Abstract
The secosteroid hormone vitamin D3 (VD3) exerts its biological actions through its cognate receptor, the Vitamin D Receptor (VDR). Vitamin D3 and VDR play a key role in bone formation and keratinocyte differentiation, exert anti-proliferative actions in human cancer, and is widely used as a chemotherapeutic agent for cancer. Additionally, VD3 promotes differentiation of human osteosarcoma cells by up-regulating genes involved in cell cycle arrest and osteoblastic differentiation. Although considerable work has been carried out in understanding the molecular mechanisms underlying the VD3-mediated differentiation of human osteosarcoma cells, the upstream regulation of VD3 signaling pathway is still unclear. In this study we demonstrate that p73 acts as an upstream regulator of VD3 mediated osteoblastic differentiation. Transcription factor p73, a p53 homolog has been shown to play a role in development and recently been termed as a tumor suppressor. Silencing p73 results in a significant reduction of VD3 mediated osteoblastic differentiation; while DNA damage induced p73 leads to an increase in VD3 mediated differentiation of osteosarcoma cells. Together, our data implicate a novel role for p73 in vitamin D mediated differentiation of human osteosarcoma cells.
Keywords: p73, Vitamin D3, Vitamin D Receptor, Osteosarcoma, Differentiation
Introduction
The vitamin D system plays a vital role in maintaining calcium and phosphate homeostasis in order to protect skeletal integrity. Apart from its classical functions, 1,25-dihydroxyvitamin D3 (VD3), the hormonally active form of vitamin D, also has potent anti-proliferative, pro-apoptotic and pro-differentiation actions in a number of malignances (1). Biological functions of VD3 are mediated by its binding to Vitamin D receptor (VDR), a nuclear transcription factor which forms a heterodimer with the retinoid × receptor and binds to vitamin D response elements (VDRE) in the promoter regions of VD3 responsive genes (2, 3).
The anti-proliferative action of VD3 and its analogues is demonstrated in many different human cancer cell lines, notably, colon, prostate and breast cancer cell lines (4). Anti-proliferative effects mediated by VD3 and VDR involve up-regulation of growth inhibitory genes including p21, p27 and IGFBP-3 (5-7) and down-regulation of pro-proliferative genes such as c-myc and BCL-2 (8).
Additionally, VD3 and its analogues also promote osteoblastic differentiation as shown by the differentiation of osteosarcoma cell lines MG-63 and SaOS2 (9). Although VD3-mediated osteoblastic differentiation is associated with activation of cell cycle inhibitors such as p21 and differentiation markers such as Osteopontin (OPN) and Osteocalcin (OCN), the exact molecular mechanisms underlying the regulation of the VDR and VD3 signaling pathway in osteosarcoma cells is unclear (10-12). Recently, we demonstrated that VDR is directly regulated by both p63 and p73, members of the p53 family (13, 14). In addition we showed that p73 is essential for induction of VDR expression upon DNA damage (14).
The p73 transcription factor has been shown to play a vital role in development and human cancers. Unlike p53-deficient mice, p73-deficient mice exhibit severe neurological defects, high mortality rate and show a runting phenotype (15). Like p53, p73 also contains transactivation (TA), DNA binding and oligomerization domains. Additionally, multiple isoforms of p73 are generated due to alternative promoter usage and 3′ splicing (16). Full length N-terminal transactivation domain containing isoforms are termed as TA isoforms (TAp73) and the isoforms that lack the TA domain, are termed ΔN isoforms (ΔNp73). The ΔNp73 isoforms promote proliferation by inducing pro-proliferative genes and by acting in a dominant negative manner towards TAp73 isoforms and p53 (17). In contrast, TAp73 isoforms are structurally and functionally similar to full length p53 and promote cell cycle arrest and apoptosis. In particular, exogenous TAp73 isoforms are capable of inducing cell cycle arrest and apoptosis in multiple cancer cell lines (18). In addition, DNA damage mediated up-regulation of TAp73 isoforms has been shown to play a crucial role in p53-independent chemosensitivity (19). Furthermore, DNA damage mediated up-regulation of TAp73 has been shown to determine the chemosensitivity of multiple cancer cells, suggesting p73 as a potential target for cancer therapeutics (20). Herein, we investigated the role of p73 in VD3-mediated osteoblastic differentiation using SaOS2 osteosarcoma cells as a model system. Our results clearly demonstrate that p73 is essential for VD3-mediated differentiation of osteosarcoma and that induction of p73 upon DNA damage enhances VD3-mediated differentiation of osteosarcoma cell lines.
Results
Silencing p73 leads to a reduction in endogenous VDR expression levels in osteosarcoma
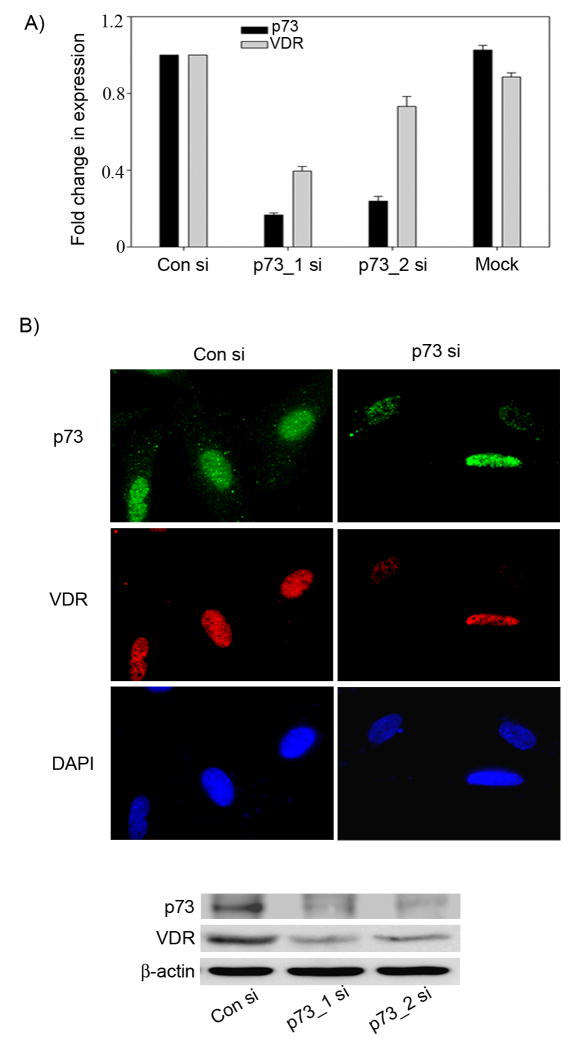
To investigate the role of p73 in VD3-mediated differentiation, we first tested whether endogenous p73 is essential for basal VDR expression. SaOS2 cells devoid of p53 were transfected with either control siRNA (con_si) or two different p73 siRNAs to rule out off target effects (p73_1 si or p73_2 si). Total RNA was extracted from these cells and subjected to real time PCR to determine the transcript levels of p73 and VDR. We observed that cells transfected with either p73_1 si or p73_2 si showed a significant reduction of basal p73 transcript levels which correlated with a reduction in basal VDR transcript levels when compared to control siRNA transfected cells (Figure 1A). Both the control siRNA transfected cells and cells without any transfection (Mock) showed similar levels of p73 and VDR, thus ruling out the possibility of any non-specific effect due to siRNA transfections (Figure 1A). Although, both siRNAs against p73 reduced the basal p73 expression levels, p73 _1 si was more potent than p73_2 si, so we employed p73_1 si for our subsequent studies. Consistent with the reduction in VDR transcript levels, we also observed a significant reduction in VDR protein levels in p73 siRNA transfected cells compared to cells transfected with con siRNA as demonstrated by immunofluorescence (IF) and western blot analyses (Figure 1B).
Figure 1. Silencing p73 results in reduction in VDR expression in osteosarcoma cells.
A) SaOS2 cells, mock transfected or transfected with two rounds of control siRNA or p73 siRNAs were harvested for total RNA at 48 hr following second round of siRNA transfection. Y-axis represents the fold change in transcript levels of VDR and p73 in cells transfected with p73 siRNA compared to control siRNA transfected cells determined by TaqMan based real time PCR. B) SaOS2 cells were transfected with control siRNA and p73 siRNA and at 48 hr post-transfection immunofluorescence was performed to detect the localization of p73 and VDR proteins as explained in materials and methods section (upper panel). Whole cell extracts prepared from SaOS2 cells were transfected with control siRNA or p73 siRNA were subjected to immunoblot analysis using VDR and β-actin specific antibodies. p73 was detected by immunoprecipitation followed by western as described in materials and methods (lower panel).
p73 activates the osteoblastic differentiation markers OPN and OCN through VDR
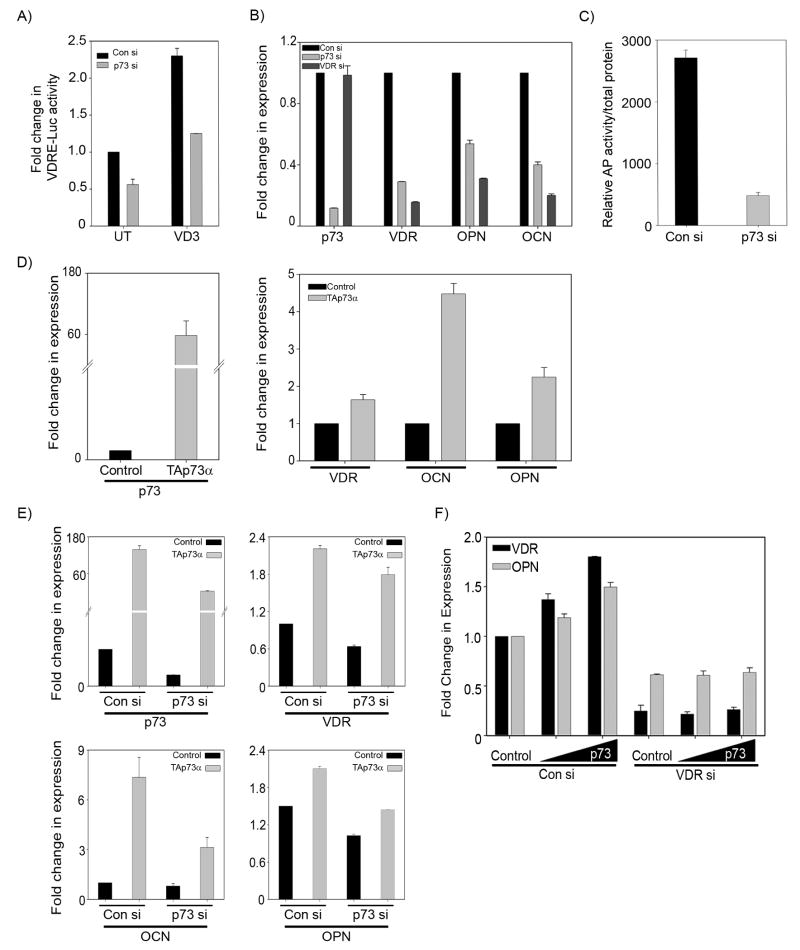
Since VD3-mediated osteoblastic differentiation is associated with the transcriptional activation of OPN and OCN through VDR, we next tested whether p73 affects the basal expression of OPN and OCN through VDR. To investigate this, we first examined whether p73 silencing affects VD3-mediated transcriptional activity in SaOS2 cells. This was examined by monitoring the VD3-mediated increase in VDRE-Luc reporter activity in the presence and absence of p73. The VDRE-Luc reporter bears four VDR responsive elements up-stream of a luciferase gene and is widely used for measuring VD3 mediated transcriptional activity. As expected, a significant increase in the VDRE-Luc activity was observed in cells transfected with control siRNA and treated with VD3 (Figure 2A). However, silencing p73 led to a significant reduction in VD3-mediated VDRE-Luc activity (Figure 2A). Next, we examined the transcript levels of OCN and OPN in SaOS2 cells transfected with control siRNA, p73 siRNA, or VDR siRNA (Figure 2B). As seen earlier in Figure 1A, a significant reduction in the transcript levels of p73 and VDR were observed with p73 siRNA (Figure 2B). In addition, silencing p73 also led to a significant reduction in basal OPN and OCN expression levels (Figure 2B). In order to determine whether p73 is able to induce differentiation markers OPN and OCN through VDR, we monitored the expression of OPN and OCN after silencing VDR. We observed that when VDR is silenced the expression of both OPN and OCN is reduced compared to control siRNA treated cells (Figure 2B). This confirms that p73 mediated VDR induction is required for induction of OPN and OCN. Since alkaline phosphatase has been shown to be regulated by VD3 and its activity is used as a marker for measuring the early stages of differentiation in osteoblasts, we examined whether silencing p73 affects alkaline phosphatase activity. We observed that upon silencing p73, the basal alkaline phosphatase activity was significantly reduced in SaOS2 cells further supporting that p73 contributes to osteoblastic differentiation (Figure 2C).
Figure 2. Silencing p73 leads to a down regulation in vitamin D transcriptional activity and VDR downstream targets.
A) SaOS2 cells transfected with either control siRNA or p73 siRNA were transfected with VDRE-Luc construct along with Renilla luciferase construct followed by treatment with 10 nM VD3 for 48 hr. Dual luciferase assays were performed to detect the VDRE-Luc activity and Y-axis represents the fold change in VDRE-Luc activity compared to control siRNA transfected cells treated with vehicle. Error bars represent standard deviation from the mean. B) SaOS2 cells were transfected twice with either control siRNA, p73 siRNA or VDR siRNA. Total RNA was harvested at 48 hr and subjected to TaqMan based real time PCR to detect the endogenous transcript levels of VDR, p73, OPN and OCN. Y-axis represents the fold change in transcript levels compared to control siRNA transfected cells. Error bars represent standard deviation from the mean. C) SaOS2 cells were transfected with control or p73 siRNA and after 36 hrs subjected to two freeze-thaw cycles for whole cell lysate extraction. Alkaline phosphatase activity was measured and normalized to total protein. D) SaOS2 cells were transfected with control vector or TAp73α expression plasmid and 24 hr post transfection transcript levels of p73, VDR, OCN and OPN were determined. Y-axis represents the fold change in transcript levels of p73, VDR, OCN and OPN compared to control vector transfected cells. Error bars represent standard deviation from the mean. E) SaOS2 cells were transfected twice with control siRNA and p73 siRNA and 24 hr post siRNA transfections, both control siRNA and p73 siRNA cells were transfected with either control vector or TAp73α expression plasmid as indicated. After 24 hrs total RNA was harvested for determination of p73, VDR, OCN and OPN transcript levels. Y-axis represents the fold change in transcript levels of p73, VDR, OCN and OPN compared to control vector transfected cells with control siRNA. Error bars represent standard deviation from the mean. F) SaOS2 cells transfected with control siRNA or VDR siRNA were transfected with vector control or increasing amounts of TAp73α. At 24 hr post-transfection, total RNA was extracted and TaqMan based real time PCR was performed to detect transcript levels of VDR and OPN. Y-axis represents the fold change in the transcript levels compared to control treated cells.
Since silencing p73 led to the down regulation in OPN and OCN, we next tested whether ectopic p73 activates VDR, OPN and OCN expression in SaOS2 cells. SaOS2 cells were transfected with control vector or TAp73α expression plasmid and transcript levels of VDR, OCN and OPN were monitored. We employed TAp73α expression for these studies, since it is the predominant isoform observed in SaOS2 cells (21). Overexpression of TAp73α was confirmed by measuring the p73 transcript (Figure 2D). A marked increase in VDR, OCN and OPN transcripts were observed in TAp73α transfected cells compared to control vector transfected cells (Figure 2D). To confirm the specificity of TAp73α mediated activation of VDR, OCN and OPN expression, we rescued the effects of silencing p73 by overexpressing TAp73α. SaOS2 cells transfected with control siRNA or p73 siRNA were transfected with either control vector or TAp73α expression plasmid and the expression of VDR, OCN and OPN was monitored. As expected, silencing p73 led to a significant reduction of p73, VDR, OCN and OPN transcript levels (Figure 2E). Interestingly, re-expression of TAp73α in p73 siRNA transfected cells reversed the inhibition of VDR, OCN, and OPN transcript levels observed upon p73 silencing (Figure 2E). Furthermore to unequivocally demonstrate that p73 exerts its effect on differentiation via VDR, SaOS2 cells transfected with either con si or VDR si were transfected with increasing doses of TAp73α expression (Figure 2F). We observed that cells transfected with con si followed by transfection with TAp73α led to a dose dependent increase in VDR as well OPN expression while TAp73α was unable to rescue the expression of OPN in cells in which VDR was silenced. Taken together, these results suggest that in SaOS2 cells p73 regulates the expression of OCN and OPN through VDR.
p73 is required for vitamin D mediated osteoblastic differentiation
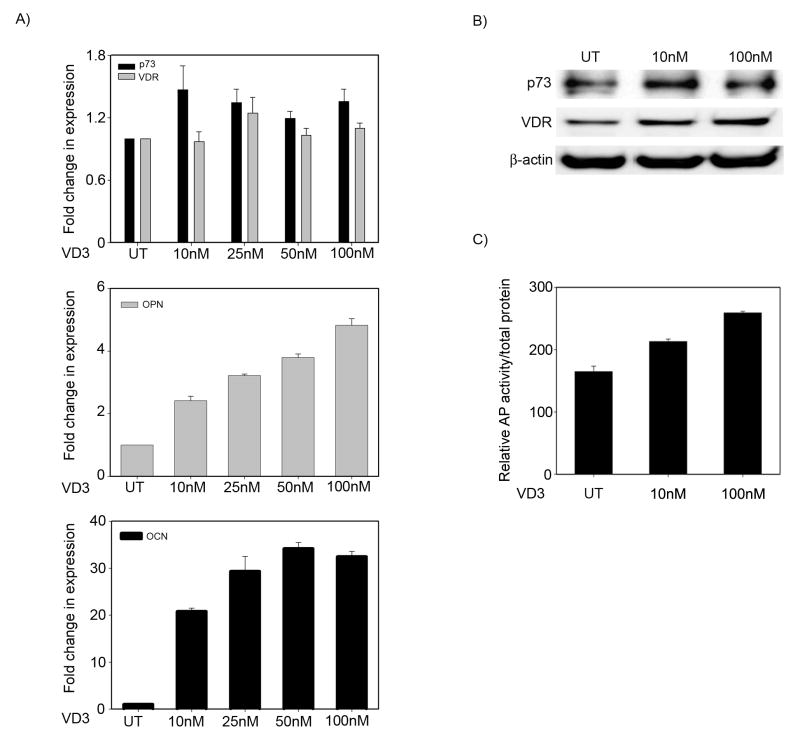
Since VD3 and VDR primarily regulate OCN and OPN genes to promote osteoblastic differentiation and p73 knockdown also led to a decrease in basal VDR, OCN, and OPN expression levels in osteosarcoma cells, we next examined whether loss of p73 affects VD3-mediated osteoblastic differentiation. To investigate this we first studied whether VD3 has any effect on endogenous p73 in SaOS2 cells. SaOS2 cells were treated with increasing concentrations of VD3 for 48 hours, and the transcript levels of p73, OCN and OPN were examined, the latter two were used as positive controls for VD3-mediated genomic effects. As expected, a dose dependant induction in both OCN and OPN (Figure 3A) transcript levels were observed with increasing concentrations of VD3. We did not, however, observe a consistently significant increase in either p73 or VDR transcript upon treatment with VD3, indicating that p73 and VDR are not transcriptionally regulated by VD3 in osteosarcoma cells (Figure 3A).
Figure 3. Vitamin D has no effect on endogenous p73 expression.
A) SaOS2 cells were treated with either vehicle (UT) or increasing concentrations of VD3 as indicated. At 48 hr post-treatment, total RNA was extracted and TaqMan based real time PCR was performed to detect the transcript levels of p73 and OPN and OCN. Y-axis represents the fold change in the transcript levels compared to vehicle treated cells. Error bars represent standard deviation from the mean. B) Cells were treated with vehicle or increasing concentrations of VD3 and at 48 hr post-treatment, protein was harvested and immunoprecipitated for p73. Both immunoprecipitated and non-immunoprecipitated samples were then subjected to western blot analysis for p73, VDR, and β-actin. C) SaOS2 cells were treated with vehicle or two different concentrations of VD3. Y-axis represents the relative AP activity normalized to total protein. Error bars represent standard deviation from the mean.
Interestingly, when effects of VD3 on p73 and VDR were studied at the protein level, we observed a slight increase in both VDR as well as p73 expression upon VD3 treatment (Figure 3B). Previous studies have shown that binding of VD3 to VDR contributes to stabilization of VDR at the protein level, which is consistent with our findings in SaOS2 cells (22). Although these results suggest the VD3 may have a modest effect on p73, this effect does not significantly contribute to the up-regulation seen in VDR upon VD3 treatment since there were no changes in VDR transcript levels by VD3. Finally, we demonstrated that VD3 treatment results in a dose dependent increase in alkaline phosphatase activity of SaOS2 cells, another measure of osteoblastic differentiation (Figure 3C).
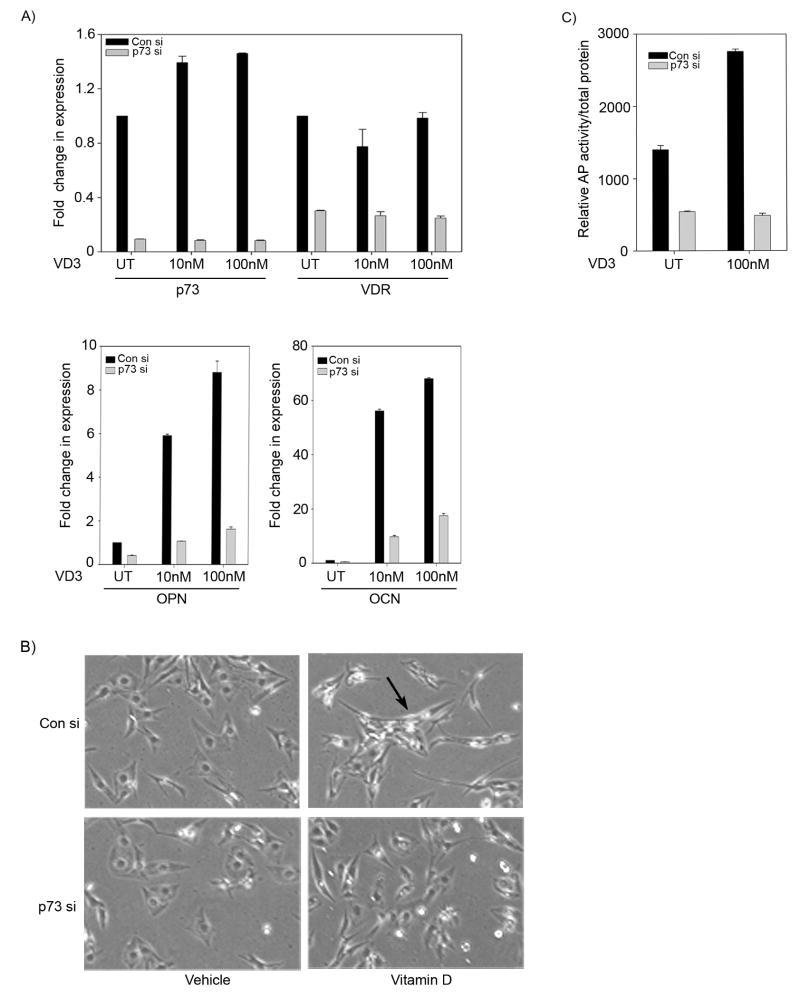
Next, to determine whether p73 plays a role in VD3-mediated osteoblastic differentiation of SaOS2 cells, we studied expression of OPN and OCN in SaOS2 cells treated with VD3 in the presence and absence of p73. As expected, p73 knockdown led to a significant decrease in p73 as well as VDR transcript levels (Figure 4A). A significant increase in OCN and OPN (Figure 4A) expression levels were observed in cells transfected with control siRNA and treated with either 10 nM or 100 nM of VD3 for 48 hrs, indicating the onset of SaOS2 cells differentiation. However, silencing p73 not only led to a decrease in VDR expression levels, but also showed a significant reduction in transcript levels of both OCN and OPN suggesting that the basal levels of VDR, under the control of endogenous p73, are sufficient for VD3 induction of OCN and OPN (Figure 4A). In addition, we also monitored the VD3-mediated morphological changes of SaOS2 cells observed upon differentiation process. We observed that cells transfected with control siRNA and treated with 10 nM VD3 exhibited a significant number of cells with elongated cytoplasmic processes and flattened fibroblastic appearance indicative of differentiation (Figure 4B, marked by an arrow). However, silencing p73 led to a reduction in the number of cells with differentiation features that were observed upon VD3 treatment (Figure 4B).
Figure 4. p73 is required for vitamin D mediated osteoblastic differentiation.
A) SaOS2 cells transfected with control siRNA or p73 siRNA were harvested and re-plated onto 6 well plates. Next day, cells were treated for 48 hr post vehicle or 10 nM or 100 nM VD3 and total RNA was extracted and transcript levels of p73, VDR, OPN and OCN were determined. Y-axis represents the fold change in transcript levels compared to control siRNA transfected cells with vehicle treatment. Error bars represent standard deviation from the mean. B) SaOS2 cells transfected with control siRNA or p73 siRNA were split and re-plated onto 6 wells. Next day, cells were treated with vehicle or 10 nM VD3 as indicated and at 48 hr post VD3 treatment morphological changes in cells were observed by phase contrast microscopy. C) SaOS2 cells were transfected with control or p73 siRNA and subsequently treated with vehicle or 100 nM VD3 for 24 hrs. Whole cell lysates were then collected and alkaline phosphatase activity was measured. Y-axis represents the relative AP activity normalized to total protein.
We next investigated whether silencing p73 could affect the VD3 mediated increase in alkaline phosphatase activity. We transfected SaOS2 cells with control or p73 siRNA, and subsequently treated the cells with vehicle or VD3. As expected cells transfected with control siRNA showed an increase in alkaline phosphatase activity when treated with VD3, which was significantly down-regulated in cells in which p73 was silenced (Figure 4C). These results clearly indicate that p73 is required for VD3 mediated differentiation of SaOS2 osteosarcoma cell lines.
DNA damage enhances vitamin D mediated osteoblastic differentiation via p73
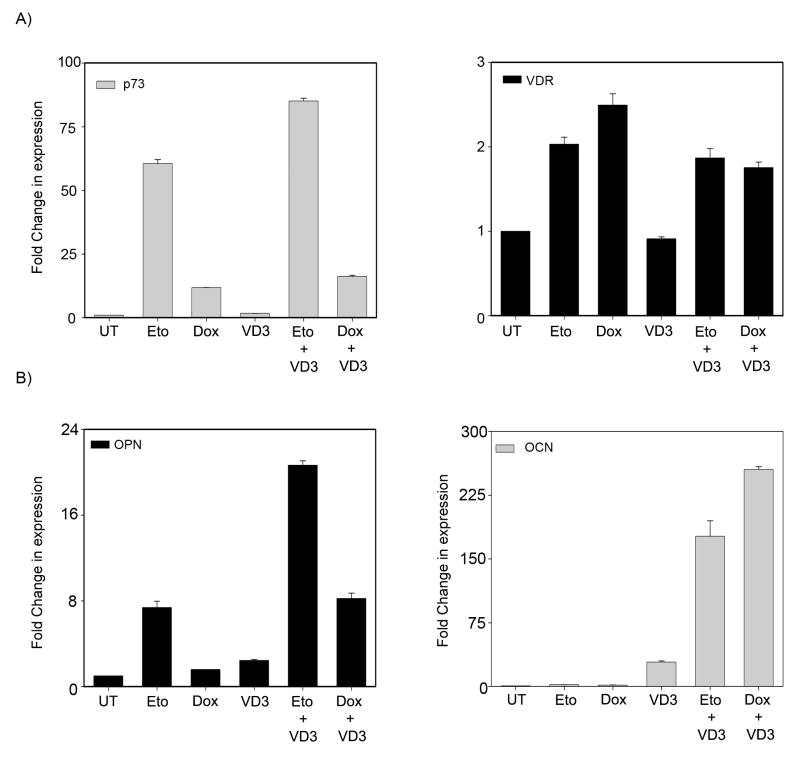
We had previously demonstrated that p73 is essential for DNA damage induced VDR expression and subsequent enhancement of VD3 transcriptional activity in H1299, a small cell lung carcinoma cell line (14). To test whether p73 mediated induction of VDR expression levels upon DNA damage can potentiate VD3-mediated differentiation of SaOS2 cells; we first verified whether DNA damage can enhance VD3 mediated induction of OCN and OPN expression levels. SaOS2 cells pretreated with etoposide or doxorubicin for 24 hrs were cultured in 10nM VD3 for 2 days. Both VDR and p73 transcript levels were significantly induced upon treatment with etoposide or doxorubicin (Figure 5A). As expected, increased transcript levels of both OPN and OCN were observed in cells treated with 10 nM VD3 alone (Figure 5B). Interestingly, cells pre-treated with etoposide or doxorubicin and cultured in VD3 showed a further increase in both OPN and OCN expression levels when compared with VD3 treatment alone (Figure 5B). These results suggest that increased p73 and VDR expression observed upon DNA damage can lead to an increase in VD3 mediated differentiation.
Figure 5. DNA damage enhances vitamin D mediated osteoblastic differentiation.
A) SaOS2 cells were pretreated for 24 hr with 4 μM etoposide or 4 μM doxorubicin and subsequently cultured with vehicle or 10 nM VD3 for an additional 48 hr. Total RNA was extracted and transcript levels of p73 & VDR (A) and OPN & OCN (B) were determined by TaqMan based real time PCR. Y-axis represents the fold change in transcript levels compared to vehicle treated (UT) cells. Error bars represent standard deviation from the mean.
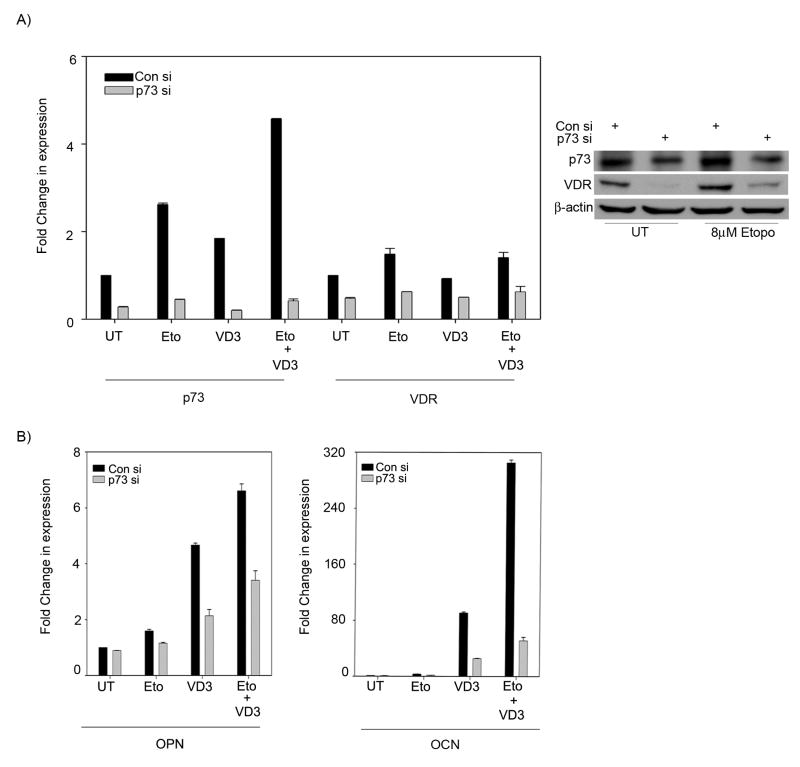
To confirm that p73 is essential for DNA damage-mediated enhancement in VD3-mediated differentiation, the transcript levels of OPN and OCN were examined in the presence and absence of p73 in cells pre-treated with etoposide followed by culturing in VD3. p73 silencing led to a reduction of VDR expression levels in cells treated with etoposide alone or in combination with VD3, indicating that p73 is essential for induction of VDR upon DNA damage in these cells (Figure 6A). Silencing p73 also led to a significant decrease in OPN and OCN expression levels in cells treated with VD3 alone or in combination with etoposide (Figure 6B). Additionally, cells transfected with p73 siRNA showed a reduced VDR expression at the protein level compared to control siRNA treated cells in absence of etoposide (Figure 6A right panel). However, etoposide treated cells even in presence of p73 siRNA showed considerable levels of VDR protein, suggesting that stabilized p73 protein upon DNA damage can induce VDR expression (Figure 6A right panel). Together, these results clearly indicate that induction of VDR by p73 upon DNA damage can enhance VD3-mediated differentiation of SaOS2 cells.
Figure 6. DNA damage potentiates vitamin D mediated differentiation via p73.
A) SaOS2 cells were transfected with either control siRNA or p73 siRNA as indicated and treated with 4 μM etoposide or left untreated as indicated. Next day, cells were either cultured in vehicle or 10 nM VD3 for additional 48 hr as indicated. Transcript levels of p73 & VDR were determined. Total protein was extracted and subjected to immunoprecipitation for p73 detection or to standard Western blot analysis for VDR and β-actin. B) Transcript levels of OPN & OCN were determined as in (A) after siRNA, drug and VD3 treatment. Y-axis represents the fold change in transcript levels compared to control siRNA transfected cells treated with vehicle. Error bars represent standard deviation from the mean.
Discussion
Osteoblasts are the main bone forming cells, which are essential for deposition of bone extracellular matrix. Osteoblasts are derived from pluripotent mesenchymal cells during embryonic development. A characteristic feature of osteoblasts is that they can be cultured in vitro to proliferate and differentiate (23). Osteoblastic differentiation is essential for proper bone formation and is mediated by multiple factors including VDR (24). The role of the VD3 in bone formation and in osteoblastic differentiation is well documented (25). Vitamin D3 has been shown to promote differentiation of mature osteoblasts through the regulation of osteoblast associated genes including osteocalcin and osteopontin (26). Osteosarcomas are the most common type of bone cancers, retaining characteristics of primary osteoblasts and are widely used for studying osteoblasts (27). Vitamin D3 and its analogues have been shown to promote the differentiation of osteosarcoma cells (28, 29). Although, VD3 exerts its biological functions on osteoblasts or osteosarcoma cells, the upstream regulation of the VD3 pathway in osteoblasts is still understudied. In this study, we demonstrated that silencing p73 resulted in down regulation of both basal VDR expression and VD3 mediated transcriptional activity in SaOS2 osteosarcoma cells (Figures 1 and 2). In SaOS2 cells ectopic TAp73α induced the expression of VDR, OCN and OPN while silencing endogenous p73 led to a significant down regulation in VDR, OCN and OPN expression levels, which were rescued by p73 overexpression (Figure 2). These results demonstrate that in osteosarcoma cells p73 modulates OCN and OPN expression by activating VDR.
Furthermore, down regulation of p73 resulted in significant down regulation in VD3 mediated induction of OPN and OCN and the differentiation of SaOS2 cells (Figure 4). These results suggest a novel upstream regulation of the vitamin D signaling pathway by p73 in osteosarcoma cells. Transforming growth factor β (TGF-β) has also been shown to play a vital role in bone remodeling (30). In particular, TGF-β has been shown to promote the proliferation of primary osteoblasts as well as shown to suppress the VD3-mediated induction of osteocalcin (31). Interestingly, transcriptional repression of TAp73 by TGF-β has been reported in transformed keratinocytes (32). Thus, it is likely that TGF-β inhibits the VD3 mediated differentiation of osteoblasts by repressing p73. Furthermore, given both VDR and p73 null mice are runted in phenotype, it will be interesting to see whether regulation of VD3 functions by p73 is essential for bone formation during embryonic development (15, 25).
Vitamin D3 and its analogues exert anti proliferative actions in different cancer cells through VDR. Since expression of p73 has been reported in the majority of human cancer cells, results from our study warrant further investigation into whether p73 is essential for VDR expression and subsequently for VD3 mediated biological functions in all VD3 responsive human cancer cell lines (33). Interestingly, adenoviral based transfer of p53 family members has been shown to promote apoptosis and cell cycle arrest in multiple cancer cell lines (34, 35). Therefore, adenoviral based gene therapy of p73 in combination with vitamin D could be used as an effective therapeutic strategy against some human cancers including osteosarcomas.
Combinatorial use of VD3 and its analogues with chemotherapeutic agents have been shown to be more potent in promoting growth inhibition than using them alone (24, 36, 37). Clinical trials are currently underway testing the combinatorial use of VD3 analogues and chemotherapeutic agents for cancer chemotherapy (38, 39). Interestingly, a wide range of chemotherapeutic agents have been shown to induce the p73 expression levels in multiple cancer cells (20, 40). Results from our study demonstrated that regulation of VDR by p73 upon DNA damage can potentiate VD3-mediated differentiation (Figure 5 & 6). Thus, these findings demonstrate a novel upstream regulation of the vitamin D signaling pathway by p73 and provide evidence for the requirement of p73 for VD3-mediated osteoblastic differentiation. Taken together, results from this study provide an insight into the combinatorial use of chemotherapeutic agents with vitamin D3 for cancer treatment, in particular, oseosarcoma.
Materials and Methods
Cell lines and sequences for siRNA
Human osteosarcoma cell line SaOS2 was obtained from ATCC and maintained in Dulbecco's modified eagle medium (DMEM) with 10% Fetal Bovine calf Serum (FBS) and 250 U of Penicillin and 250 μg of Streptomycin (PS). Sequences of siRNA oligonucleotides purchased from Qiagen (Qiagen, Vlencia, CA) were, p73_1 siRNA, sense CGGGAUGCUCAACAACCAUtt and antisense rAUGGUUGUUGAGCAUCCCGgg and p73_2 siRNA, sense CGUGACCGACGUCGUGAAAtt and antisense gtGCACUGGCUGCAGCACUUU. Sequences used for VDR siRNA are as follows, Sense 5′-GCGUCAGUGACGUGACCAAtt-3′ and antisense: 3′ggCGCAGUCACUGCACUGGUU -5′.
RNA extraction and RT-qPCR Analysis
Total RNA from cells was extracted by using eZNA RNA isolation kit (Omega Bio-tek, Norcross, GA) and 1μg of total RNA was used to synthesize cDNA by using TaqMan reverse transcription kit (Applied Biosystems, Foster city, CA). Quantitative Real-Time PCR analysis was performed by using TaqMan 2× master mix and Assays on Demand (AOD) specific for VDR (Hs_ 0017213_m1), p73 (Hs_ 00232088_m1), Osteopontin (OPN) (Hs_ 00167093_m1), and Osteocalcin (OCN) (Hs_01587813_m1) (PE Applied Biosystems, Foster City, CA).
Calcium phosphate based transfections for siRNA and Transactivation assays
For siRNA transfection, cells were seeded prior to day of transfection and at around 30- 40% confluency and siRNA oligonucleotides were transfected by using calcium phosphate method as described earlier (14). After 24 hr another round of siRNA transfections were carried out and after 48 hr cells were processed for further analysis. For VDRE-Luc transactivation assays, SaOS2 cells transfected with either control siRNA or p73 siRNA were subsequently transfected with 250 ng of VDRE-Luc construct along with Renilla luciferase construct using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). At 6 hr post transfection, cells were treated with vehicle control (UT) or 100 nM VD3 in charcoal treated media. At 48 hr, cells were harvested in PBS Lysis buffer and Dual luciferase assays were performed as per manufacture protocol (Promega, Madison, WI).
Immunoflourescense Assay
SaOS2 cells were grown on coverslips and transfected with either control siRNA or p73 siRNA and 48 hr post-transfection cells were subjected to an immunofluorescence assay. Briefly, cells were fixed with 2% paraformaldehyde and permeabilized in 0.2% Triton-X detergent. Cells were washed and blocked in PBS with 0.5% normal goat serum then incubated with mouse monoclonal anti-VDR D-6 (Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit polyclonal anti-p73 A300 (Bethyl Laboratories Inc, Montgomery, TX) antibodies. Subsequently, cells were subjected to dual immunostaining using Texas Red-conjugated anti-mouse and FITC-conjugated anti-rabbit antibodies (Jackson Laboratories, Pennsylvania, USA) and nuclei were visualized by 4,6-diamidino-2-phenylindole. Images were captured using a Leica 6000B inverted microscope.
Western Blot and Immunoprecipitation
Whole cell lysates were prepared by lysing the cells in a high salt buffer (300 mM NaCl, 100 mM Tris pH=8, 0.2 mM EDTA, 0.1% NP40, 10% glycerol) to enrich for nuclear proteins. Total protein amounts were measured using BCA (Pierce, Rockford, IL) protein detection method and equivalent amounts of protein extracts were fractionated by 10% SDS-PAGE and transferred onto polyvinylidene difluoride membranes and blocked with 5% blocking milk solution. Membranes were subsequently immunoblotted with antibodies to detect specific proteins. Monoclonal anti-VDR D-6 (Santa Cruz Biotechnology) and monoclonal anti-β-actin (Sigma, St. Louis, MO) antibodies were used to detect VDR and β-actin expression, respectively. Appropriate horseradish peroxidase-conjugated secondary antibodies (Promega, Madison, WI) were used for chemiluminescence detection with Supersignal Westpico Chemiluminescent Substrate kit (Pierce).
For immunoprecipitation studies, SaOS2 cell extracts were harvested as described above and 1mg of protein was diluted to a final concentration of 100 mM NaCl. This was precleared with 25 μl of Protein G-Sepharose beads for an hour followed by incubation with 1 μg each of p73 monoclonal antibodies Ab-4 (Thermo Fisher Scientific Inc., Fremont, CA) and IMG-259 (Imgenex, San Diego, CA) for 1 hour at 4°C. Protein G-Sepharose beads were added again and the mixture incubated an additional 2 hours at 4°C. The beads were pelleted by centrifugation, and washed 3 times with 100 mM NaCl buffer (100 mM NaCl, 100 mM Tris pH=8, 0.2 mM EDTA, 0.1% NP40, 10% glycerol). The isolated beads were resuspended in 70 uL 100 mM NaCl buffer, boiled for 5 min at 90°C and resolved by Western blot analysis as described above with rabbit polyclonal anti-p73 A300 antibody (Bethyl Laboratories).
Alkaline Phosphatase Assay
Alkaline phosphatase activity was performed as previously described (41). Briefly, cells were washed with PBS and lysed with 0.1% Triton X-100 (Sigma). The lysates were then subjected to two freeze-thaw cycles and cleared via centrifugation. 2 mg/ml of p-nitrophenol phosphate in an alkaline buffer (pH=8) (Sigma) was added to the lysates and the reaction stopped by adding 20 mM NaOH. The conversion of p-nitrophenol phosphate to p-nitrophenol was measured at 405 nM (Safire2, Tecan, San Jose, CA) and normalized to total protein.
Acknowledgments
We would like to thank Stefanie Lewis for technical assistance. This work was supported by a grant from the NCI/NIH (CA118315-2) to M.K.
Abbreviations
- VDR
Vitamin D receptor
- VDRE
Vitamin D responsive elements
- VD3
1,25 dihydroxy-vitamin D3
- OCN
osteocalcin
- OPN
osteopontin
References
- 1.Bikle DD. Vitamin D and skin cancer. J Nutr. 2004;134(12 Suppl):3472S–3478S. doi: 10.1093/jn/134.12.3472S. [DOI] [PubMed] [Google Scholar]
- 2.Cooke NE, Haddad JG. Vitamin D binding protein (Gc-globulin) Endocr Rev. 1989;10(3):294–307. doi: 10.1210/edrv-10-3-294. [DOI] [PubMed] [Google Scholar]
- 3.Nezbedova P, Brtko J. 1alpha,25-dihydroxyvitamin D3 inducible transcription factor and its role in the vitamin D action. Endocr Regul. 2004;38(1):29–38. [PubMed] [Google Scholar]
- 4.Campbell MJ, Adorini L. The vitamin D receptor as a therapeutic target. Expert Opin Ther Targets. 2006;10(5):735–48. doi: 10.1517/14728222.10.5.735. [DOI] [PubMed] [Google Scholar]
- 5.Bouillon R, Eelen G, Verlinden L, Mathieu C, Carmeliet G, Verstuyf A. Vitamin D and cancer. J Steroid Biochem Mol Biol. 2006;102(1-5):156–62. doi: 10.1016/j.jsbmb.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Huang YC, Chen JY, Hung WC. Vitamin D3 receptor/Sp1 complex is required for the induction of p27Kip1 expression by vitamin D3. Oncogene. 2004;23(28):4856–61. doi: 10.1038/sj.onc.1207621. [DOI] [PubMed] [Google Scholar]
- 7.Liu M, Lee MH, Cohen M, Bommakanti M, Freedman LP. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev. 1996;10(2):142–53. doi: 10.1101/gad.10.2.142. [DOI] [PubMed] [Google Scholar]
- 8.Banerjee P, Chatterjee M. Antiproliferative role of vitamin D and its analogs--a brief overview. Mol Cell Biochem. 2003;253(1-2):247–54. doi: 10.1023/a:1026072118217. [DOI] [PubMed] [Google Scholar]
- 9.Finch JL, Dusso AS, Pavlopoulos T, Slatopolsky EA. Relative potencies of 1,25-(OH)(2)D(3) and 19-Nor-1,25-(OH)(2)D(2) on inducing differentiation and markers of bone formation in MG-63 cells. J Am Soc Nephrol. 2001;12(7):1468–74. doi: 10.1681/ASN.V1271468. [DOI] [PubMed] [Google Scholar]
- 10.Paredes R, Arriagada G, Cruzat F, Olate J, Van Wijnen A, Lian J, et al. The Runx2 transcription factor plays a key role in the 1alpha,25-dihydroxy Vitamin D3-dependent upregulation of the rat osteocalcin (OC) gene expression in osteoblastic cells. J Steroid Biochem Mol Biol. 2004;89-90(1-5):269–71. doi: 10.1016/j.jsbmb.2004.03.076. [DOI] [PubMed] [Google Scholar]
- 11.Shen Q, Christakos S. The vitamin D receptor, Runx2, and the Notch signaling pathway cooperate in the transcriptional regulation of osteopontin. J Biol Chem. 2005;280(49):40589–98. doi: 10.1074/jbc.M504166200. [DOI] [PubMed] [Google Scholar]
- 12.Zenmyo M, Komiya S, Hamada T, Hiraoka K, Kato S, Fujii T, et al. Transcriptional activation of p21 by vitamin D(3) or vitamin K(2) leads to differentiation of p53-deficient MG-63 osteosarcoma cells. Hum Pathol. 2001;32(4):410–6. doi: 10.1053/hupa.2001.23524. [DOI] [PubMed] [Google Scholar]
- 13.Kommagani R, Caserta TM, Kadakia MP. Identification of vitamin D receptor as a target of p63. Oncogene. 2006;25(26):3745–51. doi: 10.1038/sj.onc.1209412. [DOI] [PubMed] [Google Scholar]
- 14.Kommagani R, Payal V, Kadakia MP. Differential regulation of vitamin D receptor (VDR) by the p53 Family: p73-dependent induction of VDR upon DNA damage. J Biol Chem. 2007;282(41):29847–54. doi: 10.1074/jbc.M703641200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang A, Walker N, Bronson R, Kaghad M, Oosterwegel M, Bonnin J, et al. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature. 2000;404(6773):99–103. doi: 10.1038/35003607. [DOI] [PubMed] [Google Scholar]
- 16.Kaghad M, Bonnet H, Yang A, Creancier L, Biscan JC, Valent A, et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90(4):809–19. doi: 10.1016/s0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- 17.Stiewe T, Zimmermann S, Frilling A, Esche H, Putzer BM. Transactivation-deficient DeltaTA-p73 acts as an oncogene. Cancer Res. 2002;62(13):3598–602. [PubMed] [Google Scholar]
- 18.Melino G, De Laurenzi V, Vousden KH. p73: Friend or foe in tumorigenesis. Nat Rev Cancer. 2002;2(8):605–15. doi: 10.1038/nrc861. [DOI] [PubMed] [Google Scholar]
- 19.Lissy NA, Davis PK, Irwin M, Kaelin WG, Dowdy SF. A common E2F-1 and p73 pathway mediates cell death induced by TCR activation. Nature. 2000;407(6804):642–5. doi: 10.1038/35036608. [DOI] [PubMed] [Google Scholar]
- 20.Irwin MS, Kondo K, Marin MC, Cheng LS, Hahn WC, Kaelin WG., Jr Chemosensitivity linked to p73 function. Cancer Cell. 2003;3(4):403–10. doi: 10.1016/s1535-6108(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Zheng Y, Zhu J, Jiang J, Wang J. p73 is transcriptionally regulated by DNA damage, p53, and p73. Oncogene. 2001;20(6):769–74. doi: 10.1038/sj.onc.1204149. [DOI] [PubMed] [Google Scholar]
- 22.Healy KD, Frahm MA, DeLuca HF. 1,25-Dihydroxyvitamin D3 up-regulates the renal vitamin D receptor through indirect gene activation and receptor stabilization. Arch Biochem Biophys. 2005;433(2):466–73. doi: 10.1016/j.abb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Stein GS, Lian JB, Stein JL, Van Wijnen AJ, Montecino M. Transcriptional control of osteoblast growth and differentiation. Physiol Rev. 1996;76(2):593–629. doi: 10.1152/physrev.1996.76.2.593. [DOI] [PubMed] [Google Scholar]
- 24.St-Arnaud R. The direct role of vitamin D on bone homeostasis. Arch Biochem Biophys. 2008;473(2):225–30. doi: 10.1016/j.abb.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 25.Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet. 1997;16(4):391–6. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto T, Igarashi C, Takeuchi Y, Harada S, Kikuchi T, Yamato H, et al. Stimulation by 1,25-dihydroxyvitamin D3 of in vitro mineralization induced by osteoblast-like MC3T3-E1 cells. Bone. 1991;12(1):27–32. doi: 10.1016/8756-3282(91)90051-j. [DOI] [PubMed] [Google Scholar]
- 27.Gill RK, Bell NH. Steroid receptor co-activator-1 mediates 1,25-dihydroxyvitamin D(3)-stimulated alkaline phosphatase in human osteosarcoma cells. Calcif Tissue Int. 2000;66(5):370–4. doi: 10.1007/s002230010075. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto T, Sowa Y, Ohtani-Fujita N, Tamaki T, Takenaka T, Kuribayashi K, et al. p53-independent induction of WAF1/Cip1 is correlated with osteoblastic differentiation by vitamin D3. Cancer Lett. 1998;129(1):61–8. doi: 10.1016/s0304-3835(98)00080-9. [DOI] [PubMed] [Google Scholar]
- 29.Barroga EF, Kadosawa T, Okumura M, Fujinaga T. Effects of vitamin D and retinoids on the differentiation and growth in vitro of canine osteosarcoma and its clonal cell lines. Res Vet Sci. 1999;66(3):231–6. doi: 10.1053/rvsc.1998.0265. [DOI] [PubMed] [Google Scholar]
- 30.Centrella M, McCarthy TL, Canalis E. Transforming growth factor-beta and remodeling of bone. J Bone Joint Surg Am. 1991;73(9):1418–28. [PubMed] [Google Scholar]
- 31.Kassem M, Kveiborg M, Eriksen EF. Production and action of transforming growth factor-beta in human osteoblast cultures: dependence on cell differentiation and modulation by calcitriol. Eur J Clin Invest. 2000;30(5):429–37. doi: 10.1046/j.1365-2362.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 32.Waltermann A, Kartasheva NN, Dobbelstein M. Differential regulation of p63 and p73 expression. Oncogene. 2003;22(36):5686–93. doi: 10.1038/sj.onc.1206859. [DOI] [PubMed] [Google Scholar]
- 33.Muller M, Schleithoff ES, Stremmel W, Melino G, Krammer PH, Schilling T. One, two, three--p53, p63, p73 and chemosensitivity. Drug Resist Updat. 2006;9(6):288–306. doi: 10.1016/j.drup.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Das S, Nama S, Antony S, Somasundaram K. p73 beta-expressing recombinant adenovirus: a potential anticancer agent. Cancer Gene Ther. 2005;12(4):417–26. doi: 10.1038/sj.cgt.7700803. [DOI] [PubMed] [Google Scholar]
- 35.Kunisaki R, Ikawa S, Maeda T, Nakazaki Y, Kurita R, Harata M, et al. p51/p63, a novel p53 homologue, potentiates p53 activity and is a human cancer gene therapy candidate. J Gene Med. 2006;8(9):1121–30. doi: 10.1002/jgm.945. [DOI] [PubMed] [Google Scholar]
- 36.Hershberger PA, McGuire TF, Yu WD, Zuhowski EG, Schellens JH, Egorin MJ, et al. Cisplatin potentiates 1,25-dihydroxyvitamin D3-induced apoptosis in association with increased mitogen-activated protein kinase kinase kinase 1 (MEKK-1) expression. Mol Cancer Ther. 2002;1(10):821–9. [PubMed] [Google Scholar]
- 37.Moffatt KA, Johannes WU, Miller GJ. 1Alpha,25dihydroxyvitamin D3 and platinum drugs act synergistically to inhibit the growth of prostate cancer cell lines. Clin Cancer Res. 1999;5(3):695–703. [PubMed] [Google Scholar]
- 38.Trump DL, Potter DM, Muindi J, Brufsky A, Johnson CS. Phase II trial of high-dose, intermittent calcitriol (1,25 dihydroxyvitamin D3) and dexamethasone in androgen-independent prostate cancer. Cancer. 2006;106(10):2136–42. doi: 10.1002/cncr.21890. [DOI] [PubMed] [Google Scholar]
- 39.Fakih MG, Trump DL, Muindi JR, Black JD, Bernardi RJ, Creaven PJ, et al. A phase I pharmacokinetic and pharmacodynamic study of intravenous calcitriol in combination with oral gefitinib in patients with advanced solid tumors. Clin Cancer Res. 2007;13(4):1216–23. doi: 10.1158/1078-0432.CCR-06-1165. [DOI] [PubMed] [Google Scholar]
- 40.Urist M, Tanaka T, Poyurovsky MV, Prives C. p73 induction after DNA damage is regulated by checkpoint kinases Chk1 and Chk2. Genes Dev. 2004;18(24):3041–54. doi: 10.1101/gad.1221004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ciovacco WA, Goldberg CG, Taylor AF, Lemieux JM, Horowitz MC, Donahue HJ, et al. The role of gap junctions in megakaryocyte-mediated osteoblast proliferation and differentiation. Bone. 2009;44(1):80–6. doi: 10.1016/j.bone.2008.08.117. [DOI] [PMC free article] [PubMed] [Google Scholar]