Abstract
Studies suggest that patients who live in rural areas may have worse clinical outcomes compared with patients living in urban areas. We studied whether place of residence (rural vs. urban) is associated with clinical outcomes of patients with leukemia or myelodysplastic syndrome who received an unrelated donor hematopoietic-cell transplantation (HCT). Patients’ residential ZIP code at the time of transplant was used to determine rural or urban designation based on the Rural Urban Commuting Codes. The study included 6140 patients reported to the Center for International Blood and Marrow Transplant Research from 121 US HCT centers: 1179 (19%) came from rural areas while 4961 (81%) came from urban areas. Rural and urban patients were similar in patient-, disease- and transplant-related characteristics aside from household income and distance travelled to HCT center. After adjusting for income and other significant patient, disease and transplant-related variables, the risk of overall mortality between patients residing in rural and urban areas were not statistically significant (relative risk 1.01, 95% confidence intervals 0.93–1.10, p=0.74). Similar outcomes were noted for transplant-related mortality, disease-free survival and relapse. Patient’s income, derived from US Census and based on their residential ZIP code, was independently associated with outcomes. In summary, our study showed no differences in the clinical outcomes of patients from rural or urban areas after unrelated donor HCT.
Keywords: Rural, Socioeconomic status, Unrelated donor hematopoietic cell transplantation
INTRODUCTION
Hematopoietic-cell transplantation (HCT) is a complex treatment procedure for various malignant and non-malignant hematological disorders.1–5 Patients who undergo allogeneic HCT often experience post-transplant complications which may lead to prolonged hospitalizations, readmissions or even death. Because of the high-risk nature and intensive resource dependence of allogeneic HCT, not all facilities are able to offer this treatment modality. Patients who may benefit from allogeneic HCT are often referred to larger treatment centers. In the United States, transplant centers are usually located in metropolitan or urban areas and attract a wide range of patients, including many who come from small towns and rural areas. Transplanted patients often stay in the hospital or close to the transplant center for an extended time during the peri-transplant period. Patients from rural areas or small towns usually return to their communities and referring healthcare providers for follow-up, usually after a period of observation and medical management when appropriate. These healthcare providers may or may not be oncologists and may not have the expertise in detecting complications that usually occur post-HCT.
Studies have shown that rural residents must travel from 2 to 10 times the distance of their urban counterparts to access advanced care, including HCT.6–8 Physician shortages also force many of these patients to travel great distances for specific care related to their post-transplant follow-up.9,10 Additionally, rural patients often have lower income than their urban counterparts, all of which may contribute to poor follow-up care. Rao et al. noted that patients from rural areas who received autologous HCT from a single Midwest center had a higher risk of death than urban patients who underwent the same procedure. They noted a 5% lower survival rate at 1 and 5 years post-HCT among patients from rural areas compared to patients from urban areas.8 However, they failed to detect a significant difference in the risk of death according to primary area of residence in the HLA-identical sibling HCT cohort, although this may have been due to lack of statistical power. It is not known if the characteristics and clinical outcomes of patients with acute or chronic leukemia who underwent unrelated HCT differ according to place of residence. We hypothesized that patients who live in rural areas may not be able to receive optimal post-transplant care when needed which may predispose them to have inferior clinical outcomes.
METHODS
Data source
The CIBMTR is a research affiliation of the International Bone Marrow Transplant Registry (IBMTR), Autologous Blood and Marrow Transplant Registry (ABMTR) and the National Marrow Donor Program (NMDP) that comprises a voluntary working group of more than 450 transplant centers worldwide that contribute detailed data on consecutive allogeneic and autologous HCT to a Statistical Center at the Medical College of Wisconsin in Milwaukee and the NMDP Coordinating Center in Minneapolis. Participating centers are required to report all transplants consecutively; compliance is monitored by on-site audits. Patients are followed longitudinally, with yearly follow-up. Computerized checks for errors, physicians’ review of submitted data and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are done so with a waiver of informed consent and in compliance with HIPAA regulations as determined by the Institutional Review Board and the Privacy Officer of the Medical College of Wisconsin.
Subjects
Patients who received an unrelated donor allogeneic HCT with a myeloablative preparative regimen using either a bone marrow or peripheral blood stem cell source for acute myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myeloid leukemia (CML) or myelodysplastic syndrome (MDS) between 1995 and 2004 were included in the study. The study was further limited to transplant centers located within the continental USA and to patients with available residential postal ZIP codes; 67 patients with missing ZIP codes were excluded.
All surviving recipients included in this analysis were retrospectively contacted and provided informed consent for participation in the NMDP research program. Informed consent was waived by the NMDP Institutional Review Board for all deceased recipients. Approximately 10% of surviving patients would not provide consent for use of research data. To adjust for the potential bias introduced by exclusion of non-consenting surviving patients, a corrective action plan modeling process randomly excluded appropriately the same percentage of deceased patients (n=534) using a biased coin randomization with exclusion probabilities based on characteristics associated with not providing consent for use of the data in survivors.11 The final study cohort consisted of 6140 patients representing 121 US transplant centers (Table 1).
Table 1.
Characteristics of U.S. patients who received unrelated myeloablative transplants for AML, ALL, CML and MDS from 1995–2004.
| Variable | Rural N (%) | Urban N (%) | P-value |
|---|---|---|---|
| Patient related | |||
| Number of patients | 1179 | 4961 | |
| Number of centers | 97 | 121 | |
| Age, median (range), years | 34 (<1–67) | 33 (<1–70) | 0.61 |
| Age at transplant, years | 0.96 | ||
| <1–9 | 131 (11) | 583 (12) | |
| 10–19 | 169 (14) | 700 (14) | |
| 20–29 | 195 (17) | 828 (17) | |
| 30–39 | 238 (20) | 1024 (21) | |
| 40–49 | 284 (24) | 1143 (23) | |
| ≥ 50 | 162 (14) | 683 (14) | |
| Male sex | 659 (56) | 2844 (57) | 0.37 |
| Race/Ethnicity | < 0.001 | ||
| White | 1103 (94) | 4091 (82) | |
| African-American | 42 (4) | 324 (7) | |
| Asian/Pacific Islander | 7 (1) | 131 (3) | |
| Hispanic | 27 (2) | 415 (8) | |
| Karnofsky status | 0.04 | ||
| <90 | 323 (27) | 1221 (25) | |
| ≥ 90 | 796 (68) | 3415 (69) | |
| Missing | 60 (5) | 325 (7) | |
| Median income, 2000 | $34,199 | $47,727 | <0.001 |
| Range | $11,667–63,864 | $21,109–200,001 | |
| Missing | 96 | 474 | |
| Comorbid conditions | 0.64 | ||
| 0–1 comorbidities | 1085 (92) | 4545 (92) | |
| ≥ 2 comorbidities | 94 (8) | 416 (8) | |
| Disease related | |||
| Disease | 0.17 | ||
| AML | 419 (36) | 1632 (33) | |
| ALL | 287 (24) | 1289 (26) | |
| CML | 331 (28) | 1490 (30) | |
| MDS | 142 (12) | 550 (11) | |
| Disease status at transplant | 0.71 | ||
| Early | 436 (37) | 1920 (39) | |
| Intermediate | 379 (32) | 1530 (31) | |
| Advanced | 327 (28) | 1364 (27) | |
| Unknown | 37 (3) | 147 (3) | |
| Transplant related | |||
| Time from diagnosis to transplant, median (range), months | 11 (2–309) | 11 (1–309) | 0.72 |
| Missing | 2 | ||
| Graft type | 0.37 | ||
| Bone Marrow | 976 (83) | 4051 (82) | |
| Peripheral blood | 203 (17) | 910 (18) | |
| Infused cell dose | |||
| Bone Marrow > 2 × 108 | 591 (61) | 2484 (62) | 0.61 |
| Bone Marrow 2 × 108 | 374 (39) | 1534 (38) | |
| Missing | 11 | 33 | |
| Peripheral Blood > 5 × 108 | 126 (65) | 571 (68) | 0.25 |
| Peripheral Blood 5 × 108 | 68 (35) | 272 (32) | |
| Missing | 9 | 67 | |
| HLA match status | 0.15 | ||
| Well-matched | 493 (42) | 2147 (43) | |
| Partially matched | 461 (39) | 1793 (36) | |
| Mismatched | 225 (19) | 1021 (21) | |
| Sex match (Donor/Recipient) | 0.43 | ||
| Male/Male | 437 (37) | 1877 (38) | |
| Male/Female | 298 (25) | 1142 (23) | |
| Female/Male | 222 (19) | 967 (19) | |
| Female/Female | 222 (19) | 975 (20) | |
| CMV match (Donor/Recipient | 0.11 | ||
| Negative/Negative | 412 (35) | 1641 (33) | |
| Negative/Positive | 356 (30) | 1393 (28) | |
| Positive/Negative | 161 (14) | 809 (16) | |
| Positive/Positive | 231 (20) | 1032 (21) | |
| Unknown | 19 (2) | 86 (2) | |
| Conditioning regimen | 0.36 | ||
| Bu + Cy ± other | 217 (18) | 955 (19) | |
| Cy + TBI ± other | 867 (74) | 3535 (71) | |
| TBI ± other | 42 (4) | 181 (4) | |
| Other | 53 (5) | 290 (6) | |
| Year of transplant | 0.34 | ||
| 1995–1999 | 610 (52) | 2490 (50) | |
| 2000–2004 | 569 (48) | 2471 (50) | |
| Distance to center, median (range), miles | 124 (8–4207) | 43 (<1–5194) | <0.001 |
| Distance to center | <0.001 | ||
| <17 | 3 (<1) | 1346 (27) | |
| 17–55 | 143 (12) | 1358 (27) | |
| 56–150 | 547 (46) | 999 (20) | |
| >150 | 484 (41) | 1248 (25) | |
| Missing | 2 (<1) | 10 (<1) | |
| Regiona | <0.001 | ||
| New England | 49 (4) | 332 (7) | |
| Mid-Atlantic | 95 (8) | 651 (13) | |
| South Atlantic | 132 (11) | 580 (12) | |
| East North Central | 201 (17) | 778 (16) | |
| East South Central | 136 (12) | 196 (4) | |
| West North Central | 238 (20) | 371 (7) | |
| West South Central | 146 (12) | 625 (13) | |
| Mountain | 26 (2) | 147 (3) | |
| Pacific | 156 (13) | 1281 (26) | |
| Donor search time, median (range), months | |||
| Diagnosis to preliminary search | 3 (<1–300) | 3 (<1–303) | 0.90 |
| Preliminary search to formal search | <1 (<1–77) | <1 (<1–116) | 0.06 |
| Formal search to transplant | 3 (<1–71) | 3 (<1–125) | <0.001 |
| Median follow-up of survivors, months | 63 (5–136) | 61 (3–138) | |
States comprising geographic region of transplant center:
– New England = ME, NH, VT, MA, RI, CT
– Mid-Atlantic = NY, NJ, PA
– South Atlantic = DE, MD, DC, VA, WV, NC, SC, GA, FL
– East North Central = OH, IN IL, MI, WI
– East South Central = KY, TN, AL, MS
– West North Central = MN, IA, MO, ND, SD, NE, KS
– West South Central = AR, LA, OK, TX
– Mountain = MT, ID, WY, CO, NM, AZ, UT, NV
– Pacific = WA, OR, CA, AK, HI
Study Variables
Place of residence was defined according to the Rural Urban Commuting Area (RUCA) code assigned to the ZIP code of the patient’s primary residence at the time of transplant.12 The RUCA classification was dichotomized into urban or rural designations. The RUCA defines patients’ location as either urban (≥ 50,000 residents), large rural (10,000 to 49,000 residents), small rural (2,500 to 9,999 residents), or isolated (< 2,499 residents) based on the Census Bureau’s definitions of Urbanized Areas and Urban Clusters, which in turn, rely on complex criteria including population density and population work commuting patterns. The RUCA classification system is based on the size of cities and towns and their functional relationships as measured by work commuting. For this study, rural areas included those from large rural, small rural and isolated. Information about patient race was center-reported and categorized according to the US Office of Management and Budget classification as White, African-American, Hispanic or Asian/Pacific-Islander. Patient income was estimated by the mean household income at the ZIP code level based on the 2004 US Census. The distance between the patient’s residence and the transplanting center were approximated using the Haversine approximation on the latitude and longitude of the ZIP Code.13 The package “ZIP Code deluxe”14 was used to obtain income and location data from the ZIP Code.
Disease status at transplant was classified as early, intermediate or advanced. Early disease included AML and ALL in first complete remission, CML in first chronic phase, and MDS with refractory anemia or refractory anemia with ringed sideroblasts. AML and ALL in second or greater remission or CML in accelerated phase or second or greater chronic phase was categorized as intermediate disease. Patients with advanced disease had AML and ALL in relapse or primary induction failure, CML in blast phase, or MDS with refractory anemia with excess blasts or excess blasts in transformation.
The NMDP classification of HLA matching status based on best available resolution of typing was used to categorize HLA matching status as well-matched, partially-matched or mismatched.15 Briefly, well-matched patients had no identified mismatches at HLA-A, -B, -C and -DRB1 with low/intermediate or high resolution data available at HLA-A, -B and high resolution -DRB1. Partially-matched patients had a single locus mismatch at any of the 4 loci and/or missing HLA-C data. Mismatched patients had 2 or more allele or antigen mismatches.
Outcomes and study definitions
The primary outcome of interest in this study is overall survival, (OS) defined as death from any cause. Additional outcomes evaluated included disease-free survival (DFS), relapse and treatment-related mortality (TRM). DFS was defined as survival in complete remission after HCT. Relapse was defined as disease recurrence at any site, with TRM as a competing risk. TRM was defined as death in complete remission with relapse as a competing risk.
Statistical analysis
Patient-, disease- and HCT- related characteristics were compared according to rural or urban distinction using the Chi-square statistic for categorical variables or Kruskal-Wallis test for continuous variables. Probabilities of OS and DFS were calculated using the Kaplan-Meier method. Probabilities of TRM and relapse were calculated using the cumulative-incidence function method. OS, DFS, TRM and relapse were estimated from the time of transplant.
Cox proportional hazards regression analyses were used to examine the association between place of residence and study outcomes. The following models were built to examine the association between place of residence and outcomes: 1) unadjusted model with place of residence or income alone as covariate; 2) place of residence with other statistically significant patient-, disease-, and transplant-related factors; and 3) model number 2 with income included. All variables were first examined to assure that they complied with the proportional hazards assumption. The final multivariate models were built using a forward stepwise model selection approach. Factors significant at an alpha of 5% were kept in the final model; all p-values are two-sided. The multivariate models for DFS and TRM were stratified on Karnofsky Score. Separate models limited to centers that had at least 5 rural and 5 urban patients were also performed to investigate whether volume effects could account for the findings. These models produced similar results and are not presented here. In addition, the effect of transplant center was tested in each model; the results were again similar to the analysis presented here and are therefore not included. Analyses were performed using SAS software, version 9.1 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
Table 1 shows the comparison of patient-, disease- and transplant-related characteristics according to patients’ primary area of residence. Of the 6140 patients included in the study, 1179 (19%) came from rural areas while 4961 (81%) came from urban areas. Of the 121 centers included in this study, 97 (80%) performed HCT on patients who came from rural areas. The median proportion of patients coming from rural areas was 20% per center (range 1% – 74%). Patients from rural areas were more likely than patients from urban areas to be white (94% vs. 82%), have lower median income ($34,000 vs. $48,000), and travel longer distances to the transplant center (124 miles vs. 43 miles). Rural patients were also more likely to be transplanted in the areas commonly referred to as the US Midwest region (East North and South Central, West North Central areas).
Outcome Analysis
Table 2A shows the analysis evaluating the association between place of residence and risk of death post-transplant. In the unadjusted model, rural patients were 9% more likely to die than urban patients [Relative Risk (RR) 1.09, 95% confidence interval (CI) 1.01–1.18, p = 0.02]. This association remained statistically significant after adjusting for important patient, disease and transplant-related variables (RR 1.08, 95% CI 1.00–1.17, p=0.05). However, when the models were adjusted for income in addition to other prognostic variables, the association between place of residence and risk of death was no longer significant (RR 1.01, 95% CI 0.93 – 1.10, p = 0.75). Tables 2B, 3A and 3B show no statistically significant association between place of residence and risk of TRM, treatment-failure and relapse in both unadjusted and adjusted models. Figure 1 shows the Kaplan Meier plot of the probability of survival and Figure 2 shows the cumulative incidence of TRM according to place of residence.
Table 2.
Adjusted and unadjusted models evaluating the association between place of residence and (A) Overall survival and (B) treatment-related mortality
| A. Overall Survival | ||||||
|---|---|---|---|---|---|---|
| Main Variable | Unadjusted model | Adjusted for Incomea | Adjusted for Patient, Disease and Tx Charb | Adjusted for Patient, Disease and Tx Char and Incomea,b | ||
| Urban | 1.00 | 1.00 | 1.00 | 1.00 | ||
| Rural | 1.09 (1.01–1.18) | 1.00 (0.92–1.09) | 1.08 (1.00–1.17) | 1.01 (0.93–1.10) | ||
| p=0.02 | p=0.99 | p=0.05 | p=0.75 | |||
| B. Treatment-related mortalityc | ||||||
| Main Variable | Unadjusted model | Adjusted for Incomea | Adjusted for Patient, Disease and Tx Charb | Adjusted for Patient, Disease and Tx Char and Incomea,b | ||
| Urban | 1.00 | 1.00 | 1.00 | 1.00 | ||
| Rural | 1.08 (0.98–1.18) | 0.98 (0.88–1.08) | 1.08 (0.98–1.18) | 1.00 (0.91–1.11) | ||
| p=0.10 | p=0.66 | p=0.11 | p=0.94 | |||
Income entered as quartiles: <$34,700, 34,700–43,600, 46,600–56,300, >$56,300
Adjusted for graft type and cell dose, HLA-match, patient age, disease type, disease stage, number of co-morbidities, CMV donor-recipient match, race/ethnicity, and year of transplant
Model stratified for Karnofsky performance score
Table 3.
Adjusted and unadjusted models evaluating the association between place of residence and (A) Disease-free survival and (B) Relapse
| A. Disease-free survivalc | |||||
|---|---|---|---|---|---|
| Main Variable | Unadjusted model | Adjusted for Incomea | Adjusted for Patient, Disease and Tx Charb | Adjusted for Patient, Disease and Tx Char and Incomea,b | |
| Urban | 1.00 | 1.00 | 1.00 | 1.00 | |
| Rural | 1.06 (0.98–1.14) | 0.97 (0.89–1.05) | 1.05 (0.97–1.14) | 0.99 (0.91–1.07) | |
| p=0.15 | p=0.48 | p=0.20 | p=0.76 | ||
| B. Relapse | |||||
| Main Variable | Unadjusted model | Adjusted for Incomea | Adjusted for Patient, Disease and Tx Charb | Adjusted for Patient, Disease and Tx Char and Incomea,b | |
| Urban | 1.00 | 1.00 | 1.00 | 1.00 | |
| Rural | 1.04 (0.90–1.18) | 0.98 (0.85–1.13) | 1.02 (0.89–1.16) | 0.97 (0.83–1.12) | |
| p=0.61 | p=0.78 | p=0.83 | p=0.66 | ||
Income entered as quartiles: <$34,700, 34,700–43,600, 46,600–56,300, >$56,300
Adjusted for graft type and cell dose, HLA-match, patient age, disease type, disease stage, number of co-morbidities, CMV donor-recipient match, race/ethnicity, and year of transplant
Model stratified for Karnofsky performance score
Figure 1.
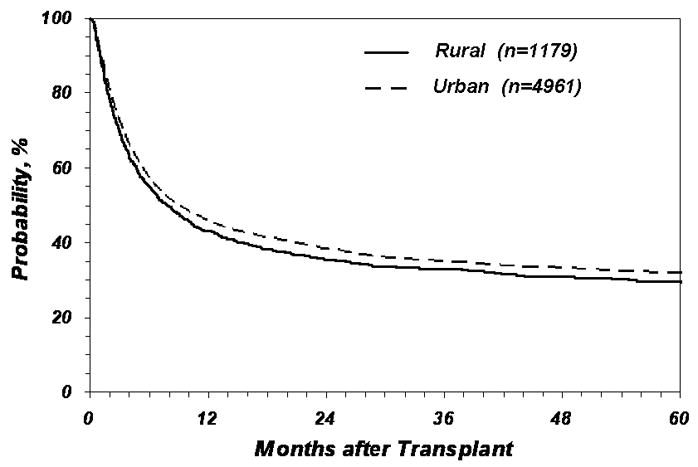
Probability of overall survival according to place of residence
Figure 2.
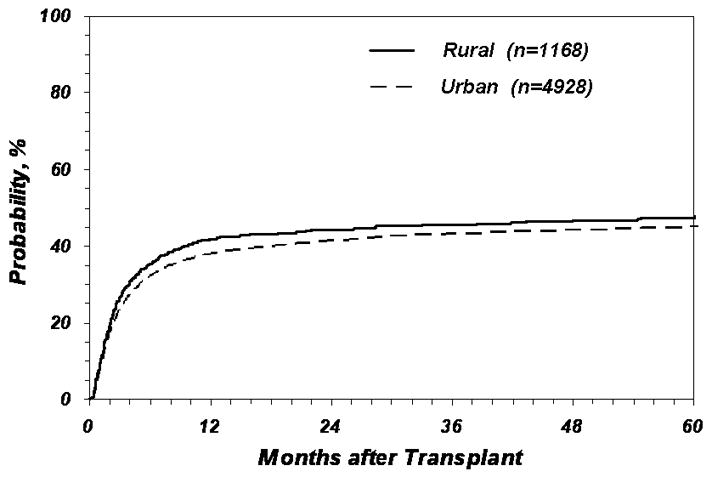
Probability of treatment-related mortality according to place of residence
Causes of Death
Table 4 shows the primary causes of death reported by HCT centers according to area of residence. The most common cause of death regardless of area of residence was disease recurrence (25%), followed by infection (20%) and multi-organ failure (18%). There is a slightly higher proportion of patients from rural areas who died from infection (all etiologies), 22% vs 19%, p = 0.05. Other causes of death appear to be similar in rates between patients from rural and urban areas.
Table 4.
Causes of death according to place of residence
| Cause of death | Rural | Urban | p-value |
|---|---|---|---|
| Graft rejection or failure | 16 (2) | 67 (2) | 0.88 |
| Infection | 183 (22) | 636 (19) | 0.05 |
| Interstitial pneumonia | 108 (13) | 402 (12) | 0.45 |
| Acute GVHD | 65 (8) | 323 (10) | 0.10 |
| Chronic GVHD | 45 (5) | 176 (5) | 0.86 |
| Recurrence or persistence of primary disease | 210 (25) | 824 (25) | 0.72 |
| Organ failure | 137 (17) | 598 (18) | 0.34 |
| Secondary malignancy | 8 (1) | 32 (1) | 0.99 |
| Hemorrhage | 31 (4) | 163 (5) | 0.16 |
| Other | 21 (3) | 91 (3) | 0.75 |
DISCUSSION
The possibility of healthcare disparities according to place of residence raises concerns about equitable access. In general, health care access has two fundamental elements: 1) ability of individuals to get care when needed in a reasonable time frame and 2) once under care, the ability of the systems to move patients across providers and through the stages of care (coordination of care) to assure good outcomes. 16,17 Although these concepts are familiar, there are very few data regarding disparities in access to care in the setting of HCT. Our study was able to examine some aspects of the second element of access: do the characteristics and clinical outcomes of patients who were able to receive unrelated HCT differ according to place of residence? Our study is the largest evaluation of whether rural or urban residence is associated with outcomes of unrelated donor HCT. We found that except for household income and distance travelled to transplant centers, rural patients who undergo unrelated HCT are generally comparable to their urban counterparts in terms of clinical outcomes. Rural patients are also similar to urban patients with respect to donor search times (preliminary to formal search), time from diagnosis to transplant, and all parameters related to the actual transplant (graft type, cell dose, level of HLA matching, etc.); all of which may have some prognostic significance. Given these overwhelming similarities, it is reassuring that there were no differences in overall survival and TRM between rural and urban patients when adjustments are made for income, disease and transplant factors.
A previous single center study8 also noted similarities in the clinical characteristics of patients who undergo HCT according to place of residence. This study also documented survival differences between rural and urban autologous patients but not recipients of HLA-identical sibling HCT. One reason why outcomes may differ in autologous transplantation but not allogeneic transplantation according to place of residence is that most patients who receive unrelated HCT and HLA-identical sibling HCT remain under the care of the transplanting physician for an extended period of time. This is not the case with autologous HCT recipients who return more quickly to their referring physicians. It is also possible that rural patients who are able to access unrelated donor HCT have resources similar to those of urban patients but are different than rural patients who were not able to access HCT
While rural/urban designation was not associated with outcome, income was associated with survival and transplant-related mortality. Other studies in different cancer populations have implicated the prognostic role of income rather consistently.18–21 Our study is not able to explore further the interaction between the two factors as both rural-urban distinction and estimated income were derived from the same ZIP code. Ideally, actual income and rural/urban designation should be collected directly from patients rather than relying on ZIP code to better explore these factors. Given the impact of income in all of the clinical outcomes studied, future studies may have to consider this socio-economic factor. Other factors linked with supportive care, including availability of caregivers, may have some mediating impact on clinical outcomes. Our study was also not able to separate patients whose HCT were paid for by Medicaid as this information is not available.
While our study represents the largest cohort to evaluate rural-urban disparity in the setting of unrelated HCT, it is limited by the retrospective observational study design. Our study was not able to address the question of whether rural patients who are likely to benefit from HCT are able to receive this treatment modality similarly to patients who reside in urban areas. This is probably the most important question regarding access that current available data is not able to answer. Our study is also not able to explore possible reasons why ZIP code-derived income was associated with outcome. However it is important to note that income may be used as a means to identify vulnerable populations who may be at risk for developing inferior outcomes. Additional studies are needed to look more closely at the process of delivery of care, including follow-up care, according to income levels and area of residence to begin to understand potential causes for inferior outcomes.
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Aetna; American Society for Blood and Marrow Transplantation; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US, Inc.; Baxter International, Inc.; Bayer HealthCare Pharmaceuticals; Be the Match Foundation; Biogen IDEC; BioMarin Pharmaceutical, Inc.; Biovitrum AB; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Canadian Blood and Marrow Transplant Group; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Centers for Disease Control and Prevention; Children’s Leukemia Research Association; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Cubist Pharmaceuticals; Cylex Inc.; CytoTherm; DOR BioPharma, Inc.; Dynal Biotech, an Invitrogen Company; Eisai, Inc.; Enzon Pharmaceuticals, Inc.; European Group for Blood and Marrow Transplantation; Gamida Cell, Ltd.; GE Healthcare; Genentech, Inc.; Genzyme Corporation; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery Co., Ltd.; The Leukemia & Lymphoma Society; Merck & Company; The Medical College of Wisconsin; MGI Pharma, Inc.; Michigan Community Blood Centers; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Nature Publishing Group; New York Blood Center; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Pall Life Sciences; PDL BioPharma, Inc; Pfizer Inc; Pharmion Corporation; Saladax Biomedical, Inc.; Schering Corporation; Society for Healthcare Epidemiology of America; StemCyte, Inc.; StemSoft Software, Inc.; Sysmex America, Inc.; Teva Pharmaceutical Industries;; THERAKOS, Inc.; Thermogenesis Corporation; Vidacare Corporation; Vion Pharmaceuticals, Inc.; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Appelbaum FR. The use of bone marrow and peripheral blood stem cell transplantation in the treatment of cancer. CA: A Cancer Journal for Clinicians. 1996;46:142–164. doi: 10.3322/canjclin.46.3.142. [DOI] [PubMed] [Google Scholar]
- 2.Bortin MM, Horowitz MM, Rimm AA. Increasing utilization of allogeneic bone marrow transplantation. Results of the 1988–1990 survey. Annals of Internal Medicine. 1992;116:505–512. doi: 10.7326/0003-4819-116-6-505. [DOI] [PubMed] [Google Scholar]
- 3.Gratwohl A, Passweg J, Baldomero H, et al. Hematopoietic stem cell transplantation activity in Europe 1999. Bone Marrow Transplant. 2001;27:899–916. doi: 10.1038/sj.bmt.1702995. [DOI] [PubMed] [Google Scholar]
- 4.Passweg JR, Rowlings PA, Armitage JO. Report from the International Bone Marrow Transplant Registry and Autologous Blood and Marrow Transplant Registry-North America. In: Cecka JM, Teraski PI, editors. Clinical Transplants. Los Angeles, CA: UCLA Tissue Typing Laboratory; 1996. pp. 117–127. [PubMed] [Google Scholar]
- 5.Eapen M. Report on state of the art in blood and marrow transplantation. International Bone Marrow Transplant Registry/Autologous Blood and Marrow Transplant Registry Newsletter. 2002;9:4–11. [Google Scholar]
- 6.Edelman MA, Menz BL. Selected comparison and implications of a national rural and urban survey on health care access, demographics, and policy issues. Journal of Rural Health. 1996;12:197–205. doi: 10.1111/j.1748-0361.1996.tb00794.x. [DOI] [PubMed] [Google Scholar]
- 7.Goodman DC, Fisher E, Stukel TA, et al. The distance to Community Medical Care and Likelihood of Hospitalization: Is closer always better? American Journal of Public Health. 1997;87:1144–1150. doi: 10.2105/ajph.87.7.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao K, Darrington D, Schumacher JJ, et al. Disparity in survival outcome after hematopoietic stem cell transplantation for hematologic malignancies according to area of primary residence. Biol Blood Marrow Transplant. 2007;13(12):1508–14. doi: 10.1016/j.bbmt.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Chan L, Hart LG, Goodman DC. Geographic access to health care for rural Medicare beneficiaries. Journal of Rural Health. 2006;22:140–146. doi: 10.1111/j.1748-0361.2006.00022.x. [DOI] [PubMed] [Google Scholar]
- 10.Martin-Mcdonald K, Rogers-Clark C, Hegney D, et al. Experiences of regional and rural people with cancer being treated with radiotherapy in a metropolitan center. International Journal of Nurse Practitioner. 2003;9:176–182. doi: 10.1046/j.1440-172x.2003.00421.x. [DOI] [PubMed] [Google Scholar]
- 11.Farag SS, Bacigalupo A, Eapen M, et al. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol Blood Marrow Transplant. 2006;12:876–884. doi: 10.1016/j.bbmt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Hart LG, Larsen EH, Lishner DM. Rural Definitions for Health Policy and Research. American Journal of Public Health. 2005;95(7):1149–1155. doi: 10.2105/AJPH.2004.042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinnott R. Virtues of the Haversine. Sky and Telescope. 1984;68:159. [Google Scholar]
- 14.Zipcode.com. US Zip Code Database - Deluxe database specifications. http://www.zip-code.com.
- 15.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aday LA, Anderson R. Development of Indices of Access to Medical Care. Ann Arbor, Michigan: Health Administration Press; 1975. [Google Scholar]
- 17.Shortell S. Continuity of Medical Care: Conceptualization and measurement. Medical Care. 1976;14:377–391. doi: 10.1097/00005650-197605000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Franzini L, Williams AF, Franklin J, Singletary SE, Theriault RL. Effects of race and socioeconomic status on survival of 1,332 black, Hispanic, and white women with breast cancer. Ann Surg Oncol. 1997;4:111–118. doi: 10.1007/BF02303792. [DOI] [PubMed] [Google Scholar]
- 19.Zell JA, Cinar P, Mobasher M, Ziogas A, Meyskens FL, Jr, Anton-Culver H. Survival for patients with invasive cutaneous melanoma among ethnic groups: the effects of socioeconomic status and treatment. J Clin Oncol. 2008;26:66–75. doi: 10.1200/JCO.2007.12.3604. [DOI] [PubMed] [Google Scholar]
- 20.Zell JA, Rhee JM, Ziogas A, Lipkin SM, Anton-Culver H. Race, socioeconomic status, treatment, and survival time among pancreatic cancer cases in California. Cancer Epidemiol Biomarkers Prev. 2007;16:546–552. doi: 10.1158/1055-9965.EPI-06-0893. [DOI] [PubMed] [Google Scholar]
- 21.Mackillop WJ, Zhang-Salomons J, Groome PA, Paszat L, Holowaty E. Socioeconomic status and cancer survival in Ontario. J Clin Oncol. 1997;15:1680–1689. doi: 10.1200/JCO.1997.15.4.1680. [DOI] [PubMed] [Google Scholar]


