Abstract
Songbirds produce high rates of song within multiple social contexts, suggesting that they are highly motivated to sing and that song production itself may be rewarding. Progress has been made in understanding the neural basis of song learning and sensorimotor processing, however little is known about neurobiological mechanisms regulating the motivation to sing. Neural systems involved in motivation and reward have been conserved across species and in songbirds are neuroanatomically well-positioned to influence the song control system. Opioid neuropeptides within these systems play a primary role in hedonic reward, at least in mammals. In songbirds, opioid neuropeptides and receptors are found throughout the song control system and within several brain regions implicated in both motivation and reward, including the medial preoptic nucleus (POM) and ventral tegmental area (VTA). Growing research shows these regions to play a role in birdsong that differs depending upon whether song is sexually-motivated in response to a female, used for territorial defense or sung as part of a flock but not directed towards an individual (undirected song). Opioid pharmacological manipulations and immunocytochemical data demonstrate a role for opioid activity possibly within VTA and POM in the regulation of song production. Although future research is needed, data suggest that opioids may be most critically involved in reinforcing song that does not result in any obvious form of immediate externally-mediated reinforcement, such as undirected song produced in large flocks or during song learning. Data are reviewed supporting the idea that dopamine activity underlies the motivation or drive to sing, but that opioid release is what makes song production rewarding.
Keywords: Reward, reinforcement, vocal communication, incentive salience, dopamine, hedonics, birdsong
For songbirds, the production of song at key developmental points and within specific social contexts is crucial for reproductive success. A majority of research on the neurobiology of birdsong is focused on sensorimotor aspects of song learning and production (see multiple reviews in Zeigler and Marler, 2004). This emphasis has revealed much about the “song control system” a discrete group of interconnected brain regions devoted to the acquisition and production of song motor patterns. However much less attention has been devoted to understanding what activates the song control system or how the brain regulates the motivation to sing. Songbirds appear highly motivated to sing within specific contexts, and it is possible that the act of singing itself is reinforcing. There is little to suggest that the motivation to sing rests within components of the song control system. Instead data suggest that highly conserved neural systems known to underlie motivation and reward interact with the song control system to ensure that a bird sings an appropriate song within an appropriate social context.
The pleasure or hedonic value associated with a particular stimulus is a powerful behavioral directive, guiding animals to engage in behaviors that are highly adaptive and critical for reproductive success, such as feeding, social or sexual behavior. Opioid neuropeptides serve as natural rewards and have been implicated in affect and social behaviors, including vocal communication (reviewed in Panksepp et al., 2004). In the present review data are presented that suggest opioids may also play an important role in motivation and reward associated with song production. The first half of this review is devoted to defining motivation and reward, the neural systems that underlie motivation and reward, and data supporting the idea that the act of song production is rewarding. Once this interpretive framework has been established, the second half of this review focuses in detail on what is known about opioid participation in song production and a case is made that opioids play a crucial, possibly context-specific role in the motivation to sing.
Context differences in the motivation to sing
Birds sing at high rates within distinct social contexts, including during song acquisition, when in large social flocks, and when singing to attract a mate or to repel a competitor (Catchpole and Slater, 1995b). What motivates a male to sing can differ depending upon both hormonal state and social context. Interestingly, what serves to reinforce or reward song may also differ context-dependently. I will emphasize in this review two motivationally and functionally distinct types of song which can be used to tease apart the role of motivation and reward systems in song production: sexually-motivated and undirected song.
What motivates song differs context-dependently
In both seasonally breeding and opportunistically breeding songbirds, males with high testosterone (T) often respond to the introduction of a female conspecific with high rates of song production accompanied by courtship displays (Ball et al., 2008; Catchpole and Slater, 1995b; Wingfield and Farner, 1993). In some species, after a male attracts and pairs with an individual female for the breeding season, a general decrease is observed in song output (e.g., European starlings (Sturnus vulgaris); Cuthill and Hindmarsh, 1985; Eens et al., 1994). However, males often resume singing at high rates immediately prior to each copulation (Eens and Pinxten, 1990; Eens and Pinxten, 1995; Pinxten and Eens, 1997). Song directed towards females by males with high T concentrations can be considered highly sexually-motivated and possibly goal-directed (with the goal of attracting a mate).
In contrast to sexually-motivated song, male songbirds also sing at high rates for purposes other than mate attraction. For example, some seasonally breeding songbirds sing at high rates outside of the breeding season, when T concentrations are low, as part of large communal flocks (e.g., song sparrows (Melospiza melodia) and European starlings; Riters et al., 2000; Smith et al., 1997). However, at this time males do not sing at high rates in response to the introduction of a female (e.g., European starlings; Riters et al., 2000). Exactly what motivates birds to sing in large flocks is not clear. Song is not directed towards individual conspecifics, but may be important for the maintenance of social contact, dominance rank or song learning (e.g., white-throated sparrows (Zonotrichia albicollis) and European starlings; Catchpole and Slater, 1995a; Hausberger et al., 1995; Wiley et al., 1993). Opportunistically breeding songbirds, such as male zebra finches (Taeniopygia guttata), also sing at high rates while in large social flocks. Song in these groups can be sexually-motivated and directed towards a female; however, males also sing high rates of song that is not directed towards a specific individual. This form of song is common outside the breeding season, is generally ignored by potential recipients, not used to immediately attract females or repel competitors, and not clearly directed towards a specific individual (Dunn and Zann, 1996; Zann and Bamford, 1996). In zebra finches this type of song is often described as undirected (Dunn and Zann, 1996; Jarvis et al., 1998), and I will adopt this terminology here.
What rewards song may also differ context-dependently
Observations of songbirds also suggest that what rewards a male for singing may differ depending upon the context in which song is produced. Specifically, sexually-motivated song often results in immediate reinforcement in the form of copulation. Thus song in this context may be rewarded to a large extent through attraction of a female and neurochemicals released in association with copulation. Unlike sexually-motivated song, undirected song is not immediately followed by any obvious form of externally-mediated reinforcement, yet males continue to sing at high rates, suggesting song may be tightly linked to internal reward systems. Overall, given that song during the breeding season can be highly sexually motivated and reinforced by copulation, brain regions involved in sexual motivation and incentive drive (i.e., motivated behavior stimulated by an external factor, or incentive) are likely to participate in song produced within this context. In contrast, although undirected song is produced at high rates, no clear song eliciting incentive has been identified, thus brain areas involved in sexual motivation or incentive drive would not be expected to play as critical a role in the regulation of song in this context. One possibility is that song in this context is maintained primarily through internal reinforcement. If true, it would be expected that neural reward systems would be especially important during undirected song production.
The neural regulation of motivation and reward
The neural basis of motivation and reward has been intensely studied in rodent model systems. These studies reveal highly evolutionarily conserved neural circuits and neurochemicals to underlie motivation and reward associated with feeding, sexual behavior, and the use of drugs of abuse. Although dopamine activity has received a majority of research attention, opioid activity within the mesolimbic system also plays an important role in motivation and reward (Agmo and Gomez, 1991; Agmo and Gomez, 1993; Devine and Wise, 1994; Smith and Berridge, 2007). This system originates in the ventral tegmental area and includes projections to the nucleus accumbens, amygdala, hippocampus, septum and bed nucleus of the stria terminalis. The involvement of other dopamine and opioid rich neural systems has received less attention; however, the incertohypothalamic system, which includes projections from the zona incerta to the medial preoptic area and paraventricular nucleus, and reciprocally connects with nuclei of the mesolimbic system, also plays an important role in motivation and reward (Band and Hull, 1990; Hull et al., 1995; Hull et al., 1999; Moses et al., 1995; Panzica et al., 1996; Pfaus and Phillips, 1991; van Furth et al., 1995a). These systems have been highly conserved and are similar in birds and mammals (Bharati and Goodson, 2006; Goodson, 2005; and as reviewed below).
Motivation and reward are tightly linked, yet distinct components of behavior. For example, if a behavior such as song production is associated with reward (i.e. pleasure or hedonic value), this behavior is more likely to be repeated. Should the reward be great, the animal is likely to express the behavior at high rates and produce the behavior even in the face of obstacles, reflecting a high level of motivation. Based on studies of drugs of abuse and naturally rewarded behaviors such as feeding and sexual behavior in rats, it appears that although closely related, reward and motivation involve distinct neurochemical modulators. The neurotransmitter dopamine has long been implicated in reward; however, mounting evidence suggests that dopamine is not the neurotransmitter underlying “liking” or reward per se (that is, it does not underlie pleasure or hedonic value). Rather, recent data strongly suggest that dopamine plays a crucial role in “wanting”, incentive drive or anticipatory components of behavior (e.g., Berridge and Robinson, 1998; Berridge, 2003; Berridge and Kringelbach, 2008; Blackburn et al., 1992; Hull et al., 1995; Kelley and Berridge, 2002; Koob, 1996; Moses et al., 1995; Panksepp et al., 2004; Pfaus and Phillips, 1991; Salamone et al., 2003; van Furth et al., 1995b). In rodents and primates, dopamine also appears important for reward prediction, and learning or stamping in relationships between a stimulus and a reward (Marcus, 2002; Schultz, 2001; Schultz, 2002; Wise, 2002; Wise, 2004a; Wise, 2004b; Wise, 2005). However, again in studies separating “liking” from “wanting” data do not strongly support the idea that dopamine functions as the final neurotransmitter of reward (reviewed in Berridge, 2007). Instead, once an animal comes into contact with an incentive or rewarding stimulus, other neurochemical systems, including opioids, appear to regulate reward.
Several studies in rats indicate a primary role for opioid neuropeptides in hedonic reward, which can result in satiety, reflected in an inhibition of motivated behaviors (Agmo and Paredes, 1988; Berridge, 2003; Burgdorf and Panksepp, 2001; Kelley and Berridge, 2002; van Furth et al., 1995b; Van Ree et al., 2000). The idea that dopamine underlies motivation, whereas as opioids underlie reward is well supported by studies of feeding, drugs of abuse, and sexual behavior. For example, opioids stimulate measures of “liking” for highly palatable food in rats (reflected in characteristic facial responses, which include lip licking and tongue protrusions). In contrast, dopamine stimulates measures of “wanting” but not “liking” (reviewed in Berridge, 2007; with the number of bar presses for food in an operant test apparatus considered a reflection of “wanting”). Furthermore, in male rats microdialysis reveals that dopamine release in the preoptic area occurs during the anticipation of copulation and during interactions with a female that result in copulation (Hull et al., 1995). In contrast, data suggest that rather than occurring in anticipation of copulation, opioid release occurs in association with ejaculation in male rats (Agmo and Berenfeld, 1990; Coolen et al., 2004; Forsberg et al., 1987; Szechtman et al., 1981). Interestingly, treating male rats with a dopamine receptor antagonist did not disrupt ejaculation-induced reward measured in a conditioned place preference paradigm. In contrast, treating male rats with an opioid receptor antagonist blocked ejaculation-induced reward (Agmo and Berenfeld, 1990). Together these results suggest that dopamine primarily underlies motivation and anticipatory components of behavior; whereas, opioids underlie reward.
Is singing rewarding?
Song production is something that cannot be manipulated in the same way as for example a food reward. That is, one cannot experimentally administer the act of singing. Therefore, it is difficult to assess the extent to which songbirds “like” or “want” to sing using traditional measures of motivation and reward such as conditioned place preference or bar pressing paradigms. Although whether the act of singing is rewarding has not been directly tested, multiple studies suggest that hearing song, including a bird’s own song, is rewarding and that a bird’s own vocal behavior can feed back to stimulate that bird’s neuroendocrine system. Nottebohm (1968) demonstrated years ago that if a young male chaffinch (Fringilla coelebs) is deafened during the subsong stage of song learning he regresses and sings a simplified form of song. When deafened as adults, songbirds can sing full song (albeit in an increasingly degraded form (e.g., zebra finches, Bengalese finches (Lonchura striata domestica); Lombardino and Nottebohm, 2000; Nordeen and Nordeen, 1992; Woolley and Rubel, 1997); however deafening results in a dramatic reduction in song rate (e.g., zebra finches, canaries (Serinus canaria), Gambel’s white-crowned sparrows (Zonotrichia leucophrys gambelii); Brenowitz et al., 2007; Horita et al., 2008; Jarvis and Nottebohm, 1997). This suggests that a lack of acoustic feedback reduces the motivation to sing, consistent with song serving as a reinforcer. Deafening however does not completely extinguish song, suggesting that perhaps song may be maintained in part through memory or reward obtained through proprioceptive aspects of singing.
Consistent with the idea that hearing song is reinforcing in songbirds, male chaffinches and female starlings have been shown to land and remain on perches triggering conspecific song playback (e.g., Gentner and Hulse, 2000; Stevenson-Hinde, 1972; Stevenson-Hinde and Roper, 1975). Furthermore, isolated young male juvenile zebra finches can be trained to peck a key for playback of conspecific song (Adret, 1993; Tchernichovski et al., 1999), suggesting again that hearing song is rewarding.
Several studies in ring doves (Streptopelia risoria), non-songbirds that display high levels of vocal behavior as part of courtship, demonstrate that the act of vocal production can be self-stimulatory. In female ring doves both auditory and proprioceptive aspects of vocal production feed back onto hypothalamic neural systems to strongly influence reproductive endocrinology and circuits richly innervated with enkephalin opioids (Cheng and Zuo, 1994; Cheng and Durand, 2004). Based on these data it is possible that self-stimulation of limbic systems through vocal production underlies reward or the motivation to communicate (reviewed in Cheng and Durand, 2004). Together, results from these studies and the fact that songbirds often sing at extremely high rates despite risk of predation and physical costs indicate that birds are highly motivated to sing and that the acoustic or proprioceptive components of the act of singing itself may be rewarding.
Opioids, social, and sexual motivation
Multiple studies including rats, Guinea pigs, primates, chicks, and dogs implicate opioid activity in the regulation of social contact (Carden et al., 1996; Herman and Panksepp, 1978; Kalin et al., 1995; Nocjar and Panksepp, 2007; Panksepp et al., 1978; Panksepp et al., 1979; Panksepp et al., 1980; Plonsky and Freeman, 1982). In general high levels of opioid activity result in a reduction in social motivation, including measures of social interest, affiliative behaviors, and vocal behavior. In contrast, low levels of opioid activity are associated with a high level of social motivation. One interpretation of these findings is that opioid release reinforces social contact, after which animals experience satiety associated with a reduction in social motivation.
Endogenous opioids are also released during copulation, and appear to regulate reward and satiety reflected in a temporarily decreased responsiveness to females immediately following copulation. Peripheral or ventricular opioid agonists generally inhibit male sexual behavior in rats (Agmo and Paredes, 1988; Pfaus and Pfaff, 1992); whereas, blocking opioid release during copulation in experienced animals (including birds and mammals) tends to produce an increase in sexual behaviors (e.g., male rats, female white-crowned sparrows, male Japanese quail (Coturnix japonica); Maney and Wingfield, 1998; Riters et al., 1999; Rodriguez-Manzo and Fernandez-Guasti, 1995), possibly reflecting an increase in an individual’s attempts to receive opioid reward normally associated with copulation.
Several brain regions within motivation and reward systems have been identified as playing a role in highly motivated social behaviors. The nucleus accumbens has been a focus of much research in rats; however, in songbirds this region has not been definitively identified and to date putative locations of the nucleus accumbens have not yet been related to song production (e.g., European starlings; Riters et al., 2005). Two regions that repeatedly have been implicated in motivation and reward and more recently in birdsong will be highlighted in this review: the medial preoptic nucleus (often referred to as POM in birds) and the ventral tegmental area (VTA). A large body of data implicates the preoptic area and VTA as important sites in which opioids regulate reward. Direct pharmacological manipulations and opioid self administration studies in rats reveal opioids to be rewarding when infused into the preoptic area and VTA (Agmo and Gomez, 1991; Agmo et al., 1994; Bozarth and Wise, 1981; Bozarth and Wise, 1983; Devine and Wise, 1994). Administering an opioid receptor antagonist directly into the medial preoptic area in rats blocked reward associated with copulation (measured using a conditioned place preference test) without suppressing copulation (Agmo and Gomez, 1993), highlighting the POM as a site in which opioids act to reward sexual activity. In male rats and Japanese quail, injections of opioid peptides into the medial preoptic area inhibit neural activity and sexual behavior (Agmo and Gomez, 1993; Kotegawa et al., 1997; van Furth et al., 1995a; van Furth et al., 1995b), suggesting sexual reward and satiety are regulated by opioid release and an accompanying reduction of activity in the preoptic area. In contrast, opioid agonists targeting VTA in rats are found to stimulate sexual motivation (Mitchell and Stewart, 1990; van Furth et al., 1995b; van Furth and van Ree, 1996). Opioids and dopamine interact within the VTA to stimulate motivated behavior in rodents (reviewed in van Furth et al., 1995b). Specifically, in rodents opioids within the VTA stimulate dopamine, and associated sexually motivated behaviors, presumably by inhibiting GABA which then disinhibits dopamine (Kalivas, 1993). Thus, in addition to evidence that opioids in VTA are rewarding, opioids may contribute to motivational components of song through interactions with dopamine in VTA.
Opioids and vocal communication
Several studies in multiple species are consistent with an inhibitory role for opioids in vocal production. Specifically, opioid blockade increased both vocal behaviors associated with the anticipation of social reward in socially experienced rats (Burgdorf and Panksepp, 2001) and vocalizations important for maintaining social contact in domestic chicks (Gallus domesticus), Guinea pigs, dogs, and rat pups. Furthermore, stimulation of mu and delta opioid receptors (receptors that bind enkephalin opioids) leads to a reduction in social contact vocalizations in multiple species (Carden et al., 1991; Carden et al., 1996; Herman and Panksepp, 1978; Panksepp et al., 1978; Panksepp et al., 1980; Winslow and Insel, 1991). In infant rhesus monkeys that had been separated from their mothers, opioid administration reduced infant vocal behavior occurring during reunion, whereas an opioid antagonist had the opposite effects (Kalin et al., 1995).
Opioids and song production
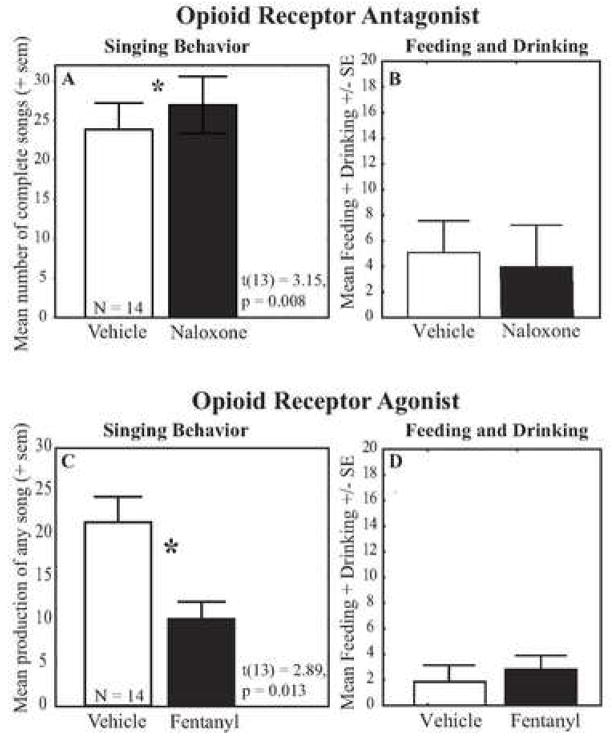
The effects of opioid receptor manipulations on birdsong have not been extensively studied, but data to date are consistent with an inhibitory role for opioids in male song production. Specifically, opioid involvement in male song was examined in castrated, T treated male European starlings singing sexually-motivated song in response to a female (Riters et al., 2005). Across four alternating test days males were treated with the opioid antagonist naloxone or vehicle. When treated with naloxone males sang significantly more complete song bouts (Fig. 1) and spent a larger proportion of time singing. Although the magnitude of the effect was not large, it was observed in the majority of birds (Fig. 2). Furthermore, males were already singing at high rates under control conditions. Therefore seeing a further increase in song after naloxone treatment strongly supports the idea that opioid blockade stimulates male song production.
Figure 1.
A) Mean number of complete song bouts produced by male starlings during 2 test days after vehicle treatment and two test days after treatment with the opioid receptor antagonist naloxone; n = 14 in each condition, B) Mean bouts of feeding and drinking over the 2 test days for vehicle and naloxone treated males; C) Mean total times a male starling initiated song during 2 test days after treatment with vehicle and 2 test days after treatment with the opioid receptor agonist fentanyl; n = 14 in each condition, D) Mean bouts of feeding and drinking over the 2 test days for vehicle and fentanyl treated males. * indicates p< 0.05. Figures A and B redrawn from data reported in (Riters et al., 2005). Figures C and D redrawn from data reported in (Schroeder and Riters, 2006).
Figure 2.
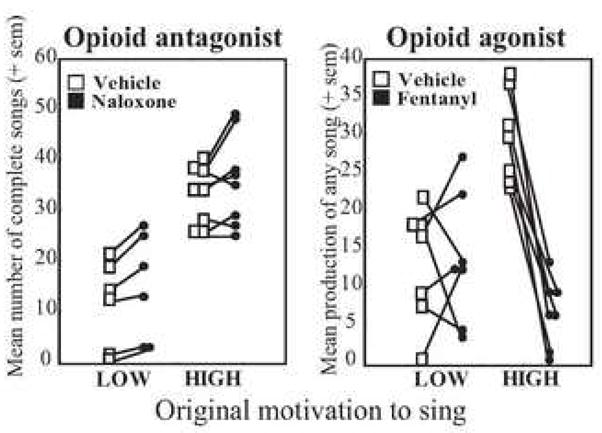
Re-plotting of data from Fig. 1 showing the results of effective doses of the opioid receptor antagonist naloxone and agonist fentanyl on measures of song production in individual starlings split into low- versus high-singing groups based on song production under vehicle treatment. Open squares show song in individuals treated with control solution. Filled circles show song in individuals treated with drug. See text for additional detail.
Using the same testing procedures, a separate group of castrated, T treated male European starlings was tested after peripheral injections of either the mu opioid receptor agonist fentanyl or vehicle (Schroeder and Riters, 2006). Fentanyl significantly suppressed production of complete or incomplete song bouts (Fig. 1), and males spent a larger proportion of time singing. The effects of naloxone and fentanyl on behavior appeared to be specific to song production. For example, neither naloxone or fentanyl significantly affected bouts of feeding and drinking (Fig. 1). Together the past opioid pharmacology studies in male starlings implicate opioids in sexually-motivated song production; however, whether such manipulations will have the same or more potent effects on undirected song (i.e., song without any obvious externally-mediated reinforcer) remains to be tested.
At first the effects of opioid receptor manipulations on song may seem counterintuitive. If it is assumed that opioid release is what makes the act of singing rewarding, why would blocking opioid receptors increase vocal production? One explanation is that animals learn to associate vocal production with resulting opioid release and reinforcement. In the absence of such reinforcement in association with vocal production (i.e., when an animal is treated with an opioid receptor antagonist) an animal will vocalize at even higher rates in an attempt to obtain the opioid reinforcement normally associated with vocal production (or in a sexually-motivated context, opioid reinforcement through copulation). This leads to the intriguing possibility in songbirds that opioid receptor blockade will have opposing effects on song production in experienced versus inexperienced singers. It may be that in contrast to the stimulatory effects of opioid receptor antagonists described in experienced male European starlings (Riters et al., 2005), opioid antagonists will inhibit song production in inexperienced singers who have not yet learned to associate the act of singing with opioid reinforcement (i.e., prior to or in the initial stages of song learning).
Evidence that individual differences in opioid receptors underlie individual differences in song production
Studying wild species in a naturalistic, dynamic social setting allows one the opportunity to examine a potentially wider range of individual variation in behavior than that observed in inbred laboratory housed species. In the opioid pharmacology study in starlings described immediately above, males displayed a range of individual differences in song rate and could easily be categorized as low- or high-singers based on the number of songs they produced during vehicle treatment. Here the individual responses to opioid pharmacological manipulations are reexamined by simply splitting the males into two approximately equal groups made up of the lowest and highest singers (Fig. 2). Interestingly, differential responses to opioid receptor pharmacological manipulations were identified for birds naturally inclined to sing at low versus high rates. Treatment with the opioid agonist fentanyl uniformly abolished song in all of the high singers. In contrast, low singers did not respond uniformly to fentanyl, with some showing increases in song production and other decreases (Fig. 2). In contrast, the opioid antagonist naloxone uniformly increased song production in low-singers, but resulted in a less uniform response in high-singers (Fig 2). These findings suggest that differences might exist in opioid receptor densities or binding affinity in individuals naturally communicating at low versus high rates. The possibility that differences in opioid receptor numbers or dynamics underlie individual differences in song production needs to be explored in future research.
Neuroanatomical basis of song
As highlighted in the introduction to this issue [ref], songbirds are unique in that they possess a specialized group of interconnected brain nuclei devoted exclusively to song, known as the song control system. Briefly, this system consists of at least two distinct neural pathways that play roles in different aspects of song behavior. A forebrain pathway is involved in song learning (e.g., Bottjer et al., 1984; Bottjer and Johnson, 1997; Doupe and Solis, 1997; Sohrabji et al., 1990), and has more recently been implicated in song perception (e.g., Brenowitz, 1991; Burt et al., 2000; Riters and Teague, 2003) and the context in which a bird sings (Hessler and Doupe, 1999; Jarvis et al., 1998). This pathway consists of a projection from HVC to Area X, to the medial nucleus of the dorsal lateral thalamus (DLM), to the lateral portions of the magnocellular nucleus of the anterior nidopallium (lMAN), which projects back to area X and on to the robust nucleus of the arcopallium (RA; for review see Bottjer and Johnson, 1997). A second pathway has been found to play a primary role in motor aspects of song production (e.g., Margoliash, 1997; Nottebohm et al., 1976). This pathway includes a projection from HVC to RA, which sends descending projections both directly to the tracheosyringeal portion of the hypoglossal nucleus (nXIIts) and indirectly to nXIIts via the dorsomedial nucleus of the nucleus intercollicularis (DM; for review see Margoliash, 1997). (For updates in avian brain nomenclature, see AvianBrain.org.)
Multiple studies in songbirds demonstrate enkephalin (both leucine- and methionine-enkephalin) opioid innervation of song control nuclei including HVC, RA, area X and lMAN across species (i.e., in zebra finches, European starlings, and song sparrows (Melospiza melodia); Ball et al., 1988; Ball et al., 1995a; Ball et al., 1995b; Bottjer and Alexander, 1995; Carrillo and Doupe, 2004; Ryan et al., 1981). The boundaries of HVC and RA can also be defined by dense mu, delta, and kappa opioid receptor subtypes, with mu and delta receptor subtypes far outnumbering kappa in dark-eyed juncos (Junco hyemalis; Gulledge and DeViche, 1995; Gulledge and Deviche, 1999). The enkephalins bind preferentially to mu and delta opioid receptors, indicating that enkephalins are well positioned to influence song behavior. Different hypotheses have been proposed on the function of opioids within the song system. For example, opioids might regulate seasonal plasticity within the song system or play a role in song learning (Gulledge and DeViche, 1995; Gulledge and Deviche, 1999); however, few studies have examined the behavioral significance of these findings.
Although a great deal is known about the specific function of nuclei of the song control system in aspects of song such as learning, sensorimotor processing, and perception, little is known about what activates this circuit, or what at a neurobiological level motivates birds to sing. Activity within the song control system can differ depending on the motivational context in which vocalizations are produced (Hessler and Doupe, 1999; Jarvis et al., 1998). Neuronal firing in area X and lMAN, and gene expression in area X, lMAN, and RA indicate that activity within these regions is higher when male zebra finches are singing alone (undirected song), compared to neural activity associated with song directed toward a conspecific (directed song) (Hessler and Doupe, 1999; Jarvis et al., 1998). However, neither lesions to lMAN or area X in adult male zebra finches disrupt song production (Bottjer et al., 1984; Nordeen and Nordeen, 1993; Nottebohm et al., 1976; Sohrabji et al., 1990). Furthermore, in male canaries, although lesions to RA and HVC result in severe deficits in song production, lesioned birds continue to assume a singing posture and display motor behaviors associated with song production, indicating an intact motivation to sing (Nottebohm et al., 1976). It is predicted that opioids act within the song control system to regulate aspects of song such as learning or sensorimotor processing, whereas motivation and reward associated with song production are regulated by opioid activity in regions outside the song control system.
Neural regulation of the motivation to sing
Given that song during the breeding season can be highly sexually motivated, brain areas outside of the song control system, such as those involved in the anticipation of copulatory behavior (e.g., POM) or motivation in general (e.g., VTA) are also likely to play an important role in this type of singing behavior. Multiple studies highlight a role for the opioid rich POM in song. The POM is largest in male starlings during, compared to outside of, the breeding season, and largest in sexually active males that sing at high rates in response to a female (Riters et al., 2000). Furthermore, in male starlings the volume of the POM relates positively to song bout length (Riters et al., 2000). Males in this study sang longer songs in spring, when a longer song bout serves to attract mates and repel competitors (Eens et al., 1991; Gentner and Hulse, 2000; Mountjoy and Lemon, 1995), providing further evidence for POM involvement in context-appropriate communication. Additional data reveal positive relationships between the numbers of cells labeled for the immediate early gene cFOS within POM and sexually-motivated song, but not undirected song in two studies on different species (European starlings and house sparrows (Passer domesticus); Heimovics and Riters, 2005; Riters et al., 2004). Lesions to the POM in male starlings block song and other courtship behaviors specifically within a sexually motivated context, but result in context-inappropriate elevations in song within more generally motivated contexts (Alger and Riters, 2006; Alger et al., 2009; Riters and Ball, 1999). Together, these data suggest that the POM plays an important role in adjusting communication so that it is context-appropriate, and they suggest that the role of POM in communication differs depending upon the social context.
Several studies also suggest the VTA differentially regulates song produced within multiple social contexts. For example, in zebra finches electrophysiological activity in VTA increased in males singing to females (Huang and Hessler, 2008; Yanagihara and Hessler, 2006). Similarly, in male starlings, cFOS in VTA correlates with song in a sexually-motivated context, but not song produced in flocks (i.e., undirected song; Heimovics and Riters, 2005). However, in male song sparrows immediate early gene data also showed correlations with song produced within an aggressive, non-sexual context (Maney and Ball, 2003), suggesting the role of VTA in song also extends to song directed at conspecifics outside a sexually-motivated context.
Overall, these data suggest differential involvement of POM and VTA in the regulation of communication within different social contexts. In birds, both the POM and VTA are densely innervated with enkephalin opioid fibers and receptors (pigeons (Columba livia), domestic chicks, dark-eyed juncos, zebra finches, European starlings; Bottjer and Alexander, 1995; Csillag et al., 1990; Deviche et al., 1993; Gale and Perkel, 2006; Reiner et al., 1989; Riters et al., 2005), suggesting these as sites in which opioids might act to regulate the motivation to sing. VTA and POM share reciprocal neuroanatomical connections in male starlings (Riters and Alger, 2004). VTA projects directly to nuclei involved in song production in canaries (HVC, RA; Appeltants et al., 2000; Appeltants et al., 2002) and sends a dense dopaminergic projection to area X in zebra finches (Lewis et al., 1981), a portion of the basal ganglia also implicated in the context in which song is produced (Hessler and Doupe, 1999; Jarvis et al., 1998). Thus, POM and VTA are rich in opioids and ideally neuroanatomically positioned to influence motivation and reward related to song production.
Evidence that opioids act within the POM and VTA to regulate song, perhaps context-dependently
In addition to pharmacological manipulations supporting a role for opioids in song (Riters et al., 2005; Schroeder and Riters, 2006), a study performed in male European starlings revealed methionine-enkephalin (mENK) opioid immunolabeled fiber densities within POM and VTA to relate positively to male song production. Song was recorded from male starlings singing sexually-motivated or undirected song (in the spring breeding season or fall, respectively). Males were sacrificed and the densities of mENK fibers labeled using immunocytochemistry were examined within 12 brain regions, including song control nucleus HVC, the nucleus intercollicularis in which opioid release may occur in response to vocal self-stimulation in ring doves (Cheng and Zuo, 1994), sites in which rats are known to self administer opioids, a possible homologue of the nucleus accumbens, and other regions heavily innervated with mENK. Of all the areas investigated significant relationships were detected only between song production and mENK fiber density within the POM and VTA (Riters et al., 2005; Fig. 3 and 4). When seasons were considered separately, for VTA positive but non-significant relationships were detected both outside and within a breeding context. Given that multiple studies implicate VTA in general motivation in rats and primates (Bals-Kubik et al., 1993; Cador et al., 1988; Devine and Wise, 1994; Druhan et al., 1990; Mogenson and Yang, 1991; Nishino et al., 1987; van Furth and van Ree, 1996), these data suggest that mENK release within VTA may motivate or reward song production within multiple social contexts. When data were analyzed by season for POM, a significant positive relationship was detected between song and mENK fiber density within POM outside, but not within the breeding season (see regression lines in Fig. 3). It is possible that these relationships were not statistically significant, because when divided by season, the range of singing was truncated for birds observed in spring (Fig. 3). However, it is also possible that mENK within the POM is more closely related to undirected song as compared to song that is sexually-motivated. Although the pharmacological data clearly implicate opioids in the regulation of sexually-motivated song (Riters et al., 2005; Schroeder and Riters, 2006), this finding is consistent with the idea that undirected song may be more critically dependent upon internal reward mechanisms than song that is sexually-motivated. In future work the effects of opioid receptor manipulations within VTA and POM on sexually-motivated and undirected song should be investigated.
Figure 3.
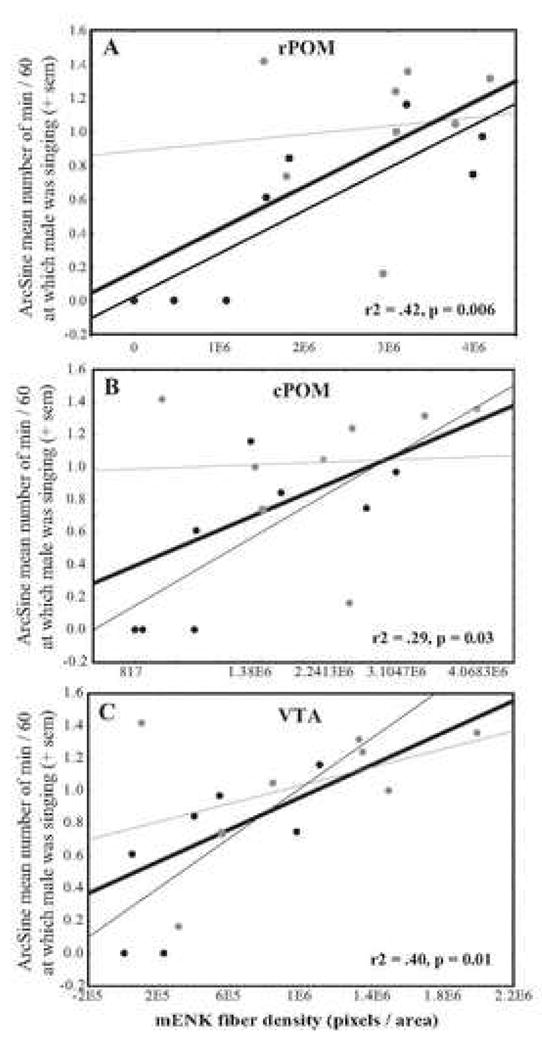
Scatterplots illustrating relationships between male song production (Mean arcsine transformed number of minutes at which each male was singing during one hour on 5 test days) and mENK fiber density in the rostral portion of the POM (rPOM), the caudal portion of POM (cPOM), and the VTA. Each dot represents data from a single male. Black dots represent data from males observed singing undirected song outside the breeding season. Gray dots represent males observed singing sexually-motivated song during the breeding season. For each scatterplot, the r2 and thick black regression line are for the regressions performed on data from breeding and non-breeding males combined. The thin dark gray line is the regression line for males singing sexually-motivated song (rPOM: r2 = 0.01, p = 0.80, cPOM: r2 = 0.005, p = 0.87, VTA: r2 = 0.20, p = 0.26). The light gray line is the regression line for males singing undirected song (VTA: r2 = 0.53, p = 0.06, rPOM: r2 = 0.70, p = 0.009, cPOM: r2 = 0.56, p = 0.03). See text for additional detail. Figure from (Riters et al., 2005).
Figure 4.
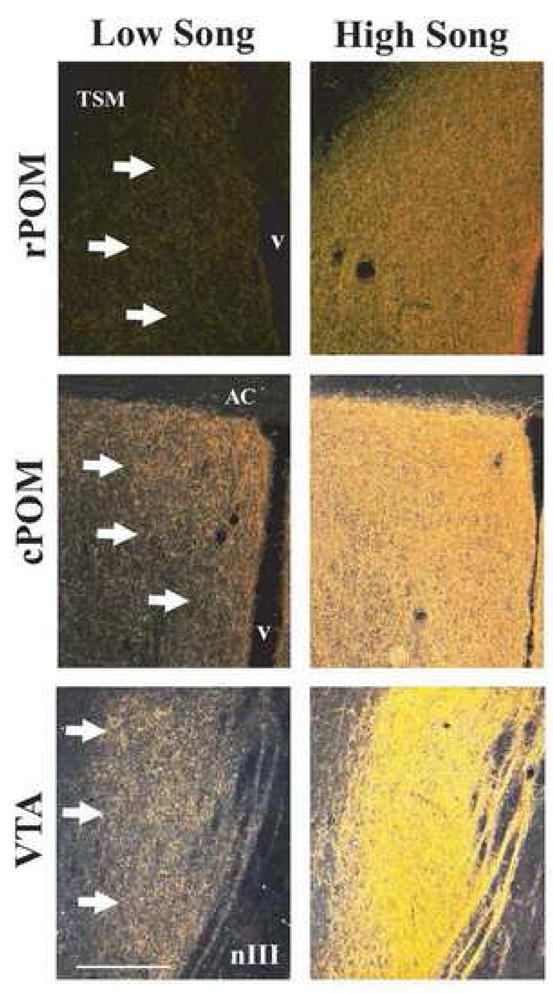
Darkfield photomicrographs illustrating mENK fiber density in the rostral portion of the POM (rPOM), the caudal portion of POM (cPOM), and the VTA of a male that did not sing and a male that sang at high levels. Arrows point to the boundaries of POM and VTA. TSM = tractus septomesencephalicus, AC = anterior commissure, v = ventricle, nIII = 3rd cranial nerve. Figure from (Riters et al., 2005).
Testosterone and context-dependent opioid regulation of the motivation to communicate
In starlings, as in other seasonally breeding songbirds, T is high in males singing sexually motivated song during the breeding season and often undetectable outside of the breeding season when males sing at high rates to maintain social contact (Dawson, 1983; Riters et al., 2000; Riters et al., 2002). Enkephalin opioid densities are affected by T or its metabolites in mammals, specifically within the preoptic area (e.g., Simerly et al., 1988; Watson et al., 1986). In songbirds, T has not been strongly linked to opioids. For example, a non-significant trend was observed for male chaffinches with high T to land and remain on perches triggering song more than males with low T (Stevenson-Hinde, 1972), hinting that the activation of neural reward systems by song may be T-dependent. Furthermore, in the study examining song and the density of mENK fibers in starlings reviewed above (Riters et al., 2005), the seasonal patterns of mENK fiber densities within POM and VTA mirrored but did not relate significantly to seasonal patterns of T concentrations. These data must be interpreted with caution, but hint that T and opioids might interact to regulate song behavior, a possibility that must be examined further.
Summary and conclusions
The idea that dopamine regulates motivated, goal-directed components of behavior, whereas other neuromodulators such as opioids underlie the actual reward or pleasure associated with a stimulus is gaining increasing support through studies examining feeding, sexual behavior, and drugs of abuse. The studies reviewed here on the role of opioids in song and multiple studies on dopamine and song (reviewed in [ref] of this issue) suggest this theory also applies to birdsong.
As reviewed above, in males with high T concentrations song in response to a female can be highly sexually-motivated, and directed towards a specific goal (a female). Song in this context can result in immediate reinforcement through attraction of, and copulation with, a mate. Dopamine within incertohypothalamic and mesolimbic systems has been specifically implicated in the regulation of goal-directed, incentive motivation. Therefore it is hypothesized that dopamine is involved in the regulation of song at times when it is highly motivated and directed towards a particular goal (i.e., a female during the breeding season). In contrast, it is expected that dopamine will play a lesser role in contexts in which communication is not highly goal-directed, such as during song learning or song produced within large social flocks (i.e., undirected song). Consistent with this hypothesis, multiple studies using a variety of techniques reveal a tight relationship between dopamine within VTA, POM, and area X (among other regions) and sexually-motivated song in zebra finches and starlings (Barclay and Harding, 1990; Heimovics and Riters, 2008; Heimovics et al., 2009; Huang and Hessler, 2008; Rauceo et al., 2008; Sasaki et al., 2006; Schroeder and Riters, 2006). Whether the role of these regions in song generalizes to “goal-directed” song that is not sexually-motivated must be examined in future work.
Undirected communication has important functions (e.g., vocal learning or maintaining social contact) and must somehow be reinforced to be maintained, however an immediate externally-mediated reward resulting from this type of song is not apparent. Therefore, it is possible that undirected song is reinforced internally, perhaps by opioid activity within incertohypothalamic or mesolimbic neural systems (Cheng and Durand, 2004). Furthermore, within a breeding context, prior to any experience with a female, it may be that reinforcement associated with song plays a role in initiating song. Therefore, it is expected that opioids might be important for internal reinforcement of vocal communication in both sexually-motivated and undirected contexts but that opioids may be most critically involved in regulating undirected song, which has no obvious external reinforcer. In support of these predictions, converging data indicate that opioid rich brain areas involved in motivation and reward (including POM and VTA), differentially regulate sexually motivated versus undirected song production (e.g., starlings, house sparrows, and zebra finches; Alger and Riters, 2006; Heimovics and Riters, 2005; Huang and Hessler, 2008; Riters et al., 2000; Riters et al., 2004; Yanagihara and Hessler, 2006). Furthermore, although opioids have been found to relate to song production in both sexually-motivated and undirected contexts in starlings (Riters et al., 2005; Schroeder and Riters, 2006), there is some evidence that mENK immunolabeling densities in POM are more tightly linked to undirected song (Riters et al., 2005; Schroeder and Riters, 2006). Together these data suggest that dopamine activity may underlie the motivation or drive to sing, but that opioid release is what reinforces song production.
The role of opioids in song to date has only been examined in association with song produced during mate attraction and in large social flocks; however, song plays a role in social interactions within many additional contexts. For example, another primary function of male song during the breeding season is territorial defense (Catchpole and Slater, 1995b). Song in this context is elicited by the presence of an intruding male and may be considered “goal-directed”, with the goal of repelling a competitor. One possibility is that song in this context is negatively reinforced (i.e., strengthened through reduction of negative agonistic interactions with a male). Data to date in male European starlings suggest that song used to repel competitors relates to dopamine D1 receptors within a subset of brain regions that is partially distinct from the regions in which D1 receptors are found to relate to sexually-motivated song (Heimovics et al., 2009). These data suggest context-dependent differences in the neural circuits in which dopamine acts to influence song. The role of opioids in territorial song has yet to be explored. Studies examining the role of opioids and dopamine in song produced in additional distinct social contexts that differ in terms of what motivates song and potentially what reinforces song (such as the context of territorial defense) are needed to explore further the hypothesis that dopamine underlies the motivation to sing but that opioid release is what makes song production rewarding.
Acknowledgments
Support from the National Institute of Mental Health MH080225 is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adret P. Operant conditioning, song learning and imprinting to taped song in the zebra finch. Animal Behaviour. 1993;46:149–159. [Google Scholar]
- Agmo A, Paredes R. Opioids and sexual behavior in the male rat. Pharmacol Biochem Behav. 1988;30:1021–34. doi: 10.1016/0091-3057(88)90135-9. [DOI] [PubMed] [Google Scholar]
- Agmo A, Berenfeld R. Reinforcing properties of ejaculation in the male rat: role of opioids and dopamine. Behav Neurosci. 1990;104:177–82. doi: 10.1037//0735-7044.104.1.177. [DOI] [PubMed] [Google Scholar]
- Agmo A, Gomez M. Conditioned place preference produced by infusion of Met-enkephalin into the medial preoptic area. Brain Res. 1991;550:343–6. doi: 10.1016/0006-8993(91)91339-3. [DOI] [PubMed] [Google Scholar]
- Agmo A, Gomez M. Sexual reinforcement is blocked by infusion of naloxone into the medial preoptic area. Behav Neurosci. 1993;107:812–8. doi: 10.1037//0735-7044.107.5.812. [DOI] [PubMed] [Google Scholar]
- Agmo A, Gomez M, Irazabal Y. Enkephalinase inhibition facilitates sexual behavior in the male rat but does not produce conditioned place preference. Pharmacol Biochem Behav. 1994;47:771–8. doi: 10.1016/0091-3057(94)90276-3. [DOI] [PubMed] [Google Scholar]
- Alger SJ, Riters LV. Lesions to the medial preoptic nucleus differentially affect singing and nest box-directed behaviors within and outside of the breeding season in European starlings (Sturnus vulgaris) Behav Neurosci. 2006;120:1326–36. doi: 10.1037/0735-7044.120.6.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger SJ, Maasch SN, Riters LV. Lesions to the medial preoptic nucleus affect immediate early gene immunolabeling in brain regions involved in song control and social behavior in male European starlings. European Journal of Neuroscience. 2009 doi: 10.1111/j.1460-9568.2009.06637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appeltants D, Absil P, Balthazart J, Ball GF. Identification of the origin of catecholaminergic inputs to HVc in canaries by retrograde tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Chem Neuroanat. 2000;18:117–33. doi: 10.1016/s0891-0618(99)00054-x. [DOI] [PubMed] [Google Scholar]
- Appeltants D, Ball GF, Balthazart J. The origin of catecholaminergic inputs to the song control nucleus RA in canaries. Neuroreport. 2002;13:649–53. doi: 10.1097/00001756-200204160-00023. [DOI] [PubMed] [Google Scholar]
- Ball GF, Faris PL, Hartman BK, Wingfield JC. Immunohistochemical localization of neuropeptides in the vocal control regions of two songbird species. J Comp Neurol. 1988;268:171–80. doi: 10.1002/cne.902680204. [DOI] [PubMed] [Google Scholar]
- Ball GF, Absil P, Balthazart J. Peptidergic delineations of nucleus interface reveal a sex difference in volume. Neuroreport. 1995a;6:957–60. doi: 10.1097/00001756-199505090-00002. [DOI] [PubMed] [Google Scholar]
- Ball GF, Absil P, Balthazart J. Assessment of volumetric sex differences in the song control nuclei HVC and RA in zebra finches by immunocytochemistry for methionine enkephalin and vasoactive intestinal polypeptide. Brain Res. 1995b;699:83–96. doi: 10.1016/0006-8993(95)00875-q. [DOI] [PubMed] [Google Scholar]
- Ball GF, Riters LV, MacDougall-Shackleton SA, Balthazart J. Sex differences in brain and behavior and the neuroendocrine control of the motivation to sing. In: Zeigler HP, Marler P, editors. Neuroscience of Birdsong. Cambridge University Press; New York: 2008. pp. 320–331. [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–95. [PubMed] [Google Scholar]
- Band LC, Hull EM. Morphine and dynorphin(1–13) microinjected into the medial preoptic area and nucleus accumbens: effects on sexual behavior in male rats. Brain Res. 1990;524:77–84. doi: 10.1016/0006-8993(90)90494-v. [DOI] [PubMed] [Google Scholar]
- Barclay SR, Harding CF. Differential modulation of monoamine levels and turnover rates by estrogen and/or androgen in hypothalamic and vocal control nuclei of male zebra finches. Brain Res. 1990;523:251–62. doi: 10.1016/0006-8993(90)91494-2. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Pleasures of the brain. Brain Cogn. 2003;52:106–28. doi: 10.1016/s0278-2626(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 2008;199:457–80. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharati IS, Goodson JL. Fos responses of dopamine neurons to sociosexual stimuli in male zebra finches. Neuroscience. 2006;143:661–70. doi: 10.1016/j.neuroscience.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn JR, Pfaus JG, Phillips AG. Dopamine functions in appetitive and defensive behaviours. Prog Neurobiol. 1992;39:247–79. doi: 10.1016/0301-0082(92)90018-a. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:901–3. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Alexander G. Localization of met-enkephalin and vasoactive intestinal polypeptide in the brains of male zebra finches. Brain Behav Evol. 1995;45:153–77. doi: 10.1159/000113547. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Johnson F. Circuits, hormones, and learning: vocal behavior in songbirds. J Neurobiol. 1997;33:602–18. doi: 10.1002/(sici)1097-4695(19971105)33:5<602::aid-neu8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Intracranial self-administration of morphine into the ventral tegmental area in rats. Life Sci. 1981;28:551–5. doi: 10.1016/0024-3205(81)90148-x. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Neural substrates of opiate reinforcement. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7:569–75. doi: 10.1016/0278-5846(83)90027-1. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA. Altered perception of species-specific song by female birds after lesions of a forebrain nucleus. Science. 1991;251:303–5. doi: 10.1126/science.1987645. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA, Lent K, Rubel EW. Auditory feedback and song production do not regulate seasonal growth of song control circuits in adult white-crowned sparrows. J Neurosci. 2007;27:6810–4. doi: 10.1523/JNEUROSCI.1248-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J. Tickling induces reward in adolescent rats. Physiol Behav. 2001;72:167–73. doi: 10.1016/s0031-9384(00)00411-x. [DOI] [PubMed] [Google Scholar]
- Burt JM, Lent KL, Beecher MD, Brenowitz EA. Lesions of the anterior forebrain song control pathway in female canaries affect song perception in an operant task. J Neurobiol. 2000;42:1–13. doi: 10.1002/(sici)1097-4695(200001)42:1<1::aid-neu1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Cador M, Kelley AE, Le Moal M, Stinus L. d-Ala-met-enkephalin injection into the ventral tegmental area: effect on investigatory and spontaneous motor behaviour in the rat. Psychopharmacology (Berl) 1988;96:332–42. doi: 10.1007/BF00216059. [DOI] [PubMed] [Google Scholar]
- Carden SE, Barr GA, Hofer MA. Differential effects of specific opioid receptor agonists on rat pup isolation calls. Brain Res Dev Brain Res. 1991;62:17–22. doi: 10.1016/0165-3806(91)90185-l. [DOI] [PubMed] [Google Scholar]
- Carden SE, Hernandez N, Hofer MA. The isolation and companion comfort responses of 7- and 3-day-old rat pups are modulated by drugs active at the opioid receptor. Behav Neurosci. 1996;110:324–30. doi: 10.1037//0735-7044.110.2.324. [DOI] [PubMed] [Google Scholar]
- Carrillo GD, Doupe AJ. Is the songbird Area X striatal, pallidal, or both? An anatomical study. J Comp Neurol. 2004;473:415–37. doi: 10.1002/cne.20099. [DOI] [PubMed] [Google Scholar]
- Catchpole C, Slater P. Bird song: Biological themes and variations. Cambridge University Press; Cambridge: 1995a. [Google Scholar]
- Catchpole C, Slater PJB. Bird song: biological themes and variations. New York NY USA: Cambridge University Press, Cambridge, [England];; 1995b. [Google Scholar]
- Cheng MF, Zuo M. Proposed pathways for vocal self-stimulation: met-enkephalinergic projections linking the midbrain vocal nucleus, auditory-responsive thalamic regions and neurosecretory hypothalamus. J Neurobiol. 1994;25:361–79. doi: 10.1002/neu.480250403. [DOI] [PubMed] [Google Scholar]
- Cheng MF, Durand SE. Song and the limbic brain: a new function for the bird’s own song. Ann N Y Acad Sci. 2004;1016:611–27. doi: 10.1196/annals.1298.019. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Fitzgerald ME, Yu L, Lehman MN. Activation of mu opioid receptors in the medial preoptic area following copulation in male rats. Neuroscience. 2004;124:11–21. doi: 10.1016/j.neuroscience.2003.10.045. [DOI] [PubMed] [Google Scholar]
- Csillag A, Bourne RC, Stewart MG. Distribution of mu, delta, and kappa opioid receptor binding sites in the brain of the one-day-old domestic chick (Gallus domesticus): an in vitro quantitative autoradiographic study. J Comp Neurol. 1990;302:543–51. doi: 10.1002/cne.903020310. [DOI] [PubMed] [Google Scholar]
- Cuthill I, Hindmarsh AM. Increase in starling song activity with removal of mate. Animal Behaviour. 1985;33:326–328. [Google Scholar]
- Dawson A. Plasma gonadal steroid levels in wild starlings (Sturnus vulgaris) during the annual cycle and in relation to the stages of breeding. Gen Comp Endocrinol. 1983;49:286–94. doi: 10.1016/0016-6480(83)90146-6. [DOI] [PubMed] [Google Scholar]
- Deviche P, Cotter P, Gulledge CC. Identification, partial characterization, and hypothalamic distribution of kappa, mu, and delta opioid receptors in a passerine songbird (Junco hyemalis) Brain Res. 1993;614:220–6. doi: 10.1016/0006-8993(93)91038-t. [DOI] [PubMed] [Google Scholar]
- Devine DP, Wise RA. Self-administration of morphine, DAMGO, and DPDPE into the ventral tegmental area of rats. J Neurosci. 1994;14:1978–84. doi: 10.1523/JNEUROSCI.14-04-01978.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe AJ, Solis MM. Song- and order-selective neurons develop in the songbird anterior forebrain during vocal learning. J Neurobiol. 1997;33:694–709. [PubMed] [Google Scholar]
- Druhan JP, Fibiger HC, Phillips AG. Amphetamine-like stimulus properties produced by electrical stimulation of reward sites in the ventral tegmental area. Behav Brain Res. 1990;38:175–84. doi: 10.1016/0166-4328(90)90015-7. [DOI] [PubMed] [Google Scholar]
- Dunn AM, Zann RA. Undirected song in wild zebra finch flocks: context and effects of mate removal. Ethology. 1996;102:529–539. [Google Scholar]
- Eens M, Pinxten R. Extra-pair courtship in the starling, Sturnus vulgaris. IBIS. 1990;132:618–619. [Google Scholar]
- Eens M, Pinxten R, Verheyen RF. Male song as a cue for mate choice in the European starling. Behaviour. 1991;116:210–238. [Google Scholar]
- Eens M, Pinxten R, Verheyen RF. Variation in singing activity during the breeding cycle of the European starling Sturnus vulgaris. Belgian Journal of Zoology. 1994;124:167–174. [Google Scholar]
- Eens M, Pinxten R. Inter-sexual conflicts over copulations in the European starling: evidence for the female mate-guarding hypothesis. Behavioral Ecology Sociobiology. 1995;36:71–81. [Google Scholar]
- Forsberg G, Wiesenfeld-Hallin Z, Eneroth P, Sodersten P. Sexual behavior induces naloxone-reversible hypoalgesia in male rats. Neurosci Lett. 1987;81:151–4. doi: 10.1016/0304-3940(87)90356-9. [DOI] [PubMed] [Google Scholar]
- Gale SD, Perkel DJ. Physiological properties of zebra finch ventral tegmental area and substantia nigra pars compacta neurons. J Neurophysiol. 2006;96:2295–306. doi: 10.1152/jn.01040.2005. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH. Female European starling preference and choice for variation in conspecific male song. Anim Behav. 2000;59:443–458. doi: 10.1006/anbe.1999.1313. [DOI] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge CC, DeViche P. Autoradiographic localization of opioid receptors in vocal control regions of a male passerine bird (Junco hyemalis) J Comp Neurol. 1995;356:408–17. doi: 10.1002/cne.903560308. [DOI] [PubMed] [Google Scholar]
- Gulledge CC, Deviche P. Age- and sex-related differences in opioid receptor densities in the songbird vocal control system. J Comp Neurol. 1999;404:505–14. [PubMed] [Google Scholar]
- Hausberger M, Richard-Yris M-A, Henry L, Lepage L, Schmidt I. Song sharing reflects the social organization in a captive group of European starlings (Sturnus vulgaris) Journal of Comparative Psychology. 1995;109:222–241. [Google Scholar]
- Heimovics SA, Riters LV. Immediate early gene activity in song control nuclei and brain areas regulating motivation relates positively to singing behavior during, but not outside of, a breeding context. J Neurobiol. 2005;65:207–224. doi: 10.1002/neu.20181. [DOI] [PubMed] [Google Scholar]
- Heimovics SA, Riters LV. Evidence that dopamine within motivation and song control brain regions regulates birdsong context-dependently. Physiol Behav. 2008;95:258–66. doi: 10.1016/j.physbeh.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimovics SA, Cornil CA, Ball GF, Riters LV. D1-like dopamine receptor density in nuclei involved in social behavior correlates with song in a context-dependent fashion in male European starlings. Neuroscience. 2009:962–973. doi: 10.1016/j.neuroscience.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman BH, Panksepp J. Effects of morphine and naloxone on separation distress and approach attachment: evidence for opiate mediation of social affect. Pharmacol Biochem Behav. 1978;9:213–20. doi: 10.1016/0091-3057(78)90167-3. [DOI] [PubMed] [Google Scholar]
- Hessler NA, Doupe AJ. Social context modulates singing-related neural activity in the songbird forebrain. Nat Neurosci. 1999;2:209–11. doi: 10.1038/6306. [DOI] [PubMed] [Google Scholar]
- Horita H, Wada K, Jarvis ED. Early onset of deafening-induced song deterioration and differential requirements of the pallial-basal ganglia vocal pathway. Eur J Neurosci. 2008;28:2519–32. doi: 10.1111/j.1460-9568.2008.06535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YC, Hessler NA. Social modulation during songbird courtship potentiates midbrain dopaminergic neurons. PLoS ONE. 2008;3:e3281. doi: 10.1371/journal.pone.0003281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Du J, Lorrain DS, Matuszewich L. Extracellular dopamine in the medial preoptic area: implications for sexual motivation and hormonal control of copulation. J Neurosci. 1995;15:7465–71. doi: 10.1523/JNEUROSCI.15-11-07465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Lorrain DS, Du J, Matuszewich L, Lumley LA, Putnam SK, Moses J. Hormone-neurotransmitter interactions in the control of sexual behavior. Behav Brain Res. 1999;105:105–16. doi: 10.1016/s0166-4328(99)00086-8. [DOI] [PubMed] [Google Scholar]
- Jarvis ED, Nottebohm F. Motor-driven gene expression. Proc Natl Acad Sci U S A. 1997;94:4097–102. doi: 10.1073/pnas.94.8.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Scharff C, Grossman MR, Ramos JA, Nottebohm F. For whom the bird sings: context-dependent gene expression. Neuron. 1998;21:775–88. doi: 10.1016/s0896-6273(00)80594-2. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Lynn DE. Opiate systems in mother and infant primates coordinate intimate contact during reunion. Psychoneuroendocrinology. 1995;20:735–42. doi: 10.1016/0306-4530(95)00023-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res Brain Res Rev. 1993;18:75–113. doi: 10.1016/0165-0173(93)90008-n. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–11. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Hedonic valence, dopamine and motivation. Mol Psychiatry. 1996;1:186–9. [PubMed] [Google Scholar]
- Kotegawa T, Abe T, Tsutsui K. Inhibitory role of opioid peptides in the regulation of aggressive and sexual behaviors in male Japanese quails. J Exp Zool. 1997;277:146–54. doi: 10.1002/(sici)1097-010x(19970201)277:2<146::aid-jez6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Ryan SM, Arnold AP, Butcher LL. Evidence for a catecholaminergic projection to area X in the zebra finch. J Comp Neurol. 1981;196:347–54. doi: 10.1002/cne.901960212. [DOI] [PubMed] [Google Scholar]
- Lombardino AJ, Nottebohm F. Age at deafening affects the stability of learned song in adult male zebra finches. J Neurosci. 2000;20:5054–64. doi: 10.1523/JNEUROSCI.20-13-05054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney DL, Wingfield JC. Neuroendocrine suppression of female courtship in a wild passerine: corticotropin-releasing factor and endogenous opioids. J Neuroendocrinol. 1998;10:593–9. doi: 10.1046/j.1365-2826.1998.00238.x. [DOI] [PubMed] [Google Scholar]
- Maney DL, Ball GF. Fos-like immunoreactivity in catecholaminergic brain nuclei after territorial behavior in free-living song sparrows. J Neurobiol. 2003;56:163–70. doi: 10.1002/neu.10227. [DOI] [PubMed] [Google Scholar]
- Marcus E, editor. Neuron. 2002. [Google Scholar]
- Margoliash D. Functional organization of forebrain pathways for song production and perception. J Neurobiol. 1997;33:671–93. doi: 10.1002/(sici)1097-4695(19971105)33:5<671::aid-neu12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Stewart J. Facilitation of sexual behaviors in the male rat associated with intra-VTA injections of opiates. Pharmacol Biochem Behav. 1990;35:643–50. doi: 10.1016/0091-3057(90)90302-x. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Yang CR. The contribution of basal forebrain to limbic-motor integration and the mediation of motivation to action. Adv Exp Med Biol. 1991;295:267–90. doi: 10.1007/978-1-4757-0145-6_14. [DOI] [PubMed] [Google Scholar]
- Moses J, Loucks JA, Watson HL, Matuszewich L, Hull EM. Dopaminergic drugs in the medial preoptic area and nucleus accumbens: effects on motor activity, sexual motivation, and sexual performance. Pharmacol Biochem Behav. 1995;51:681–6. doi: 10.1016/0091-3057(94)00437-n. [DOI] [PubMed] [Google Scholar]
- Mountjoy DJ, Lemon RE. Female choice for complex song in the European starling. Behavioral Ecology and Sociobiology. 1995;38:65–71. [Google Scholar]
- Nishino H, Ono T, Muramoto K, Fukuda M, Sasaki K. Neuronal activity in the ventral tegmental area (VTA) during motivated bar press feeding in the monkey. Brain Res. 1987;413:302–13. doi: 10.1016/0006-8993(87)91021-3. [DOI] [PubMed] [Google Scholar]
- Nocjar C, Panksepp J. Prior morphine experience induces long-term increases in social interest and in appetitive behavior for natural reward. Behav Brain Res. 2007;181:191–9. doi: 10.1016/j.bbr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Nordeen KW, Nordeen EJ. Auditory feedback is necessary for the maintenance of stereotyped song in adult zebra finches. Behav Neural Biol. 1992;57:58–66. doi: 10.1016/0163-1047(92)90757-u. [DOI] [PubMed] [Google Scholar]
- Nordeen KW, Nordeen EJ. Long-term maintenance of song in adult zebra finches is not affected by lesions of a forebrain region involved in song learning. Behav Neural Biol. 1993;59:79–82. doi: 10.1016/0163-1047(93)91215-9. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165:457–86. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Herman B, Conner R, Bishop P, Scott JP. The biology of social attachments: opiates alleviate separation distress. Biol Psychiatry. 1978;13:607–18. [PubMed] [Google Scholar]
- Panksepp J, Najam N, Soares F. Morphine reduces social cohesion in rats. Pharmacol Biochem Behav. 1979;11:131–4. doi: 10.1016/0091-3057(79)90002-9. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Bean NJ, Bishop P, Vilberg T, Sahley TL. Opioid blockade and social comfort in chicks. Pharmacol Biochem Behav. 1980;13:673–83. doi: 10.1016/0091-3057(80)90011-8. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Nocjar C, Burgdorf J, Panksepp JB, Huber R. The role of emotional systems in addiction: a neuroethological perspective. Nebr Symp Motiv. 2004;50:85–126. [PubMed] [Google Scholar]
- Panzica GC, Viglietti-Panzica C, Balthazart J. The sexually dimorphic medial preoptic nucleus of quail: a key brain area mediating steroid action on male sexual behavior. Front Neuroendocrinol. 1996;17:51–125. doi: 10.1006/frne.1996.0002. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Phillips AG. Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat. Behav Neurosci. 1991;105:727–43. doi: 10.1037//0735-7044.105.5.727. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Pfaff DW. Mu-, delta-, and kappa-opioid receptor agonists selectively modulate sexual behaviors in the female rat: differential dependence on progesterone. Horm Behav. 1992;26:457–73. doi: 10.1016/0018-506x(92)90014-m. [DOI] [PubMed] [Google Scholar]
- Pinxten R, Eens M. Copulation and mate-guarding patterns in polygynous European starlings. Animal Behaviour. 1997;54:45–58. doi: 10.1006/anbe.1996.0432. [DOI] [PubMed] [Google Scholar]
- Plonsky M, Freeman PR. The effects of methadone on the social behavior and activity of the rat. Pharmacol Biochem Behav. 1982;16:569–71. doi: 10.1016/0091-3057(82)90417-8. [DOI] [PubMed] [Google Scholar]
- Rauceo S, Harding CF, Maldonado A, Gaysinkaya L, Tulloch I, Rodriguez E. Dopaminergic modulation of reproductive behavior and activity in male zebra finches. Behav Brain Res. 2008;187:133–9. doi: 10.1016/j.bbr.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Brauth SE, Kitt CA, Quirion R. Distribution of mu, delta, and kappa opiate receptor types in the forebrain and midbrain of pigeons. J Comp Neurol. 1989;280:359–82. doi: 10.1002/cne.902800304. [DOI] [PubMed] [Google Scholar]
- Riters LV, Absil P, Balthazart J. Effects of naloxone on the acquisition and expression of appetitive and consummatory sexual behavior in male Japanese quail. Physiol Behav. 1999;66:763–73. doi: 10.1016/s0031-9384(99)00014-1. [DOI] [PubMed] [Google Scholar]
- Riters LV, Ball GF. Lesions to the medial preoptic area affect singing in the male European starling (Sturnus vulgaris) Horm Behav. 1999;36:276–86. doi: 10.1006/hbeh.1999.1549. [DOI] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Duffy DL, Balthazart J, Ball GF. Seasonal changes in courtship song and the medial preoptic area in male European starlings (Sturnus vulgaris) Horm Behav. 2000;38:250–61. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Ball GF. Seasonal changes in the densities of alpha(2) noradrenergic receptors are inversely related to changes in testosterone and the volumes of song control nuclei in male European starlings. J Comp Neurol. 2002;444:63–74. doi: 10.1002/cne.10131. [DOI] [PubMed] [Google Scholar]
- Riters LV, Teague DP. The volumes of song control nuclei, HVC and lMAN, relate to differential behavioral responses of female European starlings to male songs produced within and outside of the breeding season. Brain Research. 2003;978:91–8. doi: 10.1016/s0006-8993(03)02771-9. [DOI] [PubMed] [Google Scholar]
- Riters LV, Alger SJ. Neuroanatomical evidence for indirect connections between the medial preoptic nucleus and the song control system: possible neural substrates for sexually motivated song. Cell Tissue Res. 2004;316:35–44. doi: 10.1007/s00441-003-0838-6. [DOI] [PubMed] [Google Scholar]
- Riters LV, Teague DP, Schroeder MB, Cummings SE. Vocal production in different social contexts relates to variation in immediate early gene immunoreactivity within and outside of the song control system. Behav Brain Res. 2004;155:307–18. doi: 10.1016/j.bbr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Riters LV, Schroeder MB, Auger CJ, Eens M, Pinxten R, Ball GF. Evidence for opioid involvement in the regulation of song production in male European starlings. Behavioral Neuroscience. 2005;119:245–255. doi: 10.1037/0735-7044.119.1.245. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manzo G, Fernandez-Guasti A. Opioid antagonists and the sexual satiation phenomenon. Psychopharmacology (Berl) 1995;122:131–6. doi: 10.1007/BF02246087. [DOI] [PubMed] [Google Scholar]
- Ryan SM, Arnold AP, Elde RP. Enkephalin-like immunoreactivity in vocal control regions of the zebra finch brain. Brain Res. 1981;229:236–40. doi: 10.1016/0006-8993(81)90763-0. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote S, Weber SM. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J Pharmacol Exp Ther. 2003;305:1–8. doi: 10.1124/jpet.102.035063. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Sotnikova TD, Gainetdinov RR, Jarvis ED. Social context-dependent singing-regulated dopamine. J Neurosci. 2006;26:9010–4. doi: 10.1523/JNEUROSCI.1335-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder MB, Riters LV. Pharmacological manipulations of dopamine and opioids have differential effects on sexually motivated song production in male European starlings. Physiology and Behavior. 2006;88:575–584. doi: 10.1016/j.physbeh.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Schultz W. Reward signaling by dopamine neurons. Neuroscientist. 2001;7:293–302. doi: 10.1177/107385840100700406. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–63. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Simerly RB, McCall LD, Watson SJ. Distribution of opioid peptides in the preoptic region: immunohistochemical evidence for a steroid-sensitive enkephalin sexual dimorphism. Journal of Comparative Neurology. 1988;276:442–459. doi: 10.1002/cne.902760309. [DOI] [PubMed] [Google Scholar]
- Smith GT, Brenowitz EA, Beecher MD, Wingfield JC. Seasonal changes in testosterone, neural attributes of song control nuclei, and song structure in wild songbirds. J Neurosci. 1997;17:6001–10. doi: 10.1523/JNEUROSCI.17-15-06001.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci. 2007;27:1594–605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Nordeen EJ, Nordeen KW. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biology. 1990;53:51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- Stevenson-Hinde J. Effects of early experience and testosterone on song as a reinforcer. Anim Behav. 1972;20:430–5. doi: 10.1016/s0003-3472(72)80004-6. [DOI] [PubMed] [Google Scholar]
- Stevenson-Hinde J, Roper R. Individual differences in reinforcing effects of song. Anim Behav. 1975;23:729–734. [Google Scholar]
- Szechtman H, Hershkowitz M, Simantov R. Sexual behavior decreases pain sensitivity and stimulated endogenous opioids in male rats. Eur J Pharmacol. 1981;70:279–85. doi: 10.1016/0014-2999(81)90161-8. [DOI] [PubMed] [Google Scholar]
- Tchernichovski O, Lints T, Mitra PP, Nottebohm F. Vocal imitation in zebra finches is inversely related to model abundance. Proc Natl Acad Sci U S A. 1999;96:12901–4. doi: 10.1073/pnas.96.22.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth WR, van Emst MG, van Ree JM. Opioids and sexual behavior of male rats: involvement of the medial preoptic area. Behav Neurosci. 1995a;109:123–34. doi: 10.1037//0735-7044.109.1.123. [DOI] [PubMed] [Google Scholar]
- van Furth WR, Wolterink G, van Ree JM. Regulation of masculine sexual behavior: involvement of brain opioids and dopamine. Brain Res Brain Res Rev. 1995b;21:162–84. doi: 10.1016/0165-0173(96)82985-7. [DOI] [PubMed] [Google Scholar]
- van Furth WR, van Ree JM. Sexual motivation: involvement of endogenous opioids in the ventral tegmental area. Brain Res. 1996;729:20–8. doi: 10.1016/s0006-8993(96)00225-9. [DOI] [PubMed] [Google Scholar]
- Van Ree JM, Niesink RJ, Van Wolfswinkel L, Ramsey NF, Kornet MM, Van Furth WR, Vanderschuren LJ, Gerrits MA, Van den Berg CL. Endogenous opioids and reward. Eur J Pharmacol. 2000;405:89–101. doi: 10.1016/s0014-2999(00)00544-6. [DOI] [PubMed] [Google Scholar]
- Watson REJ, Hoffmann GE, Wiegand SJ. Sexually dimorphic opioid distribution in the preoptic area: manipulation by gonadal steroids. Brain Research. 1986;398:157–163. doi: 10.1016/0006-8993(86)91261-8. [DOI] [PubMed] [Google Scholar]
- Wiley RH, Piper WH, Archawaranon M, Thompson EW. Singing in relation to social dominance and testosterone in white-throated sparrows. Behaviour. 1993;127:175–190. [Google Scholar]
- Wingfield JC, Farner DS. Endocrinology of reproduction in wild species. In: Farner DS, King JR, Parkes KC, editors. Avian Biology. Vol. 9. Academic Press; London: 1993. pp. 163–247. [Google Scholar]
- Winslow JT, Insel TR. Endogenous opioids: do they modulate the rat pup’s response to social isolation? Behav Neurosci. 1991;105:253–63. doi: 10.1037//0735-7044.105.2.253. [DOI] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–40. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine and food reward: back to the elements. Am J Physiol Regul Integr Comp Physiol. 2004a;286:R13. doi: 10.1152/ajpregu.00590.2003. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004b;5:483–94. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wise RA. Forebrain substrates of reward and motivation. J Comp Neurol. 2005;493:115–21. doi: 10.1002/cne.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley SM, Rubel EW. Bengalese finches Lonchura Striata domestica depend upon auditory feedback for the maintenance of adult song. J Neurosci. 1997;17:6380–90. doi: 10.1523/JNEUROSCI.17-16-06380.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara S, Hessler NA. Modulation of singing-related activity in the songbird ventral tegmental area by social context. Eur J Neurosci. 2006;24:3619–27. doi: 10.1111/j.1460-9568.2006.05228.x. [DOI] [PubMed] [Google Scholar]
- Zann RA, Bamford M. The zebra finch: A synthesis of field and laboratory studies. Oxford University Press; 1996. [Google Scholar]
- Zeigler HP, Marler P, editors. Behavioral Neurobiology of Birdsong. New York Academy of Sciences; New York: 2004. [Google Scholar]