acute GVHD is higher than that seen in the HLA-matched unrelated setting [32]. The cumulative probability of NRM of 47% at 2 years in the current study is also higher than that seen in the HLA-matched unrelated setting. Additionally, in the current study acute GVHD contributed to death in 9 of 26 non-relapse deaths (35%), and 6 of 7 (86%) grade IV gastrointestinal episodes were associated with gut GVHD which directly or indirectly caused mortality.
As might be expected, there was concern that the prolonged CSP and MMF administration in the present protocol caused an increased risk of infection. Indeed, the incidence of infection was increased compared to our previous results in unrelated HCT (the documented rates per 100 patients days of viral, fungal, and bacterial infection were 0.86, 0.26, and 1.05, respectively, in the previous study [32]). In 12 of 26 (46%) patients, the causes of NRM were associated with infection.
Since excess immunosuppression can lead to high rates of relapse and infection due to delayed immune reconstitution, an optimal prophylactic regimen for GVHD in HLA-mismatched or unrelated HCT has been explored by other investigators. Alemtuzumab (total 50, 100 mg/person or 1.2 mg/kg) has been applied in HLA haploidentical related, and HLA-matched and mismatched unrelated HCT [40–43]. Some investigators reported that alemtuzumab-containing regimens were highly effective in preventing chronic as well as acute GVHD without an increased risk of relapse [40,41]. However, high relapse rates, particularly in patients with active disease at HCT (5 of 8 patients [63%]) [42] and high infection rates (serious infective complication 62.2%) [43] due to a delay in immune reconstitution were also reported. Furthermore, in a recent publication, we reported that HLA-haploidentical nonmyeloablative related HCT with high-dose posttransplantation cyclophosphamide showed an acceptable sustained engraftment rate of more than 95% and better control of acute GVHD (34% for grades II–IV and 6% for grades II–IV 6%) without increased severe infection. However, a relatively high relapse rate of 51% at 1 year was seen [44]. In our previous retrospective study, extensive chronic GVHD but not grade II to IV acute GVHD was significantly associated with a decreased risk of relapse or progression without increased NRM [45]. These data indicate that an intensified prophylaxis of acute GVHD but not chronic GVHD by an additional immunosuppressive agent such as low-dose alemtuzumab [46] or sirolimus [47] may be a reasonable strategy to improve outcomes.
The observed overall survivals in all the three groups stratified by HCT-CI score were inferior to recipients of HLA-matched related and/or unrelated HCT. These data suggested that HLA-class I disparity could be another independent outcome factor in the nonmyeloablative setting.
Immune reconstitution was similar to that after HLA-matched unrelated donor nonmyeloablative HCT [48], with the exception of slower recovery of CD8+ T cells and B cells. Both naive and memory/effector CD8+ T cells recovered slowly, suggesting both reduced thymopoiesis as well as reduced peripheral expansion, possibly due to the fact that many patients had GVHD (clinical or subclinical) and were treated with prolonged immunosuppression drugs. The slow recovery of B cells may also be due to the fact that many patients had GVHD, as GVHD and/or its treatment hamper B-lymphopoiesis [49,50]. The slow recovery of CD8+ T cells and B cells might have contributed to the relatively high infection rates.
In conclusion, this study demonstrated the feasibility of nonmyeloablative HCT from HLA-class I mismatched donors using fludarabine and low-dose TBI conditioning. While almost 30% of patients experienced long-term survival with this approach, there was a high incidence of acute GVHD and NRM decreased survival compared to fully HLA-matched unrelated patients. Future studies of more intense early prophylaxis of GVHD in this HLA-class I mismatched setting may decrease severe acute GVHD and improve survival.
Acknowledgments
Grant support: This study was supported by grants CA 18029 and CA15704 from the National Cancer Institute and HL36444 from the National Heart, Lung, and Blood Institute. Study also supported in part by a research grant from Berlex, Inc. Hirohisa Nakamae is funded by the Graduate School of Medicine, Osaka City University, Osaka, Japan. Jan Storek is funded by Canada Research Chairs Program and Alberta Heritage Foundation for Medical Research. Benedetto Bruno is funded by Regione Piemonte: Ricerca Finalizzata 2005, 2006, 2007.
The authors wish to thank the research nurses Michelle Bouvier and Hsien-Tzu Chen, data manager Gresford Thomas, and trial coordinator Jennifer Freese for their invaluable help in making the study possible. The authors are grateful to Mohamed L Sorror, MD for HCT-CI scores and Mika Nakamae, MD for review of data. The authors also wish to thank Helen Crawford, Bonnie Larson and Sue Carbonneau for manuscript preparation, and the transplant teams, physicians, nurses, and support personnel for their care of patients on this study.
Footnotes
Authors’ conflicts of interest: None of the authors have any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Finke J, Schmoor C, Lang H, Potthoff K, Bertz H. Matched and mismatched allogeneic stem-cell transplantation from unrelated donors using combined graft-versus-host disease prophylaxis including rabbit anti-T lymphocyte globulin. J Clin Oncol. 2003;21:506–513. doi: 10.1200/JCO.2003.03.129. [DOI] [PubMed] [Google Scholar]
- 2.Sedlacek P, Formankova R, Keslova P, et al. Low mortality of children undergoing hematopoietic stem cell transplantation from 7 to 8/10 human leukocyte antigen allele-matched unrelated donors with the use of antithymocyte globulin. Bone Marrow Transplant. 2006;38:745–750. doi: 10.1038/sj.bmt.1705524. [DOI] [PubMed] [Google Scholar]
- 3.Morishima Y, Sasazuki T, Inoko H, et al. The clinical significance of human leukocyte antigen (HLA) allele compatibility in patients receiving a marrow transplant from serologically HLA-A, HLA-B, and HLA-DR matched unrelated donors. Blood. 2002;99:4200–4206. doi: 10.1182/blood.v99.11.4200. [DOI] [PubMed] [Google Scholar]
- 4.Petersdorf EW, Gooley TA, Anasetti C, et al. Optimizing outcome after unrelated marrow transplantation by comprehensive matching of HLA class I and II alleles in the donor and recipient. Blood. 1998;92:3515–3520. [PubMed] [Google Scholar]
- 5.Petersdorf EW, Hansen JA, Martin PJ, et al. Major-histocompatibility-complex class I alleles and antigens in hematopoietic-cell transplantation. N Engl J Med. 2001;345:1794–1800. doi: 10.1056/NEJMoa011826. [DOI] [PubMed] [Google Scholar]
- 6.Storb R, Yu C, Wagner JL, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048–3054. [PubMed] [Google Scholar]
- 7.Maris MB, Niederwieser D, Sandmaier BM, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102:2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 8.Niederwieser D, Maris M, Shizuru JA, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101:1620–1629. doi: 10.1182/blood-2002-05-1340. [DOI] [PubMed] [Google Scholar]
- 9.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 10.Feinstein L, Sandmaier B, Maloney D, et al. Nonmyeloablative hematopoietic cell transplantation: replacing high-dose cytotoxic therapy by the graft-versus-tumor effect. Ann NY Acad Sci. 2001;938:328–339. [PubMed] [Google Scholar]
- 11.Maris MB, Sandmaier BM, Storer BE, et al. Allogeneic hematopoietic cell transplantation after fludarabine and 2 Gy total body irradiation for relapsed and refractory mantle cell lymphoma. Blood. 2004;104:3535–3542. doi: 10.1182/blood-2004-06-2275. [DOI] [PubMed] [Google Scholar]
- 12.Sorror ML, Maris MB, Sandmaier BM, et al. Hematopoietic cell transplantation after nonmyeloablative conditioning for advanced chronic lymphocytic leukemia. J Clin Oncol. 2005;23:3819–3829. doi: 10.1200/JCO.2005.04.569. [DOI] [PubMed] [Google Scholar]
- 13.Hansen JA, Mickelson EM, Choo SY, et al. Clinical bone marrow transplantation: Donor selection and recipient monitoring. In: Rose NR, De Macario EC, Fahey JL, Friedman H, Penn GM, editors. Manual of Clinical Laboratory Immunology. Washington: American Society for Microbiology; 1992. pp. 850–866. [Google Scholar]
- 14.Dupont B, Yang SY. Histocompatibility. In: Forman SJ, Blume KG, Thomas ED, editors. Bone Marrow Transplantation. Boston: Blackwell Scientific Publications; 1994. pp. 22–40. [Google Scholar]
- 15.Petersdorf EW, Smith AG, Mickelson EM, Martin PJ, Hansen JA. Ten HLA-DR4 alleles defined by sequence polymorphisms within the DRB1 first domain. Immunogenetics. 1991;33:267–275. doi: 10.1007/BF00230505. [DOI] [PubMed] [Google Scholar]
- 16.Petersdorf EW, Anasetti C, Martin PJ, et al. Limits of HLA mismatching in unrelated hematopoietic cell transplantation. Blood. 2004;104:2976–2980. doi: 10.1182/blood-2004-04-1674. [DOI] [PubMed] [Google Scholar]
- 17.Anasetti C, Amos D, Beatty PG, et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma. N Engl J Med. 1989;320:197–204. doi: 10.1056/NEJM198901263200401. [DOI] [PubMed] [Google Scholar]
- 18.Kahl C, Storer BE, Sandmaier BM, et al. Relapse risk among patients with malignant diseases given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2007;110:2744–2748. doi: 10.1182/blood-2007-03-078592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 21.Sullivan KM, Agura E, Anasetti C, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250–259. [PubMed] [Google Scholar]
- 22.Storek J, Dawson MA, Storer B, et al. Immune reconstitution after allogeneic marrow transplantation compared with blood stem cell transplantation. Blood. 2001;97:3380–3389. doi: 10.1182/blood.v97.11.3380. [DOI] [PubMed] [Google Scholar]
- 23.Storek J, Zhao Z, Lin E, et al. Recovery from and consequences of severe iatrogenic lymphopenia (induced to treat autoimmune diseases) Clinical Immunology. 2004;113:285–298. doi: 10.1016/j.clim.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein U, Kuppers R, Rajewsky K. Human IgM+IgD+ B cells, the major B cell subset in the peripheral blood, express V kappa genes with no or little somatic mutation throughout life. Eur J Immunol. 1993;23:3272–3277. doi: 10.1002/eji.1830231232. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki I, Milner ECB, Glas AM, et al. Immunoglobulin heavy chain variable region gene usage in bone marrow transplant recipients: Lack of somatic mutation indicates a maturational arrest. Blood. 1996;87:1873–1880. [PubMed] [Google Scholar]
- 26.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–1689. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 28.Dumont-Girard F, Roux E, van Lier RA, et al. Reconstitution of the T-cell compartment after bone marrow transplantation: restoration of the repertoire by thymic emigrants. Blood. 1998;92:4464–4471. [PubMed] [Google Scholar]
- 29.Storek J, Witherspoon RP, Storb R. T cell reconstitution after bone marrow transplantation into adult patients does not resemble T cell development in early life. Bone Marrow Transplant. 1995;16:413–425. [PubMed] [Google Scholar]
- 30.Okumura M, Fujii Y, Inada K, Nakahara K, Matsuda H. Both CD45RA+ and CD45RA− subpopulations of CD8+ T cells contain cells with high levels of lymphocyte function-associated antigen-1 expression, a phenotype of primed T cells. J Immunol. 1993;150:429–437. [PubMed] [Google Scholar]
- 31.Okumura M, Fujii Y, Takeuchi Y, Inada K, Nakahara K, Matsuda H. Age-related accumulation of LFA-1high cells in a CD8+CD45RAhigh T cell population. Eur J Immunol. 1993;23:1057–1063. doi: 10.1002/eji.1830230512. [DOI] [PubMed] [Google Scholar]
- 32.Maris MB, Sandmaier BM, Storer BE, et al. Unrelated donor granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cell transplantation after nonmyeloablative conditioning: the effect of postgrafting mycophenolate mofetil dosing. Biol Blood Marrow Transplant. 2006;12:454–465. doi: 10.1016/j.bbmt.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 33.Sasazuki T, Juji T, Morishima Y, et al. Effect of matching of class I HLA alleles on clinical outcome after transplantation of hematopoietic stem cells from an unrelated donor. N Engl J Med. 1998;339:1177–1185. doi: 10.1056/NEJM199810223391701. [DOI] [PubMed] [Google Scholar]
- 34.Flomenberg N, Baxter-Lowe LA, Confer D, et al. Impact of HLA class I and class II high resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplant outcome. Blood. 2004;104:1923–1930. doi: 10.1182/blood-2004-03-0803. [DOI] [PubMed] [Google Scholar]
- 35.Greinix HT, Fae I, Schneider B, et al. Impact of HLA class I high-resolution mismatches on chronic graft-versus-host disease and survival of patients given hematopoietic stem cell grafts from unrelated donors. Bone Marrow Transplant. 2005;35:57–62. doi: 10.1038/sj.bmt.1704741. [DOI] [PubMed] [Google Scholar]
- 36.Kernan NA, Bartsch G, Ash RC, et al. Analysis of 462 transplantations from unrelated donors facilitated by The National Marrow Donor Program. N Engl J Med. 1993;328:593–602. doi: 10.1056/NEJM199303043280901. [DOI] [PubMed] [Google Scholar]
- 37.McGlave P, Bartsch G, Anasetti C, et al. Unrelated donor marrow transplantation therapy for chronic myelogenous leukemia: initial experience of the National Marrow Donor Program. Blood. 1993;81:543–550. [PubMed] [Google Scholar]
- 38.Szydlo R, Goldman JM, Klein JP, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997;15:1767–1777. doi: 10.1200/JCO.1997.15.5.1767. [DOI] [PubMed] [Google Scholar]
- 39.Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068. [PubMed] [Google Scholar]
- 40.Das-Gupta EP, Russell NH, Shaw BE, Pearce RM, Byrne JL. Long-term outcome of unrelated donor transplantation for AML using myeloablative conditioning incorporating pretransplant Alemtuzumab. Biol Blood Marrow Transplant. 2007;13:724–733. doi: 10.1016/j.bbmt.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 41.Thomson KJ, Morris EC, Bloor A, et al. Favorable long-term survival after reduced-intensity allogeneic transplantation for multiple-relapse aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27:426–432. doi: 10.1200/JCO.2008.17.3328. [DOI] [PubMed] [Google Scholar]
- 42.Kanda Y, Oshima K, Asano-Mori Y, et al. In vivo alemtuzumab enables haploidentical human leukocyte antigen-mismatched hematopoietic stem-cell transplantation without ex vivo graft manipulation. Transplantation. 2005;79:1351–1357. doi: 10.1097/01.tp.0000158718.49286.14. [DOI] [PubMed] [Google Scholar]
- 43.Chakraverty R, Peggs K, Chopra R, et al. Limiting transplantation-related mortality following unrelated donor stem cell transplantion by using a nonmyeloablative conditioning regimen. Blood. 2002;99:1071–1078. doi: 10.1182/blood.v99.3.1071. [DOI] [PubMed] [Google Scholar]
- 44.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, post-transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baron F, Maris MB, Sandmaier BM, et al. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol. 2005;23:1993–2003. doi: 10.1200/JCO.2005.08.136. [DOI] [PubMed] [Google Scholar]
- 46.Morris E, Thomson K, Craddock C, et al. Outcomes after alemtuzumab-containing reduced-intensity allogeneic transplantation regimen for relapsed and refractory non-Hodgkin lymphoma. Blood. 2004;104:3865–3871. doi: 10.1182/blood-2004-03-1105. [DOI] [PubMed] [Google Scholar]
- 47.Ho VT, Aldridge J, Kim HT, et al. Comparison of tacrolimus and sirolimus (Tac/Sir) versus tacrolimus, sirolimus, and mini-methotrexate (Tac/Sir/MTX) as acute graft-versus-host disease prophylaxis after reduced-intensity conditioning allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:844–850. doi: 10.1016/j.bbmt.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baron F, Storer B, Maris MB, et al. Unrelated donor status and high donor age independently affect immunologic recovery after nonmyeloablative conditioning. Biol Blood Marrow Transplant. 2006;12:1176–1187. doi: 10.1016/j.bbmt.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 49.Storek J, Witherspoon RP, Webb D, Storb R. Lack of B cell precursors in marrow transplant recipients with chronic graft-versus-host disease. Am J Hematol. 1996;52:82–89. doi: 10.1002/(SICI)1096-8652(199606)52:2<82::AID-AJH3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 50.Storek J, Wells D, Dawson MA, Storer B, Maloney DG. Factors influencing B-lymphopoiesis after allogeneic hematopoietic cell transplantation (Brief Report) Blood. 2001;98:489–491. doi: 10.1182/blood.v98.2.489. [DOI] [PubMed] [Google Scholar]