Abstract
This study examined whether the interaction between the serotonin transporter promoter region (5-HTTLPR) and brain-derived neurotrophic factor (BDNF) Val66Met polymorphisms was associated with hypothalamic-pituitary-adrenal (HPA) axis reactivity to stress. A community sample of 144 preschool-aged children was genotyped and exposed to stress-inducing laboratory tasks. Salivary cortisol was obtained at four time points during a standardized laboratory assessment before and after stressors involving separation from a parent and frustrating tasks. Children homozygous for the short-5-HTTLPR allele and carrying the Met-BDNF allele evidenced a significantly lower initial level of cortisol, followed by a positive increase in cortisol in response to the laboratory stressors. In contrast, children who were homozygous for the short-5-HTTLPR and the Val-BDNF alleles evidenced a greater decline in cortisol in response to the laboratory stressors. Findings indicated that the BDNF gene moderated the association between 5-HTTLPR and children’s biological stress responses, suggesting that epistatic effects play a role in individual differences in stress regulation, and possibly genetic vulnerability to stress-related disorders.
Keywords: HPA axis reactivity, cortisol, serotonin transporter gene, BDNF, stress, multilevel modeling
Despite the evidence for a significant genetic contribution to psychiatric diseases, attempts at identifying candidate genes have been largely unsuccessful (e.g., Munafo, Durrant, Lewis, & Flint, 2009; Risch et al., 2009). However, more consistent findings have emerged from studies examining genes associated with intermediate phenotypes of psychiatric diseases (Gottesman & Gould, 2003), such as increased amygdala activation in response to aversive stimuli (Munafo, Brown, & Hariri, 2008) and anxiety-related personality traits (Lesch et al.,1996; Munafo, Clark & Flint, 2005). Likewise, the hypothalamic-pituitary-adrenal (HPA) axis, one of the body’s main biological systems mediating the neuroendocrine response to stress, has been hypothesized as an intermediate phenotype for stress-related disorders (Flint & Munafo, 2007; Hasler, Drevets, Manji, & Charney, 2004).
Abnormalities in HPA axis responses to stress have been documented in numerous stress-related disorders (Ehlert, Gaab, Heinrichs, 2001; McEwen, 2008), including depression (Burke, Davis, Otte, & Mohr, 2005; Lopez-Duran, Kovacs, & George, 2009), anxiety disorders (Bremner et al., 2003; Leyton et al., 1996), schizophrenia (Jansen et al., 1998), substance use disorders (Lovallo, Dickensheets, Myers, Thomas, & Nixon, 2000) and a number of negative health outcomes (McEwen, 2008). During acute stress, there is an increase in the secretion of cortisol, an adrenocortical steroid hormone, and in secretions from the corticotropin-releasing factor. However, chronic HPA axis activation, referred to as allostatic load, has been shown to lead to disruptions in the regulation and negative feedback of the HPA axis, resulting in adverse biological and health consequences, including immune system dysfunction, neuronal damage in the hippocampus, diabetes, hypertension and psychiatric diseases (McEwen, 2008; Meyer, Chrousos, & Gold, 2001).
Twin studies examining the heritability of HPA axis function have demonstrated a significant hereditary component in basal free cortisol levels (Bartels, de Geus, Kirschbaum, Sluyter, & Boomsma, 2003) and in response to a laboratory stressor (Federenko, Nagamine, Hellhammer, Wadhwa, & Wust, 2004). Only recently have studies begun to investigate the influence of genetic polymorphisms on HPA axis function. One gene that has received considerable attention in studies examining the genetic vulnerability to life stress, and that has been hypothesized to influence HPA axis function, is the serotonin transporter (5-HTT) gene, specifically a polymorphism in the promoter region of this gene (5-HTTLPR) (Caspi et al., 2003; Uher & McGuffin, 2008). Animal studies have provided evidence that 5-HTT impacts HPA activity (Li et al., 1999; Barr et al., 2004; Jiang, Wang, Luo, & Li, 2008). In addition, recent studies in humans have found that the short allele of 5-HTTLPR, which leads to reduced transcription of 5-HTT relative to the long allele (Lesch et al., 1995), is associated with higher basal cortisol levels (Chen, Joormann, Hallmayer, & Gotlib, 2009; O’Hara et al, 2007; Wust et al., 2009) and greater cortisol reactivity (Alexander et al., 2009; Gotlib, Joormann, Minor, & Hallmayer, 2008; Jabbi et al., 2007). Some findings suggest that this relation differs by gender and/or specific features of neuroendocrine function (Jabbi et al., 2007; Wust et al., 2009); however, this remains unclear given the limited data.
In addition to 5-HTT, brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family of growth factors, is hypothesized to influence the body’s neuroendocrine response to stress, and is central to models that view neurobiological responses to stress as playing a key role in understanding the pathophysiology of stress-related disorders (Duman & Monteggia, 2006). BDNF is involved in the development of the central nervous system (CNS) and the regulation of basic neuronal function (Bath & Lee, 2006), and also protects neurons from the damaging effects of stress (Bergstrom, Jayatissa, Mork, & Wiborg, 2008). Animal research has shown that BDNF influences HPA axis activity in rats (Givalois et al., 2004; Naert, Ixart, Tapia-Arancibia, & Givalois, 2006). These findings suggest that BDNF may be involved in mechanisms underlying HPA axis function in humans.
Most attention has focused on a valine (Val) to methionine (Met) substitution at codon 66 (Val66Met) in the BDNF gene. The Met-BDNF allele is associated with reduced BDNF activity (Chen et al., 2004) and reduced hippocampal volumes (Frodl et al., 2007), and has been linked to stress-related symptoms in animals (Chen et al., 2006) and humans (Kim et al., 2007; Wichers et al., 2008) [for conflicting findings, see Lang et al., 2005; Sen et al., 2003]. In addition, two recent studies in humans have reported an association between the BDNF Val66Met polymorphism and HPA axis activity in adults. Schule et al. (2006) found that homozygous Met-BDNF carriers evidenced greater HPA axis activity. Similarly, Shalev et al. (2009) found that females with a Met-BDNF allele evidenced a greater rise in cortisol is response to a laboratory challenge than Val-BDNF homozygotes, whereas the opposite was found for males. Taken together, both 5-HTTLPR and BDNF appear to be possible candidate genes underlying stress reactivity.
Importantly, 5-HTT and BDNF interact at intracellular and intercellular levels (Duman, Heninger, & Nestler, 1997). Evidence suggests that these genes have a synergistic influence on HPA axis functioning. For example, 5-HTT knockout mice bred with BDNF heterozygous mice evidence increased stress hormones compared to mice with knockouts in only one system (Ren-Patterson et al., 2005). In addition, two studies found that the combination of the short-5-HTTLPR allele and the Met-BDNF allele predicted depressive symptomatology in the presence of environmental adversity in children and adults (Kaufman et al., 2006; Kim et al., 2007); however, conflicting findings suggest that the Met-BDNF allele has a protective effect on the impact of the short-5-HTTLPR allele on brain morphology (Pezawas et al., 2008). Nevertheless, these findings raise the possibility that BDNF may interact with 5-HTTLPR to influence HPA axis reactivity to stress, thereby rendering certain individuals more vulnerable to life stress. However, no studies have directly examined this gene-gene interaction on HPA axis function.
This study aimed to add to the limited research examining the effects of 5-HTTLPR and BDNF genes on HPA axis reactivity. We were interested in advancing the understanding of epistasis as it relates to HPA axis functioning, particularly during early childhood, which appears to be a critical period in the development of the HPA axis system (Gunnar & Vazquez, 2006). We hypothesized that the interaction between 5-HTTLPR and BDNF Val66Met would be associated with individual differences in children’s cortisol responses to laboratory stressors. Specifically, we hypothesized that children homozygous for the short-5-HTTLPR allele and carrying the Met-BDNF allele would exhibit a greater increase in cortisol reactivity to laboratory stressors than other genotype groups, consistent with findings from animal (Ren-Patterson et al., 2005) and human studies (Kaufman et al., 2006; Kim et al., 2007).
No prior research has examined the influence of genetic polymorphisms on HPA axis functioning in young children. Understanding the genetic factors involved in the early regulation and development of the HPA axis is particularly important as the stress system affects the development, organization, and plasticity of many neural systems (Gunnar & Quevedo, 2007), and provides a means to understanding early-emerging developmental trajectories of adjustment and maladjustment.
Method
Participants
The sample was obtained from a consecutive series of 166 children who were recruited from a larger community sample participating in a study on temperament and risk for depression (N = 559). Of the 166 children asked to participate in the cortisol and genetic assessment, 156 children completed both the laboratory salivary cortisol samplings and provided buccal swabs for genetic analysis. Of the 156 children, 12 children were excluded, yielding a final sample of 144 children (50% female): one child whose BDNF genotyping yielded discrepant results (described below) and 11 children of non-Caucasian or unknown ethnicity were excluded. We chose to restrict our sample to an ethnically homogenous sample (i.e., Caucasian only) because ethnicity-related differences in allelic frequencies have been reported for these genes (Kim et al., 2007; Shimizu, Hashimoto, & Iyo, 2004). Given that the optimal approach to addressing the effects of ethnicity-related or other population-related genetic differences are controversial, we chose a conservative approach to limit sources of bias (Hutchison, Stallings, McGeary, & Bryan, 2004; Wacholder, Rothman, & Caporaso, 2002).1
The sample was recruited using a commercial mailing list. Children between the ages of 3 and 4 years, with no significant medical or developmental disabilities, and who lived with at least one biological parent were eligible. The mean age of the children from the subsample was 43.2 months (SD = 2.4). Most of the participants came from middle class, two-parent (98.1%) families. Children were of average cognitive ability as indexed by the Peabody Picture Vocabulary Test (Dunn & Dunn, 1997; M = 105.04, SD = 14.08) and the Expressive One-Word Picture Vocabulary Test (Brownell, 2000; M = 100.48, SD = 12.47). The Committees on Research Involving Human Subjects at Stony Brook University approved and oversaw the study. Families were compensated financially.
Procedures
The child and one parent attended an initial laboratory session that started at either 1000h (69.4%) or 1400h. During the initial laboratory visit, children participated with a female experimenter in 12 standardized tasks selected from the Laboratory Temperament Assessment Battery (Lab-TAB; Goldsmith, Reilly, Lemery, Longley, & Prescott, 1995), which includes tasks designed to elicit a range of behavioral and emotional expressions from the child. Parents were asked to refrain from feeding their child for one hour prior to coming to the laboratory, and from giving their child caffeinated products for two hours prior, and dairy products 15 minutes prior, to the session, as these factors are known to alter cortisol values (Gunnar & Talge, 2008).
The timing of the salivary cortisol samples was determined based on findings that salivary cortisol levels reflect the level of stress experienced in the prior 20–40 minutes (Dickerson & Kemeny, 2004; Gunnar & Talge, 2008) and on previous studies using similar stress-inducing paradigms, which have been shown to be sensitive to individual differences in cortisol reactivity in preschool-age children (Luby et al., 2003; Talge, Donzella, & Gunner, 2008). In order to obtain a baseline sample, the first sample was collected 20 minutes following adaptation to the laboratory during which time the child played quietly with the experimenter. This period of adaptation was based on previous developmental neuroendocrine research in young children (Gunnar & Talge, 2008; Talge et al., 2008). The second sample was collected 30 minutes following the Stranger Approach task of the Lab-TAB, during which the child was separated from his/her parent and a stranger entered the room. The third salivary cortisol sample was taken 60 minutes after the Stranger Approach task, which was 30 minutes after a qualitatively different frustration-inducing laboratory stressor (i.e., the child not being able to unlock a transparent box with a desirable toy inside). The final sample was collected 20 minutes after the final Lab-TAB task, which was another frustration-inducing laboratory stressor. During this task, the child was left alone with a wrapped empty box to open, under the pretense that an appealing toy was inside. After two minutes, the experimenter returned and explained that she forgot to put the child’s gifts in the box and presented the gifts to the child. Lastly, during a scheduled break in between tasks, the child’s buccal cells were collected for genetic analysis.
Measures
Salivary cortisol
Saliva for cortisol determination was obtained by having children dip 2 in. long cotton dental rolls into .025g of cherry Kool-Aid® mix. Children then placed the cotton in their mouths until saturated. These procedures are known to have little-to-no effect on cortisol concentrations given the assay procedures used (Talge, Donzella, Kryzer, Gierens, & Gunnar, 2005). The wet cotton was collected and then expressed into vials for storage at −20 C until assayed. Samples were later shipped to the Biochemistry Laboratory at the University of Trier, Germany. Samples were assayed in duplicate, using a time-resolved fluorescence immunoassay with flourometric end point detection (DELFIA). Inter- and intra-assay coefficients of variation are 7.1% – 9.0% and 4.0% – 6.7%, respectively.
As indicated above, four samples were taken in the laboratory and the timing of the cortisol samples was based on the presumed stress-inducing qualities of the tasks. The initial sample was taken after consent and acclimation to the laboratory (0 min), 30 minutes after the Stranger Approach task (+60 min after the initial sample), 60 minutes after Stranger Approach (+90 min), and 20 minutes after the final Lab-TAB task (+130 min).
Genotyping
Children’s buccal cells were collected for genetic analysis by rubbing the inside of each participant’s cheek with two swabs (Epicentre Biotechnologies MasterAmp™ Buccal Swab Kits, Madison, WI). Genomic DNA was successfully extracted for all 156 children. For the purposes of another study, two laboratories independently genotyped 92.3% of the sample. There was 100% agreement for the 5-HTTLPR polymorphism and 1 discrepant genotype for BDNF Val66Met. This subject was excluded, as were 11 children of non-White or unknown ethnicity, leaving a sample of 144 children for the present study.
The genotypes for 5-HTTLPR and BDNF were obtained following previously published protocols (Hunnerkopf, Strobel, Gutknecht, Brocket, & Lesch, 2007; Lesch et al., 1996). Briefly, for 5-HTTLPR, the polymorphic region was amplified by polymerase chain reaction (PCR) with oligonucleotide primers: forward-5’-GAG GGA CTG AGC TGG ACA AC-3’; reverse-5’-GCA GCA GAC AAC TGT GTT CAT C-3’. PCR started with an initial denaturation at 95° C for 3 minutes, followed by 45 seconds at 95° C, 45 seconds at 61.2° C, 45 seconds at 72° C for 30 cycles, and a final extension at 72° C for 3 minutes. PCR products were separated on a 3% agarose gel containing ethidium bromide, and bands were visualized under UV light. For the 5-HTTLPR genotype, the resulting product with 585 bp carries the short allele (s), whereas the resulting product with the 629 bp carries the long allele (l). Of the 144 children, 28 (19.4%) were homozygous for the short allele (s/s), 71 (49.3%) were heterozygous (s/l), and 45 (31.2%) were homozygous for the long allele (l/l). The distribution of genotypes is in Hardy-Weinberg equilibrium. Our primary 5-HTTLPR genotype analyses compared children who were homozygous for the short allele to those with at least one copy of the long allele (s/s vs. s/l or l/l), given previous findings that youth homozygous for the s allele exhibited heightened cortisol reactivity (Gotlib et al., 2008; Jabbi et al., 2007). However, following Lesch et al. (1996), we also compared participants with at least one short allele to those who were homozygous for the long allele on HPA axis reactivity (s/s or s/l vs. l/l).
For BDNF, the polymorphic region was first amplified by PCR with oligonucleotide primers: forward-5’-AAA GAA GCA AAC ATC CGA GGA CAA-3’; reverse-5’-ATT CCT CCA GCA GAA AGA GAA GAG G-3’. PCR started with an initial denaturation at 95° C for 3 minutes, followed by 45 seconds at 95° C, 45 seconds at 62° C, 45 seconds at 72° C for 35 cycles, and a final extension at 72° C for 3 minutes. The resulting 274 bp fragment was incubated for three hours with the restriction enzyme NlaIII (New England Biolabs, Ipswich, MA). The resulting digested PCR products were separated on a 5% agarose gel containing ethidium bromide, and bands were visualized under UV light and designated as Met or Val. In our sample, 94 children (65.3%) were homozygous for the Val/Val genotype, 41 (28.5%) were heterozygous, and 9 (6.2%) were homozygous for the Met/Met genotype, which is consistent with reports of the allelic distribution in White samples (Shimizu et al., 2004); the distribution of genotypes is in Hardy-Weinberg equilibrium. Analyses contrasted children with at least one Met allele with those with the Val/Val genotype, as the Met variant is associated with alterations in brain anatomy, memory, and reduced neuronal BDNF secretory activity than the Val/Val genotype (Bath & Lee, 2006; Chen et al., 2006).
Potential confounds
Several factors were assessed as potential confounds on children’s cortisol levels: age, gender, time of laboratory visit, hours of sleep the night prior to the laboratory sampling, observer-rated activity level during the laboratory visit, and parent-reported internalizing (α = .84) and externalizing problems (α = .90) as assessed using the Child Behavior Checklist/1½–5 (CBCL/1½ –5; Achenbach & Rescorla, 2000). Child activity level (the vigor, energy, and the extent of the child’s physical movements) was coded on a single scale for each episode of the Lab-TAB and averaged across episodes to examine the influence of activity level on children’s cortisol levels. Both the internal consistency across episodes (α = .73) and interrater reliability (intraclass correlation coefficient [ICC] = .85, assessed on a subsample of 28 cases) were acceptable.
Data analysis strategy
Cortisol data were log10 transformed prior to analysis to correct for positive skew (Gunnar & Talge, 2008) and treated as the dependent variable. Three cortisol outliers (>44 nmol/L) were removed from the data: one from the initial sample and two from the final sample.
Estimates of children’s cortisol reactivity based on each child’s four cortisol samples over the course of the laboratory session were obtained using multilevel growth curve modeling. A two-level multilevel analysis (Singer & Willet, 2003) was performed to model children’s cortisol activity in response to the laboratory stressors and to examine associations between genetic polymorphisms and the components of the growth curve defining individual differences in cortisol reactivity (i.e., intercept, linear slope, and quadratic curvature). Multilevel or mixed effects modeling was selected as it accounts for the nested data structure (cortisol levels nested within individuals) and adjusts for correlated error within each level. The multilevel model was estimated using the Hierarchical Linear Modeling (HLM) statistical program version 6 (SSI Inc., Lincolnwood, IL).
Two levels of analysis were estimated. Level 1 estimates individual-level change in cortisol over time. Specifically, we estimated each child’s cortisol levels at arrival to the laboratory (i.e., intercepts) and his/her change in cortisol in response to the laboratory stressors (i.e., linear slope and quadratic curvature). Both linear and quadratic growth models were used to examine a within-subjects regression of an individual’s cortisol reactivity onto the time of each assessment. Level 2 examines person-level differences in change. On the second level, we examined person-level genetic predictors and their interaction on cortisol activity, along with several covariates that may influence cortisol levels. The person-level covariates included: time of visit, hours of sleep the night prior to the assessment, activity level, and internalizing and externalizing problems. Any significant covariates were included in the final model to test whether they account for the relation between genetic polymorphisms and cortisol activity.
Three adjustments were made to the data to ease interpretation of the results. First, time was anchored at the first cortisol sample (time = 0) so that the cortisol intercepts would reflect the average individual’s cortisol level at arrival to the laboratory. Second, all Level 2 between-person variables were centered at their grand mean. Third, we used a pairwise missing data procedure to handle any missing data at Level 1 so the sample size varied slightly by analysis. N’s are reported for each analysis.
Results
Descriptive analyses
Table 1 shows the means, standard deviations, and N’s for the covariates and cortisol levels in nanomoles per liter (nmol/L) for the total sample and by 5-HTTLPR×BDNF genotypes. We examined whether the 5-HTTLPR (ss, sl, ll), BDNF (met/met, met/val, val/val), and 5-HTTLPR×BDNF genotypes differed on any confounding variable listed in Table 1. No significant differences between genotypes were observed.
Table 1.
Descriptive statistics of sample
| Variable | Total sample |
5-HTTLPR s/s and BDNF Val/Met or Met/Met |
5-HTTLPR s/s and BDNF Val/Val |
5-HTTLPR s/l or l/l and BDNF Val/Met or Met/Met |
5-HTTLPR s/l or l/l and BDNF Val/Val |
|---|---|---|---|---|---|
| N | 144 | 9 | 19 | 41 | 75 |
| Gender (male/female) | 72/72 | 8/1 | 8/11 | 18/23 | 38/37 |
| Age (months) | 43.50 (2.79) | 43.44 (3.91) | 44.63 (3.30) | 43.37 (2.71) | 43.29 (2.54) |
| 1000h visit/ 1400h visit | 100/44 | 6/3 | 15/4 | 29/12 | 50/25 |
| CBCL internalizing problems | 9.01 (6.42) | 8.00 (4.17) | 8.75 (6.88) | 9.09 (5.20) | 9.27 (7.21) |
| CBCL externalizing problems | 12.66 (7.22) | 15.09 (9.45) | 11.94 (6.80) | 11.37 (6.19) | 13.32 (7.67) |
| Global activity score | 1.58 (.31) | 1.62 (.24) | 1.48 (.34) | 1.57 (.34) | 1.61 (.30) |
| Hours of sleep | 10.28 (1.44) | 10.56 (.85) | 9.97 (1.12) | 10.42 (1.32) | 10.24 (1.64) |
| Cortisol level at time 1 (nmol/L) | 4.134 (5.829) | 2.048 (.852) | 3.687 (4.605) | 5.487 (7.954) | 3.794 (4.993) |
| N = 143 | N = 9 | N = 19 | N = 40 | N = 75 | |
| Cortisol level at time 2 (nmol/L) | 3.840 (5.703) | 2.752 (1.978) | 2.114 (.8040) | 5.532 (8.471) | 3.485 (4.566) |
| N = 144 | N = 9 | N = 19 | N = 41 | N = 75 | |
| Cortisol level at time 3 (nmol/L) | 3.807 (3.959) | 3.369 (1.179) | 3.583 (4.364) | 4.776 (5.940) | 3.364 (2.387) |
| N = 144 | N = 9 | N = 19 | N = 41 | N = 75 | |
| Cortisol level at time 4 (nmol/L) | 5.193 (3.423) | 4.348 (2.121) | 5.785 (2.118) | 5.920 (4.715) | 4.768 (2.949) |
| N = 142 | N = 9 | N = 19 | N = 39 | N = 75 |
Notes. 5-HTTLPR = serotonin transporter promotor region polymorphism: s = short and l = long. BDNF = brain-derived neurotrophic factor Val66Met polymorphism: Val = valine and Met = methionine. CBCL = Parent-reported Child Behavior Checklist 1½ –5; nmol/L = nanomoles/Liter.
Multilevel analyses examining differences in cortisol response activity
As seen in Table 2, the baseline trajectory model demonstrated that there was a significant linear decrease in cortisol values from arrival to the laboratory to children’s cortisol levels following the separation stressor (sample 2). Following the separation stressor, children’s cortisol levels began to rise for sample 3 (30 minutes after a frustrating task) and sample 4 (20 minutes after the last Lab-TAB task), as indicated by a significant positive quadratic effect (i.e., concave up). The growth curve measuring children’s cortisol reactivity and the salivary cortisol values, obtained in this investigation, were similar to other studies of preschoolers using analogous stress-inducing laboratory tasks conducted at similar times of the day (Luby et al., 2003; Talge et al., 2008), and similar decreases in response to stressors have been observed in other studies (e.g., Gotlib et al., 2008; Gunnar, Frenn, Wewerka, & Van Ryzin, 2009; for a review, see Gunnar, Talge, & Herrera, 2009).
Table 2.
Univariate multilevel analyses of associations between potential confounds and cortisol activity
| Fixed effect | β | SE | T | P | N |
|---|---|---|---|---|---|
| Cortisol intercept | .466 | .025 | 18.400 | .000 | 144 |
| Time of visit | −.104 | .051 | −2.069 | .040 | 144 |
| Age | −.015 | .008 | −1.963 | .051 | 144 |
| Gender | −.060 | .050 | −1.195 | .235 | 144 |
| Hours of sleep | −.016 | .015 | −1.043 | .299 | 133 |
| Global activity level | .016 | .066 | .240 | .811 | 144 |
| CBCL internalizing problems | .001 | .003 | .321 | .749 | 136 |
| CBCL externalizing problems | .000 | .003 | .014 | .988 | 136 |
| Cortisol linear slope | −.087 | .017 | −5.248 | .000 | 144 |
| Time of visit | .057 | .036 | 1.569 | .119 | 144 |
| Age | .004 | .006 | .851 | .397 | 144 |
| Gender | .025 | .033 | .770 | .443 | 144 |
| Hours of sleep | .022 | .008 | 2.594 | .011 | 133 |
| Global activity level | .018 | .050 | .347 | .729 | 144 |
| CBCL internalizing problems | −.003 | .003 | −1.119 | .266 | 136 |
| CBCL externalizing problems | −.000 | .003 | −.092 | .927 | 136 |
| Cortisol quadratic curvature | .030 | .004 | 8.006 | .000 | 144 |
| Time of visit | −.015 | .008 | −1.913 | .057 | 144 |
| Age | −.000 | .001 | −.301 | .764 | 144 |
| Gender | −.008 | .008 | −1.005 | .317 | 144 |
| Hours of sleep | −.005 | .002 | −2.693 | .008 | 133 |
| Global activity level | −.004 | .022 | −.438 | .662 | 144 |
| CBCL internalizing problems | .001 | .001 | 1.439 | .152 | 136 |
| CBCL externalizing problems | .000 | .001 | .266 | .791 | 136 |
| Random effect of unconditional growth curve |
Variance Component |
SD | χ2 | P | df |
| Level 1 intercept | .081 | .284 | 1094.736 | .000 | 143 |
| Linear slope | .026 | .163 | 432.862 | .000 | 143 |
| Quadratic curvature | .001 | .037 | 427.915 | .000 | 143 |
Notes. All fixed effects are robust standard errors. All Level 1 predictors are uncentered. Level 2 variables are grand mean centered. SE = standard error; SD = standard deviation; Time of visit: 1000h = 0 and 1400h = 1; Gender: Males = 0 and Females = 1; CBCL = parent-report Child Behavior Checklist 1½–5.
As seen in Table 2, the random error terms associated with the intercept, linear, and quadratic components were significant, demonstrating variability among children’s cortisol activity across the visit, which supports the examination of between-person predictors of each of these components.
Potential confounds in children’s cortisol activity
Prior to examining the genetic predictors of the growth curve, we examined potential confounds. As seen in Table 2, time of laboratory visit, age, and hours of sleep were significantly associated with components of the growth curve, whereas gender, children’s internalizing and externalizing symptoms and activity level were not significantly associated with the growth curve. The significant association between time of visit (1000h vs. 1400h) and the intercept of the growth curve reflects the diurnal rhythm in cortisol levels across the day (i.e., higher levels after awakening and lower levels later in the day).
Genetic polymorphisms and cortisol reactivity
For each analysis, we included the following Level 2 covariates: time of visit, age, gender, and hours of sleep. We did not observe any significant interactions with gender. Results were similar whether hours of sleep were included as a covariate or not; therefore, we removed it from analyses as it limited the sample size as a result of missing data for that variable. We examined the main effects of 5-HTTLPR and BDNF on children’s cortisol reactivity. Along with time of visit, age, and gender, both 5-HTTLPR and BDNF were included at each Level 2 parameter (i.e., intercept, linear slope, quadratic curvature). BDNF was not significantly associated with any component of the growth curve, and 5-HTTLPR s/s alleles were significantly associated with a greater positive increase in curvature.
The main effect for 5-HTTLPR was qualified by the interaction between 5-HTTLPR and BDNF. As seen in Table 3, the interaction between 5-HTTLPR and BDNF was significantly associated with the intercept, slope and curvature of the growth curve. In order to probe the interaction, we created four Level 2 dummy variables to capture the following genotypes: (1) 5-HTTLPR s/s and BDNF Val/Met or Met/Met (N=9); (2) 5-HTTLPR s/s and BDNF Val/Val (N=19); (3) 5-HTTLPR s/l or l/l and BDNF Val/Met or Met/Met (N=41); (4) 5-HTTLPR s/l or l/l and BDNF Val/Val (N=75).
Table 3.
Multilevel model of associations between 5-HTTLPR and BDNF genotypes and children’s cortisol activity
| Fixed effect | β | SE | T | P | |
|---|---|---|---|---|---|
| Cortisol intercept | .466 | .024 | 19.297 | .000 | |
| Time of visit | −.081 | .050 | −1.622 | .125 | |
| Age | −.014 | .008 | −1.638 | .103 | |
| Gender | −.078 | .051 | −1.541 | .125 | |
| 5-HTTLPR | .004 | .071 | .063 | .951 | |
| BDNF | .087 | .066 | 1.326 | .187 | |
| 5-HTTLPR×BDNF | −.301 | .113 | −2.656 | .009 | |
| Cortisol linear slope | −.087 | .016 | −5.580 | .000 | |
| Time of visit | .038 | .035 | 1.085 | .280 | |
| Age | .007 | .005 | 1.365 | .175 | |
| Gender | .040 | .033 | 1.210 | .229 | |
| 5-HTTLPR | −.140 | .043 | −3.259 | .002 | |
| BDNF | −.025 | .038 | −.642 | .521 | |
| 5-HTTLPR×BDNF | .272 | .102 | 2.670 | .009 | |
| Cortisol quadratic curvature | .030 | .004 | 8.630 | .000 | |
| Time of visit | −.010 | .007 | −1.416 | .159 | |
| Age | −.001 | .001 | −.838 | .404 | |
| Gender | −.010 | .007 | −1.332 | .185 | |
| 5-HTTLPR | .038 | .009 | 4.226 | .000 | |
| BDNF | .006 | .009 | .649 | .517 | |
| 5-HTTLPR×BDNF | −.060 | .023 | −2.641 | .010 | |
| Random effect of unconditional growth curve |
Variance Component |
SD | χ2 | P | |
| Level 1 intercept | .076 | .275 | 985.294 | .000 | |
| Linear slope | .023 | .152 | 377.517 | .000 | |
| Quadratic curvature | .001 | .034 | 360.028 | .000 | |
Note. N = 144; for t tests, df = 137. All fixed effects are with robust standard errors. All Level 1 predictors are uncentered; Level 2 variables are grand mean centered. SE = standard error; SD = standard deviation; Time of visit: 1000h = 0 and 1400h = 1; Gender: Males = 0 and Females = 1; 5-HTTLPR = serotonin transporter promotor region polymorphism: s/l and l/l = 0 and s/s = 1; BDNF = brain-derived neurotrophic factor Val66Met polymorphism: Val/Val = 0 and Val/Met and Met/Met = 1.
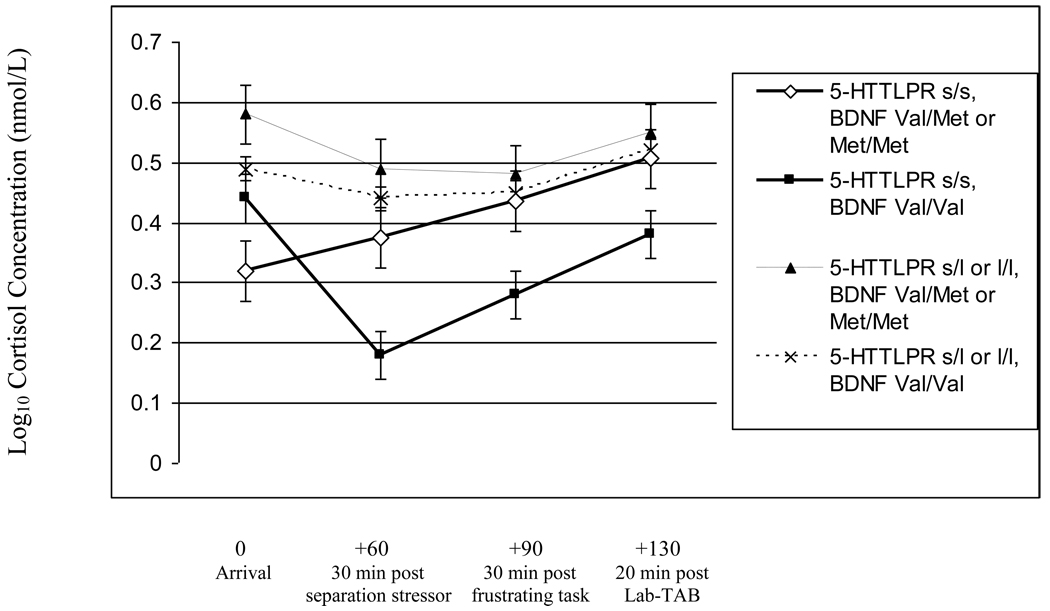
As seen in Figure 1, children homozygous for the short-5-HTTLPR allele and carrying the Met-BDNF allele had significantly lower cortisol levels at arrival compared to children homozygous for both the short-5-HTTLPR and the Val-BDNF alleles (β = −.169, SE = .081, t(25) = −2.089, p = .047); children carrying the long-5-HTTLPR and the Met-BDNF alleles (β = −.261, SE = .078, t(47) = −3.331, p = .002); and children carrying the long-5-HTTLPR and who were homozygous for the Val-BDNF alleles (β = −.181, SE = .061, t(81) = −2.990, p = .004);. In addition, children homozygous for the short-5-HTTLPR allele and carrying the Met-BDNF allele demonstrated continuously increasing cortisol levels across the lab visit as indicated by significant associations with the slope (β = .238, SE = .092, t(25) = 2.586, p = .016) and curvature (β = −.053, SE = .019, t(103) = −2.696, p = .009) compared to children homozygous for both the short-5-HTTLPR and the Val-BDNF alleles.
Figure 1.
Multilevel regression coefficients indicating children’s log10 transformed cortisol levels in nanomoles/Liter (nmol/L) as a function of serotonin transporter gene polymorphism (5-HTTLPR) and brain-derived neurotrophic factor (BDNF) genotypes (s indicates short allele and l indicates long allele; Val indicates valine and Met indicates methionine). Regression coefficients were adjusted to account for time of cortisol data collection. Bars reflect standard error of measurement.
In contrast, children homozygous for both the short-5-HTTLPR and the Val-BDNF alleles had greater initially declining cortisol levels followed by an attenuated rise in cortisol compared to children who were homozygous for the short-5-HTTLPR allele and carrying the Met-BDNF allele; children carrying the long-5-HTTLPR allele and the Met-BDNF allele; and children carrying the long-5-HTTLPR allele and who were homozygous for the Val-BDNF allele. Specifically, children homozygous for the short-5-HTTLPR and the Val-BDNF alleles had a greater decline in cortisol levels compared to children carrying the long-5-HTTLPR allele and the Met-BDNF allele, as evidenced by significant associations with the slope (β = −.102, SE = .085, t(57) = −2.106, p = .039) and curvature (β = .032, SE = .010, t(57) = 3.115, p = .003) of the growth curve. Likewise, children homozygous for the short-5-HTTLPR and the Val-BDNF alleles also had a greater decline in cortisol levels compared to children carrying the long-5-HTTLPR allele and who were homozygous for the Val-BDNF allele, as evidenced by significant associations with the slope (β = −.125, SE = .043, t(91) = −2.928, p = .005) and curvature (β = .035, SE = .010, t(91) = 3.943, p = .000) of the growth curve.
No other genotypes significantly differed from one another. The 5-HTTLPR-BDNF interaction was not observed when the s/s and s/l groups were combined and compared to the l/l group. Lastly, given that the time of the laboratory session was a significant confound, we repeated our analyses with participants who attended the 1000h laboratory session only (~ 70% of sample), and results were similar.
Discussion
This study is the first to demonstrate the epistatic effects of 5-HTTLPR and BDNF on HPA axis activity. In a community sample of preschoolers, we found that the homozygous short-5-HTTLPR genotype was associated with different patterns of stress reactivity as a function of BDNF. Children homozygous for the short-5-HTTLPR and carrying the Met-BDNF allele evidenced a lower initial cortisol level followed by a positive increase in cortisol from the laboratory stressors. In contrast, children who were homozygous for both the short-5-HTTLPR allele and the Val-BDNF allele exhibited a greater decline in cortisol in response to the laboratory stressor. Children carrying the long-5-HTTLPR genotype did not significantly differ from one another as a function of BDNF and exhibited flat growth curves, with non-significant effects. Our findings demonstrated that genetic polymorphisms influenced individual differences in reactivity and/or regulation of the HPA axis in response to a laboratory stressor.
In order to understand the differential cortisol response patterns we observed, we need to address issues that arise when assessing cortisol reactivity in young children (Gunnar & Talge, 2008; Gunnar, Talge, et al., 2009). In developmental neuroendocrine research, studies have typically failed to show a mean positive increase in cortisol responses to laboratory stressors from late infancy throughout middle childhood (Gunnar, Talge, et al., 2009). It has been hypothesized that this may reflect a stress-hyporesponsive period in the development of the HPA system. Alternatively, it may be a function of the inadequacy of laboratory stress-inducing paradigms employed with young children, which are confined by ethical and developmental constraints (Gunnar, Talge, et al., 2009). Additionally, obtaining pre-stress baseline cortisol samples in developmental research is particularly difficult as the time required to achieve a valid baseline in young children remains unclear and young children typically cannot tolerate long laboratory sessions that incorporate extended adaptation periods (Gunnar & Talge, 2008). We chose to incorporate a developmentally appropriate adaptation period (Talge et al., 2008); however, it is possible that it was not sufficient and may reflect cortisol responses to coming to the lab or other unknown factors present prior to the laboratory visit; therefore, consistent with recent developmental neuroendocrine research, we interpret the initial sample, along with the subsequent cortisol samples, as part of the continuous reactivity of the HPA axis (Gunnar & Talge, 2008).
In spite of these methodological issues present in developmental neuroendocrine studies, researchers have sought to delineate individual differences in children’s cortisol reactivity in order to identify individuals who may be more vulnerable to heightened or prolonged HPA activation (Gunnar & Quevedo, 2007; Gunnar & Vazquez, 2006). Specifically, a large body of research has established the influence of social-environmental factors (parenting, peers, maltreatment) and within-person factors (i.e., emotions and behaviors) on individual differences in children’s HPA axis reactivity (Gunnar & Donzella, 2002; Gunnar & Quevedo, 2007). However, none of the prior research has investigated the role of genetic polymorphisms on children’s HPA axis reactivity, which is particularly important given the significant heritability of the HPA axis (Bartels et al., 2003; Frederenko et al., 2004), and as both genetic and environmental factors likely play a role in HPA axis reactivity (e.g., Alexander et al., 2009).
The study provided some support for our hypothesis that children homozygous for the short-5-HTTLPR and carrying the Met-BDNF allele would demonstrate greater cortisol reactivity than other children. This genotype group exhibited a positive cortisol increase in response to the laboratory stressors, whereas the other genotype groups evidenced decline in cortisol or were unresponsive to the laboratory stressors. These findings are consistent with the hypothesis that the HPA axis is a mechanism through which these genes influence stress sensitivity at the behavioral level (Kaufman et al., 2006; Kim et al., 2007). Interestingly, this same subgroup of children demonstrated a similar pattern of cortisol responses found in depressed preschoolers (Luby et al., 2003), which suggests that these responses coincide with increasing risk. These genes were also related to lower initial cortisol levels during the pre-stress sampling. While this finding is contrary to our hypothesis, a similar finding has been reported in animals. Peer-raised 5-HTTLPR heterozygous macaques evidenced blunted pre-stress HPA-axis activity, as well as increased stress reactivity (Barr et al., 2004). On the one hand, this may be indicative of abnormalities of the HPA system (Gunnar & Vazquez, 2001). On the other hand, this differential responsivity may indicate that these genes have greater plasticity or susceptibility to context. In other words, the ss-5-HTTLPR/Met-BDNF genotype is less reactive under low stress conditions and more reactive under high stress conditions (Belsky et al., 2009; Ellis & Boyce, 2008).
We also found that children homozygous for the short-5-HTTLPR and the Val-BDNF allele evidenced greater decreases in cortisol in response to the stressors. When our findings are put in the context of gene X environment interactions involving 5-HTTLPR and BDNF (Kaufman et al., 2006; Kim et al., 2007), it appears that this subgroup may be more resilient to environmental adversity. Likewise, a recent study by Gunnar, Frenn, and colleagues (2009) found that children who were able to maintain normal growth patterns despite exposure to early life stress evidenced reduced cortisol responsiveness to a laboratory stressor, which they interpret as a reflection of resiliency. Lastly, children carrying a long5-HTTLPR allele, regardless of BDNF genotype, were unresponsive to the laboratory stressors, which complements findings that the long allele is associated with less brain activation to environmental threat (Munafo et al., 2008).
In this study, the relation of 5-HTTLPR and BDNF genotypes with HPA axis activity in preschoolers did not differ by gender, as has been previously reported in some adult populations (Jabbi et al., 2007; Shalev et al., 2009; Wust et al., 2009). Nevertheless, our findings are consistent with research demonstrating that gender differences in HPA axis function typically do not emerge until later in development, usually coinciding with puberty (Gunnar & Vazquez, 2006; Gunnar, Wewerka, Frenn, Long, & Griggs, 2009; Kirschbaum, Wust, & Hellhammer, 1992; Knutsson et al., 1997; Kudielka, Buske-Kirschbaum, Hellhammer, & Kirschbaum, 2004). Therefore, it is possible that the influence of genes on HPA axis function may vary across development, particularly around puberty, and differ by gender.
This study had several limitations. First, our sample was small, which is problematic as our findings were based on groups stratified by gene-gene interactions. We cannot rule out the possibility that the results are due to chance; therefore, our findings require replication. The limited sample size also precluded any examination of three-way interactions with gender or environmental factors. Third, we did not analyze recently reported subtypes of the 5-HTTLPR long alleles that may have functional significance for stress reactivity (Wendland, Martin, Kruse, Lesch, & Murphy, 2006). Fourth, our sample was ethnically homogenous. We cannot generalize results to economically disadvantaged groups or other racial/ethnic groups. Fifth, salivary cortisol response to stress was our only measure of HPA axis regulation. Future research should assess multiple aspects of HPA axis function, including basal cortisol secretion (e.g., the cortisol awakening response), adrenocorticotropic hormone (ACTH) levels, and dexamethasone nonsuppression. Lastly, the study was cross-sectional. Long-term follow-up is necessary to test the hypothesis that these factors contribute to stress sensitivity or resiliency at the behavioral level and in the development of stress-related disorders.
In sum, a large body of research has demonstrated that stress and the HPA axis play a critical role in numerous psychiatric and medical diseases (McEwen, 2008); therefore, understanding factors that influence HPA axis function, specifically stress sensitivity, is of critical importance. This study is the first to demonstrate the epistatic effects of 5-HTTLPR and BDNF on HPA axis reactivity to stress and is also the first to investigate the genetics of HPA axis functioning in very young children. The presence of these associations during early childhood is particularly noteworthy, as exposure to adversity during early development appears to contribute to lasting neurobiological changes that increase risk for psychopathology in adulthood (Brown & Harris, 2008; Heim, Newport, Mletzko, Miller, & Nemeroff, 2008). At the same time, other early environmental factors, such as nurturing care-giving environments, may protect developing neurobiological systems, even in those at high genetic risk (Kaufman et al., 2006). Future research should work to identify both genetic and environmental factors contributing to the development of stress regulation across the lifespan, which may help delineate how stress increases disease susceptibility in certain individuals.
Acknowledgements
The authors would like to thank Bonnie Donzella and Megan R. Gunnar (University of Minnesota) for consultation on cortisol data collection procedures; Andrea Gierens and the neuroendocrine laboratory at the University of Trier, Germany for assaying the salivary cortisol samples; and Haroon I. Sheikh and Shiva M. Singh (University of Western Ontario) for their efforts and support in genotyping for quality control purposes.
Role of the funding sources
This work was supported by the following grants: National Institute of Mental Health grants RO1 MH069942 (DNK) and F31 MH075484-01A2 (LRD), the Society for a Science of Clinical Psychology Dissertation Grant Award (LRD), the Emeritus Faculty Dissertation Award (LRD), NSF Grant BCS-0224221 (TC), and a GCRC Grant no. M01-RR10710 to Stony Brook University from the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Findings were similar when non-Caucasian children or children of unknown ethnicity were included in all analyses.
Conflict of interest
Authors reported no biomedical financial interests or potential conflicts of interest.
Contributor Information
Lea R. Dougherty, Department of Psychology, Stony Brook University.
Daniel N. Klein, Department of Psychology, Stony Brook University.
Eliza Congdon, Department of Psychology, Stony Brook University..
Turhan Canli, Department of Psychology, Stony Brook University..
Elizabeth P. Hayden, Department of Psychology, University of Western Ontario.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool Form & Profiles. Burlington, VT: University of Vermont, Department of Psychiatry; 2000. [Google Scholar]
- Alexander N, Kuepper Y, Schmitz A, Osinsky R, Kozyra E, Hennig J. Gene-environment interactions predict cortisol responses after acute stress: Implications for the etiology of depression. Psychoneuroendocrinology. 2009;34:1294–1303. doi: 10.1016/j.psyneuen.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, et al. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biological Psychiatry. 2004;55:733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Bartels M, de Geus EJC, Kirschbaum C, Sluyter F, Boomsma DI. Heritability of daytime cortisol levels in children. Behavioral Genetics. 2003;33:421–433. doi: 10.1023/a:1025321609994. [DOI] [PubMed] [Google Scholar]
- Bath KG, Lee FS. Variant BDNF (Val66Met) impact on brain structure and function. Cognitive, Affective, and Behavioral Neuroscience. 2006;6:79–85. doi: 10.3758/cabn.6.1.79. [DOI] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Molecular Psychiatry. 2009;14:746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom A, Jayatissa MN, Mork A, Wiborg O. Stress sensitivity and resilience in the chronic mild stress rat model of depression: An in situ hybridization study. Brain Research. 2008;1196:41–52. doi: 10.1016/j.brainres.2007.12.025. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam E, Vermetten E, Adil J, Khan S, Nazeer A, et al. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroedocrinology. 2003;28:733–750. doi: 10.1016/s0306-4530(02)00067-7. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris TO. Depression and the serotonin transporter 5-HTTLPR polymorphism: A review and a hypothesis concerning gene-environment interaction. Journal of Affective Disorders. 2008;111:1–12. doi: 10.1016/j.jad.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Brownell R. Expressive One-Word Picture Vocabulary Test. Novato, CA: Academic Therapy Publications; 2000. [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chen MC, Joorman J, Hallmayer J, Gotlib IH. Serotonin transporter polymorphism predicts waking cortisol in girls. Psychoneuroendocrinology. 2009;34:681–686. doi: 10.1016/j.psyneuen.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. Journal of Neuroscience. 2004;24:4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Archives of General Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biological Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody Picture Vocabulary Test. 3rd ed. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Ehlert U, Gaab J, Heinrichs M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: The role of the hypothalamic-pituitary-adrenal axis. Biological Psychology. 2001;57:141–152. doi: 10.1016/s0301-0511(01)00092-8. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT. Biological sensitivity to context. Current Directions in Psychological Science. 2008;17:183–187. [Google Scholar]
- Federenko IS, Nagamine M, Hellhammer DH, Wadhwa PD, Wust S. The heritability of hypothalamus pituitary adrenal axis responses to psychosocial stress is context dependent. Journal of Clinical Endocrinology & Metabolism. 2004;89:6244–6250. doi: 10.1210/jc.2004-0981. [DOI] [PubMed] [Google Scholar]
- Flint J, Munafò MR. The endophenotype concept in psychiatric genetics. Psychological Medicine. 2007;37:163–180. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Schüle C, Schmitt G, Born C, Baghai T, Zill P, et al. Association of the brain-derived neurotrophic factor Val66Met polymorphism with reduced hippocampal volumes in major depression. Archives of General Psychiatry. 2007;64:410–416. doi: 10.1001/archpsyc.64.4.410. [DOI] [PubMed] [Google Scholar]
- Givalois L, Naert G, Rage F, Ixart G, Arancibia S, Tapia-Arancibia L. A single brain-derived neurotrophic factor injection modifies hypothalamo-pituitary-adrenocortical axis activity in adult male rats. Molecular and Cellular Neuroscience. 2004;27:280–295. doi: 10.1016/j.mcn.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Reilly J, Lemery KS, Longley S, Prescott A. Laboratory Temperament Assessment Battery: Preschool version. 1995 (unpublished manuscript). [Google Scholar]
- Gotlib I, Joormann J, Minor K, Hallmayer J. HPA axis reactivity: A mechanism underlying associations among 5-HTTLPR, stress, and depression. Biological Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Frenn K, Wewerka SS, Van Ryzin MJ. Moderate versus severe early life stress: Associations with stress reactivity and regulation in 10- 12-year-old children. Psychoneuroendocrinology. 2009;34:62–75. doi: 10.1016/j.psyneuen.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM. Neuroendocrine measures in developmental research. In: Schmidt LA, Segalowitz SJ, editors. Developmental psychophysiology: Theory, systems, and methods. New York, NY: Cambridge University Press; 2008. pp. 343–366. [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34:953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology: Developmental Neuroscience. New York, NY: Wiley Press; 2006. pp. 533–577. [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus-pituitary-adreanl activity over the transition to adolescence: Normative changes and associations with puberty. Development and Psychopathology. 2009;21:69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney D. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Hünnerkopf R, Strobel A, Gutknecht L, Brocke B, Lesch KP. Interaction between BDNF Val66Met and dopamine transported gene variation influences anxiety-related traits. Neuropsychopharmacology. 2007;32:2552–2560. doi: 10.1038/sj.npp.1301383. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Stallings M, McGreary J, Bryan A. Population stratification in the candidate gene study: Fatal threat or red herring? Psychological Bulletin. 2004;130:66–79. doi: 10.1037/0033-2909.130.1.66. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Korf J, Kema IP, Hartman C, van der Pompe G, Minderaa RB, Ormel J, den Boer JA. Convergent genetic modulation of the endocrine stress response involves polymorphic variations of 5-HTT, COMT, and MAOA. Molecular Psychiatry. 2007;12:483–490. doi: 10.1038/sj.mp.4001975. [DOI] [PubMed] [Google Scholar]
- Jansen LMC, Gispen-de Wied CC, Gademan PJ, De Jonge RCJ, van der Linden JA, Kahn RS. Blunted cortisol response to a psychosocial stressor in schizophrenia. Schizophrenia Research. 1998;33:87–94. doi: 10.1016/s0920-9964(98)00066-8. [DOI] [PubMed] [Google Scholar]
- Jiang X, Wang J, Luo T, Li Q. Impaired hypothalamic-pituitary-adrenal axis and its feedback regulation in serotonin transporter knockout mice. Psychoneuroendocrinology. 2009;34:317–331. doi: 10.1016/j.psyneuen.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglan-Palumberi H, Grasso D, Lipschitz D, Houshyar S, et al. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biological Psychiatry. 2006;59:673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Kim YH, Yoon JS. Interactions between life stressors and susceptibility genes (5-HTTLPR and BDNF) on depression in Korean elders. Biological Psychiatry. 2007;62:423–428. doi: 10.1016/j.biopsych.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wüst S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosomatic Medicine. 1992;54:648–657. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Knutsson U, Dahlgren J, Marcus C, Rosberg S, Bronnegard M, Stierna P, et al. Circadian cortisol rhythms in healthy boys and girls: Relationship with age, growth, body composition and pubertal development. Journal of Clinical Endocrinology & Metabolism. 1997;82:536–540. doi: 10.1210/jcem.82.2.3769. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29:83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- Lang UE, Hellweg R, Kalus P, Bajbouj M, Lenzen KP, Sander T, et al. Association of a functional BDNF polymorphism and anxiety-related personality traits. Psychopharmacology. 2005;180:95–99. doi: 10.1007/s00213-004-2137-7. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengal D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Gross J, Franzek E, Wolozin BL, Riederer P, Murphy DL. Primary structure of the serotonin transporter in unipolar depression and bipolar disorder. Biological Psychiatry. 1995;37:215–223. doi: 10.1016/0006-3223(94)00147-U. [DOI] [PubMed] [Google Scholar]
- Leyton M, Belanger C, Martial J, Beaulieu S, Corin E, Pecknold J, et al. Cardiovascular, neuroendocrine, and monoaminergic responses to psychological stressors: Possible differences between remitted panic disorder patients and healthy controls. Biological Psychiatry. 1996;40:353–360. doi: 10.1016/0006-3223(95)00452-1. [DOI] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Van de Dar L, Lesch K, Murphy D. Reduction of 5-hydroxytryptamine (5-HT)(1A)-mediated temperature and neuroendocrine responses and 5-HT (1A) binding sites in 5-HT transporter knockout mice. Journal of Pharmacology and Experimental Therapeutics. 1999;291:999–1007. [PubMed] [Google Scholar]
- Lopez-Duran NL, Kovacs M, George CJ. Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology. 2009;34:1272–1283. doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcoholism: Clinical and Experimental Research. 2000;24:651–658. [PubMed] [Google Scholar]
- Luby JL, Heffelfinger A, Mrakostsky C, Brown C, Hessler M, Spitznagel E. Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric and no-disorder comparison groups. Archives of General Psychiatry. 2003;60:1248–1255. doi: 10.1001/archpsyc.60.12.1248. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer SE, Chrousos GP, Gold PW. Major depression and the stress system: A lifespan perspective. Development and Psychopathology. 2001;13:563–578. doi: 10.1017/s095457940100308x. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: A meta-analysis. Biological Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Clark T, Flint J. Does measurement instrument moderate the association between the serotonin transporter gene and anxiety-related personality traits? A meta-analysis. Molecular Psychiatry. 2005;10:415–419. doi: 10.1038/sj.mp.4001627. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Durrant C, Lewis G, Flint J. Gene x environment interactions at the serotonin transporter locus. Biological Psychiatry. 2009;65:211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Naert G, Ixart G, Tapia-Arancibia L, Givalois L. Continuous i.c.v. infusion of brain-derived neurotrophic factor modifies hypothalamic-pituitary-adrenal activity, locomotor activity and body temperature rhythms in adult male rats. Neuroscience. 2006;139:779–789. doi: 10.1016/j.neuroscience.2005.12.028. [DOI] [PubMed] [Google Scholar]
- O’Hara R, Schroder CM, Mahadevan R, Schatzberg AF, Lindley S, Fox S, et al. Serotonin transporter polymorphism, memory and hippocampal volume in the elderly: Association and interaction with cortisol. Molecular Psychiatry. 2007;12:544–555. doi: 10.1038/sj.mp.4001978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Goldman AL, Verchinski BA, Chen G, Kolachana BS, et al. Evidence of biologic epistasis between BDNF and SLC6A4 and implications for depression. Molecular Psychiatry. 2008;13:709–716. doi: 10.1038/mp.2008.32. [DOI] [PubMed] [Google Scholar]
- Ren-Patterson RF, Cochran LW, Holmes A, Sherrill S, Huang SJ, Tolliver T, et al. Loss of brain-derived neurotrophic factor gene allele exacerbates brain monoamine deficiencies and increases stress abnormalities of serotonin transporter knockout mice. Journal of Neuroscience Research. 2005;79:756–771. doi: 10.1002/jnr.20410. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. Journal of the American Medical Association. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schule C, Zill P, Baghai TC, Eser D, Zwanzger P, et al. Brain-derived neurotrophic factor Val66Met polymorphism and dexamethasone/CRH test results in depressed patients. Psychoneuroendocrinology. 2006;31:1019–1025. doi: 10.1016/j.psyneuen.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Sen S, Nesse RM, Stoltenberg SF, Li S, Gleiberman L, Chakravarti A, et al. A BDNF coding variant is associated with the NEO personality inventory domain neuroticism, a risk factor for depression. Neuropsychopharmacology. 2003;28:397–401. doi: 10.1038/sj.npp.1300053. [DOI] [PubMed] [Google Scholar]
- Shalev I, Lerer E, Israel S, Uzefovsky F, Gritsenko I, Mankuta D, Ebstein RP, Kaitz M. BDNF Val66Met polymorphism is associated with HPA axis reactivity to psychosocial stress characterized by genotype and gender interactions. Psychoneuroendocrinology. 2009;34:382–388. doi: 10.1016/j.psyneuen.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Iyo M. Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: The possibility to explain ethnic mental traits. American Journal of Medical Genetics. 2004;162B:122–123. doi: 10.1002/ajmg.b.20118. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- Talge NM, Donzella B, Gunnar MR. Fearful temperament and stress reactivity among preschool-aged children. Infant and Child Development. 2008;17:427–445. doi: 10.1002/icd.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talge NM, Donzella B, Kryzer EM, Gierens A, Gunnar MR. It’s not that bad: Error introduced by oral stimulants in salivary cortisol research. Developmental Psychobiology. 2005;47:369–376. doi: 10.1002/dev.20097. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Molecular Psychiatry. 2008;13:131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- Wacholder S, Rothman N, Caporaso N. Counterpoint: Bias from population stratification is not a major threat to the validity of conclusions from epidemiological studies of common polymorphisms and cancer. Cancer, Epidemiology, Biomarkers and Prevention. 2002;11:513–520. [PubMed] [Google Scholar]
- Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping for four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Molecular Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Wichers M, Kenis G, Jacobs N, Myin-Germeys I, Schuers K, Mengelers R, et al. The psychology of psychiatric genetics: Evidence that positive emotions in females moderate genetic sensitivity to social stress associated with the BDNF Val66Met polymorphism. Journal of Abnormal Psychology. 2008;117:699–704. doi: 10.1037/a0012909. [DOI] [PubMed] [Google Scholar]
- Wust S, Kumsta R, Treutlein J, Frank J, Entringer S, Schulze TG, Rietschel M. Sex-specific association between the 5-HTT gene-linked polymorphic region and basal cortisol secretion. Psychoneuroendocrinology. 2009;34:972–982. doi: 10.1016/j.psyneuen.2009.01.011. [DOI] [PubMed] [Google Scholar]