Abstract
We have previously shown that Toll-like receptor (TLR) agonists cooperate with CD40 to generate CD8 T cell responses exponentially larger than the responses generated with traditional vaccine formulations. We have also shown that combined TLR agonist/anti-CD40 immunization uniquely induces the upregulation of CD70 on antigen bearing dendritic cells (DCs). In contrast, immunization with either a TLR agonist or a CD40 stimulus alone does not significantly increase CD70 expression on DCs. Furthermore, the CD8+ T cell response generated by combined TLR agonist/anti-CD40 immunization is dependent on the expression of CD70 by DCs, as CD70 blockade following immunization dramatically decreases the CD8 T cell response. Here we show that other innate pathways, independent of the TLRs, can also cooperate with CD40 to induce potent, CD70 dependent, CD8 T cell responses. These innate stimuli include Type I IFN (IFN) and αgalactosylceramide (αGalCer) or αC-GalCer, glycolipids that are presented by a nonclassical class I MHC molecule, CD1d, and are able to activate NKT cells. Furthermore, this combined IFN/antiCD40 immunization generates protective memory against bacterial challenge with Listeria monocytogenes. Together these data indicate the importance of assessing CD70 expression on DCs as a marker for the capacity of a given vaccine formulation to potently activate cellular immunity. Our data indicate that optimal induction of CD70 expression requires a coordinated stimulation of both innate (TLR, IFN, αGalCer) and adaptive (CD40) signaling pathways.
Introduction
Toll-like receptor (TLR) stimulation elicits a pro-inflammatory response involving the induction of the transcription factor NFκB and MAP kinases, leading to the production of pro-inflammatory cytokines such as TNFα, IL-12, IL-6, and IL-1 by dendritic cells (DCs) and macrophages(1, 2). Triggering of TLRs 3, 4, 7, and 9 also leads to production of Type I Interferons (IFN), cytokines which have been shown to be important in the generation of adaptive immunity (3–10). Given the ability of TLRs to induce the production of these proinflammatory responses, TLR agonists are generally thought to be useful vaccine adjuvants (11). Indeed, TLR agonists are relatively successful at augmenting humoral immune responses (12), However, when used to enhance the generation of cellular immune responses, TLR agonists by themselves have largely been a disappointment (13–22). Indeed, when used alone as a vaccine adjuvant, we previously showed that they are unable to generate cellular immune responses capable of protecting against viruses or other intracellular infections (23).
The paucity with which TLR agonists initiate cellular immunity reveals the fact that the precise quantity and/or quality of DC maturation that promotes the highest magnitude T cell response is still poorly understood. In general there is a prevailing view that “more is better”. Beyond that however, a precise characterization of how much of which costimulatory molecules are required for optimal T cell expansion is as of yet unknown, a somewhat surprising fact given the degree of effort that is put into investigating both DC and T cell activation. CD70 is a costimulatory marker expressed primarily by antigen presenting cells (APCs)(24). It is a member of the TNF ligand superfamily and binds to its receptor CD27, which is expressed mainly on T cells but is also expressed on B cells and NK cells. CD70 has been shown to be important for the priming of CD8 T cell responses(23, 25–32) and blockade of CD70/CD27 interactions often dramatically reduces the generated CD8 T cell response(23, 25, 26, 28–30). Importantly, CD70 has also been shown to be upregulated on APCs during some natural infections, and is known in this setting to be important for CD8 T cell priming(30). Additionally, the constitutive expression of CD70 on immature DCs is enough to overcome tolerance and prime CD8+ T cells that can infiltrate into tumor sites and induce tumor regression(27). Given the potency of CD70 for inducing cellular immunity, an established method for inducing its expression on DCs would be of great benefit for the purposes of vaccine development.
Toward this end, we previously demonstrated that immunization with both a TLR agonist and an agonistic antiCD40 antibody induces a degree of CD8+ T cell expansion that is 10–50 fold greater than that observed in response to immunization in the presence of either agonist alone(14, 23). The magnitude of these T cell responses often matches or even exceeds that seen in response to infectious agents such as LCMV or Listeria monocytogenes (18–21). When comparing the phenotype of dendritic cells stimulated with a TLR agonist, anti-CD40, or both, we observed that upregulation of the TNF ligand superfamily member CD70 on both CD8 and CD11b DC subsets was unique to only the combined TLR agonist/antiCD40 stimulus(23). The CD8 T cell response generated by the combined TLR agonist/antiCD40 stimulus was dependent on this DC CD70 expression, since blocking CD70 from its receptor CD27, dramatically reduced the CD8 T cell response. Thus, CD70 expression in vivo is uniquely regulated by the combined stimulation of a TLR and CD40.
Here we show that the innate signaling pathways able to elicit the generation of potent CD70-dependent CD8 T cell responses in combination with CD40 are not limited to the TLRs. We demonstrate that Type I IFN (IFN) or NKT ligands (αGalCer or αC-GalCer) similarly induce the upregulation of CD70 on DCs when used in combination with antiCD40, leading to the exponential expansion of antigen specific T cells. While αGalCer alone can induce an increase in CD70 expression on DCs in vivo(29), maximal CD70 expression, leading to maximal CD8+ T cell expansion, is induced only when used in combination with antiCD40 antibody. In contrast to αGalCer but similar to the TLR agonists(23), IFN alone induces no CD70 expression at all, but synergizes effectively with anti-CD40 to induce CD70 upregulation and the subsequent induction of CD8+ T cell memory that is protective against infectious challenge. Therefore, multiple innate pathways (TLRs, Type I IFN, NKT agonists) are able to work in synergy with CD40 to generate large CD8 T cell responses through a CD70-dependent mechanism, demonstrating the importance of CD70 as a marker in identifying vaccine strategies with efficacy in generating cellular immunity.
Results
Type I IFN and α-C-GalCer can work synergistically with anti-CD40 to generate enhanced CD8 T cell responses
Combined Toll-like receptor (TLR) and CD40 stimulation along with specific antigen can induce a synergistic enhancement of the specific CD8 T cell response (14, 23). A curious feature of the CD8+ T cell responses following this immunization is its variable dependence on IFN (14). TLR agonists that induce IFN (for TLRs 3, 7, 9), generate a CD8+ T cell response that is highly IFN-dependent. In contrast, for TLR agonists that do not induce IFN (for TLRs 2, 1/2, 2/6), the ensuing CD8+ T cell response is IFN-independent. The simplest explanation for this observation is that, following its production from an IFN-inducing TLR stimulation, the IFN is actually responsible for synergizing with antiCD40 for the induction of such robust cellular immunity. This hypothesis predicts that immunization with combined IFN and anti-CD40 might produce the same exponential expansion of CD8+ T cells as a combined TLR agonist and anti-CD40 immunization. To test this hypothesis, we determined if recombinant IFN could replace the TLR agonist and act in combination with CD40 to generate CD8 T cell responses.
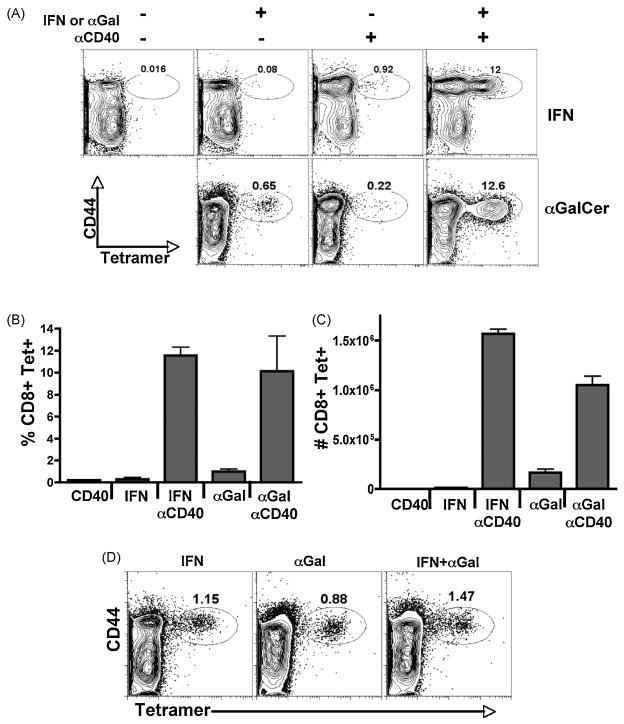
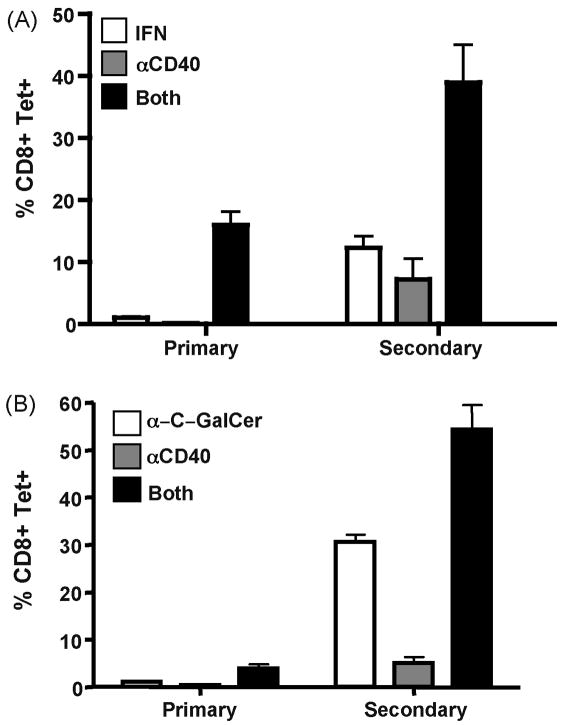
C57BL/6 wild type mice were immunized with antigen in combination with recombinant IFN (IFNα11, accession number AY225954), antiCD40, or both. We found that indeed, combined recombinant IFN/antiCD40 induced the synergistic expansion of antigen specific CD8+ T cells over the use of either stimulus alone, as measured by Kb-SIINFEKL tetramer staining of the CD8+ T cells seven days after immunization in both blood (not shown) and spleen (Figure 1A). This synergistic increase of specific CD8+ T cells was observed in both percent (Figure 1B) and total numbers (Figure 1C) of antigen specific CD8+ T cells in the spleen. In contrast, immunization with either IFN alone or anti-CD40 alone induced minimal CD8 T cell responses (<2% of CD8+ T cells being Tetramer+) (Figure 1A). The production of IFNγ from the T cells mirrored that seem by tetramer staining in that a significant percentage of IFNγ producing cells (based on intracellular staining) was observed only in response to combined IFN/antiCD40 immunization (not shown). Thus, combined IFN/antiCD40 immunization induced a CD8+ T cell response, similar in both magnitude and functionality, to that previously observed in response to TLR agonist/antiCD40 immunization (14, 23), indicating that the TLR stimulus can be removed entirely and replaced by Type I IFN.
Figure 1.
Type I IFN and α-C-GalCer work in combination with antiCD40 to generate enhanced CD8 T cell responses. Wild type C57/BL6 mice were immunized with recombinant IFN (4X106 Units), αGalCer (2ug), anti-CD40 (50ug), IFN/anti-CD40, or αGalCer/anti-CD40. Seven days later the specific CD8 T cell response was analyzed by tetramer stain in the spleen for percentage (A, B) and total numbers (C) of CD8+ Tetramer+ T cell responses. Each bar in B and C is the average of 3 mice per treatment, with the error bars indicating the standard deviation, and is representative of at least 4 experiments performed. D) Wild type mice were immunized with IFN, α-GalCer or both. Seven days later the CD8 T cell response was measured in the spleen as in A. The data is representative of 2 independent experiments.
In conjunction with our previously published data (14, 23), these data show that two different innate stimuli (TLRs or Type I IFN) can synergize with anti-CD40 to produce exponentially larger CD8+ T cell responses than can be elicited from any dose of either stimulus alone. These results lead us to speculate that synergy with innate receptor pathways might be a broad principle by which CD40 operates to induce potent cellular immunity. To examine this hypothesis, we decided to use the NKT cell agonist, α-galactosylceramide (αGalCer). NKT ligands such as αGalCer and αC-GalCer are potent activators of innate immunity (33–35), stimulating NKT cells to produce a variety of innate cytokines (36, 37), and leading to the potent activation of DCs (38). Furthermore, αGalCer has been shown to successfully elicit detectable CD8+ T cell responses against model antigens when used as a vaccine adjuvant (25, 29). We therefore examined whether or not these NKT cell activators might also synergize with anti-CD40 and result in an exponentially greater expansion of the CD8+ T cell response. Wild type B6 mice were immunized with antigen in combination with αGalCer, antiCD40, or both. Similar to previously published results (29), αGalCer alone elicited a detectable CD8+ T cell response, similar in magnitude to that observed in response to antiCD40 alone (0.5–2%) (Figure 1A). However, combined αGalCer/antiCD40 immunization synergized to produce an exponentially greater CD8 T cell response in percentage (Figure 1B) and total numbers (Figure 1C), and in their functional capacity to produce IFNγ (not shown). This synergy appears to be unique to combinations between innate stimuli and CD40, as combined IFN and αGalCer promoted only an additive increase in the CD8+ T cell response over the use of either stimulus alone (Figure 1D). This is particularly interesting since T cell responses to administration of αGalCer alone have already been shown to be dependent on CD40-CD40L interactions ((29) and see Figure 2). Thus, even for innate stimuli which function as adjuvants through engagement of the CD40-CD40L pathway, the addition of an overt CD40 stimulus is required for optimal CD8+ T cell expansion to be observed. These data further support the hypothesis that innate stimuli are broadly synergistic, not with each other, but with CD40 stimulation for the induction of potent cellular immunity.
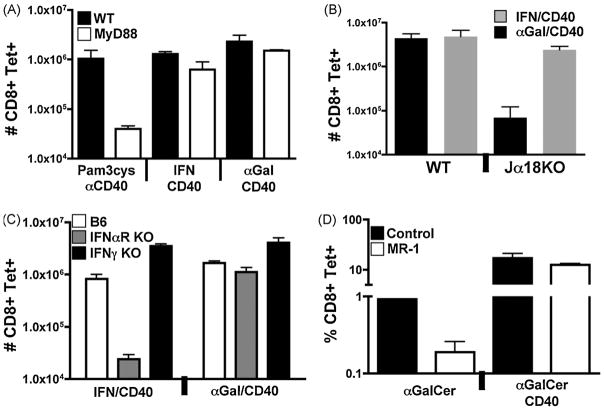
Figure 2.
IFN/antiCD40 and αGalCer/antiCD40 and TLR/antiCD40 elicit CD8+ T cell responses via non-overlapping innate signaling pathways. A–C) Wild type B6, MyD88−/−, IFNαR−/−, IFNγ−/−, and Jα18−/− mice were immunized with the various combinations of IF, αGalCer, or both as described in Figure 1. The T cell responses were measured by tetramer stain of spleen cells, as described in the Materials and Methods, six days after immunization. Total numbers of tetramer staining CD8+ T cells was calculated also as described in the Materials and Methods. Each bar is the average of 2–3 mice with the error bar indicating the range or standard deviation, respectively. The data shown is representative of 2–4 experiments. D) B6 mice were injected with either αGalCer alone or in combination with antiCD40, as marked. These injections were given either without or with 400ug of the antiCD40L antibody MR-1. Six days later, the percent of tetramer staining CD8+ T cells out of total CD8+ T cells in the spleen were calculated by FACS analysis. Each bar is the average of 3 mice with the error bar indicating the standard deviation. The data shown is representative of 2 experiments.
IFN/anti-CD40 and α-GalCer/anti-CD40 and TLR/antiCD40 elicit CD8+ T cell responses via non-overlapping innate signaling pathways
Our data are consistent with the conclusion that multiple innate pathways (TLR, IFN, and αGalCer) intersect with the CD40 pathway to elicit exponential CD8+ T cell expansion. However, many innate receptor families have common signaling elements and downstream mediators, so it is formally possible that our apparent triggering of different innate receptor families was really eliciting some common signaling component which then synergized with CD40 stimulation. We therefore determined the degree to which each innate/antiCD40 combination was dependent upon TLR, IFN or NKT signaling pathways. To determine if the IFN/antiCD40 and αGalCer/antiCD40 combinations induce enhanced CD8 T cell responses through TLR related pathways, we immunized MyD88−/− mice with antiCD40 in combination with IFN, αGalCer, or the MyD88-dependent TLR1/2 agonist Pam3cysSK4 (14, 23). We again analyzed the CD8+ T cell response seven days later by tetramer stain. As expected, the T cell response to Pam3cysSK4/αCD40 immunization was significantly compromised in the MyD88−/− host (Figure 2A). In contrast, the magnitude of the responses to either IFN/antiCD40 orαGalCer/antiCD40 was unaffected in MyD88−/− mice relative to their wild type counterparts. These data indicate that both IFN/antiCD40 and αGalCer/antiCD40 function truly independently of the MyD88 signaling pathway common to both TLRs and IL-1/18.
αGalCer activates innate immunity, and eventual adaptive immunity, in an NKT cell dependent fashion (38). To verify the requirement for NKT cells in the enhanced T cell response to αGalCer/antiCD40, we immunized Jα18 KO mice with either combined IFN/antiCD40 or αGalCer/antiCD40. Jα18−/− mice are deficient in NKT cells due to a deletion within the canonical NKT cell-specific T cell receptor (34). Seven days after immunization, we evaluated the number of antigen specific CD8 T cells by tetramer stain, and saw that the T cell response to immunization with αGalCer/antiCD40 was indeed severely blunted in the Jα18 KO mice (Figure 2C).
Upon activation with αGalCer, NKT cells are known to both produce copious amounts of cytokines such as IFNγ and IL-4, as well as increase their expression of CD40L (36, 37). To determine whether either of these NKT cell functions were necessary for the elevated CD8+ T cell response to combined αGalCer/antiCD40, we performed experiments in type I IFN (IFNαR−/−) or type II IFN (IFNγ−/−) deficient mice, and in mice injected with the CD40L blocking antibody MR-1. As anticipated, a maximal T cell response to vaccination with combined IFN/antiCD40 was dependent on the type I IFN receptor but not IFNγ (Figure 2B). In contrast, the T cell response to α-GalCer/αCD40 was independent of both type I and type II interferon (Figure 2B). In addition, though the T cell response to immunization with αGalCer alone (29) was sharply reduced in the presence of CD40L blockade, the response to αGalCer/antiCD40 was unaffected (Figure 2D). We conclude from these experiments that while NKT cells are required, their capacity to augment CD8+ T cell responses to combined αGalCer/antiCD40 is independent of their production of IFNγ or their expression of CD40L. Of note, αGalCer/antiCD40 generated an elevated CD8+ T cell response in IFNαR−/− mice while IFN/antiCD40 elicited a response in NKT deficient mice. With the data from the MyD88−/− mice, our data collectively confirm our conclusion that multiple innate pathways (TLR, IFN, and αGalCer) independently intersect with the CD40 pathway to elicit exponential CD8+ T cell expansion.
IFN and αGalCer work with antiCD40 to induce the synergistic upregulation of CD70 on DCs
We previously showed that combined TLR/anti-CD40 immunization uniquely induced the expression of CD70 on dendritic cells (DCs) while immunization with either a TLR stimulus or anti-CD40 alone did not (23). We were next interested in determining whether or not combined IFN/antiCD40 and αGalCer/antiCD40 immunizations were similarly capable of inducing elevated DC expression of CD70, particularly in light of the fact that αGalCer alone has been shown to induce some degree of DC CD70 expression on its own (29). Wild type mice were immunized with either innate stimulus, alone or in combination with anti-CD40, and at 12, 24, or 36 hours, the splenic DCs were isolated and their phenotype analyzed by flow cytometry. The surface phenotype of both CD11b+CD11c+ and CD8+CD11c+ dendritic cell subsets were analyzed for expression of multiple costimulatory molecules including CD80, CD86, and the TNFL family members OX40L, 41BBL, CD30L, and CD70.
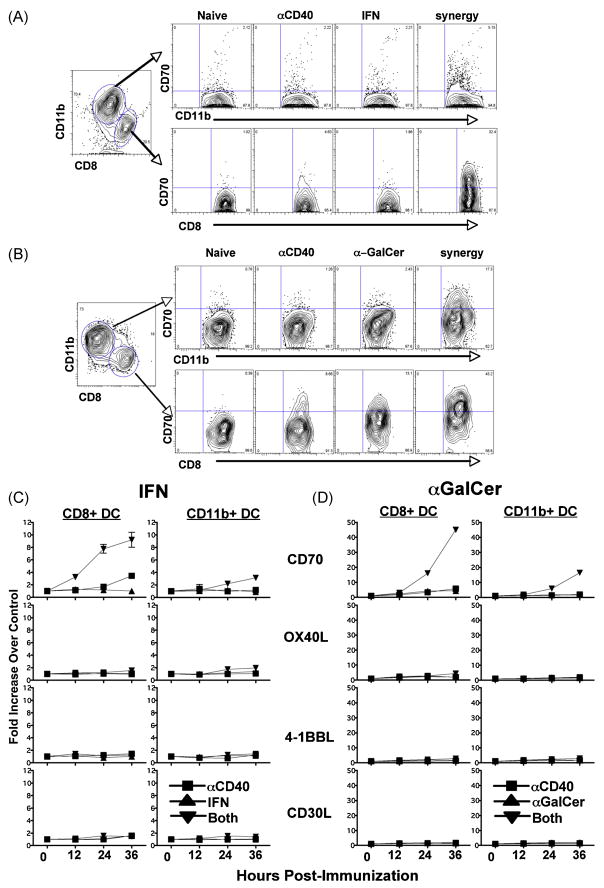
Consistent with previous reports (23, 26, 29), immunization with αGalCer or antiCD40 induced a detectable, though minimal, increase in CD70 expression on the CD8+CD11c+ DC subset, while IFN did not appear to influence DC CD70 expression at all (Figure 3A and B). In contrast, both combined IFN/antiCD40 and αGalCer/antiCD40 immunization induced robust upregulation of CD70 on both the CD11b+CD11c+ and CD8+CD11c+ DC subsets. In the case of αGalCer/antiCD40, the increase in CD70 expression was upwards of 40 fold on the CD8+ DC subset, as compared to DCs in unimmunized mice (Figure 3D). IFN/antiCD40 was also able to induce a slight upregulation of OX40L on the CD11b DC subset, though not nearly to the same degree as CD70 upregulation (Figure 3C). The expression of the other TNFL family members examined were largely unaffected by either combined immunization (Figure 3C and D). Therefore, IFN/antiCD40 and αGalCer/antiCD40 are similar to combined TLR agonist/antiCD40 immunization with respect to the quality and quantity of DC CD70 expression. It is again interesting to note that DC CD70 expression in response to αGalCer alone is also dependent on signaling through the CD40-CD40L pathway, as it can be blocked with the injection of MR-1 (data not shown and (29)). This suggests first that the induction of CD70 expression on DCs is largely, though not exclusively (39) dependent on signaling through the CD40 pathway. Second, the data reinforce our previous conclusion (23) that maximal DC CD70 expression requires coordinated and combined innate receptor/CD40 stimulation, and firmly place IFN and αGalCer on the list of innate stimuli that have the capacity to synergize with antiCD40 for this purpose.
Figure 3.
IFN and αGalCer work in combination with anti-CD40 to upregulate CD70 DCs. Mice were immunized with IFN, aGalCer, antiCD40 and their varying combinations as described in Figure 1. The zero time point indicates uninjected controls. At 12, 24, 36 hrs, DCs were harvested as previously described (23) and stained to identify the expression of the given TNF ligands on either CD8+ or CD11b+ DC subsets. Representative FACS plots showing both DC subsets following either kinds of injections are shown in A and B. C and D) The Y axis indicates the fold increase in the MFI of the expression in the given TNF ligand over the uninjected control from 3 independent experiments, with error bars indicating the standard deviation.
The Enhanced CD8 T cell response generated by the combined IFN/anti-CD40 and glycolipid/anti-CD40 immunizations is CD70-dependent
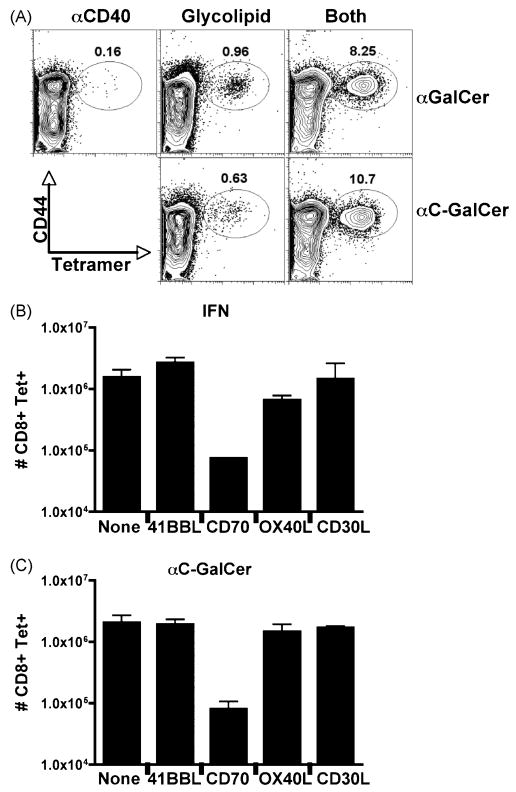
Other glycolipids have been identified that activate NKT cells and downstream adaptive responses to varying degrees (37). One variant of αGalCer, αC-GalCer, is thought to more efficiently augment Th1 responses as compared to αGalCer (35). We observed that αC-GalCer was indistinguishable from αGalCer in its capacity to elicit CD8+ T cell responses, alone or in combination with antiCD40 (Figure 4A). Indeed, in all aspects (magnitude of the T cell response, dependence on NKT cells, independence of CD40L, capacity to induce maximal DC CD70 expression) the response to αC- GalCer/antiCD40 was indistinguishable from the response to αGalCer/antiCD40 (Figure 4A and data not shown). Thus, at least for the generation of CD8+ T cell responses, synergy with antiCD40 appears to be applicable to NKT-activating glycolipids in general rather than being a specific property of only αGalCer.
Figure 4.
CD8 T cell responses generated by IFN/anti-CD40 or NKT glycolipid/CD40 are CD70-dependent. A) B6 mice were immunized with 2ug αGalCer or 2ug of the variant αC-GalCer alone or in combination with antiCD40. Six days later, antigen specific T cell responses in the spleen were measured by tetramer stain. The data shown is representative of independent experiments with 2–3 mice per immunization per experiment. B and C) Mice were immunized as described in Figure 1 with (A) combined IFN/CD40 (4×106 units IFN) or (B) αC-GalCer/anti-CD40. The day before and the day of immunization, the mice were injected IP with 250ug blocking antibodies to the indicated TNF ligand family member. Control mice were injected with PBS (No Ab). Seven days after immunization, CD8 T cell responses in the spleen were analyzed by tetramer stain as described in Figure 1. Bar graphs indicate the average of 2 mice per treatment with the error bars representing the range of responses observed. The data shown is representative of at least 3 experiments performed.
The CD8+ T cell response to combined TLR agonist/antiCD40 immunization is highly dependent on the CD70 expressed by the antigen bearing DCs (23). Since CD70 is upregulated on dendritic cells following immunization with anti-CD40 in combination with IFN, αGalCer (Figure 3) or αC-GalCer (data not shown), it was obvious to examine whether or not the CD8 T cell response that is generated by these immunizations is also CD70 dependent. Blocking antibodies to CD70 as well as the other TNFL family members 41BBL, CD30L, and OX40L were injected into mice the day before and the day of immunization with either combined IFN/anti-CD40 (Figure 4B) or αC-GalCer/anti-CD40 (Figure 4C) immunization. As with combined TLR/CD40 immunization (23) only CD70 blockade decreased the CD8 T cell response to either immunization, while blockade of the other TNFL family members did not significantly affect the CD8 T cell response. These data show that once again, CD70 plays a central role in the generation of the potent CD8 T cell responses created following immunization with combined IFN/antiCD40 and NKT glycolipid/antiCD40 combinations.
IFN/anti-CD40 and α-C-GalCer/anti-CD40 immunizations generate enhanced memory responses
We next sought to determine if both IFN/antiCD40 and NKT glycolipid/antiCD40 could similarly produce competent immune memory. Wild type C57BL/6 mice were immunized with the various combinations of IFN, αC-GalCer, and antiCD40, and the T cell responses measured in the peripheral blood by tetramer staining seven days after immunization. As before, mice immunized with either combined IFN/antiCD40 or αC- GalCer/antiCD40 generated CD8+ T cell responses that were approximately 10 fold greater than the response to any single stimulus alone (Figure 5). Two hundred and thirty days after this initial immunization, the mice were challenged with a Vaccinia virus that expresses ovalbumin (VV-ova), and the peak of the secondary response was measured by tetramer staining five days later. While all immunizations elicited memory CD8+ T cell responses as gauged by their ability to mount a stronger response to VV- ova challenge as compared to a naive host, combined IFN/antiCD40 and αC- GalCer/antiCD40 generated a secondary response significantly greater than immunization with any single stimulus alone. It is again interesting to note that the best single stimulus for the generation of memory (αC-GalCer alone with ~30% of CD8+ T cells being Tetramer+) is also the best single stimulus for eliciting some degree of DC CD70 expression during priming (Figure 3 and (25, 29)). However, the secondary response to combined αC-GalCer/anti-CD40 immunization was again significantly greater than the response to αC-GalCer alone (~55% CD8+ Tetramer+ T cells).
Figure 5.
Combined IFN/anti-CD40 and αC-GalCer/anti-CD40 immunization generate superior immune memory. Mice were immunized against ovalbumin with the indicated combinations of IFN, αC-GalCer, and anti-CD40 as described in Figure 1. Seven days later the primary response was analyzed via tetramer stain from peripheral blood as described above. 230 days later, the mice were boosted with 5×106 pfu of Vaccinia-ova and the secondary ova-specific T cell responses in the peripheral blood measured 5 days later via tetramer staining. Graphs indicate the average percent tetramer staining cells out of total CD8+ T cells from 3 mice per immunization. Data is representative of 2 experiments performed.
Importantly, these secondary responses are able to fully protect the host against infectious challenge (Figure 6). C57BL/6 mice were immunized with antigen in the context of IFN +/− anti-CD40 and 75 days after this primary immunization, the mice were challenged with 2×105 CFU of a recombinant strain of Listeria monocytogenes that expresses ovalbumin (LM-ova). Mice immunized with either anti-CD40 alone, or IFN in combination with a dose of anti-CD40 (1ug anti-CD40) that has no synergistic effect on the primary CD8+ T cell response (not shown), were unable to protect against bacterial challenge. However, a combination IFN/antiCD40 (50ug anti-CD40) that does synergistically increase the primary T cell expansion, was able to fully protect the mice against challenge with LM-ova. Similar protection against Vaccinia replication was observed in mice given a VVova challenge after primary immunization with ova in the context of combined αGalCer/antiCD40 or IFN/antiCD40 (not shown). Collectively, we conclude from these experiments that the induction of CD70 on antigen bearing DCs by combined IFN/antiCD40 or NKT glycolipid/antiCD40 ultimately facilitates the generation of superior primary T cell expansion and protective immune memory.
Figure 6.
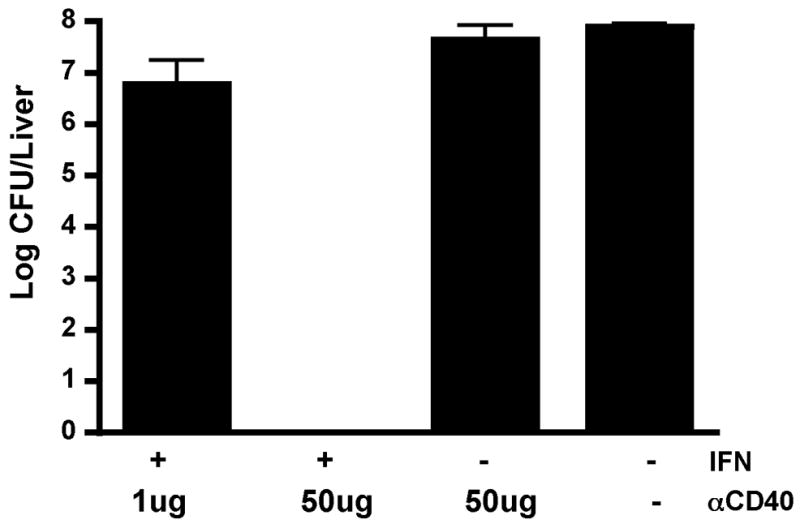
IFN/anti-CD40 synergy can protect against challenge with Listeria monocytogenes. Mice were immunized against ovalbumin in combination with IFN, antiCD40, or both, as shown. Two doses of antiCD40 were used; a low dose (1ug) that does not synergize to promote exponential CD8+ T cell expansion (not shown), and a high dose (50ug) that does synergize with IFN to promote exponential CD8+ T cell expansion as shown in Figure 1. Mice were challenged 75 days later with 2×105 CFU LMova and three days after LM challenge, the bacteria burden was determined by plating liver homogenates on BHI agar. The data shows the average between 2 mice per treatment, error bars indicating the range between the two mice, and is representative of 2 experiments performed.
Discussion
Many years ago, Charlie Janeway first referred to an adjuvant as the Immunologist’s “dirty little secret” (40). Paraphrased, this “secret” was the fact that the generation of an effective immune response to a foreign antigen required the concomitant inoculation of an inflammatory component; ie. the use of an adjuvant. In the absence of this adjuvant, one was more likely to produce tolerance to the antigen of interest rather than immunity (41). As a result, much effort has concentrated on what constitutes the best adjuvant for eliciting maximal immunity. It is generally agreed upon that an adjuvant will be something that activates the cells and factors of the innate immune system (40, 42–44). The traditional Complete Freunds Adjuvant (CFA) consists of heat-killed bacteria in an oil and water emulsion, an exceedingly inflammatory adjuvant effective for inducing antibodies, but not as effective at eliciting cellular immunity. The last decade has seen the identification of numerous innate signaling pathways (45–47), providing vaccinologists with many possible molecular pathways for their adjuvants to target. Despite the degree of effort put into adjuvant design and development, there is still no consensus on what, quantitatively, makes a good adjuvant. Specifically, the molecular signature of an effective adjuvant is not agreed upon, without which we can hardly hope to make significant progress toward the larger goal of developing stronger adjuvants that elicit more robust immune memory.
It is this gap in the collective knowledge that our data addresses by firmly establishing at least one molecular determinant of an adjuvant with the capacity to elicit potent cellular immunity, namely DC CD70 expression. The ability of CD70 expression to induce such potent cellular immunity is of particular interest since vaccines that can induce this kind of immunity have been hard to come by. For the most part, vaccines that do elicit a cellular response tend to be attenuated infectious agents, which when used clinically on large, genetically diverse populations are compounded by problems over production, storage, and reversion to virulence. Similar to our previously published data (23), our data here show that the combination of molecular components targeting innate (TLR, IFN, NKT ligand) and adaptive (CD40) receptor family members work in concert to generate large CD8 T cell responses. Our identification of CD70 expression as the common mechanism behind the potency of these combined immunizations explains much about the failure of other vaccine adjuvants to sufficiently promote cellular immunity. Immunization with either innate-targeting or adaptive-targeting stimuli alone does not induce the generation of CD8 T cell responses anywhere near the magnitude to which the combination does (Figure 1 and (14, 23)). This appears to be due to the inability of TLR, IFN, αGalCer or CD40 stimulation alone to induce maximal upregulation of the TNFL superfamily member, CD70, on dendritic cells. With this in mind, it is not a coincidence that the glycolipids are both the best single agent for inducing a CD8+ T cell responses and for inducing some degree of DC CD70 expression ((29) and Figure 3). While the eventual outcome of each innate/antiCD40 combination is the same (the induction of CD70 expression on DCs) these different combinations achieve this common endpoint via distinct molecular mechanism(s). We have identified a minimum of 3 independent means by which this is achieved; i) type I IFN dependent, Type II IFN, TLR and NKT independent, ii) TLR dependent, type I/II IFN and NKT independent, and iii) NKT dependent, type I/II IFN and TLR independent (Figure 2). The fact that a single costimulatory pathway (CD70) is independently integrated downstream of such a diversity of innate/antiCD40 combinations speaks to the central importance of this pathway in controlling potent cellular immunity. In support of this, it is interesting to note that multiple infectious agents appear to generate CD8+ T cell responses by a similar CD70-dependent mechanism (30). Thus, our data presented here and previously (23) have identified multiple vaccine combinations which, by non-infectious means, utilize the same costimulatory pathway that so potently generates cellular immunity against natural infections.
From a purely practical standpoint, we believe the significance of our findings are two fold. First, our data demonstrate that the induction of potent cellular immunity is preceded by the expression of CD70 on DCs. Thus, future efforts in vaccine adjuvant design and development should be targeted toward identifying agents that induce DC CD70 expression in vivo. Second, this induction of CD70 on DCs is rarely (in the case of TLRs, IFN or CD40) or poorly (in the case of α-GalCer) accomplished by targeting single innate or adaptive signaling pathways. Collectively our data suggest that even targeting multiple innate pathways is still insufficient for maximally utilizing the potency of CD70 in eliciting cellular immunity. The nature of innate activating agents such as polyIC (23) and zymosan (data not shown) effectively make this point. It is well documented that in vivo, polyIC stimulates both the TLR3 and mda5 pathways (48, 49). Similarly, zymosan stimulates innate responses through both TLR2/6 and the C-type lectin, Dectin-1 (50, 51). Despite each agent stimulating two independent innate activating pathways, neither polyIC nor zymosan causes an increase in CD70 expression, though they do increase expression of other costimulatory molecules on DCs such as CD80 and CD86 ((23) and data not shown). Indeed, similar to combined IFN/αGalCer as shown in Figure 1, even using combined Pam3cysSK4/PolyIC, which stimulate 3 innate pathways (TLR1/2, TLR3, mda5), will not result in enhanced CD8+ T cell responses (23) or increased DC CD70 expression (not shown) over that expressed following challenge with either agent alone. Thus, the expression of TNF ligands such as CD70 is under much tighter control than that of CD80 and CD86, likely due to its capacity to produce such potent immunity. Full utilization of this potent costimulatory molecule clearly requires the coordinated participation of both innate and adaptive receptor agonists. While this new reality makes the experimental pursuit of novel vaccine adjuvants more complicated, we believe it will also make these pursuits more fruitful.
Our data conclusively show that multiple innate stimulatory pathways integrate with CD40 to produce adaptive immunity through the induction of CD70 (Figure 3 and (23)). Furthermore, the adaptive response to multiple infectious agents is similarly controlled by the induction of CD70 ((30) and PJS and RMK unpublished data). The larger goal of vaccine development is to gain a greater understanding of what most effectively promotes the transition between innate and adaptive immune responses. Our data collectively show the importance of CD70 in controlling this transition. Thus, besides its use as a tool for vaccine development, the analysis of CD70 and the agents that induce its expression will likely uncover important components of this intersection between innate and adaptive immunity, ultimately enhancing our understanding of basic immune mechanisms as well.
Materials and Methods
Mice and injections
C57BL/6 mice purchased from NCI, or MyD88−/−, IFNαR−/−, and Ja18−/− mice bred in house (34), were immunized with 0.1–0.2 mg of ovalbumin as previously described (14). Whole protein was injected (i.v.) in combination with either recombinant IFNα (isolated in house), 2ug αC-galactosylceramide (provided by the NIH), 2ug α-Galactosylceramide (purchased from Alexis Biochemicals, cat# 306-027-M001), the anti-CD40 antibody FGK45 (50 μg), or their combinations. Ovalbumin was purchased from Sigma Corporation and contaminating LPS removed using a TritonX-114 LPS-detoxification methodology as previously described (52). 250 ug CD70, 41BBL, OX40L, and CD30L blocking antibodies(53–56) (Kind gifts from Hideo Yagita, Juntendo University, Tokyo, Japan) were injected i.v. the day before and the day of immunization.
Antibodies
CD11c (clone HL3), CD11b (clone M1/70), CD8 (clone 53-6.7), and CD70 (clone FR70), staining antibodies were purchased from BD Biosciences. CD30L (clone RM153), OX40L (clone RM134L), 4-1BBL (clone TKS-1), CD44 (clone IM7), and B220 (clone RA3-6B2) staining antibodies were purchased from eBioscience. Purified blocking antibodies against CD70 (clone FR70), OX40L (clone RM134L), CD30L (clone RM153), and 41BBL (clone TKS-1), and CD40L (MR-1) were kind gifts from Hideo Yagita, Juntendo University, Tokyo, Japan and produced as previously described (57).
Recombinant IFNα
An IFNα sequence was cloned from polyIC-stimulated B cell cDNA in the Kappler/Marrack laboratory at National Jewish Medical and Research Center. The sequence obtained was homologous to IFNα11 (gene bank accession number AY225954) except for a change from the glycosylation sequence NAT (residues 101-103) to NAN. It is unclear whether or not this represents a novel IFN subtype or is simply a PCR induced error, but regardless, this IFNα subtype was selected because it has no glycosylation sequences and can therefore be expressed in insect cells by baculovirus infection without concern for aberrant glycosylation. A TCR Cα epitope tag was added to the C-terminus for affinity purification purposes and the sequence was cloned into the p10 promotor site of the pBac vector (Invitrogen). Recombinant baculovirus was produced and after infection of Hi5 cells, recombinant IFNα was purified from the supernatant by affinity and size chromatography. The activity of the IFNα was measured and tittered based on the upregulation of class I MHC on B3K0508 cells in vitro. International Units (U) were calculated based on a comparison of this titration to one made with an IFN standard obtained from PBL Biomedical Laboratories, Piscataway, NJ (data not shown). Mice were injected i.v. with antigen and approximately 107 units of recombinant IFNα with and without concomitant anti-CD40 injection.
Cell preparation
Seven days after primary challenge (i.v.), PBLs were isolated via dorsal aorta bleed. Spleens were also removed and homogenized into single-cell suspensions. RBCs were lysed using an ammonium chloride buffer followed by washing. Cells were resuspended in RPMI (Biosource International), 2% heat-inactivated FBS (Biosource International), 1 mM sodium pyruvate, 0.1 mM non-essential amino acids, 1% PenStrep and 1% L-glutamine (Sigma). Spleen cells were resuspended at approximately 2–4 × 107 cells/mL. PBLs were resuspended in 500 μL. 50–100 μL of cells were used in subsequent tetramer stains.
Enrichment and Phenotype of Dendritic Cells
C57BL/6 mice were challenged i.v. with recombinant IFNα, αGalCer, anti-CD40, or the combinations. 12–36 hours after challenge, spleens were removed and dendritic cells were extracted as described in (2). For dendritic cell phenotype, 2.5 × 106 cells were stained with CD11c, CD11b, CD8, and an antibody specific for the indicated activation marker. Five- or 6-color flow cytometry was performed on a CyAn LX flow cytometer (DakoCytomation) and analyzed with Flowjo computer software.
Tetramer staining and flow cytometry
Cells were stained with Kb/ovalbumin tetramer as previously described (58), plated in 96-well plates and stained with tetramer for 1.5 hours at 37°C. Antibodies against CD8, CD44, and B220 were added and the cells incubated for 20 minutes at 37°C. The cells were washed, fixed, and resuspended in FACS buffer for flow cytometric analysis. Five-or 6-color flow cytometry was performed on a CyAn LX flow cytometer (DakoCytomation) and analyzed with Flowjo computer software. Data was gated on CD8+B220- events and analyzed for tetramer staining by the activation marker CD44, the antigen specific cells being CD44 high.
Protection and memory experiments
Mice were injected i.v. with the indicated vaccine, and between 50–230 days after primary immunization mice were challenged with either 2 × 105 CFU recombinant Listeria monocytogenes expressing ovalbumin (LM-ova)(59) or with 5×106 PFU Vaccinia virus(60). For LM-ova challenge, liver was removed three days post-challenge, and plated on BHI plates. They were incubated 37 degrees overnight and CFUs were determined the following day. For Vaccinia-B8R or Vaccinia-Ova challenge, ovaries were harvested five days post-challenge and Log PFU per mouse was determined following a plaque assay with BS-C-1 cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edwards AD, Diebold SS, Slack EM, Tomizawa H, Hemmi H, Kaisho T, Akira S, Reis e Sousa C. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur J Immunol. 2003;33:827–833. doi: 10.1002/eji.200323797. [DOI] [PubMed] [Google Scholar]
- 2.Edwards AD, Manickasingham SP, Sporri R, Diebold SS, Schulz O, Sher A, Kaisho T, Akira S, Reis e Sousa C. Microbial recognition via Toll-like receptor-dependent and -independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J Immunol. 2002;169:3652–3660. doi: 10.4049/jimmunol.169.7.3652. [DOI] [PubMed] [Google Scholar]
- 3.Zhu J, Huang X, Yang Y. Type I IFN signaling on both B and CD4 T cells is required for protective antibody response to adenovirus. J Immunol. 2007;178:3505–3510. doi: 10.4049/jimmunol.178.6.3505. [DOI] [PubMed] [Google Scholar]
- 4.Hervas-Stubbs S, Rueda P, Lopez L, Leclerc C. Insect baculoviruses strongly potentiate adaptive immune responses by inducing type I IFN. J Immunol. 2007;178:2361–2369. doi: 10.4049/jimmunol.178.4.2361. [DOI] [PubMed] [Google Scholar]
- 5.Fink K, Lang KS, Manjarrez-Orduno N, Junt T, Senn BM, Holdener M, Akira S, Zinkernagel RM, Hengartner H. Early type I interferon-mediated signals on B cells specifically enhance antiviral humoral responses. Eur J Immunol. 2006;36:2094–2105. doi: 10.1002/eji.200635993. [DOI] [PubMed] [Google Scholar]
- 6.Le Bon A, Durand V, Kamphuis E, Thompson C, Bulfone-Paus S, Rossmann C, Kalinke U, Tough DF. Direct stimulation of T cells by type I IFN enhances the CD8+ T cell response during cross-priming. J Immunol. 2006;176:4682–4689. doi: 10.4049/jimmunol.176.8.4682. [DOI] [PubMed] [Google Scholar]
- 7.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 8.Havenar-Daughton C, Kolumam GA, Murali-Krishna K. Cutting Edge: The direct action of type I IFN on CD4 T cells is critical for sustaining clonal expansion in response to a viral but not a bacterial infection. J Immunol. 2006;176:3315–3319. doi: 10.4049/jimmunol.176.6.3315. [DOI] [PubMed] [Google Scholar]
- 9.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson LJ, Kolumam GA, Thomas S, Murali-Krishna K. Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. J Immunol. 2006;177:1746–1754. doi: 10.4049/jimmunol.177.3.1746. [DOI] [PubMed] [Google Scholar]
- 11.Pulendran B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol Rev. 2004;199:227–250. doi: 10.1111/j.0105-2896.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 12.Durand V, Wong SY, Tough DF, Le Bon A. Shaping of adaptive immune responses to soluble proteins by TLR agonists: a role for IFN-alpha/beta. Immunol Cell Biol. 2004;82:596–602. doi: 10.1111/j.0818-9641.2004.01285.x. [DOI] [PubMed] [Google Scholar]
- 13.Tritel M, Stoddard AM, Flynn BJ, Darrah PA, Wu CY, Wille U, Shah JA, Huang Y, Xu L, Betts MR, Nabel GJ, Seder RA. Prime-boost vaccination with HIV-1 Gag protein and cytosine phosphate guanosine oligodeoxynucleotide, followed by adenovirus, induces sustained and robust humoral and cellular immune responses. J Immunol. 2003;171:2538–2547. doi: 10.4049/jimmunol.171.5.2538. [DOI] [PubMed] [Google Scholar]
- 14.Ahonen CL, Doxsee CL, McGurran SM, Riter TR, Wade WF, Barth RJ, Vasilakos JP, Noelle RJ, Kedl RM. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J Exp Med. 2004;199:775–784. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhee EG, Mendez S, Shah JA, Wu CY, Kirman JR, Turon TN, Davey DF, Davis H, Klinman DM, Coler RN, Sacks DL, Seder RA. Vaccination with heat-killed leishmania antigen or recombinant leishmanial protein and CpG oligodeoxynucleotides induces long-term memory CD4+ and CD8+ T cell responses and protection against leishmania major infection. J Exp Med. 2002;195:1565–1573. doi: 10.1084/jem.20020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wille-Reece U, Wu CY, Flynn BJ, Kedl RM, Seder RA. Immunization with HIV-1 Gag protein conjugated to a TLR7/8 agonist results in the generation of HIV-1 Gag-specific Th1 and CD8+ T cell responses. J Immunol. 2005;174:7676–7683. doi: 10.4049/jimmunol.174.12.7676. [DOI] [PubMed] [Google Scholar]
- 17.Chen ZM, O’Shaughnessy MJ, Gramaglia I, Panoskaltsis-Mortari A, Murphy WJ, Narula S, Roncarolo MG, Blazar BR. IL-10 and TGF-beta induce alloreactive CD4+CD25- T cells to acquire regulatory cell function. Blood. 2003;101:5076–5083. doi: 10.1182/blood-2002-09-2798. [DOI] [PubMed] [Google Scholar]
- 18.Busch DH, Pamer EG. T lymphocyte dynamics during Listeria monocytogenes infection. Immunol Lett. 1999;65:93–98. doi: 10.1016/s0165-2478(98)00130-8. [DOI] [PubMed] [Google Scholar]
- 19.Busch DH, Pamer EG. T cell affinity maturation by selective expansion during infection. J Exp Med. 1999;189:701–710. doi: 10.1084/jem.189.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butz E, Bevan MJ. Dynamics of the CD8+ T cell response during acute LCMV infection. Adv Exp Med Biol. 1998;452:111–122. doi: 10.1007/978-1-4615-5355-7_13. [DOI] [PubMed] [Google Scholar]
- 21.Butz EA, Bevan MJ. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tscharke DC, Karupiah G, Zhou J, Palmore T, Irvine KR, Haeryfar SM, Williams S, Sidney J, Sette A, Bennink JR, Yewdell JW. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. J Exp Med. 2005;201:95–104. doi: 10.1084/jem.20041912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanchez PJ, McWilliams JA, Haluszczak C, Yagita H, Kedl RM. Combined TLR/CD40 stimulation mediates potent cellular immunity by regulating dendritic cell expression of CD70 in vivo. J Immunol. 2007;178:1564–1572. doi: 10.4049/jimmunol.178.3.1564. [DOI] [PubMed] [Google Scholar]
- 24.Tesselaar K, Xiao Y, Arens R, van Schijndel GM, Schuurhuis DH, Mebius RE, Borst J, van Lier RA. Expression of the murine CD27 ligand CD70 in vitro and in vivo. J Immunol. 2003;170:33–40. doi: 10.4049/jimmunol.170.1.33. [DOI] [PubMed] [Google Scholar]
- 25.Taraban VY, Rowley TF, Al-Shamkhani A. Cutting edge: a critical role for CD70 in CD8 T cell priming by CD40-licensed APCs. J Immunol. 2004;173:6542–6546. doi: 10.4049/jimmunol.173.11.6542. [DOI] [PubMed] [Google Scholar]
- 26.Taraban VY, Rowley TF, Tough DF, Al-Shamkhani A. Requirement for CD70 in CD4+ Th cell-dependent and innate receptor-mediated CD8+ T cell priming. J Immunol. 2006;177:2969–2975. doi: 10.4049/jimmunol.177.5.2969. [DOI] [PubMed] [Google Scholar]
- 27.Keller AM, Schildknecht A, Xiao Y, van den Broek M, Borst J. Expression of costimulatory ligand CD70 on steady-state dendritic cells breaks CD8+ T cell tolerance and permits effective immunity. Immunity. 2008;29:934–946. doi: 10.1016/j.immuni.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Bullock TN, Yagita H. Induction of CD70 on dendritic cells through CD40 or TLR stimulation contributes to the development of CD8+ T cell responses in the absence of CD4+ T cells. J Immunol. 2005;174:710–717. doi: 10.4049/jimmunol.174.2.710. [DOI] [PubMed] [Google Scholar]
- 29.Taraban VY, Martin S, Attfield KE, Glennie MJ, Elliott T, Elewaut D, Van Calenbergh S, Linclau B, Al-Shamkhani A. Invariant NKT cells promote CD8+ cytotoxic T cell responses by inducing CD70 expression on dendritic cells. J Immunol. 2008;180:4615–4620. doi: 10.4049/jimmunol.180.7.4615. [DOI] [PubMed] [Google Scholar]
- 30.Schildknecht A, Miescher I, Yagita H, van den Broek M. Priming of CD8+ T cell responses by pathogens typically depends on CD70-mediated interactions with dendritic cells. Eur J Immunol. 2007;37:716–728. doi: 10.1002/eji.200636824. [DOI] [PubMed] [Google Scholar]
- 31.Rowley TF, Al-Shamkhani A. Stimulation by soluble CD70 promotes strong primary and secondary CD8+ cytotoxic T cell responses in vivo. J Immunol. 2004;172:6039–6046. doi: 10.4049/jimmunol.172.10.6039. [DOI] [PubMed] [Google Scholar]
- 32.Tesselaar K, Gravestein LA, van Schijndel GM, Borst J, van Lier RA. Characterization of murine CD70, the ligand of the TNF receptor family member CD27. J Immunol. 1997;159:4959–4965. [PubMed] [Google Scholar]
- 33.Chen G, Schmieg J, Tsuji M, Franck RW. Efficient synthesis of alpha-C-galactosyl ceramide immunostimulants: use of ethylene-promoted olefin cross-metathesis. Organic letters. 2004;6:4077–4080. doi: 10.1021/ol0482137. [DOI] [PubMed] [Google Scholar]
- 34.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 35.Schmieg J, Yang G, Franck RW, Tsuji M. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand alpha-Galactosylceramide. J Exp Med. 2003;198:1631–1641. doi: 10.1084/jem.20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009;9:28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 37.Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Curr Opin Immunol. 2008;20:358–368. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujii S, Shimizu K, Hemmi H, Fukui M, Bonito AJ, Chen G, Franck RW, Tsuji M, Steinman RM. Glycolipid alpha-C-galactosylceramide is a distinct inducer of dendritic cell function during innate and adaptive immune responses of mice. Proc Natl Acad Sci U S A. 2006;103:11252–11257. doi: 10.1073/pnas.0604812103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Deusen K, Rajapakse R, Bullock T. CD70 expression by dendritic cells plays a critical role in the immunogenicity of CD40-independent, CD4+ T cell dependent, licensed CD8+ T cell responses. Journal of Leukocyte Biology. 2009 doi: 10.1189/jlb.0809535. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 42.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 43.Medzhitov R, Janeway CA., Jr Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 44.Medzhitov R, Janeway CA., Jr Innate immune recognition and control of adaptive immune responses. Semin Immunol. 1998;10:351–353. doi: 10.1006/smim.1998.0136. [DOI] [PubMed] [Google Scholar]
- 45.Creagh EM, O’Neill LA. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 2006;27:352–357. doi: 10.1016/j.it.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 46.Kawai T, Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- 47.Miyake K. Nucleic acid-sensing Toll-like receptors: beyond ligand search. Adv Drug Deliv Rev. 2008;60:782–785. doi: 10.1016/j.addr.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 49.Gitlin L, Barchet W, Gilfillan S, Cella M, Beutler B, Flavell RA, Diamond MS, Colonna M. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci U S A. 2006;103:8459–8464. doi: 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003;197:1119–1124. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adam O, Vercellone A, Paul F, Monsan PF, Puzo G. A nondegradative route for the removal of endotoxin from exopolysaccharides. Anal Biochem. 1995;225:321–327. doi: 10.1006/abio.1995.1161. [DOI] [PubMed] [Google Scholar]
- 53.Akiba H, Oshima H, Takeda K, Atsuta M, Nakano H, Nakajima A, Nohara C, Yagita H, Okumura K. CD28-independent costimulation of T cells by OX40 ligand and CD70 on activated B cells. J Immunol. 1999;162:7058–7066. [PubMed] [Google Scholar]
- 54.Futagawa T, Akiba H, Kodama T, Takeda K, Hosoda Y, Yagita H, Okumura K. Expression and function of 4–1BB and 4–1BB ligand on murine dendritic cells. Int Immunol. 2002;14:275–286. doi: 10.1093/intimm/14.3.275. [DOI] [PubMed] [Google Scholar]
- 55.Oshima H, Nakano H, Nohara C, Kobata T, Nakajima A, Jenkins NA, Gilbert DJ, Copeland NG, Muto T, Yagita H, Okumura K. Characterization of murine CD70 by molecular cloning and mAb. Int Immunol. 1998;10:517–526. doi: 10.1093/intimm/10.4.517. [DOI] [PubMed] [Google Scholar]
- 56.Shimozato O, Takeda K, Yagita H, Okumura K. Expression of CD30 ligand (CD153) on murine activated T cells. Biochem Biophys Res Commun. 1999;256:519–526. doi: 10.1006/bbrc.1999.0336. [DOI] [PubMed] [Google Scholar]
- 57.Florido M, Borges M, Yagita H, Appelberg R. Contribution of CD30/CD153 but not of CD27/CD70, CD134/OX40L, or CD137/4–1BBL to the optimal induction of protective immunity to Mycobacterium avium. J Leukoc Biol. 2004;76:1039–1046. doi: 10.1189/jlb.1103572. [DOI] [PubMed] [Google Scholar]
- 58.Kedl RM, Rees WA, Hildeman DA, Schaefer B, Mitchell T, Kappler J, Marrack P. T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med. 2000;192:1105–1113. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitchell T, Kappler J, Marrack P. Bystander virus infection prolongs activated T cell survival. J Immunol. 1999;162:4527–4535. [PubMed] [Google Scholar]