Abstract
Background
To perform a colonoscopy, the endoscopist maneuvers the colonoscope through a series of loops by applying force to the insertion tube. Colonoscopy insertion techniques are operator dependent but have never been comprehensively quantified.
Objective
To determine whether the Colonoscopy Force Monitor (CFM), a device that continually measures force applied to the insertion tube, can identify different force application patterns among experienced endoscopists.
Design
Observational study of 6 experienced endoscopists performing routine diagnostic and therapeutic colonoscopy in 30 patients.
Setting
Outpatient ambulatory endoscopy center.
Patients
Adult male and female patients between 30 and 75 years of age undergoing routine colonoscopy.
Interventions
CFM monitoring of force applied to the colonoscope insertion tube during colonoscopy.
Main Outcome Measurements
Maximum and mean linear and torque force, time derivative of force, combined linear and torque vector force, and total manipulation time.
Results
The CFM demonstrates differences among endoscopists for maximum and average push/pull and mean torque forces, time derivatives of force, combined push/torque force vector, and total manipulation time. Endoscopists could be grouped by force application patterns.
Limitations
Only experienced endoscopists using conscious sedation in the patients were studied. Sample size was 30 patients.
Conclusions
This study demonstrates that CFM allows continuous force monitoring, characterization, and display of similarities and differences in endoscopic technique. CFM has the potential to facilitate training by enabling trainees to assess, compare, and quantify their techniques and progress.
Colonoscopy at varying intervals is recommended for colorectal cancer screening.1–4 In 2002, approximately 14 million screening, diagnostic, and therapeutic colonoscopies were performed.5 Although alternative technologies for screening are available or are under development, colonoscopy remains the preferred method.6–9 Colonoscopy requires a skilled operator to perform colonoscopy in a safe and effective manner.10–13 The quality of an endoscopic examination is defined by a number of parameters including the time to reach the cecum, withdrawal time, patient satisfaction, and adenoma detection rates.11,14 Training qualifications in endoscopy are based on the number of procedures performed and supervisor assessment of skill.15 Methods to quantify technique could prove useful as another measure of performance and skill acquisition. Previous studies defined some of the forces used by endoscopists but did not evaluate force monitoring as a more robust tool for understanding and monitoring technique.16,17 The Colonoscopy Force Monitor (CFM) is a new device that records, transmits, displays, stores, and analyzes all forces and torques applied to the insertion tube of the colonoscope. This initial study is designed to determine whether continuous recording of force by using the CFM can demonstrate differences in endoscopic technique among experienced endoscopists.
MATERIALS AND METHODS
Study design and protocol
The study was designed as an observational study to measure and compare operator examination patterns. The clinical protocol was reviewed and approved by the Western Institutional Review Board, Tacoma, Washington. In brief, 6 experienced endoscopists practicing in an ambulatory endoscopy center (Chevy Chase Endoscopy, Chevy Chase, Md) were recruited to participate in the study. Each experienced endoscopist was board certified in gastroenterology and had performed more than 10,000 colonoscopies. Before participating in the study, each endoscopist was given instruction in the use of the CFM. All adult male and female patients between the ages of 30 and 75 who were American Society of Anesthesiologists class 2 or lower presenting to the Chevy Chase Endoscopy Center between August and September 2008 for screening or diagnostic colonoscopy were considered for inclusion. Patients were excluded if colonic pathology in the opinion of the endoscopist could interfere with the colonoscopy, if they were pregnant, or if they required propofol for sedation. Each colonoscopy was performed by using standard methods for colonoscope insertion and withdrawal. The CFM was adapted to the Olympus PCF-160 colonoscope (Olympus Corp, Center Valley, PA), which was used for all procedures. Routine patient and procedure-related data were obtained including patient history and physical examination, standard procedure report and nursing notes, vital signs, pulse oximetry, electrocardiogram rhythm strip, assessment of level of consciousness, and pain perception. Conscious sedation in varying doses was administered by using a combination of a narcotic (meperidine or fentanyl) and sedative hypnotic (midazolam). In addition, the patient, the endoscopists, and the nurse assistant rated procedure pain severity on a scale of 1 to 4 (1 = no pain, 2 = mild pain, 3 = moderate pain, 4 = severe pain). Recorded CFM data were analyzed after all examinations were completed. Endoscopists were also asked to record the hand comfort, manipulation, and engage/disengage function performance of the device after each procedure on a Likert scale of 1 (poor) to 10 (exceptional).
CFM system
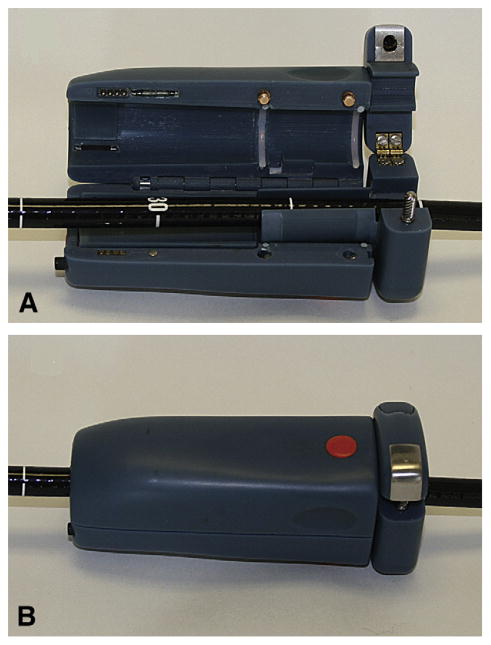
The CFM system consists of 3 components: (1) hand-held wireless colonoscope attachment with force- and torque-measuring ability (Fig. 1), (2) laptop computer with Bluetooth wireless communication, and (3) docking station to recharge the batteries. The handheld colonoscope insertion tube attachment is designed so that the endoscopist can maintain a conventional hand position over the instrument. The main components inside the CFM are 2 load cells, 4 rechargeable AAA batteries, a custom printed circuit board with built-in Bluetooth radio, and a push button colonoscope grip/release system. To attach or remove the CFM, the user simply opens the dual clam shells (Fig. 1A) and loads the colonoscope insertion tube. The physician manipulates the colonoscope, and the measured push/pull force and torque are wirelessly transmitted to a nearby computer at a rate of 7.5 measurements per second. The ergonomic asymmetrical plastic body of the CFM provides a secure grip for the gloved hand of the endoscopist. The endoscopist can reposition the CFM by activating the grip release mechanism and sliding the device along the shaft. The CFM is a nonsignificant risk device and underwent standard high-level disinfection between procedures. The CFM system software provides calibration of the device, collects and records the data, and displays the results of the measurements.
Figure 1.
Loading the colonoscope shaft into CFM. A, Dual clam shell is open and the insertion tube is placed in a central channel. B, The device is closed and ready for force monitoring.
Parameter calculations and statistical analysis
Parameter calculations
Continuous force recording permits the calculation of more than 20 parameters representing the magnitude, direction, rate of force application, and various derivatives and combinations of these main variables. Table 1 is a list of 7 parameters determined to have low cross-correlation and to be representative of the technique used by the endoscopists. The maximum forces applied as push F1 and pull F2 and maximum torques applied as clockwise F3 and counterclockwise F4 are calculated as the average of the highest 5 recorded values. The time derivative of force is a measure of the rate of change of force, that is, how fast the operator applies force to the insertion tube. The mean force rate P5 was calculated according to
TABLE 1.
Colonoscopy force monitor calculated parameter to characterize examination
| Parameter no. | Parameter name, units | Comments |
|---|---|---|
| 1 | Maximum push force, N | Averaged for 5 readings |
| 2 | Maximum pull force, N | Averaged for 5 readings |
| 3 | Maximum torque clockwise, N·m | Averaged for 5 readings |
| 4 | Maximum torque counterclockwise, N·m | Averaged for 5 readings |
| 5 | Mean push/pull force rate (average force rate), N/s | Data with force time derivative >0 |
| 6 | Mean torque rate (average torque rate), N·m/s | Data with torque time derivative >0 |
| 7 | Examination time, s | Net colonoscope insertion tube manipulation time |
where Fi is a set of recorded linear forces applied to the colonoscope insertion tube expressed in Newtons (N); n is the number of records in the data set. The data include the positive time derivative of force corresponding to the increase of applied force. The mean right and left torque rate P6 was calculated according to:
where Ti is a set of recorded torques applied to the colonoscope shaft expressed in Newton meters (N·m); n is the number of records in the data set. The data include the positive time derivatives in both directions. Operator manipulation time was measured as the duration of manipulation of the instrument exclusive of therapeutic maneuvers.
Cluster analysis
To differentiate and classify operator examination patterns, a hierarchical cluster analysis was performed by using an integral parameter vector (V), calculated for each examination by using MATLAB 6.1 (MathWorks, Natick, Mass). V was calculated as an 11-dimensional space based on the 7 parameters listed in Table 1 as well as the mean push force, mean pull force, mean torque and mean push, and torque vector. V is defined as
where n is parameter number, ξn is the component unit vector along n coordinate, Pn is the absolute value of the n parameter. Cluster analysis partitions a set of operators into groups in such a way that the profiles of operators in the same cluster are similar and the profiles of operators in different clusters are distinct.
To classify further the pattern of experienced endoscopists, an ordinal scale was established based on the mean value Pmk for each of the 7 parameters. The mean value Pmk was calculated for all 30 examinations (k is parameter number from 1 to 7) and Pmkg for each operator group (g is the group number from 1 to 3). The parameter Pk for the group g was scaled as low for force and torque parameter and short for time if Pmkg was 0.8·Pmk or less. The parameter Pk for the group g was scaled as medium for force, torque, and time if Pmkg was between 0.8·Pmk and 1.2·Pmk. The parameter Pk for the group g was scaled as high for force and torque parameter and long for time if Pmkg was 1.2·Pmk or greater.
Statistical analysis
Observational data for each parameter are calculated by using MATLAB 6.1 (MathWorks) and are represented as box plots with samples grouped for each operator. To determine whether there were differences among operators, 1-way analysis of variance with a Bonferroni adjustment was performed by using SYSTAT 12 (Systat Software, Inc, Chicago, Ill). A significance level of .05 (P ≤ .05) was chosen as an indication that parameter distributions were different among operators. A significant result indicated that at least 2 of the subgroups (operators) differed significantly.
RESULTS
A total of 30 patients had complete force monitoring data and were included in the study. Ten patients were female and 20 were male. The mean age was 55.2 ± 10.6 years of age (range 37–74). A normal colon was found in 13 patients and diverticulosis in 8 patients. Ten patients had polyps and 1 patient had a malignancy. The mean dose of meperidine was 45.4 ± 11.5 mg; 2 patients received fentanyl (100 mg). The mean dose of midazolam was 3.5 ± 1.6 mg. The mean time to reach the cecum was 10.1 ± 5.0 minutes (range 4–22 minutes). The mean pain score was 1.4 ± 0.6 (1 = no pain, 2 = mild pain, 3 = moderate pain, 4 = severe pain). The most common indication for colonoscopy was screening. The results for medication dose, the time to reach the cecum, and pain score were similar to those reported previously.18 Operator rating of CFM performance based on comfort, ease of manipulation, and engage/disengage function was 7.5 ± 1.3, 6.7 ± 1.5, and 5.9 ± 2.0, respectively.
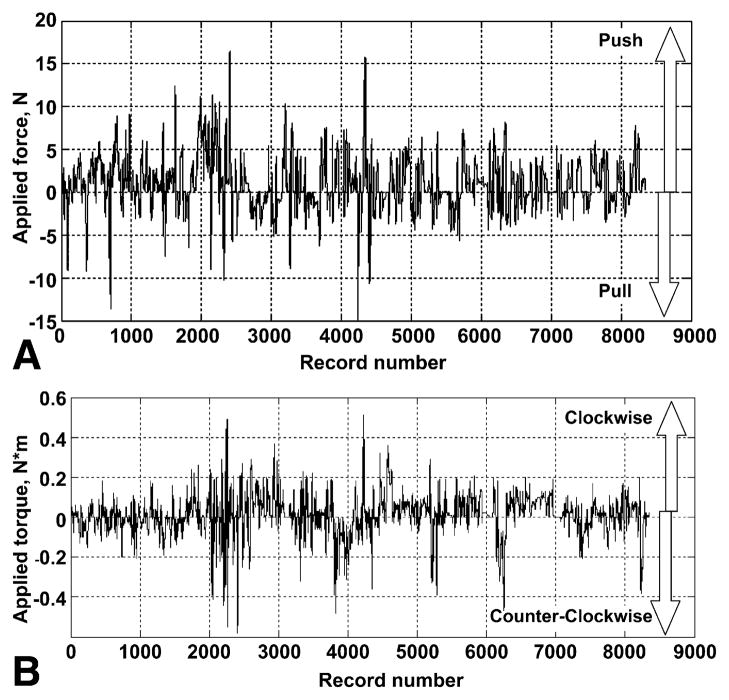
Figure 2 illustrates a typical patient CFM recording of push/pull (Fig. 2A) and torque forces (Fig. 2B). Because this analysis is focused on manipulation patterns for insertion and withdrawal, time periods when therapeutic procedures were performed were excluded. Force application varies considerably during the procedure as the endoscopist maneuvers through the segments of the colon and inspects the surface. The vast majority of axial forces are between 5 N and −5 N and for torque forces between 0.2 N·m and −0.2 N·m (data not shown).
Figure 2.
Primary data recorded by CFM. A, Push (positive) and pull (negative) force. B, Clockwise (positive) and counterclockwise (negative) torque. Each record represents a single data point, with a data acquisition rate of 7.5 records per second.
Figure 3 is a box plot representation of the total time of force application calculated for each endoscopist and is based on the individual case recordings. These data demonstrate the differences among endoscopists in the duration of manipulation and the range of those differences. Figure 4 is an overview of the sample distribution for the maximum force applied by each of 6 operators for 5 cases. These data demonstrate a clear divergence of the data for each individual operator seen for each calculated parameter. Because force is measured continually, it is possible to calculate the time derivative of force and a vector representing the application of force and torque. The time derivative of force represents how quickly the operator applies axial and torque forces to the colonoscope (Fig. 5). There was significant segregation of the time derivative of force application with operators 3, 4, and 5 applying force more quickly than operators 1, 2, and 6.
Figure 3.
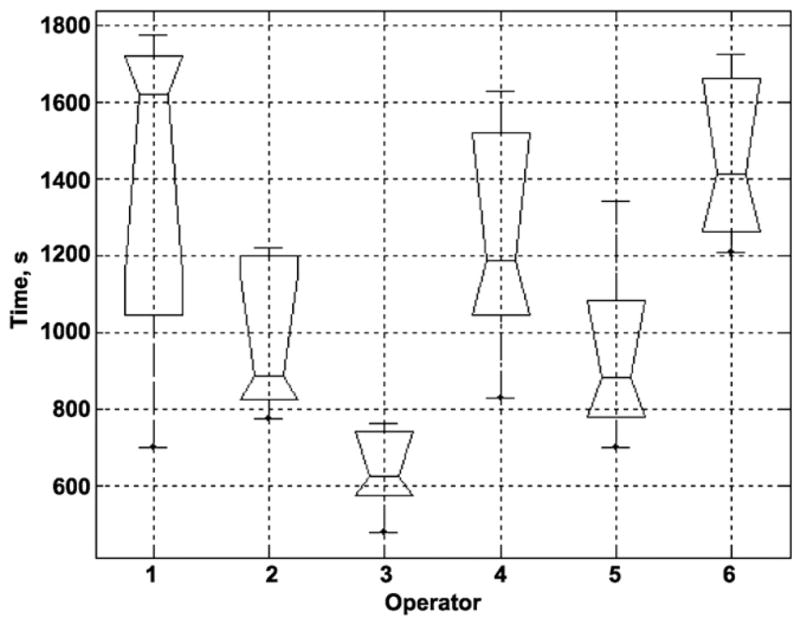
Box plot of operator manipulation time. All time intervals when the endoscopist stopped for biopsy, polypectomy, or other procedures were excluded.
Figure 4.
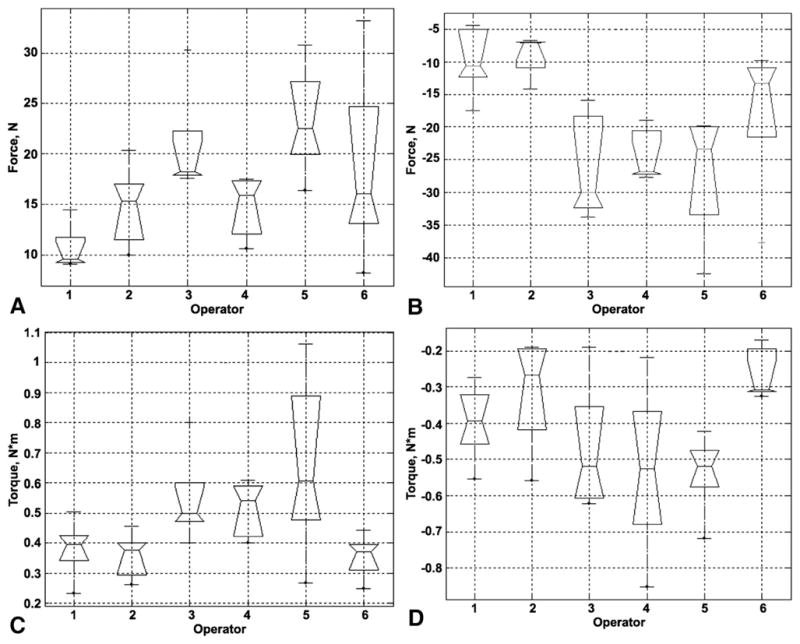
Box plots for force application, each box plot represents 5 completed cases. Maximum force for each parameter is calculated as the average of the highest 5 recorded values. Maximum push force (A), maximum pull force (B), maximum torque clockwise (C), maximum torque counterclockwise (D).
Figure 5.
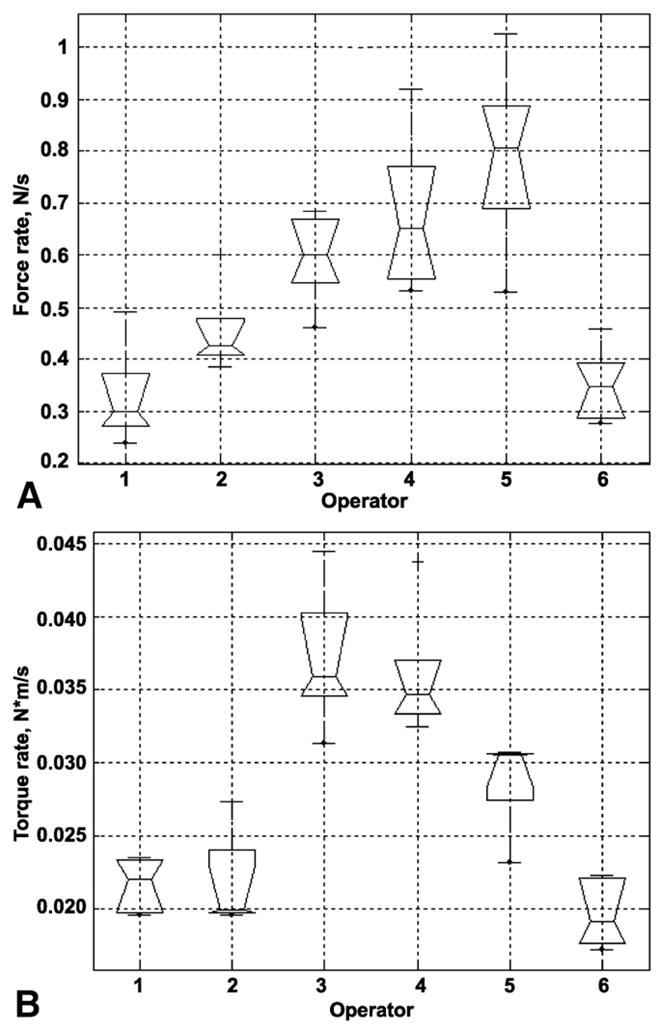
Box plots for the average time derivative of force each of the 6 operators. Each box plot represents 5 completed cases. The time derivative of force is calculated as a rate of change of the force (see Methods). A, Mean push/pull force rate. B, Mean torque rate right and left.
The box plot representations of the parameters illustrated in Figures 3 through 5 suggest that there are significant differences among the experienced endoscopists. To determine whether the divergence is the result of differences among operators or within patients, 1-way analysis of variance with a Bonferroni adjustment was performed for all 7 main parameters. Table 2 illustrates that there was no significant difference (P > .05) among endoscopists for parameter 3 (maximum torque clockwise) and parameter 4 (maximum torque counterclockwise). In contrast, endoscopists differed significantly in maximum push/pull, mean push/pull, mean torque rate, and examination time. To understand how force application patterns among endoscopists could be grouped, a hierarchical cluster analysis was performed by using a vector that represents 11 parameters with low cross-correlations. The results of this analysis are shown in Figure 6. The numbers along the horizontal axis represent the indices of the operators (Z). The links between objects are represented as upside-down U-shaped lines. The cluster analysis identified 3 distinct groups. Group 1 included operators 1, 2, and 6, group 2 included operators 4 and 5, and group 3 included operator 3.
TABLE 2.
One-way analysis of variance for 7 parameters with a Bonferroni correction for post hoc analysis
| Parameter | Max push | Max pull | Max torque clockwise | Max torque counterclockwise | Push/pull rate | Torque rate | Exam time |
|---|---|---|---|---|---|---|---|
| P value* (Bonferroni) | .016 | .012 | .41 | .14 | .001 | .001 | .002 |
Max, Maximum; Exam, examination.
P < .05 indicates a significant difference among endoscopists.
Figure 6.
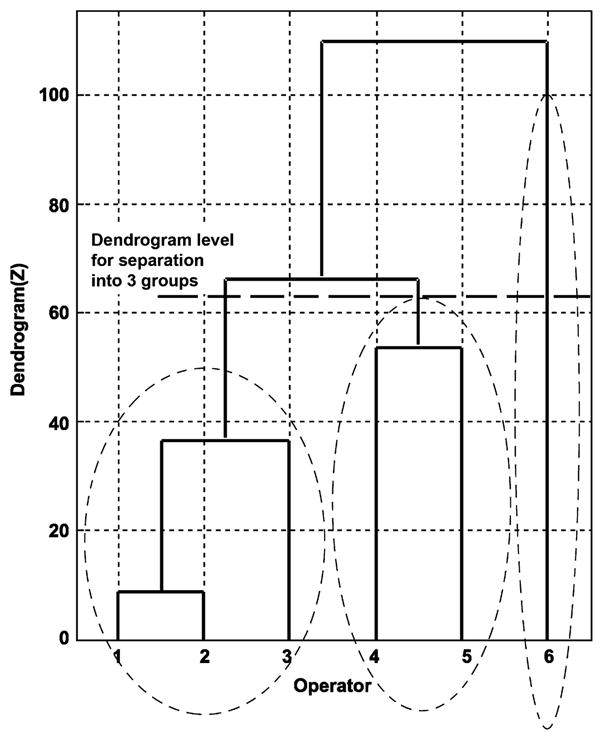
Cluster analysis of 6 operators based on 11 low-correlation parameters. The numbers along the horizontal axis represent the indices of the operators (Z). The links between objects are represented as upside-down U-shaped lines. The cluster analysis identified 3 distinct groups.
To simplify further the representation of the differences among endoscopists, an ordinal scale was developed that separated the forces into low, medium, and high and manipulation time into short, medium, and long (see Methods). Table 3 demonstrates the differences in force application pattern for the 3 endoscopist groups identified in the cluster analysis. For example, the group of endoscopists that had longer manipulation time had lower force application patterns. In contrast, endoscopists who spent less time manipulating the insertion tube used higher magnitude forces and force application rates, ie, pushed or pulled harder and moved faster.
TABLE 3.
Characteristics of groups using an ordinal scale for each parameter
| Group | Time | Max push | Max clockwise torque | Max counterclockwise torque | Avg push/pull force rate | Max pull | Avg torque rate |
|---|---|---|---|---|---|---|---|
| 1 | Long | Low | Low | Low | Low | Low | Low |
| 2 | Medium | Medium | Medium | High | High | High | Medium |
| 3 | Short | Medium | Medium | Medium | Medium | High | High |
Max, Maximum; Avg, average.
Each group was defined by cluster analysis. Group 1 represents endoscopists 1, 2, and 6; group 2 represents endoscopists 4 and 5; and group 3 includes endoscopist 3.
DISCUSSION
This study demonstrates that continuous force monitoring is a sensitive technique that allows accurate quantification of neurokinetic differences among experienced endoscopists. In addition, the CFM can characterize and classify endoscopic techniques, even among experienced endoscopists whose clinical outcomes are similar as measured by the time to reach the cecum, the medication dose, and the pain score. This study illustrates the potential utility of force monitoring as a clinical tool to identify optimal methods for the performance of colonoscopy as well as guidelines for training.
This study used a set of 7 parameters to demonstrate the substantial differences in the complex behavior pattern of experienced endoscopists (Figs. 3–5). For example, operators 1, 4, and 6 manipulate the instrument for longer periods and apply lower force than operators 2, 3, and 5 (Table 3). Precise characterization of force provides an altogether different perspective on procedure performance than existing clinical parameters such as the time to reach the cecum, the medication dose, or the pain score.14,19–21 This tool has the potential to answer important clinical questions.22 Do operators with lower force application reach the cecum later? Does a longer manipulation time represent a method of movement that produces less pain, requires less medication, or represents a more complete examination? Are there better methods for handling the insertion and withdrawal of the instrument? To establish best practices, larger powered studies will be necessary to create pooled normative data as well as methods for characterization of individual endoscopists. In particular, the relationship among force application, speed of colonoscopy, pain, and analgesia requirement can be quantified more precisely. Determining the relationship between force and injury will be more difficult because of the low frequency of significant injury such as perforation and splenic trauma.23–25
Individual force characterization may be used to evaluate repetitive-use injury by examining the relationship between the CFM neurokinetic signature and muscle use patterns.26 Although the maximum torque forces do not differ, the descriptive data (Fig. 4) suggest that the range of values does. For example, operator 5 used a much greater range of maximum clockwise than maximum counterclockwise torque; that is, an endoscopist could identify and possibly modify a behavior pattern based on comparison with an established metric. Operator 5 could choose to reduce the rate at which the push/pull force is applied (Fig. 5) or the maximum push/pull force (Fig. 4). The overall and specific patterns can be compared by using descriptive statistics (Figs. 3–5) and techniques of multivariate analysis such as cluster analysis (Fig. 6) and ordinal scales (Table 3). Comparisons can be based on multiparametric views of the technique. The operator in group 3 used higher maximum push/pull forces than operators in group 1. Operator 4 used a higher and wider range of maximum counterclockwise torque than operators 1 and 6 (Fig. 3). The ability to characterize and compare permits the design and development of feedback systems that could change behavior if those patterns are demonstrated to be counterproductive, injurious, or outside a validated benchmark.
Because the CFM was able to demonstrate highly significant differences in technique among experienced endoscopists, it is likely that these differences will also be identified in those with less experience. Thus, CFM has the potential to facilitate training by enabling trainees to assess and compare their techniques and quantify progress.27–29 The CFM technology could supplement existing methods such as simulators and magnetic endoscope imaging for education and training.30–32 Force application patterns could establish quantitative benchmarks for skill acquisition.15,27,33,34 Expert practices for maneuvering through critical segments such as the sigmoid colon and flexures can be defined and used to identify optimal training standards.14 Broader studies designed to correlate force measures with clinical endpoints such as the time to reach the cecum, the total colonoscopy time, polyp detection rates, endoscopist characteristics, the patient population, sedation methods, and endoscope design can establish the necessary standards for training and continuous quality improvement. Finally, device development could be improved by adding force measurement as a quantitative method of instrument assessment.
In conclusion, these data demonstrate that the CFM represents a unique advance in endoscopic technology. It is a clinically practical tool that distinguishes and characterizes endoscopist force application patterns. The CFM provides a unique neurokinetic signature for an endoscopist that is independent of patient characteristics. Further exploration of CFM-described force application patterns is likely to improve colonoscopy training and evaluation; establish normative data and improve quality assessment; lead to better understanding of the impact of patient characteristics on technique; quantify the relationship among force, pain, and sedation; expand the understanding of force application and repetitive-use injury; and improve colonoscope design. Advances in fabrication, design, and data representation could establish the CFM as a low-cost tool for improving colonoscopy training and quality.
Capsule Summary
What is already known on this topic
The colonoscopy force monitor (CFM) records, transmits, displays, stores, and analyzes all forces and torques applied to the insertion tube of the colonoscope.
What this study adds to our knowledge
In an observational study of 6 experienced endoscopists performing routine colonoscopy in 30 patients, CFM used a set of 7 parameters to demonstrate differences in performance patterns, which could be useful in identifying optimal methods for training and performance guidelines.
Acknowledgments
The authors acknowledge the attendings and the nursing and technical staff at Chevy Chase Endoscopy, Chevy Chase, Maryland, and Dr. Michael Brimacombe, Robert Wood Johnson School of Medicine, New Brunswick, New Jersey, for his help with study design and statistical analysis.
Abbreviation
- CFM
Colonoscopy Force Monitor
Footnotes
Presented at Digestive Disease Week; May 30 to June 4, 2009, Chicago, Illinois (Gastrointest Endosc 2009;69:AB213-AB214).
DISCLOSURE: Grant support from the National Institute of Diabetes and Digestive and Kidney Diseases grant 2R44 DK068936-02A1. The following authors disclosed financial relationships relevant to this publication: L. Y. Korman: Patent holder, Artann Laboratories; S. Tsuryupa: Patent holder and employee, Artann Laboratories; A. Sarvazyan: Patent holder and employee, Artann Laboratories; V. Egorov: Employee of Artann Laboratories; B. Corbin: Employee of Artann Laboratories; N. Sarvazyan: Employee of Artann Laboratories. M. Anderson disclosed no financial relationship relevant to this publication.
References
- 1.Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 2.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–60. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 3.Seeff LC, Manninen DL, Dong FB, et al. Is there endoscopic capacity to provide colorectal cancer screening to the unscreened population in the United States. Gastroenterology. 2004;127:1661–9. doi: 10.1053/j.gastro.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman D. Screening, surveillance, and prevention of colorectal cancer. Gastrointest Endosc Clin N Am. 2008;18:595–605. xi. doi: 10.1016/j.giec.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Seeff LC, Richards TB, Shapiro JA, et al. How many endoscopies are performed for colorectal cancer screening? Results from CDC’s survey of endoscopic capacity. Gastroenterology. 2004;127:1670–7. doi: 10.1053/j.gastro.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 6.Rex DK, Johnson DA, Anderson JC, et al. American college of gastroenterology guidelines for colorectal cancer screening 2008. Am J Gastroenterol. 2009;104:739–50. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 7.Rex DK. Colonoscopy: the dominant and preferred colorectal cancer screening strategy in the United States. Mayo Clinic Proc. 2007;82:662–4. doi: 10.4065/82.6.662. [DOI] [PubMed] [Google Scholar]
- 8.Marbet UA, Bauerfeind P, Brunner J, et al. Colonoscopy is the preferred colorectal cancer screening method in a population-based program. Endoscopy. 2008;40:650–5. doi: 10.1055/s-2008-1077350. [DOI] [PubMed] [Google Scholar]
- 9.Robertson RH, Burkhardt JH, Powell MP, et al. Trends in colon cancer screening procedures in the US Medicare and Tricare populations: 1999–2001. Prev Med. 2006;42:460–2. doi: 10.1016/j.ypmed.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Clarke GA, Jacobson BC, Hammett RJ, et al. The indications, utilization and safety of gastrointestinal endoscopy in an extremely elderly patient cohort. Endoscopy. 2001;33:580–4. doi: 10.1055/s-2001-15313. [DOI] [PubMed] [Google Scholar]
- 11.Rex DK. Quality in colonoscopy: cecal intubation first, then what? Am J Gastroenterol. 2006;101:732–4. doi: 10.1111/j.1572-0241.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- 12.Zauber AG, Winawer SJ. High-quality colonoscopies must be an integral part of screening and surveillance programs. Gastroenterology. 2006;130:620–1. doi: 10.1053/j.gastro.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2006;101:873–85. doi: 10.1111/j.1572-0241.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 14.Rex DK. Who is the best colonoscopist? Gastrointest Endosc. 2007;65:145–50. doi: 10.1016/j.gie.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 15.Principles of training in gastrointestinal endoscopy. From the ASGE. American Society for Gastrointestinal Endoscopy. Gastrointest Endosc. 1999;49:845–53. [PubMed] [Google Scholar]
- 16.Appleyard MN, Mosse CA, Mills TN, et al. The measurement of forces exerted during colonoscopy. Gastrointest Endosc. 2000;52:237–40. doi: 10.1067/mge.2000.107218. [DOI] [PubMed] [Google Scholar]
- 17.Wu TK. Occult injuries during colonoscopy. Measurement of forces required to injure the colon and report of cases. Gastrointest Endosc. 1978;24:236–8. doi: 10.1016/s0016-5107(78)73520-0. [DOI] [PubMed] [Google Scholar]
- 18.Korman J, Brandt L, Marino G, et al. Colonoscopy Skill Score: Results of Expert Skill Testing [abstract] Gastrointest Endosc. 2007;65:AB327. [Google Scholar]
- 19.Aslinia F, Uradomo L, Steele A, et al. Quality assessment of colonoscopic cecal intubation: an analysis of 6 years of continuous practice at a university hospital. Am J Gastroenterol. 2006;101:721–31. doi: 10.1111/j.1572-0241.2006.00494.x. [DOI] [PubMed] [Google Scholar]
- 20.Radaelli F, Meucci G, Sgroi G, et al. Technical performance of colonoscopy: the key role of sedation/analgesia and other quality indicators. Am J Gastroenterol. 2008;103:1122–30. doi: 10.1111/j.1572-0241.2007.01778.x. [DOI] [PubMed] [Google Scholar]
- 21.Tran Cao HS, Cosman BC, Devaraj B, et al. Performance measures of surgeon-performed colonoscopy in a Veterans Affairs medical center. Surg Endosc. 2009 doi: 10.1007/s00464-009-0358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enoka R. Neuromechanics of human movement. 4. Champaign, IL: Human Kinetics; 2008. [Google Scholar]
- 23.Arora G, Mannalithara A, Singh G, et al. Risk of perforation from a colonoscopy in adults: a large population-based study. Gastrointest Endosc. 2009;69:654–64. doi: 10.1016/j.gie.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Bokemeyer B, Bock H, Huppe D, et al. Screening colonoscopy for colorectal cancer prevention: results from a German online registry on 269000 cases. Eur J Gastroenterol Hepatol. 2009;21:650–5. doi: 10.1097/meg.0b013e32830b8acf. [DOI] [PubMed] [Google Scholar]
- 25.Skipworth JR, Raptis DA, Rawal JS, et al. Splenic injury following colonoscopy–an underdiagnosed, but soon to increase, phenomenon? Ann R Coll Surg Engl. 2009;91:W6–11. doi: 10.1308/147870809X400994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shergill AK, Asundi KR, Barr A, et al. Pinch force and forearm-muscle load during routine colonoscopy: a pilot study. Gastrointest Endosc. 2009;69:142–6. doi: 10.1016/j.gie.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 27.Cohen J, Cohen SA, Vora KC, et al. Multicenter, randomized, controlled trial of virtual-reality simulator training in acquisition of competency in colonoscopy. Gastrointest Endosc. 2006;64:361–8. doi: 10.1016/j.gie.2005.11.062. [DOI] [PubMed] [Google Scholar]
- 28.Eversbusch A, Grantcharov TP. Learning curves and impact of psycho-motor training on performance in simulated colonoscopy: a randomized trial using a virtual reality endoscopy trainer. Surg Endosc. 2004;18:1514–8. doi: 10.1007/s00464-003-9264-9. [DOI] [PubMed] [Google Scholar]
- 29.Yi SY, Ryu KH, Na YJ, et al. Improvement of colonoscopy skills through simulation-based training. Stud Health Technol Inform. 2008;132:565–7. [PubMed] [Google Scholar]
- 30.Shah SG, Thomas-Gibson S, Brooker JC, et al. Use of video and magnetic endoscope imaging for rating competence at colonoscopy: validation of a measurement tool. Gastrointest Endosc. 2002;56:568–73. doi: 10.1067/mge.2002.128133. [DOI] [PubMed] [Google Scholar]
- 31.Williams C, Guy C, Gillies D, et al. Electronic three-dimensional imaging of intestinal endoscopy. Lancet. 1993;341:724–5. doi: 10.1016/0140-6736(93)90489-4. [DOI] [PubMed] [Google Scholar]
- 32.Gerson LB, Van Dam J. Technology review: the use of simulators for training in GI endoscopy. Gastrointest Endosc. 2004;60:992–1001. doi: 10.1016/s0016-5107(04)02219-9. [DOI] [PubMed] [Google Scholar]
- 33.Sedlack RE, Kolars JC. Colonoscopy curriculum development and performance-based assessment criteria on a computer-based endoscopy simulator. Acad Med. 2002;77:750–1. doi: 10.1097/00001888-200207000-00041. [DOI] [PubMed] [Google Scholar]
- 34.Thomas-Gibson S, Bassett P, Suzuki N, et al. Intensive training over 5 days improves colonoscopy skills long-term. Endoscopy. 2007;39:818–24. doi: 10.1055/s-2007-966763. [DOI] [PubMed] [Google Scholar]