Abstract
Objective
IL-27 has stimulatory and regulatory immune functions and is expressed in rheumatoid arthritis synovium. We investigated the effects of IL-27 on human osteoclastogenesis to determine whether IL-27 can stimulate or attenuate osteoclast-mediated bone resorption that is a hallmark of rheumatoid arthritis.
Methods
Osteoclasts were generated from blood-derived human CD14+ cells. The effects of IL-27 on osteoclast formation were evaluated by counting the number of TRAP+ multinucleated cells and measuring expression of osteoclast-related genes. The induction of NFATc1 and the activation of signaling pathways downstream of RANK were measured by immunoblotting. The expression of key molecules implicated in osteoclastogenesis (NFATc1, RANK, costimulatory receptors, ITAM-harboring adaptors) was measured by real time RT-PCR. Murine osteoclast precursors were obtained from bone marrow. Responsiveness to IL-27 of synovial fluid macrophages derived from RA patients was also tested.
Results
IL-27 inhibited human osteoclastogenesis, suppressed the induction of NFATc1, downregulated expression of RANK and TREM-2, and inhibited RANKL-mediated activation of ERK, p38 and NF-κB in osteoclast precursors. Synovial fluid macrophages derived from RA patients were refractory to the effects of IL-27. In contrast to humans, IL-27 only moderately suppressed murine osteoclastogenesis, likely due to low expression of the IL-27 receptor subunit WSX-1 on murine osteoclast precursors.
Conclusion
IL-27 inhibits human osteoclastogenesis by a direct mechanism suppressing responses of osteoclast precursors to RANKL. Our findings suggest that in addition to its well-known anti-inflammatory effects, IL-27 plays a homeostatic role in restraining bone erosion. This homeostatic function is compromised under conditions of chronic inflammation such as RA synovitis.
Keywords: Osteoclastogenesis, Cytokines, Interleukins, RANKL, Rheumatoid Arthritis
IL-27 is a member of the IL-12 family of the heterodimeric cytokines that also includes IL-12, IL-23 and IL-35 (1-3). It is comprised of EBI3 (EBV-induced protein 3) and p28 subunits that share similarity with, respectively, the p40 and p35 subunits of IL-12 (4). The IL-27 receptor is a heterodimer composed of a WSX-1 subunit (also termed TCCR, T cell cytokine receptor), which confers ligand specificity, and the gp130 signaling subunit that is also utilized by the IL-6 family of cytokines (5). IL-27 activates the Jak-STAT signal transduction pathway in a context dependent manner, depending on cell type and activation state. In resting lymphocytes, IL-27 activates STAT1, STAT3, STAT5 and low amounts of STAT4 (6), whereas activation of STAT1 is decreased in fully activated CD4+ T cells relative to resting cells (7). In myeloid cells IL-27 induces phosphorylation of STAT1 and STAT3 (5, 8, 9) and we have recently reported that in human monocytes IL-27 has a STAT1-dominant effect (10). In contrast, we have found that murine BMDM are minimally responsive to IL-27.
IL-27 plays both activating and regulatory roles in immune responses (2). IL-27 is produced early during innate responses and augments the induction of Th1 responses (4, 6). In contrast, later in the evolution of an immune response IL-27 suppresses Th1 polarization and inhibits Th17 and Th2 differentiation (6, 11-14), in part by inducing IL-10 production (15-17). The role of IL-27 in arthritis and other inflammatory or infectious diseases has been explained on the basis of its effects on T cell differentiation (1-3). IL-27 can play either a pathogenic or a protective role in murine models of inflammatory arthritis, depending upon the model and underlying pathogenic mechanisms. In adjuvant-induced arthritis and proteoglycan-induced arthritis, both considered Th1-mediated diseases, IL-27 is pathogenic, consistent with its known Th1-inducing effects (18, 19). In contrast, in collagen-induced arthritis, which is Th17-mediated (and where Th1 responses can actually be protective), IL-27 was protective and proposed as a potential treatment for arthritis (20). IL-27 is expressed in human rheumatoid arthritis (20), which has been considered a Th1-mediated disease, but recent evidence suggests a role for Th17 responses in disease pathogenesis (21). Thus, it is important to determine whether IL-27 plays a pathogenic or protective role in human RA.
Rheumatoid arthritis is characterized by chronic synovial inflammation and bone destruction and a hallmark of disease is bone erosions mediated by osteoclasts (22). In the last few years, significant breakthroughs have improved our understanding of mechanisms that control the activity of osteoclasts under physiologic and pathologic circumstances (23, 24). Osteoclasts are large multinucleated cells created by the differentiation and fusion of myeloid lineage precursor cells, which include cells in the blood monocyte pool (25). In the microenvironment of bone, myeloid-derived osteoclast precursors are exposed to stimuli that commit them to the osteoclast lineage (26). The driving force of this commitment-differentiation process is the interaction of RANKL (a cytokine member of the TNF super-family) with its receptor RANK that is expressed on the surface of osteoclast precursors (27, 28). Effective osteoclastogenesis requires co-stimulatory calcium-mediated signals by ITAM-coupled receptors that co-operate with RANK signaling to induce NFATc1 and the downstream osteoclast differentiation program (29-32). In humans, TREM-2 is a key costimulatory receptor, as loss of function mutations in TREM-2 compromise bone remodeling and lead to Nasu Hakola disease (33, 34).
The multi-step osteoclast differentiation program is tightly controlled by hormones, cytokines and other regulatory factors. Cytokines that activate the Jak-STAT signal transduction pathway have been implicated in the regulation of osteoclastogenesis bridging immune regulation with bone remodeling. IFNs (35, 36), IL-4 (37), IL-6 (38) and IL-10 (39) inhibit osteoclast differentiation, while IL-23 promotes osteoclast formation by up-regulating RANK expression in precursor cells (40). IL-27, which belongs to the same cytokine family as IL-23, has been reported to modestly and indirectly regulate osteoclast differentiation in murine systems (40, 41), but mechanisms by which IL-27 regulates osteoclastogenesis and the role of IL-27 in human osteoclastogenesis have not been addressed.
We wished to explore the role of IL-27 in human osteoclastogenesis as this could yield insight into one facet of the potential pathogenic vs. protective roles of IL-27 in human RA. We found that IL-27 is a potent inhibitor of human osteoclastogenesis by a direct effect on osteoclast precursor cells. One mechanism that mediates the anti-osteoclastogenic function of IL-27 is abrogation of RANKL-induced c-Jun and NFATc1 expression by downregulation of the expression of RANK and inhibition of MAPK- and NF-κB signaling pathways downstream of RANK. We also found that in osteoclast precursor cells IL-27 downregulates the expression of the TREM-2 costimulatory receptor. Surprisingly, we found that synovial fluid derived macrophages from joint effusions of patients with active RA were refractory to the effects of IL-27. Our findings, combined with the reported inhibitory effects of IL-27 on Th17-mediated inflammation, suggest that IL-27 has the capacity to regulate both chronic inflammation and associated bone erosion, but this regulatory function is attenuated within the inflammatory microenvironment of the joint.
Materials and Methods
Cell culture
PBMCs from healthy volunteers’ blood leukocytes, purchased from the New York Blood Center, were obtained by density gradient centrifugation using Ficoll (Invitrogen Life Technologies). Mononuclear cells were isolated from synovial fluids derived from five patients with rheumatoid arthritis (diagnosis was based on ACR criteria). CD14+ cells were purified from fresh PBMCs and from synovial fluid derived mononuclear cells using anti-CD14 magnetic beads (Miltenyi Biotec) as recommended by the manufacturer. Purity of CD14+ cells was >97% as verified by FACS. Murine monocytes were obtained from C57BL/6J mice (The Jackson Laboratory) by density gradient centrifugation and positive selection using anti-CD11b magnetic beads (Miltenyi Biotec) as recommended by the manufacturer. Bone marrow cells and splenocytes were obtained from C57BL/6J mice and bone marrow-derived osteoclast precursors were generated by 4 days of culture on Petri dishes (Midwest Scientific, St. Louis, MO) in DMEM supplemented with 20% FBS (HyClone) and recombinant murine MCSF (20 ng/ml) (Peprotech). To measure cell viability, MTT assays were performed using an MTT assay kit (Roche Diagnostics, Indianapolis, IN), according to the manufacturer's instructions. Experiments with human cells were approved by the Hospital for Special Surgery IRB and with mouse cells by the Institutional Animal Care and Use Committee.
Osteoclast differentiation
Human CD14+ cells were incubated in α-MEM (Invitrogen) supplemented with 10% FBS (Defined, Hyclone) and 20ng/ml of hM-CSF (PeproTech) for 2 days to generate osteoclast precursors. Osteoclast precursors were incubated with 20ng/ml of M-CSF and 40ng/ml of sRANKL (PeproTech) for an additional 5 days. Cytokines were replenished every 3 days. On culture day 7, cells were fixed and stained for TRAP using the Acid Phosphatase Leukocyte diagnostic kit (Sigma, San Diego, CA) as recommended by the manufacturer. Multinucleated (> 3 nuclei) TRAP-positive osteoclasts were counted in triplicate wells of 96 well plates. Recombinant hIL-27 (3-100ng/ml) (R&D Systems) was added either from the beginning of the culture and before RANKL (IL-27 pretreatment), simultaneously with RANKL on culture day 3, or after RANKL (on culture day 5). Murine bone marrow-derived osteoclast precursors were incubated with mMCSF (20ng/ml) and sRANKL (80ng/ml) for 5 days in α-MEM and processed as described above.
Pit formation assay
Human CD14+ cells were cultured in the presence of M-CSF (20 ng/ml) without or with IL-27 (100 ng/ml) for two days, and then the cells were re-plated on dentin slices (5 × 104 cells/slice) in 96-well culture plates and stimulated as indicated. The dentin slices were washed with water, cells on dentin were removed, and the dentin slices were immersed in 1% toluidine blue O (Sigma-Aldrich) to stain resorption pits formed by mature osteoclasts.
Immunoblotting
Whole-cell extracts were prepared by lysis of cells in buffer containing 20 mM Hepes (pH 7.0), 300 mM NaCl, 10 mM KCl, 1 mM MgCl2, 0.1% Triton X-100, 0.5 mM DTT, 20% glycerol and 1 × proteinase inhibitor cocktail (Roche, Basel, Switzerland).
Nuclear extracts were prepared by incubating cells for 15 min (4°C) in buffer containing 10 mM Hepes (pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 1 × proteinase inhibitor cocktail and 1mM DTT. NP-40 was added to a final concentration of 0.2% and the lysate was centrifuged at 10,000g for 30sec. Finally, the nuclear pellet was lysed in SDS-PAGE loading buffer.
Protein levels of whole cell lysates were quantitated using the Bradford assay (Biorad, Hercules, CA). For immunoblotting 5 or 10μg of whole-cell lysates were fractioned on 7.5% and 10% polyacrylamide gels using SDS-PAGE, transferred to polyvinylidene fluoride membranes (Millipore) and incubated with specific Abs. ECL was used for detection. ERK, p-ERK, p-p38, p-IκBα, IκBα, c-Jun, pY-STAT1 and pY-STAT3 antibodies were from Cell Signaling Technology. TRAF6, TBP and p38 antibodies were from Santa Cruz Biotechnology while NFATc1, STAT1 and STAT3 antibodies were purchased from BD Transduction Laboratories.
RT-PCR and quantitative RT-PCR (qPCR)
For RT-PCR and qPCR total RNA was extracted using an RNeasy mini kit (Qiagen) and 1μg of total RNA was reverse transcribed using a First Strand cDNA Synthesis kit (Fermentas). qPCR was performed using iQ SYBR Green Supermix and iCycler iQ thermal cycler (Bio-Rad). PCR (34 cycles: 95°C 30sec/60°C 30sec/72°C 1 min.) was utilized to quantify the mRNA levels of WSX-1 in human CD14+ or CD14- cells and murine osteoclast precursors or splenocytes.
Results
IL-27 inhibits human osteoclastogenesis in a dose- and time-dependent manner
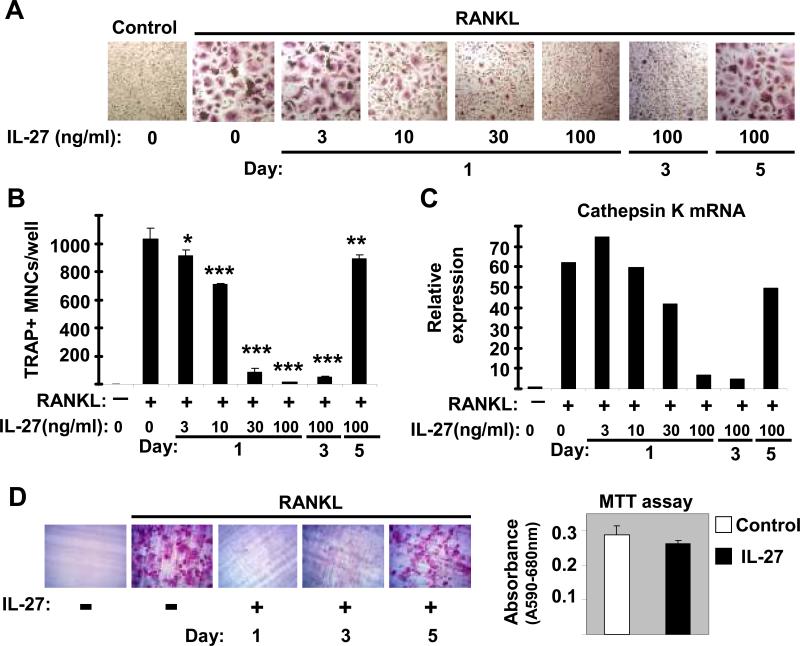
In the presence of M-CSF and RANKL myeloid-lineage precursors differentiate into osteoclasts (25), but STAT1 activation downstream of IFNs has been shown to inhibit osteoclastogenesis (42). Recently we reported that IL-27 strongly activates STAT1 in human monocytes and macrophages (10) and in this study we wished to investigate whether IL-27 has any regulatory function on human osteoclastogenesis. As expected (43), culture with M-CSF plus RANKL induced differentiation of large multinucleated TRAP+ cells that were readily apparent 5 days after RANKL addition (Figure 1A, second panel and Figure 1B). When IL-27 was added at the beginning of culture (Day 1), a dose dependent inhibition of osteoclastogenesis was observed (Figure 1A and 1B), with nearly complete inhibition of generation of multinucleated TRAP+ cells observed at doses ≥ 30ng/ml of IL-27 (p < 0.01, Student's t test). When IL-27 (100ng/ml) was added at later time points during the differentiation process, a substantial inhibition was observed when IL-27 was added on day 3 (simultaneously with addition of RANKL), while there was slight inhibition when IL-27 was added on day 5 (Figure 1A and 1B). A similar pattern of dose- and time-dependent inhibition of RANKL-induced expression of osteoclast-related genes Cathepsin K (Figure 1C) and integrin β3 (data not shown) by IL-27 was observed. Cathepsin K and integrin β3 were still expressed in the mostly mononuclear cells observed when 30 ng/ml of IL-27 was used, but were strongly downregulatd by saturating concentrations of IL-27 that nearly completely suppressed osteoclastogenesis. The functional consequence of the observed IL-27-mediated inhibition of osteoclastogenesis was assessed by resorption pit formation assay using dentin slices. As expected, the observed inhibition of osteoclastogenesis by IL-27 was reflected also by the absence of resorption pits (Figure 1D, left). Interestingly, when IL-27 was added late on day 5, the formed osteoclasts were capable of creating resorption pits (Figure 1D, left, fifth panel). The latter implies that IL-27 can not inhibit the late stages of osteoclast differentiation and function. Additionally, we found using the MTT assay that IL-27 mediates antiosteoclastogenic effects without affecting viability of osteoclast precursors (Figure 1D, right). Taken together, these observations suggest that IL-27 is a potent inhibitor of the early stages of human osteoclastogenesis by a direct effect on osteoclast precursors.
Figure 1. IL-27 inhibits human osteoclastogenesis in a dose- and time-dependent manner.
Freshly isolated human CD14+ cells were cultured with MCSF (20ng/ml) for 48h and RANKL (40ng/ml) was added on Day 3 as described in Materials and Methods. IL-27 (3, 10, 30 and 100ng/ml) was added at the initiation of cultures (Day1) or later (Day 3 or Day 5). (A and B), TRAP positive multinucleated (> 3 nuclei) cells were counted 5 days after RANKL addition and representative data of one out of three independent experiments are shown as mean ±SD from triplicate wells of 96 well plates (* = p>0.05, ** = p<0.05 and *** = p<0.01. p values were calculated by Student's t test). C, Cathepsin K mRNA was measured by using real-time PCR and normalized relative to GAPDH expression. The means ±SD of triplicate determinants in a representative experiment of three independent experiments are shown; small SDs are not readily apparent because of large inductions. D (left panels), freshly isolated human monocytes were cultured as in A and osteoclast function was measured by a resorption pit formation assay. D (right panel), Human CD14+ monocytes were cultured with MCSF (20ng/ml) in the presence or absence of IL-27 (100ng/ml) for 2days. Cell viability was measured by MTT assay. Representative results of at least three independent experiments are shown.
IL-27 abrogates RANKL-mediated induction of NFATc1
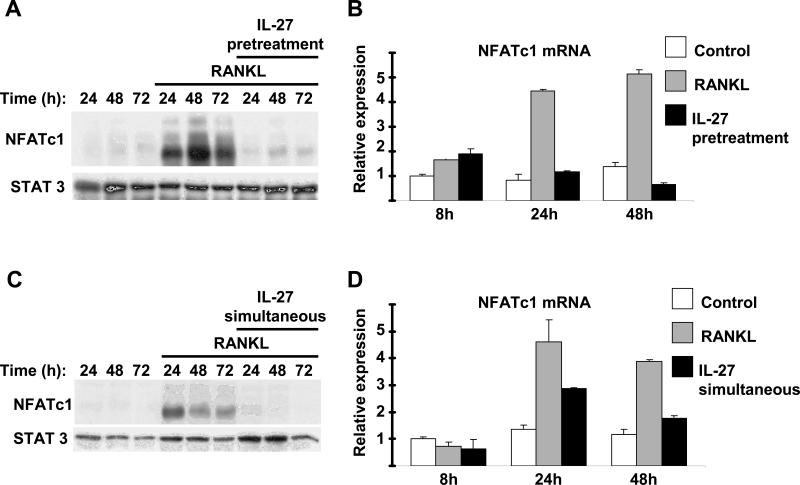
We wished to investigate mechanisms by which IL-27 inhibits osteoclast differentiation. It is well established that NFATc1 is a master regulator of osteoclastogenesis, driving the expression of genes that are crucial for the commitment and differentiation of precursor cells to osteoclasts (44, 45). We tested the effects of IL-27 on NFATc1 expression. As expected, RANKL (40ng/ml) induced within 24h NFATc1 protein expression (Figure 2A, lane 4) and the levels of NFATc1 protein remained elevated for at least 72h following RANKL stimulation (Figure 2A, lanes 4-6). Addition of IL-27 resulted in the abrogation of RANKL-induced NFATc1 protein expression for the entire 72h period of RANKL stimulation (Figure 2A, lanes 7-9). We next investigated whether IL-27 inhibits NFATc1 gene expression. The levels of NFATc1 mRNA increased following 24 and 48h stimulation with RANKL (Figure 2B). Pretreatment with IL-27 resulted in substantial suppression of NFATc1 mRNA levels (Figure 2B). A similar striking abrogation of RANKL-induced NFATc1 protein expression was observed when IL-27 was added together with RANKL (on culture day 3) (Figure 2C, lanes 4-9). Simultaneous addition of IL-27 had a similar suppressive effect on NFATc1 mRNA expression (Figure 2D). These results indicate that the abrogation of NFATc1 induction is a mechanism that mediates the inhibitory effects of IL-27 on human osteoclastogenesis.
Figure 2. IL-27 abrogates RANKL-mediated induction of NFATc1.
A and B, Freshly isolated human CD14+ cells were cultured with MCSF (20ng/ml) in the presence or absence of IL-27 (100ng/ml) for 48h and then were stimulated with RANKL. C and D, Freshly isolated human CD14+ cells were cultured with MCSF (20ng/ml) for 48h and then were stimulated with RANKL (40ng/ml) in the presence or absence of IL-27 (100ng/ml). NFATc1 protein expression 24, 48 and 72h following RANKL stimulation was measured by immunoblotting (A and C). NFATc1 mRNA was measured using real-time PCR and normalized relative to GAPDH expression (B and D). Representative results of at least three independent experiments are shown.
IL-27 inhibits RANKL-induced activation of MAPK and NF-κB pathways and suppresses RANK expression
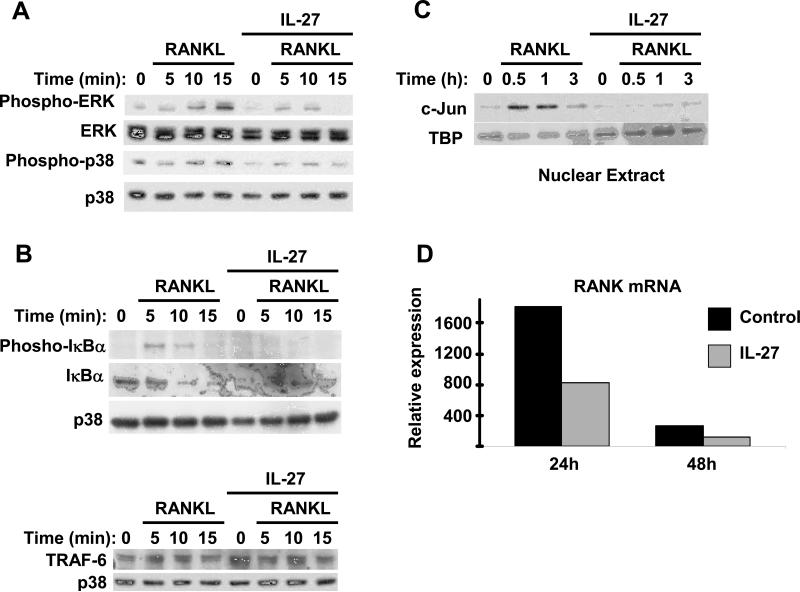
The induction of NFATc1 expression by RANK is dependent upon RANKL-induced activation of MAPK and NF-κB pathways (23, 24), and thus we investigated whether IL-27 inhibited RANK signaling. In agreement with the literature, we found that stimulation of osteoclast precursors with RANKL (40ng/ml) induced rapid phosphorylation of ERK and p38 MAPKs (Figure 3A, first and third panels, lanes 2-4). We observed strong inhibition of RANKL-induced ERK and p38 phosphorylation by IL-27 (Figure 3A, first and third panels, lanes 6-8). In addition, RANKL induced the expected phosphorylation and rapid degradation of IκBα (Figure 3B, top panels, lanes 2-4) indicating activation of the classical NF-κB pathway, while IL-27 prevented RANKL-induced IκBα phosphorylation and degradation (Figure 3B, top panels, lanes 6-8). IFNγ, the prototypic STAT1 activating cytokine, inhibits RANKL signaling in murine osteoclast precursors by inducing rapid degradation of the adapter TRAF6 that lies upstream of NF-κB and MAPK activation (35). In our system IL-27 had no effect on the expression of TRAF6 protein (Figure 3B, bottom panel). RANKL-mediated induction of AP-1 proteins, including Fos and Jun, is important for osteoclastogenesis. Induction of Fos by RANKL was not consistently observed in our human osteoclastogenesis system (data not shown). However, RANKL consistently induced c-Jun protein and this induction was detectable in nuclear extracts for at least 3h following RANKL stimulation (Figure 3C, lanes 2-4). In the presence of IL-27 the RANKL-mediated induction of c-Jun was abrogated (Figure 3C, lanes 6-8). We then investigated whether IL-27 inhibits expression of RANK, which could explain IL-27-mediated inhibition of several RANK signaling pathways. Culture with MCSF induced the expected increase in RANK mRNA that peaked at 24h and remained elevated at 48h of culture (Figure 3D, black bars). IL-27 partially suppressed M-CSF-mediated induction of RANK mRNA expression in all donors tested (>10) (Figure 3D, grey bars). When we tested the effects of IL-27 on the expression of the M-CSF receptor c-Fms, there was variability among donors in the effects of IL-27 on c-Fms mRNA levels and cell surface expression, while IL-27 strongly inhibited osteoclastogenesis in all donors tested (n >10). Because IL-27 inhibited osteoclastogenesis effectively even in donors where c-Fms expression was not affected, we conclude that an effect of IL-27 on c-Fms expression is not a major mechanism to explain the observed inhibition of osteoclastogenesis. In sum, the results demonstrate that IL-27 inhibits RANK-induced signaling that is required for NFATc1 expression, and suggest that this inhibition may be mediated in part by downregulation of RANK expression.
Figure 3. IL-27 inhibits RANKL-mediated activation of MAPK and NF-κB pathways and induction of c-Jun.
Freshly isolated human CD14+ cells were cultured with MCSF (20ng/ml) in the presence or absence of IL-27 (100ng/ml) for 48h. Control and IL27-treated cells were stimulated with RANKL (40ng/ml) for 5, 10 and 15 minutes (A and B) or for 0.5, 1 and 3h (C). Immunoblotting was used to measure threonine 202/tyrosine 204 phosphorylation of Erk1/Erk2 and threonine 180/tyrosine 182 phoshorylation of p38 (A), serine 32 phosphorylation of IκBα and total IκBα (B), total TRAF6 (B), and nuclear c-Jun and TBP proteins (C). Representative results of at least five independent experiments are shown. D, RANK mRNA was measured in control and IL-27-treated cells using real-time PCR and normalized relative to GAPDH expression. The means ±SD of triplicate determinants in a representative experiment of three independent experiments are shown; small SDs are not readily apparent because of large inductions.
IL-27 suppresses TREM-2 mRNA expression
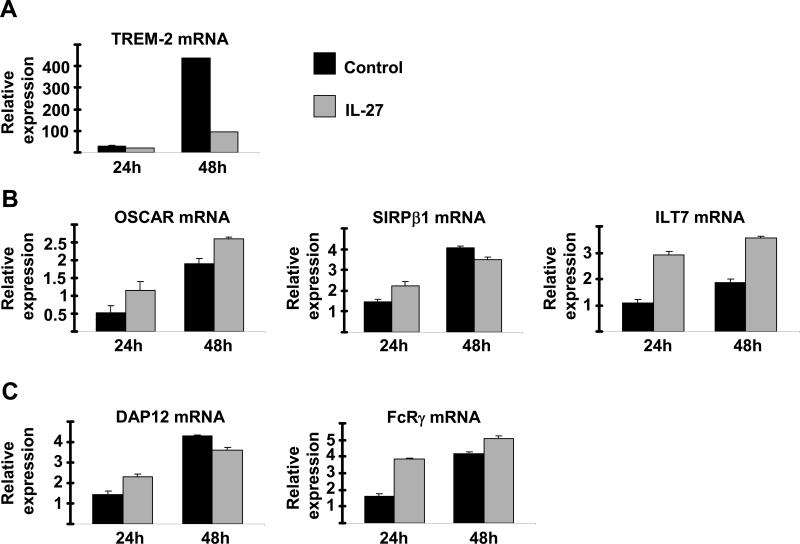
As the strong inhibition of osteoclastogenesis and NFATc1 expression by IL-27 could not be solely explained by inhibition of RANK expression, we investigated the effects of IL-27 on other pathways implicated in the induction of NFATc1 expression. The immunonoreceptors TREM-2, OSCAR, SIRPβ1 and ILT7 (homolog of murine PIR-A) and integrin αVβ3 that signal via ITAM-containing adaptors DAP12 and FcRγ cooperate with RANK signaling pathways to induce NFATc1 expression and osteoclastogenesis (29-32). We investigated the effects of IL-27 on costimulatory receptors and DAP12/FcRγ expression. The expression of TREM-2 increased during the early differentiation of osteoclast precursors, and this increase in TREM-2 expression was strongly suppressed by IL-27 (Figure 4A). In contrast, expression of OSCAR, SIRPβ1, DAP12 and FcRγ, did not substantially change during differentiation of osteoclast precursors, and was not suppressed by IL-27 (Figure 4B and 4C). ILT7 mRNA expression was moderately increased by IL-27 (Figure 4B). As costimulatory signaling is important for NFATc1 expression and TREM-2 plays a key role in costimulation of human osteoclastogenesis (33, 34), these observations suggest down-regulation of TREM-2 as a mechanism by which IL-27 suppresses NFATc1 induction and inhibits human osteoclastogenesis.
Figure 4. IL-27 inhibits TREM-2 expression.
Freshly isolated human CD14+ cells were cultured with MCSF (20ng/ml) in the presence or absence of IL-27 (100ng/ml) for 24 and 48h. TREM-2 mRNA (A), OSCAR mRNA, SIRPβ1 mRNA and ILT7 mRNA (B) and DAP12 and FcRγ mRNA (C) was measured using real-time PCR and normalized relative to GAPDH expression. Representative results of at least four independent experiments are shown.
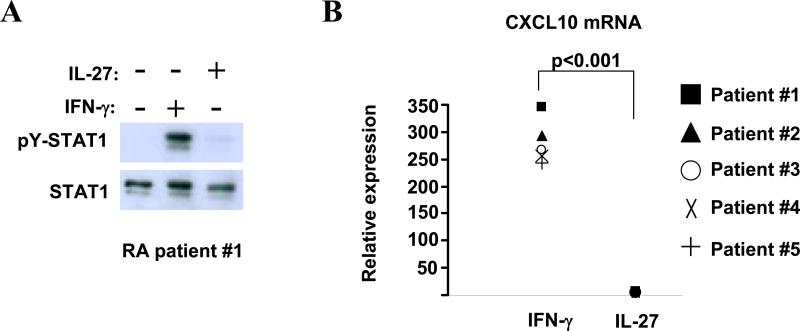
Synovial fluid macrophages derived from RA patients are refractory to IL-27
Given the above described inhibitory effects of IL-27 in human osteoclastogenesis, we wondered whether IL-27 exerts these regulatory functions in CD14+ cells derived from synovial fluid (SF) of patients with active rheumatoid arthritis. SF-derived CD14+ cells from RA patients responded to IFN-γ stimulation with activation of STAT1 (Figure 5A, first panel, lane 2) and a robust induction of CXCL10 gene expression (Figure 5B, white bar). Surprisingly, following stimulation with IL-27 we observed a very faint activation of STAT1 (Figure 5A, top panel, lane 3) that was inadequate to induce STAT1-target gene expression (including CXCL10, CXCL9, IRF-1 and STAT1, Figure 5B and data not shown; p < 0.001, Student's t test). These results were consistently observed in synovial macrophages from all five patients tested and suggest that cells derived from the joints of many patients with active RA are only minimally responsive to IL-27. The latter implies that, within the microenvironment of an inflamed joint, IL-27 may not exert its regulatory functions.
Figure 5. Synovial fluid macrophages derived from Rheumatoid Arthritis patients are refractory to IL-27.
Freshly isolated CD14+ cells derived from synovial fluid of five RA patients were stimulated for 15min (A) or 3h (B) with hIFN-γ (100U/ml) or hIL-27 (100ng/ml). A, tyrosine 701 phosphorylation of STAT1 was measured by immunoblotting. Representative results of one out of five independent experiments are shown. B, CXCL10 mRNA expression was measured using real-time PCR and normalized relative to GAPDH expression (p<0.001 by paired Student's t test, n=5).
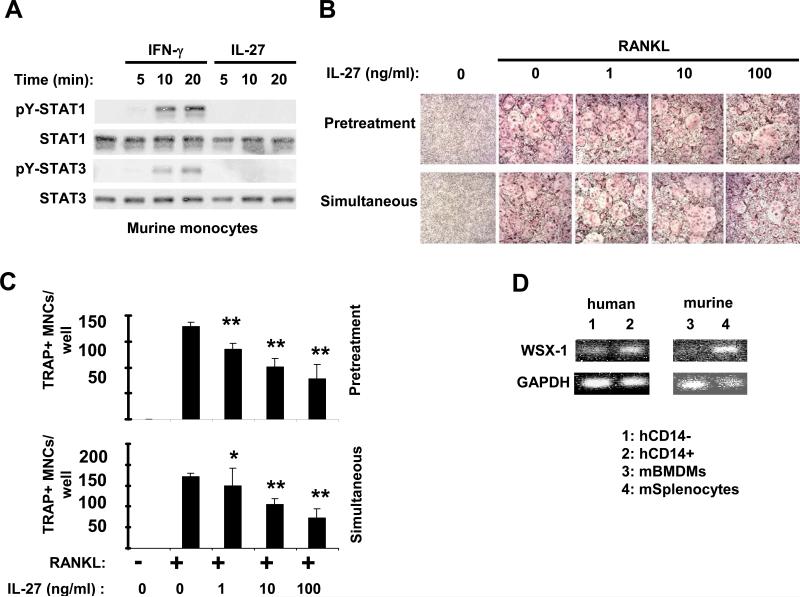
IL-27 moderately inhibits murine osteoclastogenesis due to low expression of WSX-1
We have recently reported that murine macrophages are minimally responsive to IL-27 (10) and in this study we wished to test whether IL-27 has an effect on murine osteoclast differentiation. Because we wished to use murine monocytes as osteoclast precursors (to directly compare to human monocytes), we first tested the responsiveness of murine monocytes to IL-27. Following stimulation of murine monocytes with IFN-γ (that was used as a positive control) we observed the expected activation of STAT1 and STAT3 (Figure 6A, first and third panel, lanes 2-4) and induction of classical STAT1-target genes (including IP-10, MIG, IRF-1, data not shown). Similarly to murine macrophages (10), in murine monocytes following IL-27 stimulation there was no detectable activation of STAT1 and STAT3 (Figure 6A, first and third panel, lanes 5-7) and minimal induction of STAT1-target genes (data not shown). Because murine monocytes did not differentiate efficiently into osteoclasts in our system, we used bone marrow-derived osteoclast precursors, a standard approach to study murine osteoclastogenesis, to test the effects of IL-27. As expected, addition of RANKL to murine bone marrow-derived osteoclast precursors resulted in the formation of large multinucleated TRAP+ cells within 5 days (Figure 6B, upper panels). When IL-27 was added (prior to or simultaneously with RANKL) in doses ranging from 1-100ng/ml there was a minimal to moderate inhibition of osteoclast formation (Figure 6B, panels 3-5). Similarly, the number of TRAP+ multinucleated cells (Figure 6C) and expression of osteoclast marker genes (data not shown) were only moderately lower in the presence of IL-27. In stark contrast to the human system, IL-27 had minimal effect on RANKL-mediated induction of NFATc1 expression in murine osteoclast precursors (data not shown), further supporting the notion that murine osteoclast precursors are moderately responsive to IL-27. In our recent report (10), we have shown that IL-6 induces a substantial activation of STAT-3 in murine myeloid cells, indicating that gp130, the shared receptor subunit for IL-6 and IL-27 receptors, is expressed on these cells. We next investigated the expression levels of the WSX-1 subunit of the IL-27 receptor in human and murine cells. We found that human lymphocytes and osteoclast precursors express WSX-1 mRNA at comparable levels (Figure 6D, lanes 1-2), while WSX-1 expression is low in murine osteoclast precursors compared to murine splenocytes (predominantly lymphocytes) (Figure 6D, lanes 3-4). The latter suggests that low expression of WSX-1 in murine osteoclast precursors is a potential explanation for the modest responsiveness of these cells to the effects of IL-27.
Figure 6. IL-27 is a moderate inhibitor of in vitro osteoclastogenesis in murine systems.
A, Murine monocytes were stimulated for 5, 10 and 20min with mIFN-γ (100U/ml) or mIL-27 (100ng/ml). Immunoblotting was used to measure tyrosine 701 phosphorylation of STAT1 and tyrosine 705 phosphorylation of STAT3. (B-C) Bone marrow-derived osteoclast precursors obtained from C57BL/6J mice were cultured in the presence of MCSF (20ng/ml) and RANKL (80ng/ml) was added as described in Materials and Methods. IL-27 (1, 10 and 100ng/ml) was added 1 day before RANKL (Pretreatment) or simultaneously with RANKL. (B and C), TRAP positive multinucleated (> 3 nuclei) cells were counted 5 days after RANKL addition and representative data of one out of three independent experiments are shown as mean ±SD from triplicate wells of 96 well plates (* = p>0.05 and ** = p<0.05. p values were calculated by Student's t test). D, The expression of WSX-1 m-RNA was measured by PCR in freshly isolated human CD14+ and CD14- cells and in murine BMDM and splenocytes.
Discussion
In this study we have found that IL-27 is a potent inhibitor of human osteoclastogenesis. This effect is mediated by directly targeting the early stages of differentiation of precursor cells into osteoclasts. In the presence of IL-27 we observed down-regulation of RANK and TREM-2 expression, an inhibition of MAPK- and NF-κB- pathways downstream of RANK and most importantly an abrogation of RANKL-induced c-Jun and NFATc1 expression. In stark contrast to blood-derived CD14+ cells from healthy donors, SF-derived CD14+ cells from RA patients were refractory to IL-27. Our observations, combined with the well-characterized anti-inflammatory functions of IL-27 (3), suggest that IL-27 has the potential to limit the extent of bone destruction in the setting of infection or inflammation, but that this homeostatic function of IL-27 can be compromised during chronic inflammation, such as occurs in rheumatoid arthritis.
NFATc1 is essential for osteoclast differentiation in vitro and in vivo (44, 45). We observed a remarkable suppression of NFATc1 mRNA and protein expression by IL-27 in our system. Signaling pathways downstream of RANK and immunoreceptors (including TREM-2) co-operate leading to the activation of calcium signaling, the activation of NFATc1 and finally strong induction of NFATc1 itself through an auto-amplification loop (24). In our study, the attenuation of MAPK- and NF-κB-signaling pathways downstream of RANK correspond to a mechanism that contributes to the suppression of NFATc1 expression by IL-27. IL-27 appears to regulate the levels of c-Jun protein that is induced downstream of MAPK pathways. c-Jun is the partner of c-Fos for the formation AP-1, a transcription factor essential for the induction of NFATc1 (46). A potential explanation for the inhibition of the MAPK and NF-κB signaling pathways observed in our system is the down-regulation of RANK expression by IL-27 in human osteoclast progenitors. This downregulation of RANK was partial, suggesting that it contributes to diminished responses to RANKL only under conditions where osteoclast precursors are exposed to low concentrations of RANKL (as in the case of the inflamed synovium in rheumatoid arthritis). In contrast, TREM-2 expression was effectively inhibited by IL-27, indicating that IL-27 suppresses costimulation of RANK signaling.
We have previously reported that IL-27 has a STAT1-dominant, IFNγ-like effect on human monocytes (10). IFNγ is also a powerful suppressor of osteoclast differentiation by a STAT1-mediated mechanism (42). Similar to our observations with IL-27, IFN-γ inhibits the activation of MAPK and NF-κB pathways downstream of RANK (35). IFNγ inhibits RANK signaling in murine osteoclast precursors by inducing rapid degradation of the signaling adapter TRAF6 that functions downstream of RANK (35). In our system the protein levels of TRAF6 were not reduced in IL-27 treated cells prior to or after RANKL stimulation, indicating that there was no overt degradation of TRAF6. This observation suggests that IL-27 and IFNγ inhibit osteoclastogenesis by different mechanisms, although it is possible that differences in TRAF6 protein regulation and stability between murine and human osteoclast precursors contributes to the observed differences.
In the current study we have found that SF macrophages derived from joint effusions of patients with active RA retain responsiveness to IFN-γ but are refractory to IL-27. We have recently reported that resistance to IL-27 can be induced by LPS by a p38-dependent mechanism (10). These findings suggest that inflammatory factors that activate p38 can induce a state of refractoriness to IL-27, and in this context we hypothesize that, within the inflammatory microenvironment of the joint, where p38 is known to be activated, SF macrophages are exposed to stimuli that render them refractory to the regulatory effects of IL-27. The level of refractoriness is likely to wax and wane with disease activity, but the data suggest that patients with active disease would be refractory to IL-27 therapy. However, greater understanding of mechanisms that render cells refractory to IL-27 could potentially lead to therapeutic manipulations to restore cell responsiveness to the regulatory functions of IL-27. Under such conditions, endogenous IL-27 would have a beneficial effect in suppressing bone resorption, and exogenous IL-27 might represent an effective therapy.
Recently, several differences have become apparent in the regulation of osteoclastogenesis and bone remodeling between humans and mice (47). For example, TREM-2 deficiency in humans is associated with impaired osteoclastogenesis, aberrant bone remodeling and Nasu-Hakola disease (also called polycystic lipomembranous osteodysplasia with sclerosing leukoengephalopathy) (33, 34). In contrast, mice deficient in TREM-2 do not have a clear bone phenotype in vivo and have increased osteoclastogenesis in vitro (48). Our observations add to these species differences by showing substantially less effective inhibition of osteoclast differentiation by IL-27 with mouse relative to human osteoclast precursors. One potential explanation of this difference is that murine osteoclast precursors are moderately responsive to IL-27 due to low expression of WSX-1. An additional explanation is likely related to differences in TREM-2 regulation and function in humans and mice, and the contribution of TREM-2 downregulation to the suppressive effects of IL-27 in human cells. In addition, we have shown that in human cells IL-27 is a powerful inhibitor of osteoclastogenesis directly targeting osteoclast progenitors, while our findings with murine bone marrow-derived osteoclast progenitors and evidence from other groups suggest that in murine systems IL-27 regulates osteoclast differentiation mainly indirectly by affecting cell populations other than the osteoclast precursors. It has been reported that IL-27 induces activation of STAT-1 and STAT-3 in murine osteoblasts, but no profound functional consequences were found (41). In the same study, IL-27 inhibited osteoclastogenesis indirectly possibly by regulating the functions of activated CD4+ T-cells. Along the same lines, another study identified murine Th17 cells as the exclusive osteoclastogenic subset among the known CD4+ T-cell lineages (49) and several reports suggest that IL-27 suppresses Th17 polarization (12, 13, 50).
The findings of our current study provide evidence that IL-27 is a powerful inhibitor of human osteoclastogenesis, but the perspective of using IL-27 as a potential treatment in human diseases including rheumatoid arthritis needs further investigation and careful evaluation.
Acknowledgments
This work was supported by grants from NIH (L.B.I), Niarchos Foundation (G.D.K) and Arthritis Foundation (K.H-P.M).
References
- 1.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–42. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida H, Yoshiyuki M. Regulation of immune responses by interleukin-27. Immunol Rev. 2008;226:234–47. doi: 10.1111/j.1600-065X.2008.00710.x. [DOI] [PubMed] [Google Scholar]
- 3.Batten M, Ghilardi N. The biology and therapeutic potential of interleukin 27. J Mol Med. 2007;85(7):661–72. doi: 10.1007/s00109-007-0164-7. [DOI] [PubMed] [Google Scholar]
- 4.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16(6):779–90. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 5.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, et al. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172(4):2225–31. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 6.Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2003;100(25):15047–52. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshimura T, Takeda A, Hamano S, Miyazaki Y, Kinjyo I, Ishibashi T, et al. Two-sided roles of IL-27: induction of Th1 differentiation on naive CD4+ T cells versus suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4+ T cells partially through STAT3-dependent mechanism. J Immunol. 2006;177(8):5377–85. doi: 10.4049/jimmunol.177.8.5377. [DOI] [PubMed] [Google Scholar]
- 8.Lang R. Tuning of macrophage responses by Stat3-inducing cytokines: molecular mechanisms and consequences in infection. Immunobiology. 2005;210(24):63–76. doi: 10.1016/j.imbio.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Miyazaki Y, Shinozaki Y, Yoshida H. Augmentation of antigen-presenting and Th1-promoting functions of dendritic cells by WSX-1(IL-27R) deficiency. J Immunol. 2007;179(10):6421–8. doi: 10.4049/jimmunol.179.10.6421. [DOI] [PubMed] [Google Scholar]
- 10.Kalliolias GD, Ivashkiv LB. IL-27 activates human monocytes via STAT1 and suppresses IL-10 production but the inflammatory functions of IL-27 are abrogated by TLRs and p38. J Immunol. 2008;180(9):6325–33. doi: 10.4049/jimmunol.180.9.6325. [DOI] [PubMed] [Google Scholar]
- 11.Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, et al. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19(5):645–55. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 12.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7(9):929–36. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 13.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13(6):711–8. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 14.Artis D, Villarino A, Silverman M, He W, Thornton EM, Mu S, et al. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J Immunol. 2004;173(9):5626–34. doi: 10.4049/jimmunol.173.9.5626. [DOI] [PubMed] [Google Scholar]
- 15.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8(12):1372–9. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 16.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8(12):1380–9. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 17.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8(12):1363–71. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg R, Wildbaum G, Zohar Y, Maor G, Karin N. Suppression of ongoing adjuvant-induced arthritis by neutralizing the function of the p28 subunit of IL-27. J Immunol. 2004;173(2):1171–8. doi: 10.4049/jimmunol.173.2.1171. [DOI] [PubMed] [Google Scholar]
- 19.Cao Y, Doodes PD, Glant TT, Finnegan A. IL-27 induces a Th1 immune response and susceptibility to experimental arthritis. J Immunol. 2008;180(2):922–30. doi: 10.4049/jimmunol.180.2.922. [DOI] [PubMed] [Google Scholar]
- 20.Niedbala W, Cai B, Wei X, Patakas A, Leung BP, McInnes IB, et al. Interleukin 27 attenuates collagen-induced arthritis. Ann Rheum Dis. 2008;67(10):1474–9. doi: 10.1136/ard.2007.083360. [DOI] [PubMed] [Google Scholar]
- 21.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka S, Nakamura K, Takahasi N, Suda T. Role of RANKL in physiological and pathological bone resorption and therapeutics targeting the RANKL-RANK signaling system. Immunol Rev. 2005;208:30–49. doi: 10.1111/j.0105-2896.2005.00327.x. [DOI] [PubMed] [Google Scholar]
- 24.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7(4):292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 25.Xing L, Schwarz EM, Boyce BF. Osteoclast precursors, RANKL/RANK, and immunology. Immunol Rev. 2005;208:19–29. doi: 10.1111/j.0105-2896.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 26.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 27.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 28.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95(7):3597–602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Humphrey MB, Lanier LL, Nakamura MC. Role of ITAM-containing adapter proteins and their receptors in the immune system and bone. Immunol Rev. 2005;208:50–65. doi: 10.1111/j.0105-2896.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 30.Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428(6984):758–63. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 31.Zou W, Kitaura H, Reeve J, Long F, Tybulewicz VL, Shattil SJ, et al. Syk, c-Src, the alphavbeta3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J Cell Biol. 2007;176(6):877–88. doi: 10.1083/jcb.200611083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mocsai A, Humphrey MB, Van Ziffle JA, Hu Y, Burghardt A, Spusta SC, et al. The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc Natl Acad Sci U S A. 2004;101(16):6158–63. doi: 10.1073/pnas.0401602101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cella M, Buonsanti C, Strader C, Kondo T, Salmaggi A, Colonna M. Impaired differentiation of osteoclasts in TREM-2-deficient individuals. J Exp Med. 2003;198(4):645–51. doi: 10.1084/jem.20022220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paloneva J, Mandelin J, Kiialainen A, Bohling T, Prudlo J, Hakola P, et al. DAP12/TREM2 deficiency results in impaired osteoclast differentiation and osteoporotic features. J Exp Med. 2003;198(4):669–75. doi: 10.1084/jem.20030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;408(6812):600–5. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 36.Takayanagi H, Kim S, Matsuo K, Suzuki H, Suzuki T, Sato K, et al. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature. 2002;416(6882):744–9. doi: 10.1038/416744a. [DOI] [PubMed] [Google Scholar]
- 37.Shioi A, Teitelbaum SL, Ross FP, Welgus HG, Suzuki H, Ohara J, et al. Interleukin 4 inhibits murine osteoclast formation in vitro. J Cell Biochem. 1991;47(3):272–7. doi: 10.1002/jcb.240470313. [DOI] [PubMed] [Google Scholar]
- 38.Duplomb L, Baud'huin M, Charrier C, Berreur M, Trichet V, Blanchard F, et al. Interleukin-6 inhibits receptor activator of nuclear factor kappaB ligand-induced osteoclastogenesis by diverting cells into the macrophage lineage: key role of Serine727 phosphorylation of signal transducer and activator of transcription 3. Endocrinology. 2008;149(7):3688–97. doi: 10.1210/en.2007-1719. [DOI] [PubMed] [Google Scholar]
- 39.Xu LX, Kukita T, Kukita A, Otsuka T, Niho Y, Iijima T. Interleukin-10 selectively inhibits osteoclastogenesis by inhibiting differentiation of osteoclast progenitors into preosteoclast-like cells in rat bone marrow culture system. J Cell Physiol. 1995;165(3):624–9. doi: 10.1002/jcp.1041650321. [DOI] [PubMed] [Google Scholar]
- 40.Chen L, Wei XQ, Evans B, Jiang W, Aeschlimann D. IL-23 promotes osteoclast formation by up-regulation of receptor activator of NF-kappaB (RANK) expression in myeloid precursor cells. Eur J Immunol. 2008;38(10):2845–54. doi: 10.1002/eji.200838192. [DOI] [PubMed] [Google Scholar]
- 41.Kamiya S, Nakamura C, Fukawa T, Ono K, Ohwaki T, Yoshimoto T, et al. Effects of IL-23 and IL-27 on osteoblasts and osteoclasts: inhibitory effects on osteoclast differentiation. J Bone Miner Metab. 2007;25(5):277–85. doi: 10.1007/s00774-007-0766-8. [DOI] [PubMed] [Google Scholar]
- 42.Takayanagi H, Sato K, Takaoka A, Taniguchi T. Interplay between interferon and other cytokine systems in bone metabolism. Immunol Rev. 2005;208:181–93. doi: 10.1111/j.0105-2896.2005.00337.x. [DOI] [PubMed] [Google Scholar]
- 43.Sorensen MG, Henriksen K, Schaller S, Henriksen DB, Nielsen FC, Dziegiel MH, et al. Characterization of osteoclasts derived from CD14+ monocytes isolated from peripheral blood. J Bone Miner Metab. 2007;25(1):36–45. doi: 10.1007/s00774-006-0725-9. [DOI] [PubMed] [Google Scholar]
- 44.Takayanagi H. The role of NFAT in osteoclast formation. Ann N Y Acad Sci. 2007;1116:227–37. doi: 10.1196/annals.1402.071. [DOI] [PubMed] [Google Scholar]
- 45.Aliprantis AO, Ueki Y, Sulyanto R, Park A, Sigrist KS, Sharma SM, et al. NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J Clin Invest. 2008;118(11):3775–89. doi: 10.1172/JCI35711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wagner EF, Eferl R. Fos/AP-1 proteins in bone and the immune system. Immunol Rev. 2005;208:126–40. doi: 10.1111/j.0105-2896.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 47.Takayanagi H. Mechanistic insight into osteoclast differentiation in osteoimmunology. J Mol Med. 2005;83(3):170–9. doi: 10.1007/s00109-004-0612-6. [DOI] [PubMed] [Google Scholar]
- 48.Colonna M, Turnbull I, Klesney-Tait J. The enigmatic function of TREM-2 in osteoclastogenesis. Adv Exp Med Biol. 2007;602:97–105. doi: 10.1007/978-0-387-72009-8_13. [DOI] [PubMed] [Google Scholar]
- 49.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203(12):2673–82. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7(9):937–45. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]