Abstract
Objectives
To determine whether carbonic anhydrase-IX (CA-IX) was associated with progression-free survival (PFS) and overall survival (OS) in women with high-risk, early-stage cervical cancer treated with adjuvant pelvic radiotherapy with or without radiosensitizing chemotherapy.
Methods
CA-IX expression was detected using an immunohistochemistry assay and categorized as low when ≤80% of tumor cells exhibited CA-IX staining and high when >80% tumor cells display CA-IX staining. Associations between CA-IX expression and clinical characteristics, angiogenesis marker expression and clinical outcome were evaluated.
Results
High CA-IX expression was observed in 35/166 (21.1%) of cases. CA-IX expression was not associated with age, race, stage, cell type, grade, positive margins, parametrial extensions, positive lymph nodes or lymphovascular space invasion but was associated with tumor size categorized as <2 cm, 2-2.9 cm, or ≥3 cm (high expression: 4.7% vs. 23.2% vs. 32.5%, p=0.003) and cervical invasion confined to the inner two thirds compared with the outer third of the cervix (high expression: 6.1% vs. 23.7%, p=0.028). CA-IX expression was not associated with immunohistochemical expression of p53, CD31, CD105, thrombospondin-1 or vascular endothelial growth factor-A. Women with high versus low CA-IX expression had similar PFS (p=0.053) and significantly worse OS (p=0.044). After adjusting for prognostic clinical covariates, high CA-IX expression was an independent prognostic factor for PFS (hazard ratio [HR]=2.12; 95% confidence interval [CI]=1.13 -3.95; p=0.019) and OS (HR=2.41; 95% CI=1.24-4.68; p=0.009).
Conclusions
Tumor hypoxia measured by immunohistochemical expression of CA-IX is an independent prognostic factor for both PFS and OS in high-risk, early-stage cervical cancer.
Introduction
Cervical cancer is the second-most common cause of cancer-related deaths in women worldwide, causing an estimated 273,000 deaths annually worldwide [1] and 3,870 deaths annually in the United States [2]. Of the 19,339 cases registered with Surveillance Epidemiology and End Results (SEER) program between 1996 and 2003, 51% of cervical cancers were diagnosed as local disease, with a 5-year survival rate of 92% for these women [3]. Analysis of specimens obtained from women receiving surgical treatment for early-stage cervical cancer has identified several clinical and pathologic poor prognostic factors including increased age, African-American ethnicity, human papillomavirus (HPV) 18 infection, deep cervical stromal invasion, tumor size >2 cm, lymphovascular space invasion (LVSI), nodal metastases, microscopic tumor in uterine parametrial tissues, and positive surgical margins [4-7]. Though these prognostic factors have been well established, the biologic factors associated with recurrence and survival remain largely unknown.
In the 1990s, the antigen MN was identified [8]. MN is a 54 to 58 kDa transmembrane glycoprotein, and is a member of the carbonic anhydrase gene family, and is more specifically designated carbonic anhydrase IX (CA-IX) [9]. CA-IX is a biomarker of several types of human tumors, namely carcinomas of the cervix [10-16], kidney, esophagus and stomach, colon, head and neck, lung, and breast [17-22]. CA-IX expression in cancerous tissues and its absence in normal counterparts suggest a role in carcinogenesis [23]. Not only is CA-IX emerging as an important biomarker involved in carcinogenesis but its expression appears to be induced by hypoxia [11,23]. CA-IX expression is controlled by the transcription factor, hypoxia inducible factor-1 (HIF-1) and is up-regulated in hypoxic regions of tumor tissues. CA-IX expression was associated with microvessel density and hypoxia in head and neck squamous cell cancers [20] and with tumor stage and lymph node metastasis in cervical cancer [12]. The independent prognostic significance of CA-IX expression was recently reported in breast cancer patients [22]. The prognostic significance of CA-IX expression in carcinomas of the lung and cervix has also been examined [21,11]. In the case of carcinoma of the cervix, preliminary studies have shown that CA-IX expression is up-regulated in hypoxic regions of cervical tumors and is associated with a poor prognosis [11]. However, other reports have also indicated that there was no association of CA-IX expression and clinical outcome in cervical cancer transitional cell carcinoma of the bladder, or head and neck squamous cell carcinoma [24-26].
The purpose of this study was for the Gynecologic Oncology Group (GOG) to determine whether CA-IX was associated with progression-free survival (PFS) and overall survival (OS) in women with high-risk, early-stage cervical cancer treated with adjuvant pelvic radiotherapy with or without radiosensitizing chemotherapy on a multi-center randomized phase III trial [27]. The secondary objectives of this study were to examine the relationship between CA-IX and clinical characteristics as well as the expression of p53 and other biomarkers previously reported in this cohort, including CD31, CD105, thrombospondin-1 (TSP-1) and vascular endothelial growth factor-A (VEGF-A) [40].
Materials and Methods
Eligibility Requirements
Women who participated in a multi-center randomized phase III trial (Southwest Oncology Group 8797/GOG 109/Radiation Therapy Oncology Group 91-12) between 1991 and 1996, and provided a primary tumor specimen were eligible for this translational research study. Women were required to have undergone type III radical hysterectomy and pelvic lymphadenectomy for International Federation of Gynecology and Obstetrics (FIGO) stage IA2, IB, and IIA cervical cancer with pathologic findings of lymph node metastases, parametrial involvement, or positive surgical margins prior to treatment with adjuvant pelvic radiotherapy (RT) with or without radiosensitizing chemotherapy. Women with squamous carcinoma, adenosquamous carcinoma, and adenocarcinoma histologic subtypes were included. The results of this multi-center randomized phase III trial have been previously reported [27]. All women provided written informed consent and annual approval by the institutional review board at the GOG participating institutions in accordance with federal, state, and local requirements for the treatment protocol including primary tumor block collection for research, and approval for this translational research study was obtained from the GOG, the Cancer Therapy Evaluation Program at the National Cancer Institute and the University of California, Irvine Institutional Review Board.
Treatment Regimens
Eligible women were randomized within six weeks of surgery to receive 49.3 Gy ± 4.5 Gray delivered via a standard four-field box to the para-aortic nodes in women with positive high common iliac nodes with or without 70 mg/m2 cisplatin given as a 2-hour intravenous infusion on day 1 and 1,000 mg/m2 5-FU per day for 4 consecutive days delivered as a 96-hour infusion every 21 days for a total of 4 cycles.
Follow Up and End Points
Follow-up consisted of physical examinations performed quarterly for 2 years, semiannually for 3 years, and annually thereafter. PFS was calculated as the time in months from date of enrollment to disease progression or death independent of cause, or date of last contact for those with no evidence of disease progression. OS was calculated as the time in months from date of enrollment to death independent of cause, or date of last contact for those who were still alive.
Primary Tumor Specimens
Formalin-fixed and paraffin-embedded (FFPE) pre-treatment radical hysterectomy specimens or large excisional biopsies were cut into 4 micron sections and fixed onto positively-charged glass slides. Histologic eligibility was verified by the GOG Pathology Committee, and the adequacy of FFPE primary tumor block for research was determined by the study pathologist (SYL) who examined hematoxylin and eosin-stained tissue specimens to confirm that at least 50% of each section consisted of malignant tissue.
Immunohistochemical Expression of CA-IX
Immunohistochemical staining of tissue sections with anti-CA-IX antibody was performed using a peroxidase technique with pressure cooking pretreatment [10]. Briefly, the specimens were de-paraffinized in xylene, rehydrated in graded alcohols, and rinsed in distilled, deionized water. Slides were placed in antigen retrieval citrate solution and pre-treated with pressure cooking, and then incubated with a 3% (v/v) hydrogen peroxide solution for 10 minutes and 5% (v/v) normal horse serum in phosphate buffered saline (PBS) for 20 minutes. Specimens were incubated at ambient room temperature with an anti-CA-IX mouse monoclonal antibody (M75) with 1:10,000 dilution for an hour, a 1:200 dilution of biotinylated horse anti-mouse immunoglobulin G in PBS for 30 minutes, avidin-biotin peroxidase complex (ABC Elite, Vector Laboratories, Burlingame, CA) for 30 minutes, and 3′,3′-diaminobenzidine chromogen solution for 10 minutes. Specimens were counter-stained with hematoxylin (Dako, Carpenteria, CA), rinsed in double distilled water, and mounted with Aquamount. Positive and negative controls were included in each run. CA-IX immunostaining was evaluated by one of authors (SYL) blinded to all clinical and outcome data. Specific immunohistochemical staining was defined by the presence of a brown reaction product on the plasma membrane under 400× magnification (with 10× ocular lens). Faint staining of the cytoplasm was considered negative. The intensity of immunoreactivity was scored as strong (2+) and weak (1+). Strong positivity was defined as dark brown immunoreactivity that was easily identified at a low power magnification (40× or 100×). A reasonable degree of heterogeneity of CA-IX-positive staining was noted; therefore, based on the average percentage of strongly (2+) positive cells present in the entire tissue section, the CA-IX immunoreactivity was divided into four groups: (A) >80% tumor cells positive; (B) 40 to 80% of tumor cells positive; (C) 15-39% of tumor cells positive; (D) <15% tumor cells positive or no immunoreactivity was present. We further classified tumors that were uniformly hypoxic across the entire section (Group A) from those that exhibited limited hypoxia or were not hypoxic (Groups B, C and D) as illustrated in Figure 1.
Figure 1.

Representative examples of CA-IX expression in squamous cell carcinoma of the cervix: Low expression (A): Focal CA-IX expression (brown immunostain) limited to a few neoplastic cell clusters (long arrow), the majority of carcinoma nests are negative (short arrow). High expression (B): Diffuse, strong (2+) immunoreactivity is seen in >80% of the neoplastic cells (arrow) in a single section. The stroma (◆) and areas of necrotic tumor are negative. Original magnification ×100.
Immunohistochemical Expression of VEGF-A, TSP-1, CD31, CD105 and p53
Semi-quantitative immunohistochemical (IHC) staining for vascular endothelial growth factor-A (VEGF-A, pro-angiogenesis factor), thrombospondin-1 (TSP-1, anti-angiogenesis factor), CD31 (pan endothelial marker), and CD105 (tumor-specific endothelial marker) was evaluated as previously described [28]. Briefly, p53 was detected using the N-terminal DO-1 anti-p53 clone that recognizes the major normal and mutant p53 isoforms but not the isoforms lacking the first 40 or 133 amino acids of full length p53 (Santa Cruz Biotechnology, Santa Cruz, CA). TSP-1 and p53 were categorized as negative or positive [28]. CD31 and CD105 microvessel density (MVD) “hotspots” were counted in three 20× high-power fields and categorized as low (<110 and <28) or high (≥110 and ≥28), respectively [28]. Tumoral histoscores (HS) were calculated for VEGF-A using the formula: [% cells positive × (intensity +1)] and categorized as low (<200) or high (≥200) [28].
Statistical Analysis
Biomarker and clinical data for this ancillary study were analyzed using SPSS version 14 (SPSS Inc., Chicago, IL) and SAS version 9.1 (SAS Institute, Inc., Cary, NC). CA-IX expression was evaluated and categorized as low when ≤80% of tumor cells exhibited CA-IX staining and high when >80% tumor cells display CA-IX staining. Exact testing was used to test the hypothesis of independence between CA-IX expression and clinical characteristics or biomarkers [29,30]. Estimates of the survival probabilities were calculated using the Kaplan-Meier product limit method [31], and the logrank test employed to test the null hypothesis of equality between strata [32,33]. Hazard ratios were estimated using Cox proportional hazard regression analysis [34], without or with adjustments for prognostic clinical covariates (age, race, depth of cervical invasion, parametrial extensions, positive lymph nodes and treatment) for PFS and OS. These covariates were selected for adjustment based on their documented prognostic value in cervical cancer [4-7].
Results
Of the 243 eligible and evaluable women who participated in the multi-center randomized phase III trial there were 180 women who were enrolled at GOG institutions and provided FFPE primary tumor tissue for translational research and 166 of these women were eligible for this translational research study. One hundred and fifty three of the specimens (92.2%) were from radical hysterectomies and 13 (7.8%) were from large excisional biopsies. Fourteen women were excluded for the following reasons: benign disease (n=1), low-risk, early stage disease (n=2), no FFPE tumor for testing (n=4), and inevaluable for CA-IX expression (n=7). The patient characteristics for the 166 women in this cohort are summarized in Table 1 and are representative of that observed in the entire cohort of women who participated in the phase III intergroup trial [27]. At the time of the final analyses, 110 women were alive with no evidence of disease, 5 were alive with disease progression, 43 died due to disease progression, 5 died due to a reason other than disease progression or treatment, and three died of unknown cause. Median follow-up for the 115 women who were still alive at the time of the final analysis was 105.9 (range: 2.7 to 184.8) months. The distribution of cases by treatment was as follows: 86 (51.8%) women were randomized to radiation and 80 (48.2%) women were randomized to chemoradiation.
Table 1. Relationship between CA-IX expression and clinical characteristics.
| CA-IX Expression * | Exact test | |||
|---|---|---|---|---|
| Clinical Characteristics | Cases | Low Cases (%) | High Cases (%) | p-value |
| Age ** | 0.121 | |||
| <40 | 91 | 72 (79) | 19 (21) | |
| 40-49 | 37 | 32 (86) | 5 (14) | |
| 50-59 | 23 | 18 (78) | 5 (22) | |
| 60-69 | 12 | 6 (50) | 6 (50) | |
| 70-79 | 3 | 3 (100) | 0 (0) | |
| Race and Ethnicity | 0.498 | |||
| Caucasian | 104 | 79 (76) | 25 (24) | |
| African American | 29 | 24 (83) | 5 (17) | |
| Hispanic | 25 | 20 (80) | 5 (20) | |
| Other † | 8 | 8 (100) | 0 (0) | |
| Stage | 1.000 | |||
| IB | 158 | 124 (78) | 34 (22) | |
| IIA | 8 | 7 (88) | 1 (12) | |
| Cell Type | 0.810 | |||
| Squamous Carcinoma | 135 | 107 (79) | 28 (21) | |
| Other Carcinoma †† | 31 | 24 (77) | 7 (23) | |
| Grade | 0.240 | |||
| 1 | 13 | 8 (62) | 5 (38) | |
| 2 | 80 | 63 (79) | 17 (21) | |
| 3 | 73 | 60 (82) | 13 (18) | |
| Tumor Size | 0.003 | |||
| <2 cm | 43 | 41 (95) | 2 (5) | |
| 2-2.9 cm | 82 | 63 (77) | 19 (23) | |
| ≥3 cm | 40 | 27 (68) | 13 (32) | |
| Depth of Cervical Invasion | 0.028 | |||
| Inner two-thirds ‡ | 33 | 31 (94) | 2 (6) | |
| Outer third | 131 | 100 (76) | 31 (24) | |
| Positive Margin | 1.000 | |||
| No | 156 | 123 (79) | 33 (21) | |
| Yes | 10 | 8 (80) | 2 (20) | |
| Parametrial Extensions | 1.000 | |||
| No | 107 | 84 (79) | 23 (21) | |
| Yes | 59 | 47 (80) | 12 (20) | |
| Positive Nodes | 0.451 | |||
| No | 27 | 23 (85) | 4 (15) | |
| Yes ‡‡ | 139 | 108 (78) | 31 (22) | |
| Lymphovascular Space Invasion | 0.378 | |||
| No | 41 | 30 (73) | 11 (27) | |
| Yes | 124 | 100 (81) | 24 (19) | |
Cases (row percentage × 100).
CA-IX expression was categorized as low when ≤80% of tumor cells exhibited CA-IX staining and high when >80% tumor cells display CA-IX staining.
Median age at enrollment for the entire cohort was 39.03 years.
Other includes Asian/Pacific Islander (5), Filipino (2), and Native American (1).
Other carcinoma includes 20 adenocarcinomas and 11 adenosquamous carcinomas
Five invaded the inner third and 28 invaded the middle third of the cervix.
There were 68 women with one and 71 with two or more positive lymph nodes.
Figure 1 displays representative immunostaining for CA-IX. High level expression was observed in 35/166 (21.1%) of cases when >80% tumor cells displayed strong CA-IX immunostaining (2+ intensity) that was distributed throughout the tumor nests in the entire tissue section. CA-IX expression was not associated with age, race, stage, cell type, grade, positive margins, parametrial extensions, positive lymph nodes or lymphovascular space invasion (Table 1) but was correlated with tumor size categorized as <2 cm, 2-2.9 cm, or ≥3 cm (high expression: 5% vs. 23% vs. 33%; p=0.003). Tumors with invasion into the outer third of the cervix also had higher expression of CA-IX than those confined to the inner two thirds (high expression: 24% vs. 6%, p=0.028). CA-IX expression was not associated with immunohistochemical expression of p53, CD31, CD105, TSP-1 or VEGF-A (Table 2).
Table 2. Relationship between CA-IX and other biomarkers including p53, CD31, CD105, TSP-1, and VEGFA.
| CA-IX Expression * | Exact test | ||
|---|---|---|---|
| Clinical Characteristics | Low Cases (%) | High Cases (%) | p-value |
| p53 | 0.445 | ||
| Negative | 57 (75) | 19 (25) | |
| Positive | 68 (81) | 16 (19) | |
| CD31-Microvessel Density | 1.000 | ||
| Low <110 | 81 (78) | 23 (22) | |
| High ≥110 | 44 (79) | 12 (21) | |
| CD105-Microvessel Density | 0.823 | ||
| Low <28 | 36 (78) | 10 (22) | |
| High ≥28 | 75 (80) | 19 (20) | |
| TSP-1 | 1.000 | ||
| Negative | 40 (80) | 10 (20) | |
| Positive | 87 (78) | 25 (22) | |
| VEGF-A Histoscore | 0.150 | ||
| Low <200 | 39 (72) | 15 (28) | |
| High ≥200 | 86 (83) | 18 (17) | |
Cases (row percentage × 100).
CA-IX expression was categorized as low when ≤80% of tumor cells exhibited CA-IX staining and high when >80% tumor cells display CA-IX staining.
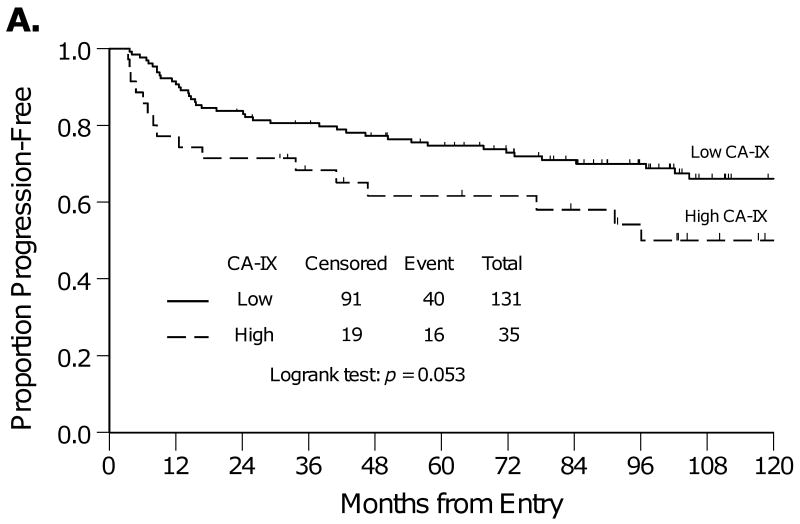
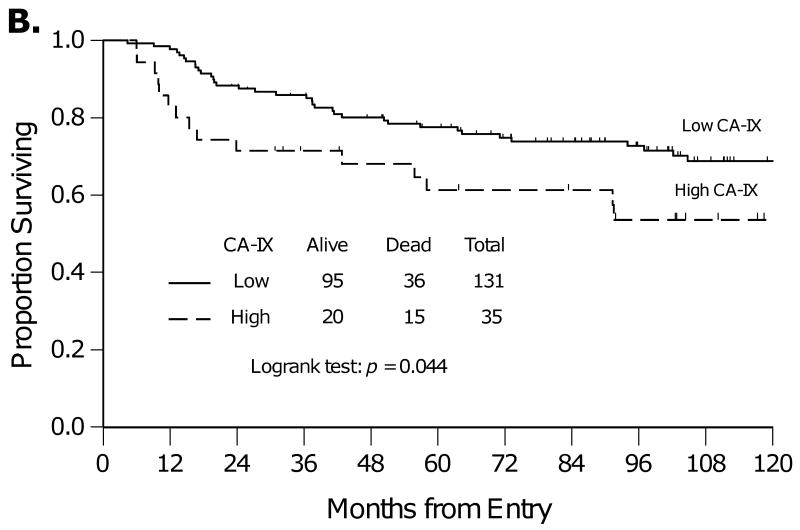
The Kaplan-Meier method was used to estimate PFS and OS for women with low compared with high CA-IX expression (Figure 2). Women with high versus low CA-IX expression had similar PFS (Figure 2A, p=0.053) and significantly worse OS (Figure 2B, p=0.044). The five-year OS was 78% vs. 61% for women with tumors with low or high CA-IX expression, respectively.
Figure 2.
Kaplan-Meier plots for progression-free survival (A) and overall survival (B) for women categorized by low or high CA-IX expression.
Unadjusted and adjusted Cox regression analyses were performed to examine the association between CA-IX expression and PFS or OS (Table 3). Women whose tumor exhibited high compared with low CA-IX expression had a similar risk of disease progression (hazard ratio [HR]=1.760; 95% confidence interval [CI]=0.985-3.144; p=0.056) and a significant increase in the risk of death (HR=1.840; 95% CI=1.007-3.362; p=0.047). After adjusting for prognostic clinical covariates, high CA-IX expression was an independent prognostic factor for PFS (HR=2.12; 95% CI=1.13-3.95; p=0.019) and OS (HR=2.41; 95% CI=1.24-4.68; p=0.009).
Table 3. Associations between CA-IX expression and progression-free survival or overall survival.
| Progression-Free Survival (PFS) | Overall Survival (OS) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biomarker Expression† | Unadjusted Cox Model | Adjusted Cox Model ‡ | Unadjusted Cox Model | Adjusted Cox Model ‡ | ||||||||
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| CA-IX | ||||||||||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| High | 1.76 | 0.99-3.14 | 0.056 | 2.12 | 1.13-3.95 | 0.019 | 1.84 | 1.01-3.36 | 0.047 | 2.41 | 1.24-4.68 | 0.009 |
| CA-IX* | ||||||||||||
| Low | 1.00 | 1.00 | 1.00 | |||||||||
| High | 1.71 | 0.94-3.11 | 0.077 | 1.82 | 0.96-3.46 | 0.067 | 1.77 | 0.95-3.29 | 0.072 | 2.07 | 1.05-4.10 | 0.037 |
| CD31-MVD* | ||||||||||||
| Low | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| High | 0.36 | 0.18-0.71 | 0.003 | 0.35 | 0.17-0.70 | 0.003 | 0.36 | 0.17-0.73 | 0.005 | 0.33 | 0.16-0.69 | 0.003 |
HR: hazard ratio; 95% CI: 95% confidence interval.
CA-IX expression was categorized as low when ≤80% of tumor cells exhibited CA-IX staining and high when >80% tumor cells display CA-IX staining. CD31 was categorized as low for tumors with a microvessel density (MVD) <110 and high for tumors with a MVD ≥110.
Adjusted for patient age, race, stage, depth of cervical invasion, parametrial extensions, positive lymph nodes and treatment.
CA-IX and CD31-MVD were both included in the Cox regression analyses for PFS and OS.
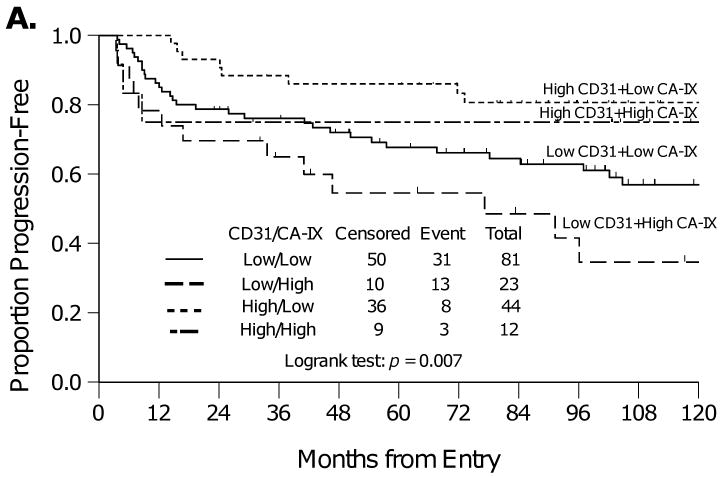
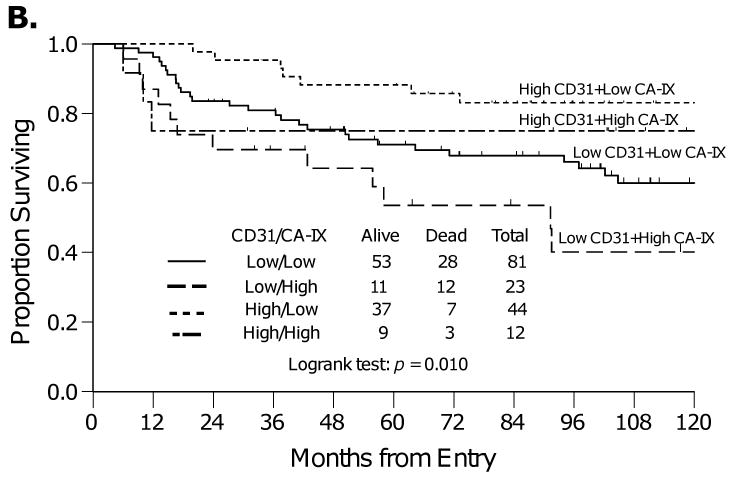
In follow up to these findings, an analysis was then performed to explore the relationship between CD31-MVD (detailed analysis previously reported in [28]) and CA-IX in this cohort to provide enhanced resolution regarding tumor vascularization and permeability within the tumor tissue from these patients, as CD31-MVD contributes a hot spot assessment of regions within the tumor that exhibit the highest degree of vascularization whereas CA-IX expression provides a score for whether or not the tumor is exhibiting uniform hypoxia throughout the entire tissue section. Categorized CD31-MVD was not correlated with categorized CA-IX expression (Table 2). Specifically, women who have tumors with high CD31-MVD (angiogenesis) and low CA-IX (hypoxia) had the best PFS (Figure 3A) and OS (Figure 3B). Those with low CD31-MVD and high CA-IX had the worst PFS and OS (Figure 3). Those with either low CD31-MVD (angiogenesis) and low CA-IX (hypoxia) or high CD31-MVD and high CA-IX had intermediate PFS and OS (Figure 3). Inclusion of both biomarkers in an adjusted Cox regression model demonstrated that after adjusting for CD31-MVD, high CA-IX expression was associated with increased risk of death (Table 3). In addition, after adjusting for CA-IX expression, high CD31-MVD was associated with a reduced risk of progression and death (Table 3).
Figure 3.
Kaplan-Meier plots for progression-free survival (A) and overall survival (B) for women categorized by CA-IX and CD31-MVD expression.
Discussion
Experimental and clinical studies have shown that hypoxic tumors are not only resistant to radiation and chemotherapy, but are also associated with genetic instability and increased risk of invasion, metastasis, and poor clinical outcome. The intrinsic biological aggressiveness of hypoxic tumors is explained, in part, by the up-regulation of a number of hypoxia-inducible genes mediated by the activation of the transcription factor, HIF-1. Investigators continue to search for reliable diagnostic, prognostic and predictive biomarkers that can assist in clinical treatment and management decisions and identify new therapeutic targets. CA-IX has recently emerged as one of the most promising endogenous markers of cellular hypoxia and has been validated directly or indirectly by various studies [11,35,36].
Although one cervical cancer study showed that CA-IX was not associated with clinical outcome [24], others demonstrate that high CA-IX expression was associated with worse survival in cervical carcinoma [11,14,15]. In this study, we confirm the observation that high CA-IX expression was associated with worse clinical outcome Our study defined high levels of CA-IX expression as more than 80% of tumor cells in entire tissue section exhibiting strong CA-IX immunoreactivity (2+ intensity) because this represents a uniform diffuse distribution of hypoxia throughout the carcinoma. Based on this definition, 21% (35/166) of the tumors exhibited high levels of CA-IX expression (uniform hypoxia). Women with high levels of CA-IX expression had an increased risk of disease progression and death. In addition, categorized CA-IX expression was an independent prognostic factor for PFS and OS after adjusting for clinical covariates with documented prognostic relevance in this cohort [6] and in other studies [4-5,7]. Differences in stage of disease, sample type and size as well as treatment regimens may influence, at least in part, the disparity in findings [24] compared with others [11,14,15] and that described herein.
CA-IX expression was not associated with age, race, stage, cell type, grade, positive margins, parametrial extensions, positive lymph nodes or lymphovascular space invasion (Table 1), consistent with prior publications[11,14]. However, CA-IX expression did correlate with categorized tumor size and the depth of cervical invasion. These findings are biologically plausible given that CA-IX expression will be induced via HIF-mediated transcription when the diffusional capacity of the tumor vasculature is exceeded as the tumor increases in size and invades deeper into the surrounding tissue [11,35-38].
It is well established that hypoxia results in the upregulation of genes that facilitate anaerobic metabolism and promote tumor vascularization (e.g. VEGF). Although the study did not show a correlation between CA-IX expression and several angiogenesis biomarkers including CD31-MVD, CD105-MVD, TSP-1 and VEGF-A or p53, an interesting association was observed between CD31-MVD, CA-IX expression and prognosis. Specifically, women who have tumors with high CD31-MVD (angiogenesis) and low CA-IX (hypoxia) had the best PFS and OS while those with low CD31-MVD (angiogenesis) and high CA-IX (hypoxia) had the worst PFS and OS (Figure 3). Those with either low CD31-MVD (angiogenesis) and low CA-IX (hypoxia) or high CD31-MVD and high CA-IX had intermediate PFS and OS (Figure 3). Adjusted Cox regression modeling demonstrated that CA-IX was a prognostic factor for OS even after adjusting for CD31-MVD. Taken together, these findings extend our previous hypothesis [28] by clarifying that improved survival is observed in the well-vascularized and well-oxygenated cervical cancers with high CD31-MVD and low CA-IX expression.
In conclusion, a high level of CA-IX expression in tumors appears to be an independent prognostic indicator in women with high-risk, early stage cervical carcinoma and may provide a number of implications for cancer patient management such as serving as an indicator of the patient selection in combination with CD31-MVD and prognostic clinical covariates for possible hypoxia-modifying therapy, bio-reductive drugs administration or combination regimens that utilize immunomodulatory agents. Moreover, CA-IX is an emerging target for cancer therapy and functional imaging [39-41], further broadening the potential clinical value of the CA-IX biomarker in the detection, treatment and management of women with cervical cancer.
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group (GOG) Administrative Office (CA 27469), the GOG Tissue Bank (CA 27469, CA 11479), and to the GOG Statistical and Data Center (CA 37517). The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: University of Alabama at Birmingham, Oregon Health Sciences University, Duke University Medical Center, Walter Reed Medical Center, Wayne State University, University of Southern California at Los Angeles, University of Pennsylvania Cancer Center, University of Miami School of Medicine, Milton S. Hershey Medical Center, Georgetown University Hospital, University of Cincinnati, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University Medical Center, Wake Forest University School of Medicine, University of California Medical Center at Irvine, Tufts-New England Medical Center, Rush University Medical Center, SUNY Downstate Medical Center, Eastern Virginia Medical School, Johns Hopkins Cancer Center, State University of New York at Stony Brook, Washington University School of Medicine, Cooper Hospital/University Medical Center, Columbus Cancer Council, M. D. Anderson Cancer Center, University of Massachusetts Medical School, Fox Chase Cancer Center, Women's Cancer Center, University of Oklahoma, University of Virginia Health Sciences Center, University of Chicago, University of Arizona and Case Western Reserve University.
The authors thank Anne Reardon for her assistance in preparing this manuscript for publication, and Dr. Mark Brady, the GOG Publications Subcommittee and Dr. John Schorge for their critical review of the manuscript and helpful suggestions.
Footnotes
Conflict of Interest Statement: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The study was presented at the Society of Gynecologic Oncologist's 40th Annual Meeting on Women's Cancer, February 5-8 2009, San Antonio, TX.
References
- 1.Parkin MD, Bray F, Ferjay J, Pisani P. Global Cancer Statistics 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Ries LAG, Melbert D, Krapcho M, et al. National Cancer Institute; Bethesda, MD: 2007. SEER Cancer Statistics Review, 1975-2004. http://seer.cancer.gov/csr/1975_2004/, based on November 2006 SEER data submission, posted to the SEER web site. [Google Scholar]
- 4.Delgado G, Bundy B, Zaino R, Sevin BU, Creasman WT, Major F. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1990;38:352–7. doi: 10.1016/0090-8258(90)90072-s. [DOI] [PubMed] [Google Scholar]
- 5.Burger RA, Monk BJ, Kurosaki T, et al. J Natl Cancer Inst. 1996;88:1361–8. doi: 10.1093/jnci/88.19.1361. [DOI] [PubMed] [Google Scholar]
- 6.Monk BJ, Wang J, Im S, et al. Rethinking the use of radiation and chemotherapy after radical hysterectomy: a clinical-pathologic analysis of a Gynecologic Oncology Group/Southwest Oncology Group/Radiation Therapy Oncology Group trial. Gynecol Oncol. 2005;96:721–8. doi: 10.1016/j.ygyno.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Im SS, Wilczynski SP, Burger RA, et al. Early stage cervical cancers containing human papillomavirus type 18 DNA have more nodal metastasis and deeper stromal invasion. Clin Cancer Res. 2003;9:4145–50. [PubMed] [Google Scholar]
- 8.Zavada J, Zavadova Z, Pastorekova S, Ciampor F, Pastorek J, Zelnik V. Expression of MaTu-MN protein in human tumor cultures and in clinical specimens. Int J Cancer. 1993;54:268–74. doi: 10.1002/ijc.2910540218. [DOI] [PubMed] [Google Scholar]
- 9.Pastorek J, Pastorekova S, Callebaut I, Mornon JP, Zelnik V, Opavsky R, et al. Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and a putative helix-loop-helix DNA binding segment. Oncogene. 1994;9:2877–88. [PubMed] [Google Scholar]
- 10.Liao SY, Brewer C, Zavada J, Pastorek J, Pastorekova S, Manetta A, et al. Identification of the MN antigen as a diagnostic biomarker of cervical intraepithelial squamous and glandular neoplasia and cervical carcinoma. Am J Pathol. 1994;145:598–609. [PMC free article] [PubMed] [Google Scholar]
- 11.Loncaster JA, Harris AL, Davidson SE, Logue JP, Hunter RD, Wycoff CC, et al. Carbonic anhydrase (CA-IX) expression, a potential new intrinsic marker of hypoxia: correlations with tumor oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res. 2001;61:6396–9. [PubMed] [Google Scholar]
- 12.Mayer A, Höckel M, Vaupel P. Carbonic anhydrase IX expression and tumor oxygenation status do not correlate at the microregional level in locally advanced cancers of the uterine cervix. Clin Cancer Res. 2005;11:7220–5. doi: 10.1158/1078-0432.CCR-05-0869. [DOI] [PubMed] [Google Scholar]
- 13.Kim JY, Shin HJ, Kim TH, Cho KH, Shin KH, Kim BK, et al. Tumor-associated carbonic anhydrase are linked to metastases in primary cervical cancer. J Cancer Res Clin Oncol. 2006;132:302–8. doi: 10.1007/s00432-005-0068-2. [DOI] [PubMed] [Google Scholar]
- 14.Kirkpatrick JP, Rabbani ZN, Bentley RC, Hardee ME, Karol S, Meyer J, et al. Elevated CAIX Expression is Associated with an Increased Risk of Distant Failure in Early-Stage Cervical Cancer. Biomarker Insights. 2008;3:45–55. doi: 10.4137/bmi.s570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S, Shin HJ, Han IO, Hong EK, Park SY, Roh JW, et al. Tumor carbonic anhydrase 9 expression is associated with the presence of lymph node metastases in uterine cervical cancer. Cancer Sci. 2007;98:329–333. doi: 10.1111/j.1349-7006.2007.00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee WY, Huang SC, Hsu KF, Tzeng CC, Shen WL. Roles for hypoxia-regulated genes during cervical carcinogenesis: Somatic evolution during the hypoxia-glycolysis-acidosis sequence. Gynecol Oncol. 2008;108:377–384. doi: 10.1016/j.ygyno.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 17.Liao SY, Aurelio ON, Jan K, Zavada J, Stanbridge EJ. Identification of the MN/CA9 protein as a reliable diagnostic biomarker of clear cell carcinoma of the kidney. Cancer Res. 1997;57:2827–31. [PubMed] [Google Scholar]
- 18.Driessen A, Landuvt W, Pastorekova S, Moons J, Goethals L, Haustermans K, et al. Expression of carbonic anhydrase IX (CAIX, a hypoxia-related protein, rather than vascular-endothelial growth factor (VEGF), a pro-angiogenic factor, correlates with an extremely poor prognosis in esophageal and gastric adenocarcinomas. Ann Surg. 2006;243:334–40. doi: 10.1097/01.sla.0000201452.09591.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saarnio J, Parkkila S, Parkklia AK, Pastoreková S, Pastorek J, Kairaluoma MI, Karttunen TJ. Immunohistochemical study of colorectal tumors for expression of novel transmembrane carbonic anhydrase, MN/CA IX, with potential value as a marker of cell proliferation. Am J Pathol. 1998;153:279–85. doi: 10.1016/S0002-9440(10)65569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beasley NJ, Wykoff CC, Watson PH, Leek R, Turley H, Gatter K, et al. Carbonic anhydrase IX, an endogenous hypoxia marker, expression in head and neck squamous cell carcinoma and its relationship to hypoxia, necrosis, and microvessel density. Cancer Res. 2001;61:5262–7. [PubMed] [Google Scholar]
- 21.Giatromanolaki A, Koukouakis MI, Sivridis E, Pastorek J, Wykoff CC, Gatter KC, Harris AL. Expression of hypoxia-inducible carbonic anhydrase-9 relates to angiogenic pathways and independently to poor outcome in non-small cell lung cancer. Cancer Res. 2001;61:7992–8. [PubMed] [Google Scholar]
- 22.Chia SK, Wykoff CC, Watson PH, Han C, Leek RD, Pastorek J, et al. Prognostic significance of a novel hypoxia-regulated marker, carbonic anhydrase IX, in invasive breast carcinoma. J Clin Oncol. 2001;19:3660–8. doi: 10.1200/JCO.2001.19.16.3660. [DOI] [PubMed] [Google Scholar]
- 23.Ivanov S, Liao SY, Ivanova A, Danilkovitch-Miaqkova A, Tarasova N, Weirich G, et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158:905–19. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hedley D, Pintillie M, Woo J, Morrison A, Birle D, Fyles A, Milosevic M, Hill R. Carbonic anhydrase IX expression, hypoxia and prognosis in patients with uterine cervical carcinomas. Clin Cancer Res. 2003;9:5666–74. [PubMed] [Google Scholar]
- 25.Hussain SA, Palmer DH, Ganesan R, Hiller L, Gregory J, Murray PG, et al. Carbonic anhydrase IX, a marker of hypoxia: correlation with clinical outcome in transitional cell carcinoma of the bladder. Oncol Report. 2004;11:1005–10. [PubMed] [Google Scholar]
- 26.Eriksen J, Overgaard J, Head and Neck Cancer Study Group (DAHANCA) Lack of prognostic and predictive value of CA IX in radiotherapy of squamous cell carcinoma of the head and neck with know modifiable hypoxia: An evaluation of the DAHANCA 5 study. Radiother Oncol. 2007;83:383–8. doi: 10.1016/j.radonc.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Peters WA, 3rd, Liu PY, Barrett RJ, 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–13. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 28.Randall LM, Monk BJ, Darcy KM, Tian C, Burger RA, Liao SY, et al. Markers of angiogenesis in high-risk, early-stage cervical cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2009;112:583–9. doi: 10.1016/j.ygyno.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher RA. On the interpretation of χ2 from contingency tables, and the calculation of P. J Royal Stat Soc. 1922;85:87–94. [Google Scholar]
- 30.Mehta CR, Patel NR. A network algorithm for performing Fisher's exact test in r × c contingency tables. J Am Stat Assoc. 1983;78:427–434. [Google Scholar]
- 31.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 32.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemotherapy Reports. 1966;50:63–70. [PubMed] [Google Scholar]
- 33.Peto R, Peto J. Asymptotically Efficient Rank Invariant Test Procedures. J Royal Stat Soc Series A (General) 1972;135:185–207. [Google Scholar]
- 34.Cox DR. Regression models and life tables. J Royal Stat Soc Series B. 1972;34:187–220. [Google Scholar]
- 35.Olive PL, Aquini-Parsons C, MacPhail SH, Liao SY, Raleigh JA, Lerman MI, Stanbridge EJ. Carbonic anhydrase 9 as an endogenous marker for hypoxic cells in cervical cancer. Cancer Res. 2001;61:8924–9. [PubMed] [Google Scholar]
- 36.Jankovic B, Aquino-Parsons C, Raleigh JA, Stanbridge EJ, Durand RE, Banath JP, et al. Comparison between pimonidazole binding Comparison between pimonidazole, oxygen electrode measurements, and expression of endogenous hypoxia markers in cancer of the uterine cervix. Cytometry B Clin Cytom. 2006;70:45–55. doi: 10.1002/cyto.b.20086. [DOI] [PubMed] [Google Scholar]
- 37.Mayer A, Höckel M, Vaupel P. Endogenous hypoxia markers in locally advanced cancers of the uterine cervic: reality or wishful thinking? Strahlenther Onkol. 2006;182:501–10. doi: 10.1007/s00066-006-1525-9. [DOI] [PubMed] [Google Scholar]
- 38.Tatum JL, Kelloff GJ, Gilliews RJ, Arbeit JM, Brown JM, Chao KS, et al. Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol. 2006;82:699–757. doi: 10.1080/09553000601002324. [DOI] [PubMed] [Google Scholar]
- 39.Pastorekova S, Kopacek J, Pastorek J. Carbonic anhydrase inhibitors and the management of cancer. Curr Top Med Chem. 2007;7:865–78. doi: 10.2174/156802607780636708. [DOI] [PubMed] [Google Scholar]
- 40.Winum JY, Rami M, Scozzafava A, Montero JL, Supuran C. Carbonic anhydrase IX: a new druggable target for the design of antitumor agents. Med Res Rev. 2008;28:445–63. doi: 10.1002/med.20112. [DOI] [PubMed] [Google Scholar]
- 41.Pastorekova S, Zatovicova M, Pastorek J. Cancer-associated carbonic anhydrases and their inhibition. Curr Pharm Des. 2008;14:685–98. doi: 10.2174/138161208783877893. [DOI] [PubMed] [Google Scholar]