Abstract
The excitatory amino acid transporters (EAATs) are a family of molecules that are essential for regulation of synaptic glutamate levels. The EAATs may also be regulated by N-glycosylation, a posttranslational modification that is critical for many cellular functions including localization in the plasma membrane. We hypothesized that glycosylation of the EAATs is abnormal in schizophrenia. To test this hypothesis, we treated postmortem tissue from the dorsolateral prefrontal and anterior cingulate cortices of patients with schizophrenia and comparison subjects with deglycosylating enzymes. We then measured the resulting shifts in molecular weight of the EAATs using Western blot analysis to determine the mass of glycans cleaved from the transporter. We found evidence for less glycosylation of both EAAT1 and EAAT2 in schizophrenia. We did not detect N-linked glycosylation of EAAT3 in either schizophrenia or the comparison subjects in these regions. Our data suggest an abnormality of posttranslational modification of glutamate transporters in schizophrenia that suggests a decreased capacity for glutamate reuptake.
Keywords: GLAST, GLT-1, EAAC1, deglycosylation, anterior cingulate cortex, dorsolateral prefrontal cortex
Introduction
Glycosylation of proteins is a posttranslational modification that plays a role in molecular trafficking, protein folding, endocytosis, receptor activation, signal transduction, and cell adhesion (Ohtsubo and Marth, 2006). Abnormalities of glycosylation can lead to a number of cellular storage disorders including Gaucher's, Niemann-Pick type C, Sandhoff's, and Tay-Sach's diseases, as well as other congenital disorders of glycosylation (Ohtsubo and Marth, 2006). Disruptions in glycosylation have also been implicated in Alzheimer's disease (Takeuchi and Yamagishi, 2009), Huntington's disease (Hung et al., 1980), and schizophrenia (Narayan et al., 2008).
Two common forms of protein glycosylation include N-linked glycosylation and O-linked glycosylation. N-linked glycosylation is the covalent linkage of oligosaccharides to asparagine residues of proteins. N-glycosyl residues are processed as proteins are trafficked through the endoplasmic reticulum and golgi. The excitatory amino acid transporters (EAATs) are N-glycosylated proteins that transport extracellular glutamate out of the synapse and thus are critical for glutamatergic signaling. However, glycosylation of the EAATs in human brain has not been evaluated.
EAAT1 is variably expressed throughout the cortex in astroglia (Rothstein et al., 1994; Chaudhry et al., 1995; Kondo et al., 1995; Lehre et al., 1995; Gegelashvili et al., 1996; Schmitt et al., 1997; Williams et al., 2005). GLAST, the rodent form of EAAT1, exists as two isoforms, 70-kDa and 64-kDa, which differ only by the degree of N-glycosylation at Asn206 and Asn195 (Conradt et al., 1995; Schulte and Stoffel, 1995). Glycosylation of this transporter may serve an important functional role because nonglycosylated GLAST does not form homomultimers, which are the native conformation of GLAST in vivo (Conradt et al., 1995). In addition, glycosylation of GLAST has been correlated with trafficking of GLAST to plasma membrane and increased glutamate uptake (Escartin et al., 2006).
EAAT2 is an astrocytic transporter responsible for the majority of glutamate uptake in the cortex. Deglycosylation of the rodent isoforms of EAAT2 (GLT-1) resulted in a ∼10-15 kDa shift in molecular weight of the monomer band (Kalandadze et al., 2004). There is a conflicting literature describing the functional effects of EAAT2 glycosylation. One group found that glycosylation-deficient GLT-1 (the rodent form of EAAT2) had a decreased rate of glutamate transport due to decreased expression in the plasma membrane (Trotti et al., 2001). This may be attributed to retention of GLT-1 in the endoplasmic reticulum, because mutant GLT-1 expressing an altered extracellular leucine-based motif is immaturely glycosylated and retained in the ER (Kalandadze et al., 2004). However, another group found no effect of N-glycosylation on the trafficking or transport activity of GLT-1 in transfected BHK cells, but increased stability at the plasma membrane, which may be critical for transporter localization in vivo (Raunser et al., 2005).
EAAT3 is a neuronal glutamate transporter expressed in the cortex. In rat C-6 glioma cells, EAAC1 (the rodent form of EAAT3) is N-glycosylated with high mannose-containing sidechains and processed into complex chains, coinciding with insertion into the plasma membrane (Yang and Kilberg, 2002). A shift of approximately 5 kDa was detected when EAAT3 immunoprecipitated from human brain synaptosomes were treated with Endoglycosidase F (Shashidharan et al., 1997).
We previously reported alterations in EAAT1 and EAAT3 protein in prefrontal cortex in schizophrenia, suggesting diminished EAAT-mediated glutamate reuptake as a part of the pathophysiology of this illness (Bauer et al., 2008). However, localization of the transporters may be as important as overall protein levels. Altered EAAT localization may lead to glutamate spillover into the extrasynaptic space and adjacent synapses, causing loss of input specificity (Overstreet et al., 1999; Tsvetkov et al., 2004; Marcaggi and Attwell, 2007). Since glycosylation is important for targeting of the EAATs to the plasma membrane, abnormal glycosylation of these proteins may play a role in schizophrenia.
Glycobiology is a growing field with an increasing number of tools. The enzyme peptide-N4-(N-acetyl-beta-glucosaminyl) asparagine amidase F (PNGase F) cleaves N-linked sugars off of proteins attached at asparagine residues. Endoglycosidase H (Endo H) cleaves hybrid and high mannose containing residues from glycoproteins, and is therefore specific to immaturely glycosylated proteins that have not been processed beyond the endoplasmic reticulum. The removal of glycans is often substantial enough to detect a change in molecular weight of proteins when measured by Western blot analysis. In this study, we assessed glycosylation of EAAT1, EAAT2, and EAAT3 through enzymatic deglycosylation in schizophrenia and a comparison group.
Materials and Methods
Subjects
Subjects from the Mount Sinai Medical Center Schizophrenia Brain Bank were studied (Table 1), including 35 individuals diagnosed with schizophrenia and 33 comparison subjects. Subjects were diagnosed with schizophrenia if the presence of schizophrenic symptoms was documented before age 40, the medical records contained evidence of psychotic symptoms and at least 10 years of psychiatric hospitalization with diagnosis of schizophrenia, and a DSM-III-R diagnosis of schizophrenia was agreed upon by two experienced clinicians. Diagnostic groups did not significantly differ for age, sex, postmortem interval, and tissue pH. Upon neuropathological examination, no evidence of Alzheimer or other neurodegenerative disease was found. The brain banking procedures were approved by the Mount Sinai School of Medicine Institutional Review Board.
Table 1.
Subject Characteristics
| Comparison Group | Schizophrenia | |||
|---|---|---|---|---|
| Region | ACC | DLPFC | ACC | DLPFC |
| N | 34 | 32 | 34 | 33 |
| Sex | 14 m / 20 f | 12 m / 20 f | 24 m / 10 f | 23 m / 10 f |
| Tissue pH | 6.4 ± 0.2 | 6.5 ± 0.2 | 6.4 ± 0.3 | 6.4 ± 0.3 |
| PMI (hours) | 8.3 ± 6.7 | 8.2 ± 6.8 | 13.4 ± 8.1 | 12.5 ± 6.7 |
| Age (years) | 78 ± 14 | 78 ± 14 | 74 ± 12 | 74 ± 12 |
| On / Off Rx | 0 / 34 | 0 / 32 | 23 / 11 | 22 / 11 |
Values presented as mean ± standard deviation
Abbreviations: anterior cingulate cortex (ACC), dorsolateral prefrontal cortex (DLPFC), male (m), female (f), antipsychotic medication (Rx), postmortem interval (PMI).
Tissue preparation
Brains were obtained after autopsy and one hemisphere was cut coronally into ∼0.8 - 1 cm3 slabs and flash frozen. Gray matter was dissected from anterior cingulate cortex (ACC) (n = 68) and dorsolateral prefrontal cortex (DLPFC) (n = 66). ACC was dissected at the level of the genu of the corpus callosum. Tissue blocks were dissected from the dorsal surface of the corpus callosum extending 12–15 mm dorsally and extending 12–15 mm laterally from the midline. DLPFC was dissected corresponding to Brodmann area 46 and measuring ≈1.5 cm along the cortical surface as described by Rajkowska and Goldman-Rakic (Rajkowska and Goldman-Rakic, 1995). Approximately 1 cm3 of frozen tissue was pulverized in liquid nitrogen, then homogenized (10% wt/vol) in 5 mM Tris-HCl (pH 7.4) with 320 mM sucrose and 1 protease inhibitor tablet (Complete mini, Roche Diagnostics, Manheim, Germany) per 10 mL for 30 sec with a polytron homogenizer (Fisher Scientific, Pittsburgh, Pennsylvania) and stored at −80°C in 0.5 mL aliquots. To determine protein concentrations, assay by the Bradford method (Bradford, 1976) was performed on these homogenates.
Deglycosylation
16 μg of protein for each sample was added to 6.7 μl 5X reaction buffer (QA Bio), 1.7 μl denaturation solution (2% SDS/ 1M β mercaptoethanol) (QA Bio), and adjusted to volume with deionized water. Samples were then incubated at 70°C for 10 min. Samples were cooled to room temperature and incubated with 1.3 μl Endoglycosidase H or 1.3 μl PNGase F and 1.7 μl 15% triton X-100 (QA Bio) at 37°C for 12 hours. Non-enzyme-treated samples were prepared identically to the enzyme-treated samples with the same buffers except that they were incubated with water instead of the deglycosylating enzymes.
Electrophoresis
NuPAGE sample reducing agent (Invitrogen), and NuPAGE LDS sample buffer (Invitrogen) were added to the samples, which were then incubated at 70°C for 10 minutes. The Novex Mini Cell NuPAGE system (Invitrogen) with 4-12% Bis-Tris gradient polyacrylamide gels (Invitrogen) was used and 8μg of protein was added per lane. A molecular mass standard was run on each gel (Kaleidescope prestained standards, BioRad). Gels were suspended in a bath of NuPAGE MES SDS running buffer (Invitrogen) with 500μl NuPAGE antioxidant (Invitrogen) during electrophoresis.
Western blot analysis
Following electrophoresis, proteins were transferred onto Immobilon-FL PVDF membranes (Millipore) using a semi-dry transfer apparatus (BioRad). After electroblot transfer, membranes were washed twice and incubated with Odyssey Blocking Buffer (Li-Cor Biosciences) for 1 hour at room temperature with rocking to block nonspecific antibody binding. Membranes were incubated with either a rabbit polyclonal antibody to EAAT1 (Santa Cruz sc-15316) diluted 1:1,000, rabbit polyclonal antibody to EAAT2 (Santa Cruz sc-15317) diluted 1:1000, mouse moloclonal antibody to EAAT3 (Chemicon MAB1578) diluted 1:1000, rabbit polyclonal antibody to EAAT3 (Santa Cruz sc-25658) diluted 1:500, or rabbit polyclonal antibody to EAAT3 (Alpha Diagnostics #EAAC11-A) diluted 1:500 in blocking buffer with 0.1% tween overnight at 4°C with rocking. Next, the membranes were washed three times for ten minutes in tris-buffered saline with 0.1% tween (TBST), then rocked for 15 minutes at room temperature with anti-rabbit or anti-mouse IR-Dye 800CW secondary antibody (Li-Cor Biosciences) diluted 1:10,000 in blocking buffer with 0.1% tween. Membranes were washed three times for 10 minutes in TBST then washed 5 times in deionized water and allowed to dry for 3-5 minutes before scanning (infrared imaging system; Li-Cor Biosciences).
Data Analysis
Membranes probed with infrared-labeled secondary antibodies were scanned using a Li-Cor Odyssey scanner, and the migration distance for each protein band was measured in pixels using the Odyssey 2.1 software package. Migration distance was converted to molecular mass by plotting the relative migration of the molecular mass standards against the log of their molecular masses, and fitting the relative migration of the bands of interest to that standard curve (Jarvie et al., 1988). Band shift was measured as molecular mass of the control band minus the molecular mass of the enzyme treated band in the adjacent lane, as described previously (Jimenez-Huete et al., 1998; Nielsen et al., 2004; Toledo et al., 2005). EAAT1 and EAAT2 migrate as both monomers and multimers (Bauer et al., 2008), and the molecular mass shifts of monomers and multimers were analyzed separately.
Statistical Analysis
All statistical analyses were performed using Statistica (StatSoft, Tulsa, Oklahoma). Outliers more than 6 standard deviations from the mean were excluded. Correlation analysis was performed to determine associations between the dependent variable, molecular mass shift and age, PMI, and pH. We analyzed deglycosylation induced changes in molecular mass using analysis of variance (ANOVA), or analysis of covariance (ANCOVA) when significant correlations were detected. To test for possible medication effects, patients with schizophrenia off antipsychotic medication for at least 6 weeks prior to death were compared to patients on antipsychotic medication within 6 weeks of death.
Results
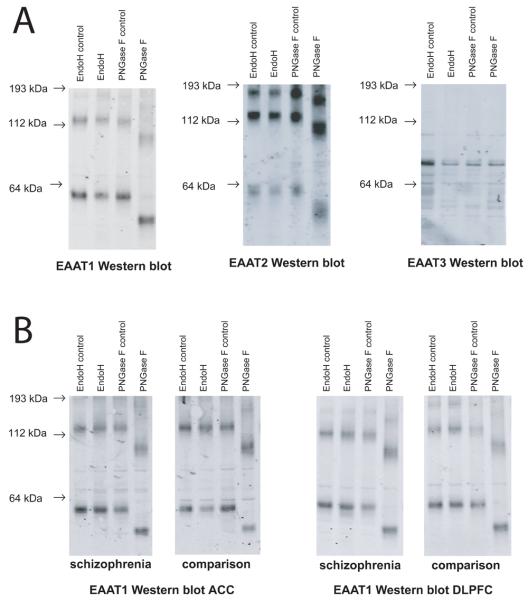
When samples were treated with EndoH, none of the transporters exhibited shifts in molecular mass of either monomeric or multimeric forms (Figure 1). When samples were treated with PNGase F, EAAT1 and EAAT2 exhibited detectable shifts in molecular mass for both monomeric and multimeric forms (Figure 1). However, EAAT3 did not shift when treated with PNGase F (Figure 1). Because we were concerned that the lack of shift could be due to a loss of an epitope following enzymatic digestion, we performed Western blots with two additional EAAT3 antibodies raised against different epitopes, and did not detect shifts in EAAT3 (data not shown).
Figure 1.
Western blots of deglycosylated EAATs. A. Western blot analysis of EAAT1, EAAT2, and EAAT3 deglycosylated with the enzymes Endoglycosidase H and PNGase F. EndoH and PNGase F lanes indicate enzyme treated samples. EndoH control and PNGase F control lanes were treated identically to the corresponding enzyme treated samples except the enzymes were omitted. Molecular masses of EAAT1 and EAAT2 monomers and multimers were shifted in the PNGase F treated lanes. No shift was detected for EAAT3. B. Western blot analysis of EAAT1 deglycosylated with EndoH and PNGase F in anterior cingulate cortex and dorsolateral prefrontal cortex from a patient with schizophrenia and a comparison subject. Abbreviations: kDa (kilodaltons), excitatory amino acid transporter (EAAT), endoglycosidase H (EndoH), peptide-N4-(N-acetyl-beta-glucosaminyl) asparagine amidase F (PNGase F).
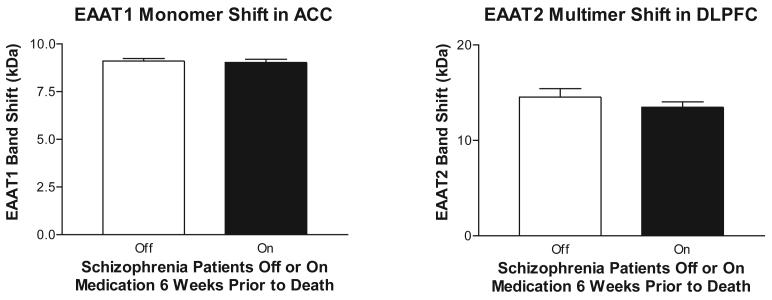
We examined the effects of PNGase treatment on monomer and multimer forms of EAAT1 and EAAT2 in schizophrenia and comparison subjects. There were generally no correlations detected between age, PMI (which differs between diagnosis groups (F(1, 66)=7.3766, p=.00843)), or pH and our dependent measures with the exception of shift of EAAT1 monomer in the DLPFC: (R= 0.28, p < 0.05). We found less of a molecular mass shift for the EAAT1 monomer in schizophrenia in the ACC (F(1, 61) = 6.40; p < 0.05) (Figure 2). We found less of a mass shift for the EAAT2 multimer in schizophrenia in the DLPFC (F(1,52) = 9.41; p < 0.05) (Figure 2). There was no effect of medication status in the subjects with schizophrenia on either of these dependent measures (EAAT1 monomer in ACC (F(1, 29) = 0.06, p = 0.80), EAAT2 multimer in DLPFC (F(1, 27) = 1.04, p = 0.32)).
Figure 2.
Molecular mass shifts of EAAT1 and EAAT2 in schizophrenia and a comparison group following enzymatic deglycosylation with PNGase F. Data expressed as means +/− standard error of the mean. Asterisks indicate a significant difference between schizophrenia and comparison subjects (p < 0.05). Abbreviations: kDa (kilodaltons), anterior cingulate cortex (ACC), dorsolateral prefrontal cortex (DLPFC), excitatory amino acid transporter (EAAT), endoglycosidase H (EndoH), peptide-N4-(N-acetyl-beta-glucosaminyl) asparagine amidase F (PNGase F).
Discussion
Previous work has demonstrated altered glycosylation of several proteins in schizophrenia. Increases in the plasma activity of the glycosylating enzyme alpha 2,6 sialyltransferase and in serum levels of alpha 2 and beta globulins have been found in schizophrenia (Varma and Hoshino, 1980; Maguire et al., 1997). In addition, a decrease in the number of cells expressing polysialated neural cell adhesion molecule (NCAM) was detected in the hilus of the hippocampus without an overall change in NCAM expression (Barbeau et al., 1995). We detected two additional proteins that have altered glycosylation in schizophrenia, suggesting that deficits in glycosylation may have a role in the pathophysiology of this illness.
We found that EAAT1 and EAAT2, but not EAAT3, are N-glycosylated in the human brain. Given that we found changes in the glial (EAAT1 and EAAT2) but not neuronal (EAAT3) transporters, it is possible that the mechanisms for glycosylation deficits in schizophrenia are glia-specific. Our EAAT3 finding is surprising, given that in the rodent EAAT3 (EAAC1) is glycosylated (Yang and Kilberg, 2002), and that another study demonstrated deglycosylation of EAAT3 in an immunoprecipitated fraction from human brain synaptosomes (Shashidharan et al., 1997). These divergent findings might be due to the type of deglycosylating enzyme used, or that immunoprecipitating EAAT3 from synaptosomes significantly enriched EAAT3, allowing detection of subtle changes that might not be apparent in tissue homogenate. It may be that a small subset of EAAT3 is glycosylated in human brain, while the majority of EAAT3 is unglycosylated, and thus not detectable with our approach. Alternatively, the absence of a molecular mass shift in EAAT3 could be due to a loss of epitopes associated with glycosylation for the EAAT3 antibodies following enzymatic digestion (Levenson et al., 2002; Holmseth et al., 2005). For example, an antibody might bind to bind to a glycosylated residue of EAAT3 and lose antiginicity if that glycan is cleaved. However, we feel this is unlikely because we did not detect a shift using any of the three EAAT3 antibodies raised to different epitopes and one of these epitopes does not contain any putative N-glycosylation sites.
We found that EAAT1 has fewer sugar residues added by N-glycosylation in schizophrenia. The change in molecular mass shift of EAAT1 was relatively small (∼5%) between schizophrenia and the comparison group. It is difficult to determine if such a small change in glycosylation is of physiological significance. However, preclinical data suggests that a small change in glycosylation can have a strong effect on glutamate uptake. For example, activation of astrocytes with ciliary neurotrophic factor (CNTF) results in a small increase in glycosylation of GLAST (∼8% shift), with increased localization of GLAST to lipid rafts at the cell surface and increased glutamate reuptake, resulting in a 67% decrease in extracellular glutamate levels upon quinolinate evoked glutamate release (Escartin et al., 2006). This suggests that the decrease in glycosylation of EAAT1 that we detected may significantly impact glutamate reuptake.
We also found evidence for less glycosylation of the other astrocytic transporter, EAAT2, in schizophrenia. The difference in molecular mass shift between groups was larger for EAAT2 (∼13%) than for EAAT1, although the functional effect of this larger shift is not known. Less glycosylation of EAAT2 might reflect decreased glutamate reuptake, since altered glycosylation of EAAT2 is associated with ER retention and decreased plasma membrane expression, and trafficking of EAAT2 to the plasma membrane is necessary for EAAT2 mediated glutamate reuptake (Trotti et al., 2001; Kalandadze et al., 2004).
One potential mechanism for the decreases in glycosylation could be altered splice variant expression. EAAT1 and EAAT2 can both be alternatively spliced to skip exon 9, which contains an ER exit motif. These splice variants are retained in the ER, and cause ER retention of any full length variants with which they dimerize (Kalandadze et al., 2004). The transporters that are retained in the ER are less glycosylated than transporters that are not retained (Kalandadze et al., 2004). Thus, it is possible that the decreases in glycosylation that we found are due to increased expression of these exon skipping variants. In fact, we found increases in the exon 9 skipping variant of EAAT2, which could explain the decrease we found in EAAT2 glycosylation (unpublished observation).
It is also possible that the changes in glycosylation we found are due to changes in the levels or activity of the glycosyl transferases that attach glycans to the proteins. Few studies have investigated glycosyl transferases in schizophrenia. An increase has been detected in the plasma activity of the glycosylating enzyme alpha 2,6 sialyltransferase (Maguire et al., 1997). An increase in activity of a glycosyl transferase is unlikely to explain a decrease in glycosylation, but it is possible that other glycosyl transferases are decreased in schizophrenia.
Since most of the patients with schizophrenia were treated with antipsychotic medications, the reductions we found in glycosylation could be due to a medication effect. However, we did not find any effects of medication on molecular mass shift when comparing patients on medication 6 weeks prior to death to patients off medication at least 6 weeks prior to death.
The reductions in EAAT1 and EAAT2 glycosylation suggest decreased plasma membrane expression of these transporters. Altered localization of EAAT1 and EAAT2, combined with the decreased EAAT1 protein expression we previously described (Bauer et al., 2008), suggest that there is decreased perisynaptic glutamate reuptake into astrocytes in schizophrenia. The glutamate transporters are important for maintaining low synaptic glutamate levels by buffering and transporting synaptic glutamate (Tong and Jahr, 1994; Tzingounis and Wadiche, 2007). Diminished perisynaptic reuptake and buffering may lead to glutamate spillover and loss of input specificity (Overstreet et al., 1999; Tsvetkov et al., 2004). Our data suggesting decreased glutamate reuptake support a hypothesis of increased synaptic glutamate levels and/or glutamate spillover in schizophrenia. Consistent with this hypothesis, EAAT1 deficient mice exhibit endophenotypes including self-neglect, social withdrawal, and impaired learning, suggesting that schizophrenia-associated rodent endophenotypes can be modeled by disruption of EAAT1-mediated glutamate reuptake (Karlsson et al., 2009). This hypothesis is further supported by a report of a subject with schizophrenia who has a partial deletion of the EAAT1 gene (Walsh et al., 2008). Finally, our data suggest that reducing synaptic glutamate could be a useful strategy in the treatment of schizophrenia. One study using an mGluR2/3 agonist, which decreases glutamate release, had antipsychotic effects in schizophrenia (Patil et al., 2007). Taken together, these data support a role for diminished glutamate reuptake in the pathophysiology of schizophrenia.
Figure 3.
Molecular mass shifts of EAAT1 and EAAT2 following enzymatic deglycosylation with PNGase F in patients with schizophrenia off or on medication 6 weeks prior to death. Data expressed as means +/− standard error of the mean.
Acknowledgments
Role of Funding Source
Funding for this study was provided by the NIMH grants MH53327, MH78378, and MH074016. The NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Supported by MH53327 (JMW), MH78378 (DEB), MH064673 & MH066392 (VH) and MH074016 (REM)
Abbreviations
- kDa
kilodaltons
- ACC
anterior cingulate cortex
- DLPFC
dorsolateral prefrontal cortex
- EAAT
excitatory amino acid transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All authors declare they have no conflicts of interest.
References
- Barbeau D, Liang JJ, Robitalille Y, Quirion R, Srivastava LK. Decreased expression of the embryonic form of the neural cell adhesion molecule in schizophrenic brains. Proc Natl Acad Sci U S A. 1995;92(7):2785–9. doi: 10.1073/pnas.92.7.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D, Gupta D, Harotunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal expression of glutamate transporter and transporter interacting molecules in prefrontal cortex in elderly patients with schizophrenia. Schizophr Res. 2008;104(13):108–20. doi: 10.1016/j.schres.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Lehre KP, van Lookeren Campagne M, Ottersen OP, Danbolt NC, Storm-Mathisen J. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15(3):711–20. doi: 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- Conradt M, Storck T, Stoffel W. Localization of N-glycosylation sites and functional role of the carbohydrate units of GLAST-1, a cloned rat brain Lglutamate/L-aspartate transporter. Eur J Biochem. 1995;229(3):682–7. doi: 10.1111/j.1432-1033.1995.tb20514.x. [DOI] [PubMed] [Google Scholar]
- Escartin C, Brouillet E, Gubellini P, Trioulier Y, Jacquard C, Smadja C, Knott GW, Kerkerian-Le Goff L, Deglon N, Hantraye P, Bonvento G. Ciliary neurotrophic factor activates astrocytes, redistributes their glutamate transporters GLAST and GLT-1 to raft microdomains, and improves glutamate handling in vivo. J Neurosci. 2006;26(22):5978–89. doi: 10.1523/JNEUROSCI.0302-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegelashvili G, Civenni G, Racagni G, Danbolt NC, Schousboe I, Schousboe A. Glutamate receptor agonists up-regulate glutamate transporter GLAST in astrocytes. Neuroreport. 1996;8(1):261–5. doi: 10.1097/00001756-199612200-00052. [DOI] [PubMed] [Google Scholar]
- Holmseth S, Dehnes Y, Bjornsen LP, Boulland JL, Furness DN, Bergles D, Danbolt NC. Specificity of antibodies: unexpected cross-reactivity of antibodies directed against the excitatory amino acid transporter 3 (EAAT3) Neuroscience. 2005;136(3):649–60. doi: 10.1016/j.neuroscience.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Hung WY, Mold DE, Tourian A. Huntington's-chorea fibroblasts. Cellular protein glycosylation. Biochem J. 1980;190(3):711–9. doi: 10.1042/bj1900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvie KR, Niznik HB, Seeman P. Dopamine D2 receptor binding subunits of Mr congruent to 140,000 and 94,000 in brain: deglycosylation yields a common unit of Mr congruent to 44,000. Mol Pharmacol. 1988;34(2):91–7. [PubMed] [Google Scholar]
- Jimenez-Huete A, Lievens PM, Vidal R, Piccardo P, Ghetti B, Tagliavini F, Frangione B, Prelli F. Endogenous proteolytic cleavage of normal and disease-associated isoforms of the human prion protein in neural and non-neural tissues. Am J Pathol. 1998;153(5):1561–72. doi: 10.1016/S0002-9440(10)65744-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalandadze A, Wu Y, Fournier K, Robinson MB. Identification of motifs involved in endoplasmic reticulum retention-forward trafficking of the GLT-1 subtype of glutamate transporter. J Neurosci. 2004;24(22):5183–92. doi: 10.1523/JNEUROSCI.0839-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson RM, Tanaka K, Saksida LM, Bussey TJ, Heilig M, Holmes A. Assessment of glutamate transporter GLAST (EAAT1)-deficient mice for phenotypes relevant to the negative and executive/cognitive symptoms of schizophrenia. Neuropsychopharmacology. 2009;34(6):1578–89. doi: 10.1038/npp.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K, Hashimoto H, Kitanaka J, Sawada M, Suzumura A, Marunouchi T, Baba A. Expression of glutamate transporters in cultured glial cells. Neurosci Lett. 1995;188(2):140–2. doi: 10.1016/0304-3940(95)11408-o. [DOI] [PubMed] [Google Scholar]
- Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci. 1995;15(3 Pt 1):1835–53. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson J, Weeber E, Selcher JC, Kategaya LS, Sweatt JD, Eskin A. Long-term potentiation and contextual fear conditioning increase neuronal glutamate uptake. Nat Neurosci. 2002;5(2):155–61. doi: 10.1038/nn791. [DOI] [PubMed] [Google Scholar]
- Maguire TM, Thakore J, Dinan TG, Hopwood S, Breen KC. Plasma sialyltransferase levels in psychiatric disorders as a possible indicator of HPA axis function. Biol Psychiatry. 1997;41(11):1131–6. doi: 10.1016/S0006-3223(96)00223-5. [DOI] [PubMed] [Google Scholar]
- Marcaggi P, Attwell D. Short- and long-term depression of rat cerebellar parallel fibre synaptic transmission mediated by synaptic crosstalk. J Physiol. 2007;578(Pt 2):545–50. doi: 10.1113/jphysiol.2006.115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan S, Head SR, Gilmartin TJ, Dean B, Thomas EA. Evidence for disruption of sphingolipid metabolism in schizophrenia. J Neurosci Res. 2008 doi: 10.1002/jnr.21822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen D, Gyllberg H, Ostlund P, Bergman T, Bedecs K. Increased levels of insulin and insulin-like growth factor-1 hybrid receptors and decreased glycosylation of the insulin receptor alpha- and beta-subunits in scrapie-infected neuroblastoma N2a cells. Biochem J. 2004;380(Pt 2):571–9. doi: 10.1042/BJ20040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126(5):855–67. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Overstreet LS, Kinney GA, Liu YB, Billups D, Slater NT. Glutamate transporters contribute to the time course of synaptic transmission in cerebellar granule cells. J Neurosci. 1999;19(21):9663–73. doi: 10.1523/JNEUROSCI.19-21-09663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, Mosolov SN, Neznanov NG, Reznik AM, Smulevich AB, Tochilov VA, Johnson BG, Monn JA, Schoepp DD. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13(9):1102–7. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cereb Cortex. 1995;5(4):307–22. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- Raunser S, Haase W, Bostina M, Parcej DN, Kuhlbrandt W. High-yield expression, reconstitution and structure of the recombinant, fully functional glutamate transporter GLT-1 from Rattus norvegicus. J Mol Biol. 2005;351(3):598–613. doi: 10.1016/j.jmb.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13(3):713–25. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Asan E, Puschel B, Kugler P. Cellular and regional distribution of the glutamate transporter GLAST in the CNS of rats: nonradioactive in situ hybridization and comparative immunocytochemistry. J Neurosci. 1997;17(1):1–10. doi: 10.1523/JNEUROSCI.17-01-00001.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte S, Stoffel W. UDP galactose:ceramide galactosyltransferase and glutamate/aspartate transporter. Copurification, separation and characterization of the two glycoproteins. Eur J Biochem. 1995;233(3):947–53. doi: 10.1111/j.1432-1033.1995.947_3.x. [DOI] [PubMed] [Google Scholar]
- Shashidharan P, Huntley GW, Murray JM, Buku A, Moran T, Walsh MJ, Morrison JH, Plaitakis A. Immunohistochemical localization of the neuron-specific glutamate transporter EAAC1 (EAAT3) in rat brain and spinal cord revealed by a novel monoclonal antibody. Brain Res. 1997;773(12):139–48. doi: 10.1016/s0006-8993(97)00921-9. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Yamagishi S. Involvement of toxic AGEs (TAGE) in the pathogenesis of diabetic vascular complications and Alzheimer's disease. J Alzheimers Dis. 2009;16(4):845–58. doi: 10.3233/JAD-2009-0974. [DOI] [PubMed] [Google Scholar]
- Toledo JR, Sanchez O, Montesino Segui R, Fernandez Garcia Y, Rodriguez MP, Cremata JA. Differential in vitro and in vivo glycosylation of human erythropoietin expressed in adenovirally transduced mouse mammary epithelial cells. Biochim Biophys Acta. 2005;1726(1):48–56. doi: 10.1016/j.bbagen.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Tong G, Jahr CE. Block of glutamate transporters potentiates postsynaptic excitation. Neuron. 1994;13(5):1195–203. doi: 10.1016/0896-6273(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Trotti D, Aoki M, Pasinelli P, Berger UV, Danbolt NC, Brown RH, Jr., Hediger MA. Amyotrophic lateral sclerosis-linked glutamate transporter mutant has impaired glutamate clearance capacity. J Biol Chem. 2001;276(1):576–82. doi: 10.1074/jbc.M003779200. [DOI] [PubMed] [Google Scholar]
- Tsvetkov E, Shin RM, Bolshakov VY. Glutamate uptake determines pathway specificity of long-term potentiation in the neural circuitry of fear conditioning. Neuron. 2004;41(1):139–51. doi: 10.1016/s0896-6273(03)00800-6. [DOI] [PubMed] [Google Scholar]
- Tzingounis AV, Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci. 2007;8(12):935–47. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- Varma R, Hoshino AY. Serum glycoproteins in schizophrenia. Carbohydr Res. 1980;82(2):343–51. doi: 10.1016/s0008-6215(00)85708-0. [DOI] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, Nord AS, Kusenda M, Malhotra D, Bhandari A, Stray SM, Rippey CF, Roccanova P, Makarov V, Lakshmi B, Findling RL, Sikich L, Stromberg T, Merriman B, Gogtay N, Butler P, Eckstrand K, Noory L, Gochman P, Long R, Chen Z, Davis S, Baker C, Eichler EE, Meltzer PS, Nelson SF, Singleton AB, Lee MK, Rapoport JL, King MC, Sebat J. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320(5875):539–43. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Williams SM, Sullivan RK, Scott HL, Finkelstein DI, Colditz PB, Lingwood BE, Dodd PR, Pow DV. Glial glutamate transporter expression patterns in brains from multiple mammalian species. Glia. 2005;49(4):520–41. doi: 10.1002/glia.20139. [DOI] [PubMed] [Google Scholar]
- Yang W, Kilberg MS. Biosynthesis, intracellular targeting, and degradation of the EAAC1 glutamate/aspartate transporter in C6 glioma cells. J Biol Chem. 2002;277(41):38350–7. doi: 10.1074/jbc.M202052200. [DOI] [PubMed] [Google Scholar]