Abstract
Tumor necrosis factor (TNF) receptor-associated factor 2 (TRAF2) and receptor interacting protein 1 (RIP1) play critical roles in activating c-Jun N-terminal kinase (JNK) and IκB kinase (IKK), as well as in inhibiting apoptosis induced by TNFα. The TRAF2 RING domain-mediated polyubiquitination of RIP1 is believed to be essential for TNFα-induced IKK activation, and the RING domain-deleted TRAF2 (TRAF2-ΔR) has been widely used as a dominant negative in transient overexpression systems to block TNFα-induced JNK and IKK activation. Here, we report that stable expression of TRAF2-ΔR at a physiological level in TRAF2 and TRAF5 double knockout (TRAF2/5 DKO) cells almost completely restores normal TNFα-induced IKK activation, but not RIP1 polyubiquitination. In addition, stable expression of TRAF2-ΔR in TRAF2/5 DKO cells efficiently inhibited TNFα-induced later-phase of prolonged JNK activation, yet failed to inhibit TNFα-induced cell death. Although the basal and inducible expression of antiapoptotic proteins in TRAF2-ΔR-expressing TRAF2/5 DKO cells was normal, the cells remained sensitive to TNFα-induced cell death because anti-apoptotic proteins were not recruited to the TNFR1 complex efficiently. Moreover, stable expression of TRAF2-ΔR in TRAF2/5 DKO cells failed to suppress constitutive p100 processing in these cells. These data suggest that: i) the TRAF2 RING domain plays a critical role in inhibiting cell death induced by TNFα, and is essential for suppressing the noncanonical NF-κB pathway in unstimulated cells; ii) RIP1 polyubiquitination is not essential for TNFα-induced IKK activation; and iii) prolonged JNK activation has no obligate role in TNFα-induced cell death.
Keywords: TNFα, TRAF2, JNK, NF-κB, apoptosis
Introduction
The TNF receptor (TNFR)-associated factor (TRAF) family of proteins consists of six members that are characterized by a highly conserved TRAF domain at the protein C-terminus. With the exception of TRAF1, the TRAFs contain an N-terminal RING domain followed by five or seven zinc-finger motifs 1; 2. These TRAFs serve as scaffold proteins and/or E3 ubiquitin ligases, and regulate signal transduction by most members of the TNFR superfamily and the interlukin-1 receptor/Toll-like receptor superfamily, resulting in activation of the c-Jun N-terminal kinase (JNK) and the inhibitor of κB (IκB) kinase (IKK) 1; 2. JNK and IKK then activate the AP-1 (e.g. c-Jun/ATF2 heterodimer) and NF-κB transcription factors, respectively, and these transcription factors in turn induce the expression of genes involved in inflammation, the immune response, cell proliferation and cell differentiation, as well as of genes that suppress death receptor- and stress-induced apoptosis 1; 3.
TNFR family members activate NF-κB through both canonical and noncanonical pathways. For example, ligation of TNFR1 activates the canonical NF-κB pathway, and ligation of Lymphotoxin β receptor (LTβR) activates the noncanonical NF-κB pathway 3; 4. In the case of TNFR1, the ligated receptor recruits the TNFR-associated death domain (TRADD) protein, which in turn recruits TRAF2, TRAF5 and receptor-interacting protein 1 (RIP1). This signaling complex then activates the IKK complex (consisting of α, β and γ subunits), leading to phosphorylation-dependent degradation of the inhibitory protein IκBα, and thereby to activation of canonical NF-κB (e.g. p65/p50 heterodimer). Although TRADD can also recruit the Fas-associated death domain (FADD) protein and caspase-8 to trigger apoptotic signalings, in normal cells, NF-κB activation leads to the expression of anti-apoptotic proteins such as cellular FLICE (caspase-8)-like inhibitory protein (cFLIP) and inhibitor of apoptosis (cIAP), which prevent caspase-8 activation induced by TNFα 4; 5. Thus, TNFα triggers apoptosis only when new protein synthesis or signaling through the NF-κB pathway is inhibited. TNFα-induced cell death is also thought to depend on prolonged JNK activation, which is also inhibited by NF-κB target genes such as cFLIP and X-linked inhibitor of apoptosis (XIAP) 6; 7. In contrast to activated TNFR1, activated LTβR directly recruits TRAF2 and TRAF3, and activates IKKα homodimers through NF-κB inducing kinase (NIK). IKKα in turn induces phosphorylation-dependent partial processing of the NF-κB p100 precursor to its p52 form, resulting in activation of noncanonical NF-κB (e.g. RelB/p52 heterodimer) 3; 4. Unlike TNFR1, LTβR does not associate with death domain-containing proteins, and thus does not directly activate pro-apoptotic caspase cascades.
TRAF2 is a prototypical member of the TRAF family, and contributes to activation of both the canonical and non-canonical NF-κB pathways 3; 4. Gene knockout studies have revealed that TRAF2 loss impairs TNFα-induced activation of JNK, but not that of IKK 8. Tada et al. have reported that whereas mouse embryonic fibroblasts (MEFs) from TRAF5-null mice respond normally to TNFα-induced activation of JNK and NF-κB, TRAF2/TRAF5 double knockout (TRAF2/5 DKO) MEFs exhibit an almost complete loss of TNFα-induced NF-κB activation. This observation suggests that TRAF2 and TRAF5 play a redundant role in IKK activation in response to TNFα stimulation 9. On the other hand, conditional knockout of TRAF2 in B-cells results in constitutive activation of the noncanonical NF-κB pathway 10. These data suggested that TRAF2 positively regulates the canonical NF-κB pathway, and negatively regulates the non-canonical NF-κB pathway. Nevertheless, in the TRAF2 field, it has been widely accepted that the TRAF2 RING domain plays an essential role in activating both the JNK/c-Jun and the IKK/NF-κB pathways 2. In addition, a RING domain-deleted form of TRAF2 (TRAF2-ΔR) has been widely used as a dominant negative inhibitor of TNFα-induced JNK and IKK activation 1; 2.
Recently, we reported that the canonical IKK complex is constitutively activated in both TRAF2 knockout (TRAF2 KO) and TRAF2/5 DKO MEFs, and that stimulation of these cells with TNFα further increases IKK activity in the absence of RIP1 polyubiquitination 11. This raised the question of what role the TRAF2 RING domain plays in activating IKK. We addressed this question by stably expressing TRAF2-ΔR at a physiological level in TRAF2/5 DKO cells, and investigating the activation status of both the canonical and non-canonical NF-κB pathways. Here we report that the TRAF2 RING domain plays a critical role in suppressing the non-canonical NF-κB pathway in resting cells and in inhibiting cell death induced by TNFα stimulation, but that neither the TRAF2 RING domain nor RIP1 polyubiquitination is essential for TNFα-induced activation of the canonical NF-κB pathway.
Results
The TRAF2 RING domain is essential for TNFα-induced c-Jun, but not NF-κB, activation
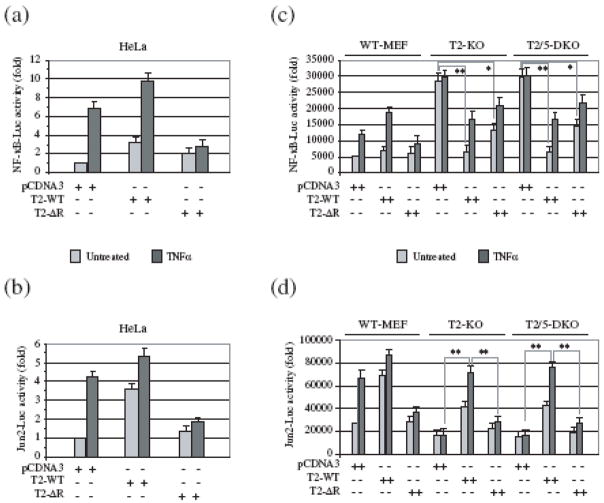
Numerous studies have shown that, in HeLa and 293T cells, transient overexpression of TRAF2-ΔR inhibits TNFα-induced activation of both NF-κB and c-Jun 1; 2. As expected, our NF-κB and c-Jun luciferase reporter gene assays revealed that expression of TRAF2-ΔR in HeLa cells indeed inhibits TNFα-induced activation of NF-κB and c-Jun (Fig. 1a, b). Recently, we have shown that TRAF2/5 DKO MEFs in fact exhibit high basal NF-κB activity, and that expression of wild-type TRAF2 (TRAF2-WT) in these cells significantly reduces this high basal NF-κB activity 11. To examine the role of the TRAF2 RING domain in regulating basal as well as inducible NF-κB activation in a TRAF2-null background, we carried out the NF-κB reporter gene assay in wild-type (WT), TRAF2 KO and TRAF2/5 DKO MEFs. As shown in Fig. 1c, both TRAF2 KO and TRAF2/5 DKO MEFs exhibited high basal NF-κB activity compared to WT MEFs, and expression of TRAF2-WT in these cells significantly suppressed this high basal NF-κB activity. Interestingly, the expression of TRAF2-ΔR also led to a suppression of basal NF-κB activity in TRAF2 KO and TRAF2/5 DKO MEFs, but to a lesser degree than did the expression of TRAF2-WT (Fig. 1c). Notably, whereas TRAF2-ΔR acted as a dominant negative inhibitor in HeLa cells (preventing TNFα-induced NF-κB activation), it failed to do so in TRAF2 KO and TRAF2/5 DKO MEFs. On the other hand, c-Jun activity — both basal and induced — was lower in TRAF2 KO and TRAF2/5 DKO MEFs than in WT MEFs, and the expression of TRAF2, but not that of TRAF2-ΔR, restored TNFα-induced c-Jun activation (Fig. 1d). Overall, these data suggest that the integrity of the TRAF2 RING domain plays an important role in efficient suppression of basal NF-κB activity in resting cells, and that it is required for TNFα-induced c-Jun, but not NF-κB, activation.
Fig. 1.
In MEFs, the TRAF2 RING domain is essential for TNFα-induced c-Jun, but not NF-κB, activation. (a-d) HeLa cells and MEFs of various genotype (Wild-type: WT-MEF; TRAF2 KO: T2-KO; and TRAF2/5 DKO: T2/5 DKO) were co-transfected with either NF-κB-Luc (a, c) or Jun2-Luc (b, d), plus pRL-TK and pCDNA3, TRAF2-WT (T2-WT) or TRAF2-ΔR (T2-ΔR). 36 hrs after transfection, cells were left untreated or treated with human TNFα (10 ng/ml; HeLa) for 6 hrs or with mouse TNFα (5 ng/ml; MEFs) for 4 hrs. The NF-κB-Luc and Jun2-Luc activities were then measured and normalized to pRL-TK activity. Data are presented as mean ± SD of the results from three independent experiments carried out in triplicate. (*) represents p< 0.05 and (**) represents p< 0.01.
The TRAF2 RING domain is not essential for TNFα-induced expression of NF-κB target genes
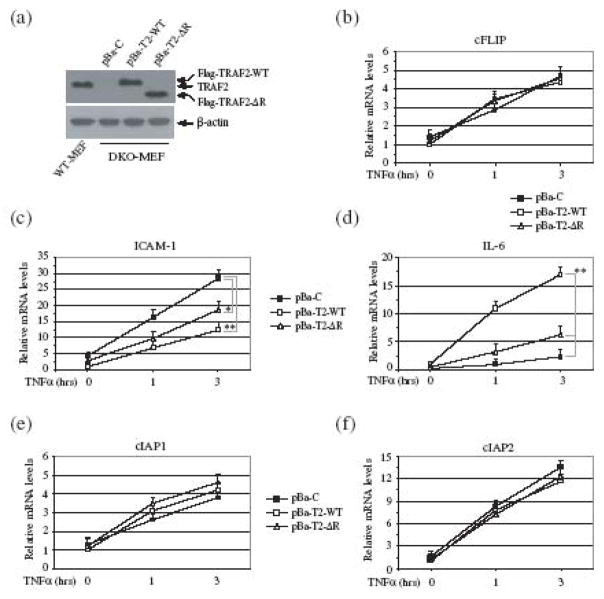
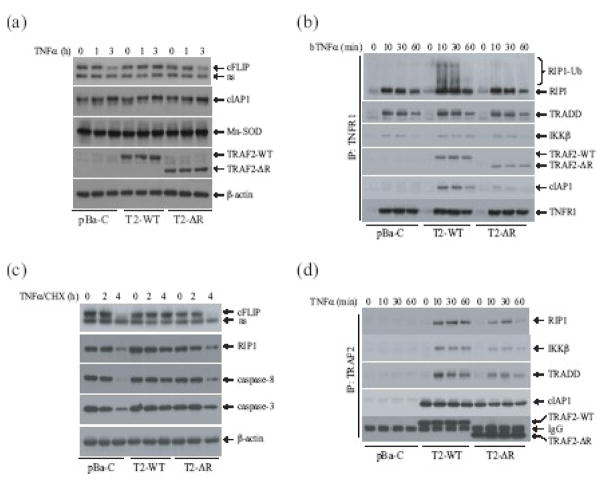
To investigate the role of the TRAF2 RING domain in a physiological setting, and to rule out the possibility that TRAF5 affects on NF-κB activation, we established TRAF2/5 DKO cell lines that stably express empty vector (pBa-C), TRAF2-WT (pBa-T2-WT) or TRAF2-ΔR (pBa-T2-ΔR) at a physiological level, as described previously (Fig. 2a) 12. Using these cells, we analyzed the expression of well-characterized NF-κB and c-Jun target genes, including ICAM-I, cFLIP, IL-6, cIAP1 and cIAP2 by quantitative real-time PCR. As expected, the basal and TNFα-induced levels of ICAM-1 expression were higher in pBa-C cells than in pBa-T2-WT cells (Fig. 2c). Consistent with our observation in the reporter gene assays, both the basal and inducible expression of ICAM-I in pBa-T2-ΔR cells was higher than that in pBa-T2-WT cells, but lower than that in pBa-C cells. In the case of cFLIP, cIAP1 and cIAP2, on the other hand, the basal and inducible expression levels in all three cell types were comparable (Fig. 2b, 2e and 2f). Of note, TNFα-induced expression of cIAP2 was much greater than that of cIAP1 and cFLIP in these cells. These data suggest that neither TRAF2 nor its RING domain has a substantial role in the NF-κB-dependent gene expression elicited by TNFα stimulation. In the case of IL-6, however, TNFα-induced expression was almost completely impaired in pBa-C cells, and significantly reduced in pBa-T2-ΔR cells compared to that in pBa-T2-WT cells (Fig. 2d).
Fig. 2.
The TRAF2 RING domain is essential for TNFα-induced IL-6, but not cFLIP and ICAM-I, expression. (a) TRAF2/5 DKO MEFs were stably transfected with empty vector (pBa-C), Flag-TRAF2-WT (pBa-T2-WT) or Flag-TRAF2-ΔR (pBa-T2-ΔR) by retroviral infection followed by puromycin selection. The expression of Flag-TRAF2-WT, Flag-TRAF2-ΔR and endogenous TRAF2 in these cell lines and WT-MEFs was then monitored by Western blotting, using an anti-TRAF2 antibody. (b-f) pBa-C, pBa-T2-WT or pBa-T2-ΔR cells were untreated or treated with mTNFα (5 ng/ml) for 1 and 3 hrs, after which the expression of ICAM-I, cFLIP, IL-6, cIAP1 and cIAP2 was determined by real-time PCR. The relative expression level of each gene is presented as the ratio between expression of the respective gene and that of the reference gene GAPDH, as mean ± SD from four independent experiments. (*) represents p < 0.05 and (**) p< 0.01.
The expression of cFLIP, cIAP1 and cIAP2 is induced by the canonical NF-κB and the PI3K/Akt pathways, whereas the expression of ICAM-I is regulated by both the canonical and noncanonical NF-κB pathways 4; 13; 14; 15; 16. The noncanonical NF-κB pathway is constitutively activated to a maximal level in TRAF2/5 DKO MEFs, and TNFα-induced activation of the canonical NF-κB pathway is not impaired in these cells. Thus, the elevated expression of ICAM-I in pBa-C cells is due to the synergistic effects of constitutive activation of the noncanonical NF-κB pathway and normal TNFα-induced activation of the canonical NF-κB pathway. The significant suppression of ICAM-1 expression in pBa-T2-WT cells is because of the suppression of both the canonical and noncanonical NF-κB pathways by TRAF2-WT in these cells under unstimulated conditions (Fig. 3a and 4a). Although stable expression of TRAF2-ΔR in TRAF2/5 DKO cells did not suppress constitutive p100 processing (Fig. 4a), it significantly inhibited the elevated basal activity of the IKK complex in these cells (Fig. 3a). Consistent with these results, the basal and inducible expression of ICAM-1 in pBa-T2-ΔR cells was in fact slightly higher than that in pBa-T2-WT cells. On the other hand, efficient expression of IL-6 requires both c-Jun and NF-κB activities, as TNFα-induced IL-6 expression is impaired in both JNK1/2 DKO and p65 KO MEFs 17; 18. Taken together with the above-mentioned published findings, our data suggest that the TRAF2 RING domain plays a critical role in c-Jun-dependent, but not NF-κB-dependent, gene expression in response to TNFα stimulation.
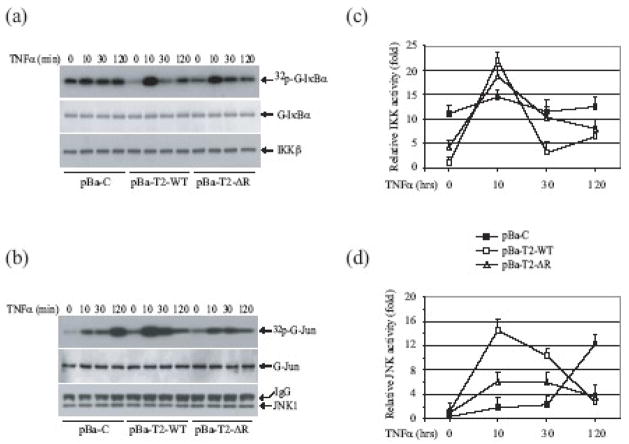
Fig. 3.
The TRAF2 RING domain is essential for TNFα-induced JNK, but not IKK, activation. (a, b) pBa-C, pBa-T2-WT and pBa-T2-ΔR cells were left untreated or were treated with mTNFα (10ng/ml) as indicated. The IKK complex (a) and JNK1 (b) were then immunoprecipitated with anti-IKKγ and anti-JNK1 antibodies, and subjected to in vitro kinase assays in which GST-IκBα1-55 and GST-jun1-87 served as substrates, respectively. The reaction mixtures were separated by SDS-PAGE, transferred onto nitrocellulose membranes and exposed to X-ray film for 4–6 hrs (32p-G-IκBα and 32p-G-jun). The same membranes were stained with Ponceau S (to detect G-IκBα and G-jun) and blotted with anti-IKKβ and anti-JNK1 antibodies, respectively. (c, d) Three sets of pBa-C, pBa-T2-WT and pBa-T2-ΔR cell lines established independently were left untreated or were treated with mTNFα (10ng/ml) as indicated, after which IKK (c) and JNK (d) kinase activities were determined as above. Data presented are the mean ± SD of IKK and JNK activities from four independent kinase assays.
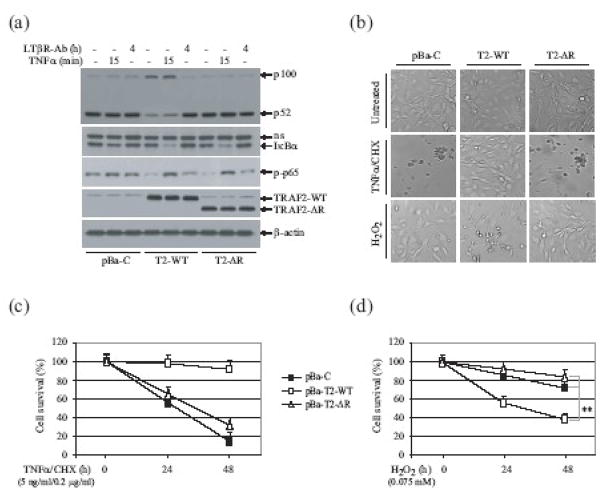
Fig. 4.
The TRAF2 RING domainis required for suppression of p100 processing in resting cells, and for inhibition of TNFα-induced cell death. (a) pBa-C, pBa-T2-WT (T2-WT) and pBa-T2-ΔR (T2-ΔR) cells were left untreated, were treated with mTNFα (10ng/ml) for 15 min, or were treated with the anti-LTβR antibody (0.5μg/ml) for 4 hrs. Thereafter, p100 processing, IκBα degradation and p65 phosphorylation were monitored by Western blotting. (b) pBa-C, pBa-T2-WT and pBa-T2-ΔR cells were left untreated, were treated with mTNFα (5ng/ml) plus CHX (0.2 μg/ml), or were treated with H2O2 (0.075 mM) for 30 hrs, at which point cell death was assessed by microscope (x200). (c, d)The indicated cells were left untreated, were treated with mTNFα/CHX (c; 5ng/ml/0.2 μg/ml), or were treated with H2O2 (d; 0.075 mM), and the rate of cell death was assessed 24 and 48 hrs later via the trypan blue exclusion assay. The data shown represent the mean ± SD of three experiments performed in triplicate. (**) represents p< 0.01.
The TRAF2 RING domain is essential for TNFα-induced JNK, but not IKK, activation
In a previous study, we reported that the canonical IKK complex is also constitutively activated in TRAF2 KO and TRAF2/5 DKO cells, due to the accumulation of NIK in these cells 11. To better assess the role of the TRAF2 RING domain in TNFα-induced JNK and IKK activation, we carried out in vitro JNK and IKK kinase assays, using GST-Jun and GST-IκBα fusion-proteins as substrates, respectively. To rule out the possibility that an IKKα homodimer affects GST-IκBα phosphorylation in the kinase assay, we immunoprecipitated the IKK complex with anti-IKKγ antibody and extensively washed the IKK-bound G-protein beads with lysis buffer containing 350 mM NaCl. As expected, TRAF2/5 DKO MEFs stably transfected with empty vector (pBa-C) exhibited high basal IKK activity, and TNFα stimulation further increased IKK activity, albeit weakly (Fig. 3a). TRAF2/5 DKO cells reconstituted with TRAF2-WT (pBa-T2-WT) exhibited low basal IKK activity, and immediate and robust IKK activation in response to TNFα stimulation (Fig. 3a). Interestingly, stable expression of TRAF2-ΔR in TRAF2/5 DKO MEFs (pBa-T2-ΔR) partially suppressed basal IKK activity, and almost completely restored TNFα-induced IKK activation. On the other hand, pBa-C, pBa-T2-WT and pBa-T2-ΔR cells did not display elevated basal JNK activity, and stimulation of these cells with TNFα induced immediate and robust JNK activation in pBa-T2-WT cells, but not in pBa-C and pBa-T2-ΔR cells (Fig. 3b). Notably, stable expression of TRAF2-WT and TRAF2-ΔR in TRAF2/5 DKO cells completely suppressed the prolonged JNK activation that occurs in TRAF2/5 DKO cells upon TNFα stimulation (Fig. 3b; 120 min time point). These results were confirmed in two independently established sets of pBa-C, pBa-T2-WT and pBa-T2-ΔR cell lines, and the JNK and IKK immunokinase assays were performed three times; the results obtained were always consistent (Fig. 3c and d). Collectively, these data demonstrated that the TRAF2 RING domain is essential for the protein’s efficient suppression of basal IKK activity in resting cells, and for TNFα-induced transient and robust activation of JNK.
TNFα induces IκBα degradation and p65 phosphorylation in TRAF2-ΔR-expressing cells
Stable expression of TRAF2-ΔR in TRAF2/5 DKO cells did not completely suppress basal IKK activity, yet almost completely restored TNFα-induced IKK activation (Fig. 3a). To further assess the role of the TRAF2 RING domain in TNFα-induced NF-κB activation, we examined IκBα degradation and p65 phosphorylation in TRAF2/5 DKO cells reconstituted with empty vector (pBa-C), TRAF2-WT (pBa-T2-WT) or TRAF2-ΔR (pBa-T2-ΔR), by Western blotting. As shown in Fig. 4a, in pBa-T2-ΔR cells TNFα stimulation caused IκBα degradation within 15 min of stimulation, to an extent comparable to that in pBa-T2-WT cells; however, TNFα failed to induce IκBα degradation to the same extent in pBa-C cells. Consistent with this finding, TNFα stimulation triggered similar levels of p65 phosphorylation in pBa-T2-WT and pBa-T2-ΔR cells (Fig. 4a). These data suggested that the TRAF2 RING domain is dispensable for TNFα-induced IκBα degradation and p65 phosphorylation.
The TRAF2 RING is essential for suppressing the non-canonical NF-κB pathway
Processing of p100 to p52, a hallmark of activation of the noncanonical NF-κB pathway, takes place constitutively in TRAF2 KO, TRAF2/5 DKO and TRAF3 KO cells, due to the accumulation of NIK in these cells 11; 19; 20; 21. To assess the role of the TRAF2 RING domain in regulating the noncanonical NF-κB pathway, we analyzed p100 processing in pBa-C, pBa-T2-WT and pBa-T2-ΔR cells, following their stimulation with the agonistic anti-LTβR antibody. As expected, in pBa-C cells p100 was constitutively processed to p52 even in the absence of stimulation, but in pBa-T2-WT cells this processing step required exposure of the cells to anti-LTβR antibody (Fig. 4a). Notably, in pBa-T2-ΔR cells, p100 was also constitutively processed to p52. These data suggest that the TRAF2 RING domain plays a critical role in suppressing the non-canonical NF-κB pathway in resting cells.
The TRAF2 RING domain is essential for inhibiting TNFα-induced cell death
TRAF2 KO and TRAF2/5 DKO cells are known to be sensitive to TNFα-induced cell death, but resistant to oxidative stress (e.g. H2O2)-induced cell death 8; 11; 22. We next examined the role of the TRAF2 RING domain in regulating cell death induced by TNFα and H2O2. We treated pBa-C, pBa-T2-WT and pBa-T2-ΔR cells with TNFα (5 ng/ml) plus cycloheximide (CHX; 0.2 μg/ml), a condition that does not cause more than 10% cell death in WT MEFs 8; 11. As shown in Fig. 4b, TNFα/CHX treatment induced cell death in pBa-C and pBa-T2-ΔR cells, but not in TRAF-WT cells, suggesting that the TRAF2 RING domain plays a critical role in inhibiting TNFα-induced cell death. To confirm these results, we repeated the cell death assays in all three sets of independently established pBa-C, pBa-T2-WT and pBa-T2-ΔR cell lines, and obtained the same results (Fig. 4c). On the other hand, when cell death assays were carried out in these cell lines following H2O2 treatment, the pBa-C and pBa-T2-ΔR cells displayed significant resistance in comparison to do pBa-T2-WT cells (Fig. 4d). Notably, we repeatedly observed that all of the pBa-T2-ΔR cell lines independently established from TRAF2/5 DKO cells are slightly resistant to TNFα/CHX- and H2O2-induced cell death in comparison to the pBa-C cell lines. It is thus likely that the prolonged JNK activation characteristic of pBa-C cell lines in response to TNFα stimulation may contribute to TNFα-induced cell death. However, we did not see statistically significant differences between the pBa-C and pBa-T2-ΔR cell lines with respect to their sensitivities to TNFα/CHX- and H2O2-induced cell death (Fig. 4c and d).
The TRAF2 RING domain is not essential for TNFα-induced expression of anti-apoptotic proteins
Although TNFα-induced IκBα degradation and NF-κB-dependent gene expression appeared to be normal in pBa-T2-ΔR cells, these cells nevertheless underwent cell death upon TNFα treatment (Fig. 4c). Mn-SOD, cIAP1 and cFLIP are among the anti-apoptotic proteins that inhibit TNFα-induced cell death, and they do so by scavenging reactive oxygen species (ROS) and by blocking caspase activation 2. Therefore, we next analyzed the expression of these anti-apoptotic proteins by Western blotting. As shown in Fig. 5a, the basal expression levels of Mn-SOD, cIAP1 and cFLIP were comparable among the pBa-C, pBa-T2-WT and pBa-T2-ΔR cell lines. When each cell type was stimulated with TNFα for 1 or 3 hrs, cIAP1 and Mn-SOD protein levels were slightly increased in all cell types, whereas cFLIP protein levels were increased in pBa-T2-WT cells but reduced in both pBa-C and pBa-T2-ΔR cells. Given that the cFLIP mRNA levels increased upon TNFα stimulation in all these cells (Fig. 2c), the decrease in cFLIP protein levels in the pBa-C and pBa-T2-ΔR cells must be due to post-translational cleavage and degradation 7. These data suggest that the expression of anti-apoptotic proteins in TRAF2-ΔR-expressing cells is not impaired, and that the TRAF2 RING domain inhibits TNFα-induced cell death independent of NF-κB activation.
Fig. 5.
The TRAF2 RING domain plays an essential role in TNFα-induced RIP1 ubiquitination and in inhibition of caspase-8 activation. (a) pBa-C, pBa-T2-WT and pBa-T2-ΔR cells were left untreated or were treated with mTNFα (10ng/ml) for 1 and 3 hrs, after which the expression levels of cFLIP, cIAP1, Mn-SOD, TRAF2-WT and TRAF2-ΔR was monitored by Western blotting. (b) pBa-C, pBa-T2-WT and pBa-T2-ΔR cells were left untreated or were treated with mTNFα (5ng/ml) plus CHX (0.2μg/ml) for 2 and 4 hrs, after which the expression and cleavage of cFLIP, RIP1, caspase-8 and caspase-3 was monitored by Western blotting. (c) pBa-C, pBa-T2-WT and pBa-T2-ΔR cells were left untreated or treated with biotinylated TNFα (bTNFα; 100ng/ml) for 10, 30 and 60 min, after which the TNFR1 complexes were pulled down with the aid of Dynabead Streptavidin. Recruitment of RIP1, TRADD, IKKβ and cIAP1 to TNFR1 was then monitored by Western blotting, using the corresponding antibodies. (d) pBa-C, pBa-T2-WT and pBa-T2-ΔR cells were left untreated or were treated with mTNFα (10ng/ml) for 10, 30 and 60 min, after which Flag-TRAF2-WT and Flag-TRAF2-ΔR were immunoprecipitated using an anti-Flag antibody. The interactions of RIP1, IKKβ, TRADD and cIAP1 with Flag-TRAF2-WT and Flag-TRAF2-ΔR were then monitored by Western blotting.
The TRAF2 RING domain is essential for inhibiting caspase-8 activation induced by TNFα
Caspase-8 plays an essential role in TNFα-induced cell death 4; 5. Therefore, we examined the effect of the TRAF2 RING domain on caspase-8 activation, as well as on cleavage of the well-known caspase-8 substrate RIP1, by Western blotting. Indeed, treatment of cells with TNFα in the presence of 0.2 μg/ml CHX clearly induced caspase-8 activation and led to the subsequent cleavage of RIP1 in both pBa-C and pBa-T2-ΔR cells, but did not have a significant effect on this in pBa-T2-WT cells (Fig. 5b). In addition, caspase-3 cleavage was clearly detected in pBa-C and pBa-T2-ΔR cells. NF-κB-dependent expression of cIAP1 and cIAP2 suppresses TNFα-induced caspase-8 activation, and cIAP1 and cIAP2 are components of the TNFR1 signaling complex 15; 23. Therefore, we further examined the recruitment of cIAP1 and cIAP2 to TNFR1 following TNFα stimulation. As shown in Fig. 5c, pull-down of the TNFR1 complex following TNFα stimulation resulted in recruitment of cIAP1 to TNFR1 in pBa-T2-WT cells but not in pBa-C cells, and this recruitment was significantly reduced in pBa-T2-ΔR cells. Interestingly, TRAF2-ΔR recruitment to TNFR1 was also slightly reduced in pBa-T2-ΔR cells compared to TRAF2-WT recruitment to TNFR1 in pBa-T2-WT cells (Fig. 5c), although the expression levels of the TRAF2-WT and TRAF2-ΔR proteins in these cells, respectively, are comparable (Fig. 2a). On the other hand, the recruitment of TRADD, RIP1 and IKKβ to TNFR1 in response to TNFα stimulation was comparable among the three cell lines of each type. Unexpectedly, when Flag-TRAF2-WT and Flag-TRAF2-ΔR were immunoprecipitated with anti-Flag antibody following TNFα stimulation, both TRAF2-WT and TRAF2-ΔR were found to equally and constitutively associated with cIAP1, before and after TNFα stimulation, and this interaction was not affected by TNFα stimulation (Fig. 5d). Although RIP1 and IKKβ were also present in the complexes pulled down by both TRAF2-WT and TRAF2-ΔR, the amount of RIP1 and IKKβ brought down with the TRAF2-ΔR complex was slightly less than that brought down with the TRAF2-WT complex (Fig. 5d). Given that both TRAF2-WT and TRAF2-ΔR equally and constitutively associated with cIAP1 regardless of TNFα stimulation, the inefficient recruitment of cIAP1 to the TNFR1 complex in pBa-T2-ΔR cells is most likely due to dissociation of cIAP1 from TRAF2-ΔR after TRAF2-ΔR is recruited to the TNFR1/TRADD complex following TNFα stimulation. It is possible that the association of TRAF2 with TRADD causes a conformational change in TRAF2, and that the intact TRAF2 RING domain plays a role in stabilizing the TRAF2-cIAP1 complex upon TRAF2 association with TRADD. As we were not able to reproducibly detect endogenous cIAP2 expression in these cells by Western blotting, it is not clear whether cIAP2 is also present in the TRAF2 complex. Nevertheless, our data suggest that the TRAF2 RING domain inhibits TNFα-induced cell death, and that it do so most likely by retaining cIAP1 (and possibly also cIAP2) in the TNFR1 signaling complex.
The TRAF2 RING domain regulates TNFα-induced RIP1 ubiquitination in vivo
RIP1 is ubiquitinated immediately upon TNFα stimulation, and this ubiquitination is believed to be catalyzed by the RING domain of TRAF2 and/or TRAF5, and to be essential for TNFα-induced NF-κB activation 24; 25; 26. In light of this model, we examined RIP1 ubiquitination in pBa-C, pBa-T2-WT and pBa-T2-ΔR cells following TNFα stimulation. As expected, TNFα induced RIP1 ubiquitination in pBa-T2-WT cells, but not in pBa-C cells (Fig. 5c). Notably, TNFα failed to induce RIP1 ubiquitination in pBa-T2-ΔR cells in spite of the fact that TNFα efficiently induced IκBα degradation and p65 phosphorylation in these cells (Fig. 4a). These data suggest that the TRAF2 RING domain plays an important role in TNFα-induced RIP1 ubiquitination, but that TRAF2-mediated RIP1 ubiquitination has no substantial role in TNFα-induced NF-κB activation.
Discussion
TRAF2 is a cytoplasmic protein believed to function as an E3 ubiquitin ligase to regulate activation of the JNK/c-Jun and IKK/NF-κB pathways in response to TNFα stimulation 4; 25; 26. Knockout of TRAF2, however, impairs TNFα-induced JNK activation without affecting the activation of IKK 8. In the field of NF-κB study, it is now widely believed that simultaneous knockout of TRAF2 and TRAF5 abolishes TNFα-induced NF-κB activation 4; 9. However, this conclusion is based on an analysis of IκBα protein levels in these cells 9. We recently reported that the canonical IKK complex is constitutively activated in TRAF2/5 DKO cells, and that TNFα stimulation further increases IKK activity in these cells in the absence of RIP1 polyubiquitination 11. This constitutive IKK activation leads to constitutive IκBα phosphorylation, degradation and resynthesis in TRAF2/5 DKO cells, effectively masking TNFα-induced immediate and complete IκBα degradation 11. Therefore, the lack of complete degradation of IκBα in TRAF2/5 DKO cells following TNFα exposure is not a consequence of impaired IKK activation, but rather of constitutive degradation and resynthesis of IκBα prior to stimulation. We also found in the previous study that the expression of a dominant negative NIK or application of a NIK-targeting siRNA in TRAF2/5 DKO cells reduced but did not completely suppress the elevated basal IKK activity, suggesting that a NIK-independent pathway leading to IKK activation may also be constitutively activated to some extent in these cells 11.
Activation of the noncanonical NF-κB pathway requires the accumulation of NIK, which is otherwise constitutively targeted by TRAF2 and TRAF3 in unstimulated cells 20; 21. TRAF2 and TRAF3 have nonredundant and complementary functions in targeting NIK for ubiquitination-dependent degradation, as the noncanonical NF-κB pathway is constitutively activated in both TRAF2-deficient and TRAF3-deficient cells 20; 21; 27. He et al have reported that the RING domain of TRAF3 must be structurally intact for its inhibition of the noncanonical NF-κB pathway 28. In the current study, we demonstrate that stable expression of TRAF2-WT in TRAF2/5 DKO cells completely suppresses the elevated basal IKK activity and inhibits constitutive p100 processing, whereas the stable expression of TRAF2-ΔR in TRAF2/5 DKO cells significantly suppresses the basal IKK activity but does not block constitutive p100 processing (Fig. 3a and 4a). This suggests that the TRAF2 RING domain plays an essential role in suppressing the noncanonical NF-κB pathway in resting cells, but is not required to suppress the canonical NF-κB pathway. In other words, constitutive activation of the canonical IKK complex in TRAF2/5 DKO cells is due to constitutive activation of not only the noncanonical, but also the canonical, NF-κB pathway, and that TRAF2-ΔR is sufficient to inhibit the canonical, but not the noncanonical, NF-κB pathway.
TRAF2-ΔR has been widely used as a dominant negative inhibitor of TNFα-induced activation of both JNK and IKK in a transient overexpression system 1; 2. However, adenoviral vector-mediated expression of TRAF2-ΔR in hepatoma cells inhibits only JNK, and not NF-κB, activation 29. In addition, lymphocytes derived from transgenic mice expressing TRAF2-ΔR exhibit normal NF-κB, but not JNK, activation in response to stimulation with TNFα and CD40L 30. These data suggest that the mode of action of transiently overexpressed TRAF2-ΔR on NF-κB activation is distinct from that of stably expressed TRAF2-ΔR. A possible explanation for this difference is that transient transfection in HeLa or 293T cells produces such a high level of TRAF2-ΔR protein that it suppresses or distorts signaling pathways that are not regulated by the RING domain of TRAF2 in a physiological setting. In the current study, we provide compelling evidence— by stably expressing TRAF2-ΔR in TRAF2/5 DKO cells at a physiological level—that the TRAF2 RING domain plays a critical role in TNFα-induced JNK activation but is not essential for TNFα-induced IKK activation and NF-κB-dependent gene expression (Fig. 3a and 3b). Similarly, the RING domain-deleted TRAF6 (TRAF6-ΔR) has also been used as a dominant negative inhibitor of IL-1-induced JNK and IKK activation in a transient overexpression system 1; 31, yet Kobayashi et al. have shown that stable expression of TRAF6-ΔR in TRAF6 KO cells fully restores IL-1- and LPS-induced NF-κB activation 32. Collectively, these data suggest that the RING domains of TRAF2 and TRAF6 are dispensable for IKK activation in a physiological setting.
The current belief in the NF-κB field is that the RING domain of TRAF2 and/or TRAF5 catalyzes K63-linked polyubiquitination of RIP1 in response to TNFα stimulation, and that this polyubiquitination of RIP1 somehow activates the canonical IKK complex 4; 9. We found that stable expression of TRAF2-ΔR in TRAF2/5 DKO cells suppresses the elevated basal IKK activity and restores TNFα-induced IKK activation and IκBα degradation without restoring RIP1 polyubiquitination. This suggests that RIP1 ubiquitination is not essential for TNFα-induced IKK activation. Recently, three groups independently generated TRADD knockout mice, and found that TNFα-induced activation of JNK and IKK is abolished in TRADD-deficient MEFs, due to impaired recruitment of RIP1 and TRAF2 to the TNFR1 complex 33; 34; 35. However, in TRADD-deficient macrophages, RIP1 is recruited to TNFR1 in response to TNFα stimulation due to its high level of expression, but is not ubiquitinated because TRAF2 is not recruited to the TNFR1 complex in the absence of TRADD. Surprisingly, RIP1 recruitment to TNFR1 in this context is sufficient to activate IKK in the absence of K63-linked RIP1 polyubiquitination 34; 35, suggesting that the efficient recruitment of RIP1 to TNFR1 in response to TNFα stimulation is sufficient to activate IKK. It has been shown that RIP1 can be recruited to the TNFR1 complex independently of TRAF2 and TRAF5, and that it directly associates with IKKγ 11; 36; 37. We found that in TRAF2/5 DKO cells reconstituted with TRAF2-ΔR, both TRADD and RIP1 are recruited normally to TNFR1 upon TNFα treatment, although RIP1 is not ubiquitinated (Fig. 5c and 5d). Therefore, it is not surprising that TNFα can activate IKK in TRAF2/5 DKO cells reconstituted with TRAF2-ΔR, even in the absence of RIP1 ubiquitination.
TNFα stimulation causes prolonged JNK activation in TRAF2/5 DKO and p65 KO cells, and this is believed to promote TNFα-induced cell death 6; 38; 39. We found that stable expression of TRAF2-ΔR in TRAF2/5 DKO cells efficiently suppresses TNFα-induced prolonged JNK activation (Fig. 3b), but fails to inhibit TNFα-induced cell death. However, we noticed that all three independently established cell lines that express TRAF2-ΔR are slightly resistant to TNFα-induced cell death compared to cell lines expressing empty vector. This suggests that prolonged JNK activation is not essential for, but may accelerate, TNFα-induced cell death.
It has now been well established that TRAF2 directly associates with and recruits cIAP1/2 to the TNFR1 complex, and that it inhibits TNFα-induced cell death independent of NF-κB activation 4; 40; 41; 42. In a transient overexpression system, it has been shown that TRAF2 associates with cIAP1/2 through its TRAF-N domain 43. Indeed, both TRAF2-WT and TRAF2-ΔR associated with cIAP1 in the cytoplasm regardless of TNFα stimulation (Fig. 5d). However, the RING domain of TRAF2 seems to be required for stable interaction between TRAF2 and cIAP1 upon their recruitment to the TNFR1 complex following TNFα stimulation (Fig. 5c and 5d). Bertrand et al. have recently shown that cIAP1/2 target RIP1 for ubiquitination 44. cIAP1 has also been shown to target TRAF2 for degradation 45. Therefore, it is possible that TRAF2 RING domain-mediated, K63-linked polyubiquitination of RIP1 serves to stabilize the TNFR1 complex by interfering with the cIAP1-mediated ubiquitination and degradation of RIP1 and TRAF2. In this scenario, in the absence of TRAF2 RING domain-mediated RIP1 ubiquitination, cIAP1 would target TRAF2-ΔR for degradation within the TNFR1 complex in pBa-T2-ΔR cells following TNFα stimulation, resulting in the dissociation of cIAP1 itself from the TNFR1 complex. Thus, the primary function of TRAF2 RING domain-mediated RIP1 ubiquitination might be to inhibit TNFα-induced cell death by preventing cIAP1-dependent degradation of TRAF2 and RIP1 within the TNFR1 complex, rather than by activating the canonical IKK complex. IKKβ KO and p65 KO cells are also sensitive to TNFα-induced cell death 4. Therefore, we believe that two events might be required for the inhibition of TNFα-induced cell death: i) NF-κB-dependent expression of anti-apoptotic proteins such as cIAP1/2 and cFLIP; and ii) TRAF2 RING domain-dependent retention of cIAP1 in the TNFR1 complex. Ablation of either event leads to caspase-8 activation and cell death in response to TNFα stimulation.
Materials and Methods
Cell lines, plasmids and reagents
HeLa cells and both WT and TRAF2/5 DKO MEFs were maintained in DMEM supplemented with 10% BCS and antibiotics. Antibodies (Abs) and reagents were purchased as follows: anti-TRAF2, anti-JNK, anti-IKKγ, anti-IKKβ, anti-cIAP1 and anti-Mn-SOD antibodies (Abs) from Santa Cruz Biotechnology (Santa Cruz, CA); anti-cFLIP Ab from Upstate Biotechnology (Lake Placid, NY); anti-IκBα and anti-caspase-8 Abs from Cell Signaling (Danvers, MA); mouse and human TNFα from Roche (Indianapolis, IN); anti-Flag Ab from Sigma Chemicals (St. Louis, MO); cocktail inhibitors of proteases and phosphatases from Pierce (Rockford, IL). Constructs encoding 2xNF-kB-Luc and Jun2-Luc reporter genes and Flag-TRAF2-WT have been previously described 11; 46. The retroviral plasmids pBabe-Flag-TRAF2-ΔR (encoding the region encompassing aa 87-501) were generated by subcloning the TRAF2-ΔR into the pBabe-puro plasmids.
Luciferase reporter gene assays
Cells cultured in 6-well plates were transfected with an NF-κB or c-Jun firefly luciferase reporter plasmid (NF-κB-Luc or Jun2-Luc; 0.2 μg), together with a Flag-TRAF2-WT or Flag-TRAF2-ΔR (0.2 μg) and a Renilla luciferase reporter plasmid (pRL-TK; 0.01 μg), using Lipofectamine 2000 reagents. 36 hrs after transfection, test cells were treated with hTNFα (10 ng/ml) or mTNFα (5 ng/ml), and protein samples were prepared at 6 (HeLa) or 4 (MEFs) hrs after treatment. The firefly and Renilla luciferase activities were then measured using the Dual-luciferase assay system according to the manufacturer’s instructions (Promega).
JNK and IKK immunokinase assays
MEF cells were treated with mTNFα (10 ng/ml) and protein samples were extracted using TNE lysis buffer (20 mM HEPES, pH 7.4, 350 mM NaCl, 1% Triton X-100, 1mM DTT, 1mM EDTA, 20% glycerol and a cocktail of protease and phosphatase inhibitors). Endogenous JNK1 or IKK complex was immunoprecipitated using anti-JNK1 or anti-IKKγ antibody, respectively, and then these samples were subjected to in vitro kinase assays, in which GST-Jun1–87 (for JNK) or GST-IκBα1–55 (for IKK) served as substrate, as described previously 46.
Preparation of retroviral supernatants and infection of TRAF2/5 DKO MEFs
TRAF2/5 DKO cell lines that stably express TRAF2-WT or TRAF2-ΔR at a physiological level were generated as described previously 11; 12. Briefly, 293T cells at 60–70% confluence were co-transfected with 2 μg of pMD.OGP (encoding gag-pol), 2 μg of pMD.G (encoding vesicular stomatitis virus G protein) and 2 μg of pBabe-Flag-TRAF2-WT or pBa-Flag-TRAF2-ΔR, using the standard calcium phosphate precipitation method. 48 hrs after transfection, the viral supernatant was collected and filtered through a 0.45 μm filter. The retroviral supernatants were diluted 3-fold with 10%FBS/DMEM, and then immediately used to infect TRAF2/5 DKO MEFs in the presence of 4 μg/ml polybrene for 6 hrs. 48 hrs after infection, cells were selected with puromycin (2.0μg/ml) for 6 days. Resistant cells were pooled and used for the functional experiments within one month of establishment.
Real-time PCR
MEF cells were treated with mTNFα (10 ng/ml), and total RNA was prepared using the RNeasy Mini Kit (Qiagen). Five μg of total RNA was treated with RQ1 RNase-free DNase for 30min at 37 °C, and then reverse transcribed using an oligo dT-primer. The resulting cDNA was subjected to quantitative real-time PCR using the Power SYBR Green AB Master Mix and an ABI Prism 7700 Sequence Detector (Applied Biosystems). Mouse GAPDH-specific primers were used to generate an internal control, and the average threshold cycle (CT) for samples in triplicate was used in the subsequent calculations. Relative expression levels of NF-κB target genes were calculated as the ratio with respect to the GAPDH expression level. The mean ± S.D. of four independent experiments was considered to be statistically significant at p < 0.05. All primers used in this study are exactly the same as reported previously 11; 12.
RIP1 ubiquitination
The TNFR1 complex was immunoprecipitated using biotinylated mouse TNFα (bTNFα) in combination with Dynabeads-Streptavidin (Invitrogen). Recombinant mTNFα was biotinylated using Sulfo-NHS-LC-Biotin (Pierce) at 1 mg/ml for 1 hr according to the manufacturer’s instructions. Unincorporated biotin was removed from bTNFα by buffer exchange into PBS on PD-10 columns (Amersham). The biological activity of bTNFα was determined by its apoptosis-inducing capacity, and was found to be comparable to nonbiotinylated mTNFα. MEFs (4×100mm cells per treatment) were treated with bTNFα for 10, 30 and 60 min. Cells were then washed twice with ice-cold PBS and lysed in TNEN lysis buffer (20 mM HEPES, pH 7.4, 250 mM NaCl, 1% Triton X-100, 0.2 % CHAPS, 1mM DTT, 2 mM EDTA, 10% glycerol, 5 mM NEM and a cocktail of protease and phosphatase inhibitors) on ice for 30 min, followed by centrifugation at 13,000 ×g for 20 min at 4°C. The TNFR1 complex was then precipitated using 30μl of Dynabeads-Streptavidin at 4°C for 4 hours. Precipitates were washed four times with the same lysis buffer containing 2 mM NEM. RIP1 recruitment to TNFR1 and its ubiquitination were then monitored by Western blotting.
Acknowledgments
We thank Hiroyasu Nakano (Juntendo University School of Medicine, Japan) for providing us with TRAF2/5 DKO cells. Support by NCI grant CA78419 (to HH) is gratefully acknowledged. None of the authors have conflicts of interest.
Abbreviations used
- cFLIP
cellular caspase-8 (FLICE)-like inhibitory protein
- CHX
cycloheximide
- IAP
inhibitor of apoptosis
- IκB
inhibitor of κB
- IKK
IκB kinase
- JNK
c-Jun N-terminal kinase
- MAPK
Mitogen-activated protein kinase
- NF-κB
nuclear factor κB
- RIP1
receptor interacting protein 1
- ROS
reactive oxygen species
- TNFα
tumor necrosis factor α
- TNFR
TNF receptor
- TRADD
TNFR associated death domain
- TRAF
TNFR associated factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bradley JR, Pober JS. Tumor necrosis factor receptor-associated factors (TRAFs) Oncogene. 2001;20:6482–91. doi: 10.1038/sj.onc.1204788. [DOI] [PubMed] [Google Scholar]
- 2.Wajant H, Henkler F, Scheurich P. The TNF-receptor-associated factor family: scaffold molecules for cytokine receptors, kinases and their regulators. Cell Signal. 2001;13:389–400. doi: 10.1016/s0898-6568(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 3.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–8. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–62. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–7. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 6.Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, Lin A. Inhibition of JNK activation through NF-kappaB target genes. Nature. 2001;414:313–7. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- 7.Nakajima A, Komazawa-Sakon S, Takekawa M, Sasazuki T, Yeh WC, Yagita H, Okumura K, Nakano H. An antiapoptotic protein, c-FLIPL, directly binds to MKK7 and inhibits the JNK pathway. Embo J. 2006;25:5549–59. doi: 10.1038/sj.emboj.7601423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeh WC, Shahinian A, Speiser D, Kraunus J, Billia F, Wakeham A, de la Pompa JL, Ferrick D, Hum B, Iscove N, Ohashi P, Rothe M, Goeddel DV, Mak TW. Early lethality, functional NF-kappaB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity. 1997;7:715–25. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- 9.Tada K, Okazaki T, Sakon S, Kobarai T, Kurosawa K, Yamaoka S, Hashimoto H, Mak TW, Yagita H, Okumura K, Yeh WC, Nakano H. Critical roles of TRAF2 and TRAF5 in tumor necrosis factor-induced NF-kappa B activation and protection from cell death. J Biol Chem. 2001;276:36530–4. doi: 10.1074/jbc.M104837200. [DOI] [PubMed] [Google Scholar]
- 10.Grech AP, Amesbury M, Chan T, Gardam S, Basten A, Brink R. TRAF2 differentially regulates the canonical and noncanonical pathways of NF-kappaB activation in mature B cells. Immunity. 2004;21:629–42. doi: 10.1016/j.immuni.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Blackwell K, Thomas GS, Sun S, Yeh WC, Habelhah H. TRAF2 suppresses basal IKK activity in resting cells and TNFalpha can activate IKK in TRAF2 and TRAF5 double knockout cells. J Mol Biol. 2009;389:495–510. doi: 10.1016/j.jmb.2009.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackwell K, Zhang L, Thomas GS, Sun S, Nakano H, Habelhah H. TRAF2 phosphorylation modulates tumor necrosis factor alpha-induced gene expression and cell resistance to apoptosis. Mol Cell Biol. 2009;29:303–14. doi: 10.1128/MCB.00699-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffmann A, Leung TH, Baltimore D. Genetic analysis of NF-kappaB/Rel transcription factors defines functional specificities. Embo J. 2003;22:5530–9. doi: 10.1093/emboj/cdg534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science. 2002;298:1241–5. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- 15.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–3. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 16.Madge LA, Kluger MS, Orange JS, May MJ. Lymphotoxin-alpha 1 beta 2 and LIGHT induce classical and noncanonical NF-kappa B-dependent proinflammatory gene expression in vascular endothelial cells. J Immunol. 2008;180:3467–77. doi: 10.4049/jimmunol.180.5.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ventura JJ, Kennedy NJ, Lamb JA, Flavell RA, Davis RJ. c-Jun NH(2)-terminal kinase is essential for the regulation of AP-1 by tumor necrosis factor. Mol Cell Biol. 2003;23:2871–82. doi: 10.1128/MCB.23.8.2871-2882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okazaki T, Sakon S, Sasazuki T, Sakurai H, Doi T, Yagita H, Okumura K, Nakano H. Phosphorylation of serine 276 is essential for p65 NF-kappaB subunit-dependent cellular responses. Biochem Biophys Res Commun. 2003;300:807–12. doi: 10.1016/s0006-291x(02)02932-7. [DOI] [PubMed] [Google Scholar]
- 19.Piao JH, Yoshida H, Yeh WC, Doi T, Xue X, Yagita H, Okumura K, Nakano H. TNF receptor-associated factor 2-dependent canonical pathway is crucial for the development of Peyer’s patches. J Immunol. 2007;178:2272–7. doi: 10.4049/jimmunol.178.4.2272. [DOI] [PubMed] [Google Scholar]
- 20.Vallabhapurapu S, Matsuzawa A, Zhang W, Tseng PH, Keats JJ, Wang H, Vignali DA, Bergsagel PL, Karin M. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat Immunol. 2008;9:1364–70. doi: 10.1038/ni.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardam S, Sierro F, Basten A, Mackay F, Brink R. TRAF2 and TRAF3 signal adapters act cooperatively to control the maturation and survival signals delivered to B cells by the BAFF receptor. Immunity. 2008;28:391–401. doi: 10.1016/j.immuni.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Shen HM, Lin Y, Choksi S, Tran J, Jin T, Chang L, Karin M, Zhang J, Liu ZG. Essential roles of receptor-interacting protein and TRAF2 in oxidative stress-induced cell death. Mol Cell Biol. 2004;24:5914–22. doi: 10.1128/MCB.24.13.5914-5922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shu HB, Takeuchi M, Goeddel DV. The tumor necrosis factor receptor 2 signal transducers TRAF2 and c-IAP1 are components of the tumor necrosis factor receptor 1 signaling complex. Proc Natl Acad Sci U S A. 1996;93:13973–8. doi: 10.1073/pnas.93.24.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee TH, Shank J, Cusson N, Kelliher MA. The kinase activity of Rip1 is not required for tumor necrosis factor-alpha-induced IkappaB kinase or p38 MAP kinase activation or for the ubiquitination of Rip1 by Traf2. J Biol Chem. 2004;279:33185–91. doi: 10.1074/jbc.M404206200. [DOI] [PubMed] [Google Scholar]
- 25.Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol. 2005;7:758–65. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-kappaB activation [corrected] Nat Cell Biol. 2006;8:398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- 27.Zarnegar BJ, Wang Y, Mahoney DJ, Dempsey PW, Cheung HH, He J, Shiba T, Yang X, Yeh WC, Mak TW, Korneluk RG, Cheng G. Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat Immunol. 2008;9:1371–8. doi: 10.1038/ni.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He JQ, Saha SK, Kang JR, Zarnegar B, Cheng G. Specificity of TRAF3 in its negative regulation of the noncanonical NF-kappa B pathway. J Biol Chem. 2007;282:3688–94. doi: 10.1074/jbc.M610271200. [DOI] [PubMed] [Google Scholar]
- 29.Liedtke C, Plumpe J, Kubicka S, Bradham CA, Manns MP, Brenner DA, Trautwein C. Jun kinase modulates tumor necrosis factor-dependent apoptosis in liver cells. Hepatology. 2002;36:315–25. doi: 10.1053/jhep.2002.34615. [DOI] [PubMed] [Google Scholar]
- 30.Lee SY, Reichlin A, Santana A, Sokol KA, Nussenzweig MC, Choi Y. TRAF2 is essential for JNK but not NF-kappaB activation and regulates lymphocyte proliferation and survival. Immunity. 1997;7:703–13. doi: 10.1016/s1074-7613(00)80390-8. [DOI] [PubMed] [Google Scholar]
- 31.Deng L, Wang C, Spencer E, Yang L, Braun A, You J, Slaughter C, Pickart C, Chen ZJ. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–61. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi N, Kadono Y, Naito A, Matsumoto K, Yamamoto T, Tanaka S, Inoue J. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. Embo J. 2001;20:1271–80. doi: 10.1093/emboj/20.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen NJ, Chio II, Lin WJ, Duncan G, Chau H, Katz D, Huang HL, Pike KA, Hao Z, Su YW, Yamamoto K, de Pooter RF, Zuniga-Pflucker JC, Wakeham A, Yeh WC, Mak TW. Beyond tumor necrosis factor receptor: TRADD signaling in toll-like receptors. Proc Natl Acad Sci U S A. 2008;105:12429–34. doi: 10.1073/pnas.0806585105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pobezinskaya YL, Kim YS, Choksi S, Morgan MJ, Li T, Liu C, Liu Z. The function of TRADD in signaling through tumor necrosis factor receptor 1 and TRIF-dependent Toll-like receptors. Nat Immunol. 2008;9:1047–54. doi: 10.1038/ni.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ermolaeva MA, Michallet MC, Papadopoulou N, Utermohlen O, Kranidioti K, Kollias G, Tschopp J, Pasparakis M. Function of TRADD in tumor necrosis factor receptor 1 signaling and in TRIF-dependent inflammatory responses. Nat Immunol. 2008;9:1037–46. doi: 10.1038/ni.1638. [DOI] [PubMed] [Google Scholar]
- 36.Zhang SQ, Kovalenko A, Cantarella G, Wallach D. Recruitment of the IKK signalosome to the p55 TNF receptor: RIP and A20 bind to NEMO (IKKgamma) upon receptor stimulation. Immunity. 2000;12:301–11. doi: 10.1016/s1074-7613(00)80183-1. [DOI] [PubMed] [Google Scholar]
- 37.Devin A, Cook A, Lin Y, Rodriguez Y, Kelliher M, Liu Z. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity. 2000;12:419–29. doi: 10.1016/s1074-7613(00)80194-6. [DOI] [PubMed] [Google Scholar]
- 38.Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y, Piao JH, Yagita H, Okumura K, Doi T, Nakano H. NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. Embo J. 2003;22:3898–909. doi: 10.1093/emboj/cdg379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, Cong R, Franzoso G. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature. 2001;414:308–13. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- 40.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, Flygare JA, Fairbrother WJ, Deshayes K, Dixit VM, Vucic D. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–81. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 41.Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, Brink R, Leverkus M, Tergaonkar V, Schneider P, Callus BA, Koentgen F, Vaux DL, Silke J. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–93. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 43.Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83:1243–52. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 44.Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Yang Y, Ashwell JD. TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature. 2002;416:345–7. doi: 10.1038/416345a. [DOI] [PubMed] [Google Scholar]
- 46.Habelhah H, Takahashi S, Cho SG, Kadoya T, Watanabe T, Ronai Z. Ubiquitination and translocation of TRAF2 is required for activation of JNK but not of p38 or NF-kappaB. Embo J. 2004;23:322–32. doi: 10.1038/sj.emboj.7600044. [DOI] [PMC free article] [PubMed] [Google Scholar]