Abstract
Unlike most mammalian species, the prairie vole is highly affiliative, forms enduring social bonds between mates, and displays biparental behavior. Over two decades of research in this species has enhanced our understanding of the neurobiological basis not only of monogamy, social attachment and nurturing behaviors, but also other aspects of social cognition. Because social cognitive deficits are hallmarks of many psychiatric disorders, discoveries made in prairie voles may direct novel treatment strategies for disorders such as autism spectrum disorder and schizophrenia. With the ongoing development of molecular, genetic and genomic tools for this species, prairie voles will likely maintain their current trajectory becoming an unprecedented model organism for basic and translational research focusing on the biology of the social brain.
The need for a mammalian model of social behavior
Impairments in the ability to engage in healthy social interactions and to form stable social attachments are common characteristics of several mental health disorders, including depression, addiction, schizophrenia and autism spectrum disorders (ASD). Identifying the neurobiological and genetic mechanisms contributing to normative social cognitive function is essential for understanding these disorders as well as for identifying potential targets for pharmacological interventions. Because the ability to form stable social attachments is rare in animals, particularly among those species typically studied in the laboratory, studies of the biological bases of complex social behaviors that recapitulate the richness of human social relationships have been limited. Thus, there is a dire need to identify model organisms ideally suited for investigating complex social behavior at a mechanistic level. Indeed, the US Department of Human Services Interagency Autism Coordinating Committee has recommended a five-year budget of $75,000,000 to support research to standardize and validate at least 20 model systems that replicate features of ASD and will allow identification of specific molecular targets or neural circuits amenable to existing or new interventions.
Few neuroscientists will argue the extraordinary value of the translational opportunities afforded by studies of biological phenomena in traditional mammalian laboratory animal models such as mouse (Mus musculus) and rat (Rattus norvegius). Several factors have ensured the dominance of these rodent models in biomedical research, including the ease of maintaining laboratory populations and the abundance of pharmacological, molecular and genomic resources available for these species. Their phylogenetic positioning relative to humans sets mice and rats apart from other model organisms (i.e. Drosophila melanogaster and Caenorhabditis elegans), particularly with regard to understanding the complexity of gene-brain-behavior relationships. However, the translational utility of a model organism is only as valuable as the traits it expresses, and though almost all eukaryotic animal models exhibit some form of social behavior, few recapitulate the richness of social behavior of humans, including our capacity to form lasting social attachments.
Here, we introduce a model organism, the prairie vole (Microtus ochrogaster), which is highly suited for the study of social behavior. We first provide an overview of the social behaviors displayed by this species and then highlight some of the areas in which prairie vole research has enlightened our understanding of the neurobiology and genetics of social bonding, parental care, the effects of early life experience on adult behaviors and the consequences of social loss or isolation. Finally, we reflect on how these studies have already impacted our understanding of social cognition in our own species
The prairie vole model
The socially monogamous prairie vole has emerged as an excellent model species for examining the neurobiology of complex social behaviors, including social bonding. Prairie voles differ considerably from more traditional laboratory models as they are exceptionally social and often form long-term, socially monogamous relationships with their mates. Both parents contribute nearly equally to nurturing the young, and in natural environments alloparental care is commonly observed [1] (Box 1; Figure 1A). By contrast, other related vole species such as the meadow vole (M. pennsylvanicus) and the montane vole (M. montanus) are relatively asocial and do not readily form social bonds, providing a useful point of comparison for identifying neurobiological and genetic systems leading to diversity in social behaviors [2]. In addition, laboratory-reared prairie vole colonies are systematically outbred, often only a few generations from the field, allowing for the study of individual variation in neurochemistry and sociobehavioral traits (Figure 1B). This inter- and intra-species diversity in brain and behavior set prairie voles apart from mice and rats, making this species an ideal model organism for studying the biological mechanisms underlying natural variation social behaviors relevant to our own species.
Text Box 1. Gene-brain-behavior relationships in an ecologically relevant context.
Although prairie voles are considered socially monogamous, genetic monogamy in which a male-female pair cohabitate and share exclusive parentage of offspring, is often not the norm. Initial observations of the socially monogamous mating system of prairie voles stemmed from field observations in which male-female pairs were repeatedly captured together within the same home ranges [52–55] and subsequent laboratory studies determined that when female prairie voles lose a mate, they often fail to take on a new partner [56]. However, in addition to male-female pairs, prairie vole populations often consist of single females (presumably survivors of male-female pairs) and communal groups with philopatric offspring. In addition, though most male prairie voles display a “resident” strategy where they form a pair bond with a female, defend a territory and assist females in the rearing of offspring, many males (~35–45%) assume a “wandering” tactic where they acquire large home ranges that overlap territories of one or more “resident” pairs [57, 58]. “Wanderer” males typically only sire offspring via extra-pair fertilizations and provide no care for their young. Thus, despite the notoriety that prairie voles receive for their monogamous behavior, genetic determination of paternity in natural and semi-natural populations has revealed that between 23–56% of litters are sired by multiple males [59, 60]. Further, even among prairie vole populations, there are significant geographic differences in social behavior. For example, prairie voles from Kansas are less social and display lower levels of physical contact between adult males and females, less spontaneous alloparental and parental behavior, and are more aggressive than Illinois prairie voles [61, 62]. However, Kansas voles are more socially and genetically monogamous than voles in an Indiana population [63] and some of these population differences are associated with differences in estrogen receptor expression in the brain [64].
The tremendous variation in social behaviors both within and between natural prairie vole populations exemplifies both the need and utility of studying the genetics and neural circuitry of social behaviors in an ecologically relevant context. For example, to determine how the reproductive tactics of “resident” males versus “wandering” males relates to V1aR expression patterns in the lateral septum and ventral pallidum, Ophir et al. (2008) used radio telemetry to track spatial use of sexually naïve adult prairie voles within semi-natural, outdoor enclosures [3]. After approximately three weeks, they assessed the paternity of litters and then examined the distribution of V1aR in adult male forebrains. Most males (74%) were classified as a member of a bonded pair, while the remaining males assumed the “wandering” tactic. Surprisingly, although AVPR1A in the ventral pallidum and lateral septum have been shown to be involved in the regulation of partner preference formation and paternal care, levels of V1aR in these regions did not correspond to male mating tactic, male mating success, nor the male’s propensity to engage in extra-pair copulations. Instead, V1aR density in the posterior cingulated/retrosplenial cortex, a region of the brain involved in spatial learning and memory predicted reproductive success in “wanderers”. Male’s with lower densities of V1aR in this brain region assumed larger home territories, sired more offspring, and were more likely to engage in extra-pair copulations. These studies emphasize the importance and exciting opportunities of coupling laboratory and field studies to more thoroughly understand the mechanistic basis of complex social behaviors.
Figure 1.
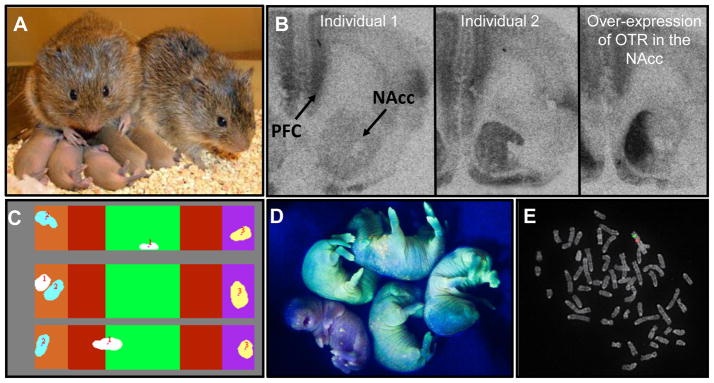
The prairie vole as a model organism. A) This hamster-sized rodent forms selective social bonds with its partner and displays bi-parental behavior both in nature and in the laboratory (photograph provided by T. Ahern). There is considerable individual variation in social behavior, which is reflected by variation in neuropeptide receptor distributions in the brain. For example, the two autoradiograms on the left of B) illustrate the individual variation in OTR binding densities in the nucleus accumbens (NAcc) of female prairie voles, variation which has been linked to variation in alloparental behavior. Artificially increasing OTR binding using viral vector mediated gene transfer (right autoradiogram) facilitates partner preference formation in female (figure adapted from [16]). Thus, prairie voles are useful models for dissecting the neurobiological basis for diversity in behavior. C) Partner preference formation can be accurately quantified in a high-throughput manner using automated behavioral analysis systems (Figure adapted from [65]). D) Lentiviral transgenesis can now be used to manipulate the genome of prairie voles as demonstrated by the green prairie vole pups that are transgenic for expression of a green fluorescent protein (photograph provided by Z. Donaldson). This technique, when applied to behaviorally relevant genes, will facilitate research into understanding the relationship between genes and behavior. E) Genomic resources are rapidly becoming available for the prairie vole, including cytogenetic maps, single nucleotide polymorphism (SNP) panels, bacterial artificial chromosome (BAC) libraries, and the full genome is slated to be sequenced by NHGRI. Shown is fluorescent in situ hybridization (FISH) localizing two prairie vole BAC clones with known homology to the mouse genome to the prairie vole X chromosome.
Prairie voles are hamster-sized Microtine rodents (typically 30–60g) that are geographically distributed throughout grasslands in central North America. Prairie voles and related vole species are easily maintained and housed in standard rodent vivariums and are also routinely studied in their natural and semi-natural habitats [3, 4] (Box 1). Both laboratory-reared and recently captured wild voles are amenable to pharmacological and genetic manipulations and classic behavioral paradigms used in mice and rats. The prototypical behavioral assay used in prairie voles is the partner preference test (PPT), which is used to quantify social attachments between mates [5]. In this test, the experimental animal and a “partner” are allowed to cohabitate for a set period of time, during which mating may or may not be permitted. Following cohabitation, the “partner” animal and an unrelated, novel “stranger” animal are tethered to opposite ends of a three-chambered arena. The test animal may freely explore the arena for three hours and is said to have formed a “partner preference” if it has spent at least twice the amount of time in contact with its “partner” versus the “stranger”. In prairie voles, mating facilitates the formation of a partner preference, but longer cohabitations without mating also lead to a partner preference [5]. Non-monogamous vole species, such as montane or meadow voles, typically do not form partner preferences even after extended periods of cohabitation. Sophisticated and extremely accurate automated technologies are now routinely used for high-throughput analysis of the PPT in real-time allowing up to 36 tests to be performed within a 24 hour period without the need for human scoring [6] (Figure 1C). As described in the following section, the PPT used in conjunction with pharmacological and genetic manipulations has been instrumental in identifying neural and genetic mechanisms underlying social attachment in voles.
Neural circuitry of social bonding
Much of our understanding of the neuronal systems involved in social bonding has stemmed from pharmacological and comparative neuroanatomical studies between monogamous and non-monogamous vole species [7, 8]. These studies have demonstrated that arginine vasopressin (AVP), oxytocin (OT), and dopamine (DA), along with their respective receptors, act within specific brain circuitry to facilitate social attachment in a gender-specific manner [9]. Although there are few species differences in the distribution of AVP and OT in the brain [10, 11], there are striking species differences in the location and density of their respective receptors [7, 8]. As the neural circuitry of pair bonding has been reviewed extensively elsewhere [e.g. 12, 13], here we provide just a brief overview of the neuronal systems that influence social bonding, including new data where appropriate.
In female voles, OT plays a critical role in regulating the formation of a partner preference by activating OT receptors (OTR) in the nucleus accumbens (NAcc) and prefrontal cortex [10, 14]. Sociosexual interactions trigger the release of OT into the NAcc from neuronal fibers that likely originate from magnocellular neurons in the hypothalamus that also project to the pituitary [10]. Infusion of OT into the brain during cohabitation with a male accelerates the development of a partner preference, while blocking OTR in the NAcc during mating prevents this behavior [14, 15]. There is remarkable individual variation in OTR density in the NAcc which may contribute to natural variation in the propensity to form a pair bond (Figure 1B). For example, over-expressing OTR within the NAcc of prairie voles using viral vector mediated gene transfer accelerates the formation of a partner preference in female prairie voles (Figure 1B) [16]. Females in non-monogamous vole species as well as mice, display very low or no OTR binding in the NAcc, while the monogamous common marmoset (Callithrix jacchus) has high densities of OTR in this region much like the prairie vole [17].
In male prairie voles, AVP plays a critical role in the regulation of partner preferences, although it is likely that OT also plays a role [18, 19]. Specifically, blocking vasopressin V1a receptors (AVP1A) in the ventral pallidum or lateral septum with antagonists prevents partner preference formation following mating [20, 21]. Although there are few species differences in the distribution of the AVP peptide, as is the case for OTR, there are significant individual and species differences in AVPR1A distribution in the brain that may contribute to variation in social behavior. For example, socially monogamous male prairie voles have high densities of AVPR1A binding in the ventral pallidum, a major output of the NAcc, while non-mongamous vole species do not [2]. Interestingly, other monogamous species, including common marmosets and the California mouse (Peromyscus californicus) also have similarly high levels of V1aR in this ventral forebrain region compared to related non-monogamous species [17, 22]. Over-expressing the gene encoding the prairie vole AVPR1A, Avpr1a, in the ventral pallidum of male meadow voles using viral vector-mediated gene transfer results in the ability of this promiscuous species to display partner preferences [23]. These studies in female and male prairie voles suggest that variation in OT and AVP receptor regulation may contribute to diversity in social behavior, a concept that may be highly relevant to disorders characterized by impairments in social cognition.
In addition to the AVP and OT systems, the neurotransmitter dopamine DA, acting within the NAcc, plays a critical role in social bond formation in both male and female prairie voles [24, 25]. D2-type DA receptor activation facilitates partner preferences, while D1-type DA receptor activation prevents partner preference formation [26]. Thus, the D1 and D2 dopamine receptors have antagonistic influences on initial pair bond formation. However, once a pair bond has been established and maintained for two weeks, males show a significant increase in the density of D1, but not D2, receptors in the NAcc [26]. This increase in D1 receptors following the initial pair bond formation may serve to prevent subsequent pair bond formation as a consequence of extra-pair copulations. Interestingly, non-monogamous meadow voles have high levels of D1 receptors in place in the NAcc even prior to mating. When antagonists are used to block these receptors, males show greater affiliate behavior towards a female after a short cohabitation period, but do not form a partner preference [26]. These data suggest that while these receptors are associated with species differences in social behaviors, they are not solely responsible.
Studies of AVP, OT and DA systems demonstrate the critical role of the brain’s reward and reinforcement circuitry in social bond formation, which has lead to a neural circuitry model of social bonding (Figure 2). Within the reward system, simultaneous activation of OTRs or AVPR1As and DA receptors may result in the establishment of an association between the social olfactory cues of the partner, and the rewarding or reinforcing aspects of copulation, reminiscent of classical conditioning. Since the reinforcing nature of drug abuse and social bonding are mediated by a common neural system, DA within the NAcc, prairie voles serve as a valuable model for understanding the interaction between social relationships and addiction [27].
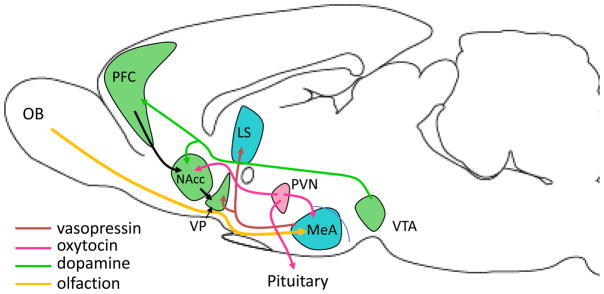
Figure 2.
A schematic illustrating the proposed neural circuitry of social bonding in prairie voles. Oxytocin (OT) neurons in the paraventricular nucleus of the hypothalamus (PVN) project to the nucleus accumbens (NAcc) as well as to the posterior pituitary [10]. Sociosexual interactions in female prairie voles stimulate the release of both OT and dopamine into the NAcc. In males, vasopressin neurons in the extended amygdala project to the ventral pallidum (VP) and the lateral septum (LS). Concurrently, olfactory signatures of the sexual partner are processed through the amygdala. It is hypothesized that the simultaneous activation of the neuropeptide and dopamine receptor systems in these regions leads to a conditioned partner preference by linking the rewarding nature of the sociosexual interaction and the olfactory signatures of the partner. Inter- and intra-species variation in OTR and AVPR1A in these regions contribute to diversity in social behaviors in voles. (Figure modified from [66].)
Neurogenetics of social bonding
Although many of the neural components underlying social bonding are beginning to be identified, little is known of the genetic regulation of these systems. Insights into the genetic mechanisms producing diversity in sociobehavioral traits have emerged from studies attempting to identify the molecular mechanisms giving rise to species differences and individual variation in AVPR1A distribution in the vole brain [28]. First identified in comparative genomic studies between prairie and montane voles, a polymorphic microsatellite element located near proximal regulatory regions of Avpr1a may contribute to the variation in AVPR1A distribution in the brain [29, 30]. Early studies suggested that variation in the length of this microsatellite might explain both species differences in social organization among voles, and individual variation in social attachment in prairie voles. For example, montane and meadow voles have considerably shorter microsatellite elements than prairie voles [30], and male prairie voles with relatively short microsatellites had lower levels of AVPR1A binding in the olfactory bulb and lateral septum, and were less likely to display a partner preference than males with longer microsatellites [31]. However, it is now known that length per se does not predict social organization of other Microtus species [29, 32], and sequence variation within the microsatellite may have a greater influence on expression than length itself [3]. The precise mechanisms by which the microsatellite element contributes to variation in receptor distribution and behavior is still poorly understood, however, as discussed below, there is now growing evidence that similar microsatellite variability in the human AVPR1A contributes to variation in human social cognition and behavior [28].
The neural regulation of parental care
Just as the prairie vole’s propensity to form pair bonds has allowed for investigation of the mechanisms underlying social bonds between mates, the biparental nature and remarkable individual variation in parental care displayed by prairie voles provides an excellent opportunity to investigate the neural mechanism underlying parental nurturing behavior. Studies of both alloparental behavior and paternal behavior have revealed that common pathways underlie pair bonding and parental nurturing behavior. This suggests that social bonding in monogamous species may have evolved through subtle tweaking of ancient neural circuits regulating parental behaviors.
Both virgin and post-partum female prairie vole adults display nurturing behavior toward infants. However, there is remarkable individual variation in the maternal-like behavior displayed by virgins, referred to as alloparental behavior. Approximately 60% of adult females display spontaneous maternal nurturing toward novel pups, while 40% either ignore or attack pups [33–35]. This intra-species variation in nurturing behavior provides a unique opportunity to elucidate the neurological mechanisms underlying maternal care. For example, virgin female prairie voles that display maternal behavior have higher densities of OTR in the NAcc than non-maternal females [33, 34]. Further, an infusion OTR antagonist into the NAcc prevents the display of spontaneous maternal behavior [33]. Although high densities of OTR within the NAcc are correlated with high levels of alloparental care in females, viral vector-mediated over-expression of OTR within the NAcc of adult female prairie voles does not result in enhanced alloparental behavior, even though partner preference formation is accelerated in those same animals [35]. These results suggest that variation in OTR density during development, or perhaps OTR in additional brain regions are contributing to the diversity in alloparental behavior.
Prairie voles are an important model for understanding the genetic and neurobiological basis of paternal care as well. Early comparative studies discovered that in prairie voles, cohabitation with a female resulted in a decrease in AVP content in the lateral septum (likely reflecting a release of AVP from neuronal fibers in the region), which was coincident with an increase in paternal responsiveness. This phenomenon was not observed in meadow voles [36, 37]. Infusion of AVP into the lateral septum increased paternal behaviors, while an AVPR1A antagonist decreased time spent tending the pups [38]. These studies suggest that, as in females, the neural mechanisms involved in pair bond formation also play an important role in the regulation of paternal care.
Early life experience and its effect on adult social behavior
There is now growing evidence that the quality of early life nurturing received can have life-long consequences on adult social behavioral tendencies. Prairie voles have proven particularly useful for investigating how early life experience can impact later life social behaviors. For example, a recent study directly manipulated the social environment in the laboratory to reflect various rearing conditions of voles from natural populations (see Text Box 1). This was accomplished by raising prairie vole pups in either single mother or biparental units. The single mother-reared pups received less parental nurturing (e.g. licking and grooming) than biparentally-reared pups. In addition, females raised in single mother units were less likely to display alloparental behavior and both male and female offspring from this group required longer cohabitation periods than biparentally reared offspring to form partner preferences [39]. Varying the family structure also altered the number of OT neurons in the paraventricular nucleus of hypothalamus. This paradigm offers an excellent opportunity to understand how early-life experience alters the neurochemistry underlying social cognition, as well as for potentially exploring gene by environment interactions, which has important implications for psychopathologies affecting social relationships. For example, women who have experienced abuse and neglect in childhood have reduced OT concentrations in their cerebrospinal fluid compared to controls [40].
Social loss, depression and heart disease
Just as early life experience can have profound effects on later life social behaviors, social experiences encountered during adult life can also have significant influences, not only on subsequent social behavior, but also on mental and physical health. Because of their highly social nature, prairie voles have become an important model for studying the consequences of social loss or social isolation on mental and physical health. When prairie voles are chronically isolated, or separated from their pair bonded partner, they display behaviors similar to those found in depression. For example, female prairie voles that are socially isolated have reduced sucrose intake, and higher plasma levels OT, AVP and corticosterone than socially housed females [41]. Cardiac disturbances including increased heart rate and reduced heart rate variability also follow social isolation [42]. Administration of OT during periods of social isolation can alleviate some of the behaviors relevant to depression including the elevated basal heart rate [43]. Prairie voles have also been used as a model of social loss. Disruption of an established pair bond leads to high levels of passive behavior (immobility) in the forced swim test and the tail suspension tests, a behavioral response reminiscent of grieving and bereavement in humans. This response appears to be mediated by the corticotrophin releasing factor (CRF) system receptor since a CRF antagonist blocked the development of social loss-induced depressive-like behavior [44]. Thus, in addition to understanding the mechanisms promoting social attachments, prairie voles are a valuable model for understanding the consequences of social loss and deprivation, which has important implications for understanding common human conditions such as a depression and bereavement.
Contributions towards understanding of human social behaviors
A growing number of studies are revealing a remarkable conservation between the mechanisms underlying social behaviors in prairie voles and social cognition in humans, particularly with regard to the OT and AVP systems [28, 45]. For example, intranasal OT administration has been shown to enhance interpersonal trust, increase eye-to-eye contact, and even enhance the ability of a person to infer the emotions of another person based on facial expression [45]. These findings have enormous implications for developing treatment strategies to ameliorate the social deficits in disorders such as ASD and schizophrenia. For example, OT infusions in high-functioning ASD subjects have been reported to enhance retention of social information [46], although much more research into this area is needed. In addition, at least three independent studies have now reported associations between polymorphisms in the human OT receptor gene (OXTR) and symptoms of ASD [45].
Further insights into the genetics of human social behaviors have stemmed from studies of the microsatellite polymorphism in the vole Avpr1a, as similar polymorphic microsatellites are found upstream of the human AVPR1A. Variation in these microsatellite elements in the human AVPR1A appear to contribute to variation in social cognition, altruistic behavior, brain activation patterns, AVPR1A mRNA levels in the hippocampus [28]. In addition, there have now been three independent reports of a genetic association between these polymorphisms in the human AVPR1A and symptoms of ASD [47–49]. One of these studies found that AVPR1A polymorphisms specifically, mediated socialization skills in the subjects with ASD [49]. Perhaps most remarkably, one of these polymorphic markers in the AVPR1A, which has been associated with altered brain activation patterns during a face processing task, has been associated with pair bonding behavior in humans, including measure of partner bonding, perceived marital problems, marital status, as well as spousal perception of marital quality [50]. These observations illustrate how taking advantage of the genetic diversity in laboratory populations of prairie voles can lead to exciting insights into how variation in gene regulation affects behavior in rodents as well as in humans.
Sociogenomics: the future of the prairie vole model
The continued trajectory of the prairie vole as a powerful model organism for understanding the social brain requires the development of genomic resources and transgenic technologies equivalent to those available in mice and rats. Progress to date toward this end is promising. Lentiviral mediated transgenesis has been used to create green fluorescent protein (GFP) transgenic voles as a proof of principle (Figure 1D) [51], and progress is being made combining this approach with shRNA technologies to silence gene expression. In addition, the prairie vole is now targeted for full-genome sequencing (http://www.genome.gov/10002154) and several genomic resources including a 10X coverage BAC library (http://bacpac.chori.org/library.php?id=481), a panel of over 700 single nucleotide polymorphisms (SNPs), a cytogenetic and genetic linkage map and a comprehensive catalogue of prairie vole gene sequences are or will be available shortly (Figure 1E).
With the ongoing development of this comprehensive prairie vole genomic toolbox, exciting frontiers in understanding the relationship between the complexities of the genome and the complexities of the social brain are rapidly becoming possible. The availability of these tools and technologies will give insights into the abundance, distribution and sequence characteristics of genes and gene families that differentiate social and asocial individuals and/or species. As prairie vole genome sequence becomes available, the ease of isolating and/or amplifying vole-specific gene sequences will allow for targeted tissue, brain region or neuron specific over-expression or knock-down of genes relevant to social behavior using transgenic technology and will aid in the development of more sophisticated technologies to knock-out or replace genes of interest. In addition, these resources will expedite the development of other genomic tools such as tiling arrays, SNP chips or genome-wide methylation screens that comprehensively span both coding and non-coding elements. When combined with traditional neuroscience techniques, these resources and technologies will open exciting new avenues in prairie vole research and will allow for discovery-based genome research and the development of additional molecular and genetic tools to dissect the relationship between the genome and the social brain in ways that previously have only been possible in traditional model organisms.
Conclusions
Although it is unlikely that studies of social behavior in prairie voles will identify the exact etiologies of human psychiatric disorders, this model organism has exceptional potential to begin to guide us in understanding the genetic pathways and neurobiological systems that regulate aspects of sociality that are often impaired in these patients. For example, although prairie voles cannot model disrupted aspects of social cognition characteristic of individuals with ASD, such as facial processing, empathy or calculating theory of mind, they do provide an unprecedented animal model for understanding the neural and genetic basis of social motivation and social processing. Even if these systems are not dysfunctional per se in psychiatric disorders, a more comprehensive understanding of them may represent an avenue for developing pharmacological targets to enhance social motivation and cognition in patients who suffer from these disorders. The past two decades of prairie vole research have led to the development of a model organism that is unparalleled for understanding the social brain. The rapid development of transgenic technologies and genomic resources coupled with unique opportunities to integrate genetic, neurobiological and behavioral ecological approaches to understand social behavior in this species will ensure that prairie voles continue to develop as a premier model organism for identifying mechanisms regulating complex social behaviors, which will directly impact the understanding of our own sociality and inform future treatment of psychiatric disorders of the social domain.
Glossary
- Alloparenting
parenting performed by individuals that are not the biological parents
- Philopatry
living groups consisting of multiple generations of related individuals
- Pair bond
a long-term selective social attachment between a mating pair that is typically associated with a shared territory and nest, but does not imply sexual fidelity
- Partner preference
a laboratory measure of an individual’s preference to associate with a partner versus a novel or opposite sex conspecific. Individuals that have formed a partner preference spend more time in close proximity to their partner than to a novel stimulus animal, however, the presence of a partner preference does not imply the establishment of a pair bond
- Social monogamy
a long-term association between a male and a female that cooperate in producing and rearing offspring. Social monogamy, unlike genetic monogamy, does not imply sexual fidelity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carter CS, et al. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- 2.Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 3.Ophir A, et al. Field tests of cis-regulatory variation at the prairie vole avpr1a locus: Association with V1aR abundance but not sexual or social fidelity. Horm Behav. 2008 doi: 10.1016/j.yhbeh.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Solomon N, Crist T. Estimates of reproductive success for group-living prairie voles, Microtus ochrogaster, in high-density populations. Anim Behav. 2008;76:881–892. [Google Scholar]
- 5.Williams J, et al. Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm Beh. 1992;26:339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- 6.Ahern T, et al. Evaluation of two automated metrics for analyzing partner preference tests. J Neurosci Methods. 2009 doi: 10.1016/j.jneumeth.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci USA. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Insel TR, et al. Patterns of vasopressin receptor distribution associated with social organization in microtine rodents. J Neurosci. 1994;14:5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Insel TR, Hulihan T. A gender-specific mechanism for pair bonding: Oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109:782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- 10.Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, et al. Immunoreactivity of central vasopressin and oxytocin pathways in microtine rodents: a quantitative comparative study. J Comp Neurol. 1996;366:726–737. doi: 10.1002/(SICI)1096-9861(19960318)366:4<726::AID-CNE11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 12.Carter CS, et al. Oxytocin, vasopressin and sociality. Prog Brain Res. 2008;170:331–336. doi: 10.1016/S0079-6123(08)00427-5. [DOI] [PubMed] [Google Scholar]
- 13.Young KA, et al. The neurobiology of social attachment: A comparative approach to behavioral, neuroanatomical, and neurochemical studies. Comp Biochem Physiol C Toxicol Pharmaco. 2008;148:401–410. doi: 10.1016/j.cbpc.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young LJ, et al. Cellular mechanisms of social attachment. Horm Beh. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- 15.Williams JR, et al. Oxytocin administered centrally facilitates formation of a partner preference in prairie voles (Microtus ochrogaster) J Neuroendocrin. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 16.Ross HE, et al. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J Neurosci. 2009;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schorscher-Petcu A, et al. Distribution of vasopressin and oxytocin binding sites in the brain and upper spinal cord of the common marmoset. Neurosci Lett. 2009;461:217–222. doi: 10.1016/j.neulet.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Cho MM, et al. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav Neurosci. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- 19.Winslow J, et al. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- 20.Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125:35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, et al. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster) Behav Neurosci. 2001;115:910–919. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- 22.Young LJ. Frank A. Beach Award. Oxytocin and vasopressin receptors and species-typical social behaviors. Horm Beh. 1999;36:212–221. doi: 10.1006/hbeh.1999.1548. [DOI] [PubMed] [Google Scholar]
- 23.Lim MM, et al. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429:754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Wang ZX. Nucleus accumbens dopamine and oxytocin interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- 25.Aragona BJ, et al. A critical role for nucleus accumbens dopamine in partner preference formation of male prairie voles. J Neurosci. 2003;23:3483–3490. doi: 10.1523/JNEUROSCI.23-08-03483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aragona BJ, et al. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9:133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- 27.Aragona B, et al. Amphetamine reward in the monogamous prairie vole. Neurosci Lett. 2007;418:190–194. doi: 10.1016/j.neulet.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donaldson Z, Young L. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 29.Hammock EAD, Young LJ. On switches and knobs, microsatellites and monogamy. Trends Genet. 2007;23:209–212. doi: 10.1016/j.tig.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Young LJ, et al. Increased affiliative response to vasopressin in mice expressing the vasopressin receptor from a monogamous vole. Nature. 1999;400:766–768. doi: 10.1038/23475. [DOI] [PubMed] [Google Scholar]
- 31.Hammock EAD, Young LJ. Microsatellite instability generates diversity in brain and sociobehavioral traits. Science. 2005;308:1630–1634. doi: 10.1126/science.1111427. [DOI] [PubMed] [Google Scholar]
- 32.Fink S, et al. Mammalian monogamy is not controlled by a single gene. Proc Natl Acad Sci USA. 2006;103:10956–10960. doi: 10.1073/pnas.0602380103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olazábal DE, Young LJ. Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience. 2006;141:559–568. doi: 10.1016/j.neuroscience.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Olazábal DE, Young LJ. Species and individual differences in juvenile female alloparental care are associated with oxytocin receptor density in the striatum and the lateral septum. Horm Beh. 2006;49:681–687. doi: 10.1016/j.yhbeh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Ross H, et al. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J Neurosci. 2009;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bamshad M, et al. Cohabitation alters vasopressin innervation and paternal behavior in prairie voles (Microtus ochrogaster) Physio Beh. 1994;56:751–758. doi: 10.1016/0031-9384(94)90238-0. [DOI] [PubMed] [Google Scholar]
- 37.Bamshad M, et al. Sex and species differences in the vasopressin innervation of sexually naive and parental prairie voles, Microtus ochrogaster and meadow voles, M. pennsylvanicus. J Neuroendocrinol. 1993;5:247–255. doi: 10.1111/j.1365-2826.1993.tb00480.x. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, et al. Role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster) Proc Natl Acad Sci USA. 1994;91:400–404. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahern TH, Young LJ. The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (Microtus ochrogaster) Front Behav Neurosci. 2009;3(17) doi: 10.3389/neuro.08.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heim C, et al. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Mol Psychiatry. 2009;10:954–958. doi: 10.1038/mp.2008.112. [DOI] [PubMed] [Google Scholar]
- 41.Grippo A, et al. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosom Med. 2007;69:149–157. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grippo AJ, et al. Social isolation disrupts autonomic regulation of the heart and influences negative affective behaviors. Biol Psychiatry. 2007;62:1162–1170. doi: 10.1016/j.biopsych.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grippo A, et al. Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology. 2009;34:1542–1553. doi: 10.1016/j.psyneuen.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bosch O, et al. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology. 2009;34:1406–1415. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heinrichs M, et al. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30:548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Hollander E, et al. Oxytocin increases retention of social cognition in autism. Biol Psychiatry. 2006;61:498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 47.Kim S, et al. Transmission disequilibrium testing of arginine vasopressin receptor 1A (AVPR1A) polymorphisms in autism. Mol Psychiatry. 2001;7:503–507. doi: 10.1038/sj.mp.4001125. [DOI] [PubMed] [Google Scholar]
- 48.Wassink TH, et al. Examination of AVPR1a as an autism susceptibility gene. Mol Psychiatry. 2004;9:968–972. doi: 10.1038/sj.mp.4001503. [DOI] [PubMed] [Google Scholar]
- 49.Yirmiya N, et al. Association between the arginine vasopressin 1a receptor (AVPR1a) gene and autism in a family-based study: mediation by socialization skills. Mol Psychiatry. 2006;11:488–494. doi: 10.1038/sj.mp.4001812. [DOI] [PubMed] [Google Scholar]
- 50.Walum H, et al. Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proc Natl Acad Sci USA. 2008;105:14153–14156. doi: 10.1073/pnas.0803081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donaldson ZR, et al. Production of germline transgenic prairie voles (Microtus ochrogaster) using lentiviral vectors. Biol Reprod. 2009 doi: 10.1095/biolreprod.109.077529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Getz L, et al. Social organization and mating system of the prairie vole, Microtus ochrogaster. In: Tamarin T, et al., editors. Social systems and population cycles in voles. Birkhauser Verlag; 1990. pp. 69–80. [Google Scholar]
- 53.Getz LL, et al. The mating system of the prairie vole Microtus ochrogaster: Field and laboratory evidence for pair bonding. Behav Ecol Sociobiol. 1981;8:189–194. [Google Scholar]
- 54.Getz L, Hoffman J. Social-organization in free-living prairie voles, Microtus ochrogaster. Behav Ecol Sociobiol. 1986;18:275–282. [Google Scholar]
- 55.Hoffman J, et al. Home range overlap and nest cohabitation of male and female prairie voles. Am Mid Nat t. 1984;112:314–319. [Google Scholar]
- 56.Pizzuto T, Getz L. Female prairie voles (Microtus ochrogaster) fail to form a new pair after loss of mate. Beh Proc. 1998;43:79–86. doi: 10.1016/s0376-6357(97)00091-0. [DOI] [PubMed] [Google Scholar]
- 57.Getz L, et al. Social organization of the prairie vole (Microtus ochrogaster) J Mammal. 1993;74:44–58. [Google Scholar]
- 58.Thomas J, Birney E. Parental care and mating system of the prairie vole, Microtus ochrogaster. Behav Ecol Sociobiol. 1979;5:171–186. [Google Scholar]
- 59.Ophir A, et al. Social but not genetic monogamy is associated with greater breeding success in prairie voles. Anim Behav. 2008;75:1143–1154. [Google Scholar]
- 60.Solomon N, Keane B. Reproductive strategies in female rodents. In: Sherman P, Wolff J, editors. Rodent socieites: and ecological and evolutionary perspective. The University of Chicago Press; 2007. pp. 42–56. [Google Scholar]
- 61.Roberts RL, et al. Role of social and endocrine factors in alloparental behavior of prairie voles (Microtus ochrogaster) Can J Zool. 1998;76:1862–1868. [Google Scholar]
- 62.Cushing B, et al. The effects of peptides on partner preference formation are predicted by habitat in prairie voles. Horm Behav. 2001;39:48–58. doi: 10.1006/hbeh.2000.1633. [DOI] [PubMed] [Google Scholar]
- 63.Solomon NG, Keane B. What’s love got to do with it?. American Society of Mammalogists Annual Meeting.2008. [Google Scholar]
- 64.Cushing B, et al. Intraspecific variation in estrogen receptor alpha and the expression of male sociosexual behavior in two populations of prairie voles. Brain Research. 2004;2004:247–254. doi: 10.1016/j.brainres.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 65.Ahern TH, et al. Evaluation of two automated metrics for analyzing partner preference tests. J Neurosci Methods. 2009;182:180–188. doi: 10.1016/j.jneumeth.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Young LJ, et al. Anatomy and neurochemistry of the pair bond. J Comp Neurol. 2005;493:51–57. doi: 10.1002/cne.20771. [DOI] [PubMed] [Google Scholar]