Abstract
We developed a panel of non-obese diabetic (NOD) mice deficient in major lysosomal cysteine proteases (cathepsins S, L and B) to identify protease enzymes essential for autoimmune diabetes. Null alleles for cathepsins (Cts) S, L or B were introgressed onto the NOD genetic background with 19 Idd markers at homozygosity. Diabetes onset was determined among females aged up to 6 months. We evaluated insulitis and sialadenitis in tissues using histology and computer assisted morphology. NOD mice deficient in Ctss or Ctsb were partially protected from diabetes with incidence at 33% and 28%, respectively, versus wild-type NOD (69%; p<0.00001). NODs lacking cathepsin L (Ctsl−/−) are completely protected from IDDM, as originally shown by others. Ctsl, Ctss, or Ctsb heterozygous mice were able to develop IDDM, although incidence levels were significantly lower for Ctsb+/− (50%) and Ctsl+/− (55%) as compared to NODs (69%; p<0.03). Ctsl−/− mice contain functional, diabetogenic T-cells and an enriched Foxp3+ regulatory T-cell population, and diabetes resistance was due to the presence of an expanded population of regulatory T-cells. These data provide additional information about the potency of the diabetogenic T-cell population in Ctsl−/− mice which were comparable in potency to wild-type NOD mice. These data illustrate the critical contribution of each of these proteases in determining IDDM in the NOD mouse and provide a useful set of models for further studies.
Keywords: cathepsin, type 1 diabetes, mouse, NOD
INTRODUCTION
The functional properties of lysosomal proteases, which are also known as cathepsins, have garnered much interest in recent years due to their therapeutic potential. Studies on the individual cathepsins have uncovered distinct and non-redundant roles for these enzymes. In our laboratory, we have shown that cathepsin L (Ctsl) and cathepsin S (Ctss) are important participants in antigen processing and presentation (1,2). The significant roles of cathepsins have been hypothesized to extend beyond the limits of antigen presentation. As demonstrated in various disease models, cathepsins may also directly process proteins involved in extracellular matrix remodeling, atherosclerosis, cerebral aneurysms, obesity, bone resorption, kidney disease and tumorigenesis (3–9).
In immunity, lysosomal cysteine proteases are involved in MHC class II antigen presentation (10). This is a highly coordinated process involving the proper maturation and trafficking of MHC class II ab heterodimers, the processing of exogenous antigens, and the loading of peptide antigens onto MHC class II molecules. Exogenous antigens and MHC class II molecules meet in the endosomal compartments. The MHC class II molecule enters the endosomal pathway by its association with the type II membrane glycoprotein, invariant chain (Ii). As a chaperone protein for MHC class II, Ii helps to facilitate its folding in the ER and also targets the Ii/MHCII complex to the endosomes via a signaling motif in its cytoplasmic tail. In the late endosomes, invariant chain is degraded in a sequential manner such that only the Class II-associated Invariant Chain Peptide (CLIP) is left seated in the peptide-binding groove.
In previous work, we showed that the lysosomal cysteine protease Ctsl is abundantly expressed in cortical thymic epithelial cells (cTEC) and that Ctsl-deficiency in two haplotypes results in a defect in positive selection specific for CD4+ T cells, suggesting that cTEC in Ctsl-deficient mice express an altered peptide repertoire (1). We proposed a novel role for cathepsin L in regulating positive selection by generating the MHC class II bound peptide ligands presented by cTEC. Since then, Maehr et al. (11) reported that Ctsl−/− NOD mice are protected from insulitis and autoimmune diabetes by virtue of a shift in the CD4+ T cell compartment favoring an elevated level of regulatory versus cytotoxic T cells. Overall, Ctsl modulates antigen presentation primarily at the level of cTEC.
On the other hand, Ctss exhibits a different expression pattern than Ctsl, with activity found in dendritic cells, macrophages and B cells (2). Also important for MHC class II maturation in antigen presenting cells, Ctss has been found to influence the presentation of class II-bound epitopes by enhancing or decreasing their presentation. At least in a collagen-induced arthritis model, the absence of Ctss seems to reduce the presentation of type II collagen, thus mitigating the disease (12). Although Ctsb does not appear to participate in MHC class II antigen processing and presentation, there is evidence suggesting that it could participate in TNF-alpha mediated liver injury (13) as well as the onset of pancreatitis (14). Here, we test and compare the roles of all three cathepsin proteins in determining susceptibility to type 1 diabetes mellitus using the NOD mouse.
We have established congenic lines for null alleles of Ctsl, Ctss and Ctsb on the NOD genetic background. We confirmed that Ctsl−/− mice are protected from type 1 diabetes (IDDM), and demonstrated a significant reduction in IDDM incidence for Ctss−/− and Ctsb−/− NOD mice. We confirm that Ctsl−/− NOD mice are CD4+ lymphopenic and have an altered ratio of regulatory to activated T cells. We extend previous data by showing that the activated T cells have the same diabetogenic potential as wild-type NOD T cells. Overall, these new congenic strains provide important tools for further studies defining the roles of cathepsin proteases in autoimmunity.
METHODS
Mice
NODscid/scid were purchased from The Jackson Laboratory (Bar Harbor, ME). NOD mice were originally obtained in 1998 as a kind gift from Charles A. Janeway, Jr. (Department of Immunology, Yale University, New Haven, CT) and have been interbred at the University of Washington through more than 30 generations (NOD/UW). Mice carrying null alleles for Ctsl, Ctss and Ctsb were originally generated using embryonic stem cells derived from a 129/Sv genetic background with implantation into C57BL/6J mice as described for Ctsl (15), Ctss (16) and Ctsb (17). Each allele was introgressed onto the NOD/UW genetic background by successive backcrossing and selection for 19 previously identified Idd loci as well as markers at or tightly linked to cathepsins S, L or B (see Supplemental Table 1). The three congenic strains have been fixed to homozygosity for linkage markers at the Idd loci and have been maintained by brother-sister mating. All animals were housed in a specific pathogen free animal facility at the University of Washington in a temperature-controlled room (25°C) with a fixed 12-hour light/dark cycle. Mice had free access to food and water. These strains are now under development for distribution at The Jackson Laboratory.
Experimental design
Mice were fed pelleted rodent chow (LabDiet 5053, Teklad, Madison, WI) containing 4% fat (w/w), 24% protein and 4.5% crude fiber. For studies of tissue inflammation, mice were monitored for body weight and urine glucose weekly. Mice exhibiting urine glucose levels (Diastix, Bayer Corp., Elkhart, IL) of greater than 250 mg/dl were deemed diabetic, and this was confirmed by blood glucose measurements (OneTouch Profile, Lifescan, Milpitas, CA). Blood glucose levels of greater than 250 mg/dl were considered diabetic and nearly all mice exhibiting IDDM had blood glucose levels greater than 375 mg/dl. Mice showing no evidence of diabetes by 6 months of age were killed and considered to be non-diabetic. At either IDDM onset or at 6 months of age, mice were killed by cervical dislocation, perfused through the heart apex with sterile PBS, and tissues collected for analyses. Tissues were fixed in 4% buffered formaldehyde and paraffin blocks prepared for sectioning. All procedures were done in accordance with current NIH guidelines and approved by the Animal Care and Use Committee of the University of Washington.
Assessment of insulitis
Pancreatic tissues were collected, fixed in formalin, sectioned (5 µm thick) and stained with hematoxylin and eosin (H&E). The degree of insulitis was determined from multiple nonsequential slides from 5 to 6 individual mice (18). Every islet on each section was scored. Each islet was assigned a score, as previously described (19): 0 = no lymphocytic infiltration; 1 = peri-insulitis; 2 = <50% islet infiltration; and 3 = >50% islet infiltration. An insulitis score for each mouse was obtained by dividing the total score for each pancreas by the number of islets examined. Data are represented as mean insulitis score ± SEM for the indicated experimental group.
Assessment of sialadenitis
Submandibular glands from 5 to 6 mice per genotype were collected, fixed in formalin, sectioned (5 µm thick) and H & E stained. The total numbers of lymphocytic foci were counted on each of 3 sections per mouse as described (20). Tissue from the same mice used for insulitis determination was used for this assessment.
Antibodies
Antibodies against mouse cell surface antigens were purchased from BD Pharmingen: PerCP-conjugated anti-CD4, FITC-conjugated anti-CD8α, PE-conjugated anti-CD25, APC-conjugated anti-streptavidin. Biotinylated anti-digoxigenin antibody was purchased from Jackson ImmunoResearch Laboratory. Anti-DTA-1 monoclonal antibody, generously provided to us by MJ Turk (Department of Microbiology and Immunology, Dartmouth Medical School, Lebanon NH), was biotinylated in-house and used for the anti-GITR depletion experiments.
Cell isolation and flow cytometry
The cell surface phenotype of thymocyte and lymphocyte populations was determined by four-color flow cytometry. Briefly, thymi, spleen, lymph nodes, and pancreatic lymph nodes were mechanically disrupted. Single cell suspensions were depleted of erythrocytes and approximately 2×106 cells were incubated in the presence of fluorescently conjugated antibodies. Binding of biotin-conjugated antibodies was detected by allophycocyanin-conjugated streptavidin. Data was collected using a FACSCalibur™ flow cytometer (Becton Dickonson) and analyzed using FlowJo™ software. Typically, 20,000 events were collected for pancreatic lymph node cell analysis, 50,000 for splenocyte analysis, and 100,000 for thymocyte analysis.
Cell transfer experiments
Erythrocyte-depleted splenocytes from Ctsl−/− NOD mice or their wild-type littermate controls were incubated with biotinylated anti-DTA-1 at 5 µg/ml. GITR-hi cells were selected from the cell suspensions using anti-biotin microbeads and an AutoMACS magnetic cell sorter (Miltenyi, Bergisch Gladbach, Germany). GITR-low or GITR-negative fractions were used for cell transfer experiments. NOD.scid mice received 10–15×106 untouched or GITR-depleted Ctsl−/− NOD or wild-type splenocytes by tail-vein injection, and were monitored for the onset of diabetes.
Statistics
Values are reported as means ± SEM. Statistical differences in diabetes incidence were assessed using the SPSS program (SPSS Inc., Chicago, Il.). Groups were compared using Chi-square analyses. In some cases the Student’s t-test was used to compare independent means. P<0.05 was accepted as statistically significant.
RESULTS
NOD mice deficient in cathepsin L, but not cathepsin S or B, exhibit complete resistance to diabetes
NOD mice develop spontaneous insulin dependent diabetes mellitus (IDDM) and the incidence and age of onset is influenced by gender and environment (21,22). For female mice, onset occurs at approximately four to six months of age and incidence ranges between 60%–100% depending upon the colony. Diabetes develops at a slower rate in males, which tend to exhibit more resistance to the disease. Because females are more susceptible to diabetes than males, we used females to access diabetes incidence among our strains.
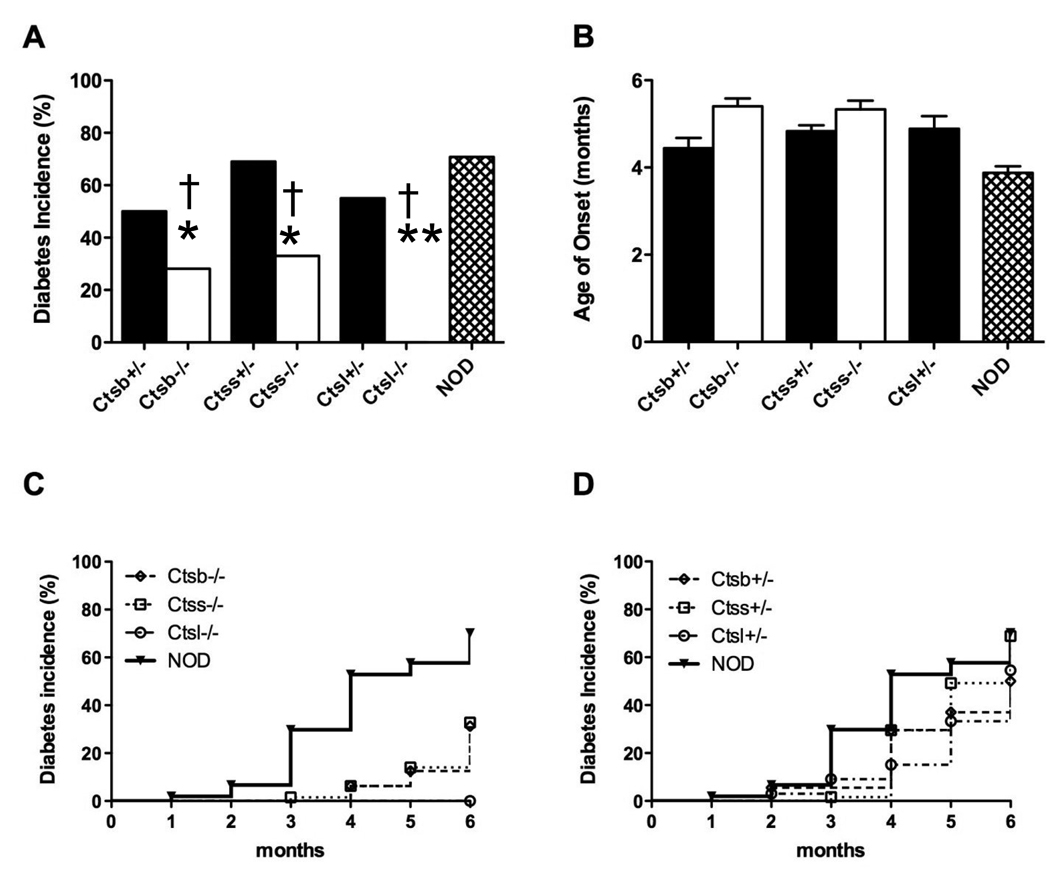
With the exception of Ctsl−/− mice, all cathepsin deficient strains developed diabetes to some extent (Figure 1). Ctss+/− matched the IDDM incidence as seen for NOD (69%), and Ctsb+/− and Ctsl+/− showed modest but significantly reduced values as compared with NOD mice (50% [p=0.0061] and 55% [p=0.035], respectively) (Figure 1A). For homozygous null cathepsin S and B congenic mice, diabetes occurred at significantly lower incidences as compared to their heterozygous littermates and NOD (p<0.00001 versus NOD and littermates). Cathepsin-deficient mice developed diabetes between 4.5 – 5.5 months of age which was later than seen for NOD (3.9 months; p<0.00001) (Figure 1B). Incidence curves for homozygous (Figure 1C) and heterozygous (Figure 1D) null mice are also shown to directly contrast the strains.
Figure 1. Cathepsin-deficient NOD mice exhibit resistance to spontaneous autoimmune diabetes mellitus.
Female cathepsin-deficient mice and their heterozygous littermate controls were monitored for diabetes and were sacrificed upon diagnosis with diabetes or at six months of age as described in Materials and Methods. (A) Diabetes incidence of cathepsin L (Ctsl), cathepsin S (Ctss)- and cathepsin B (Ctsb)-deficient mice (heterozygous, filled bars; homozygous, open bars). (B) Mean age of onset for cathepsin-deficient mice. Homozygous Catl−/− mice are completely protected from diabetes with remaining strains showing age of onset between 4 to 6 months. (C) Kaplin-Meier curves for diabetes incidence for NOD (filled circles) and homozygous cathepsin-deficient strains. Ctsl−/− (filled diamonds) are completely protected from diabetes onset. Ctss−/− (filled squares) and Ctsb−/− (filled triangles) show reduced onset levels (33% and 25%, respectively) as compared to wild-type NOD (69%). (D) Kaplin-Meier curves for diabetes incidence among cathepsin-heterozygous mice. Ctss+/− (open squares) show incidence as for the NOD strain (69%), while Ctsb+/− (open triangles) and Ctsl+/− (open diamonds) have somewhat reduced diabetes incidence (50% and 55%, respectively).
Numbers of cohorts per group monitored were (cathepsin-deficient; cathepsin-heterozygous littermate controls): Ctsl: 25;32, Ctss: 43:54, Ctsb: 60;47 and NOD: 105. *P<0.05, **p<0.00001 between NOD and cathepsin-deficient genotypes; †p<0.00001 between diabetic and non-diabetic mice within genotype. Data are presented as mean ± SEM.
Ctsl−/− NOD mice exhibited a dramatic phenotype, marked by complete resistance to diabetes. As the absence of diabetes could be due to retarded kinetics of the disease, Ctsl−/− mice were maintained and monitored over a longer period of time. Mice that were allowed to age up to ten months of age never developed diabetes, indicating that our failure to observe diabetes in these mice was not due to delayed kinetics.
Insilitis and peripheral lymphocytic infiltration occurs in NOD mice deficient in cathepsin B or S
Type 1 diabetes mellitus in humans and NOD mice is characterized by progressive stages of lymphocytic infiltration of pancreatic islets, eventually resulting in islet destruction and the onset of IDDM (23). However, infiltration of pancreatic islets does not necessarily result in islet cell pathology and loss of islet cell function as has been observed in a closely related strain, NOR (24). Therefore, we sought to determine whether we could detect the presence of mononuclear cell infiltrates in the pancreatic tissues of cathepsin-deficient NOD mice.
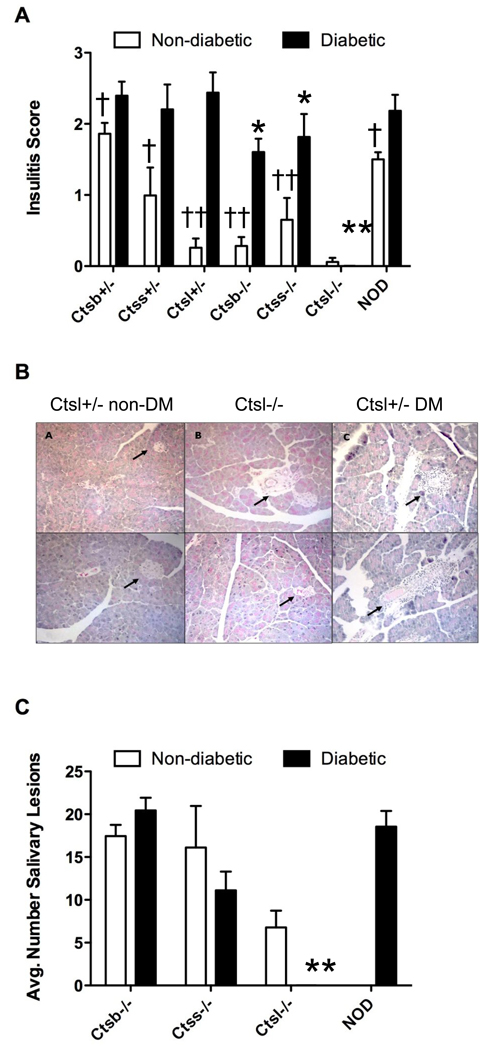
Histological analysis of pancreatic sections revealed that overall, diabetic cathepsin deficient mice had insulitis scores comparable to that of the diabetic NOD parental strain (Figure 2A), although values were significantly reduced for homozygous cathepsin S and B-deficient mice (p<0.03 versus NOD). Nondiabetic mice also showed measurable insulitis which was significantly reduced as compared to their diabetic counterparts (p<0.00001 to p<0.025 [Figure 2A]). Ctsl-deficient congenic mice were set apart from the other strains in that insulitis was absent in non-diabetic animals (Figure 2A, B).
Figure 2. Insulitis and sialadenitis among cathepsin-deficient NOD mice.
Five to 6 mice from each genotype were analyzed for lymphocytic infiltration by histological analyses as described in Materials and Methods. (A) Insulitis is markedly reduced in Catl−/− NOD mice. Pancreata were harvested from mice and fixed in 10% formalin. Pancreatic tissue sections were processed for hematoxilin and eosin (H&E) staining to assess mononuclear cell infiltration. Pancreatic sections were graded for insulitis, based on a scale from 0 to 3; 0=no insulitis, 1=peri-insulitis, 2 =<50% infiltration, 3 =>50% infiltration. (B) Pancreatic sections from nondiabetic (non-DM) cathepsin L-sufficient, cathepsin L-sufficient and control diabetic (DM) Ctsl+/− NOD mice demonstrating insulitis only in the control strain. (C) Sialadenitis is comparable among diabetic and nondiabetic cathepsin-deficient and NOD strains. Inflammation tended to be reduced in Ctsl−/− nondiabetic mice but differences from NOD did not reach significance. *P<0.03 and **p<0.00001 between diabetic NOD and diabetic cathepsin-deficient strains, n=5–6 mice per genotype. †P<0.03 and ††p<0.00001 within genotype between non-diabetic and diabetic mice. Data are presented as mean ± SEM.
To test whether cathepsin deficiency influenced autoimmune responses at additional tissue sites as seen for NOD (20), we scored salivary gland tissues for sialadenitis in homozygous cathepsin deficient genotypes at 6 months of age. Sialadenitis was noted in both diabetic and non-diabetic mice (Figure 2C), the extent of which was comparable between NOD and cathepsin S and B strains (12–20 lesions per mouse). Values tended to be lower (~6.5 lesions/mouse) for nondiabetic Ctsl−/− mice. Overall, cathepsin deficient mice experience peripheral infiltration.
The reduced insulitis observed in each homozygous cathepsin-deficient group (Figure 2A) led us to examine whether the pancreata of these mice could possibly be resistant to infiltration. It has been suggested that cathepsins are involved in extracellular matrix remodeling, and thereby can potentially influence the accessibility of the pancreas to autoreactive islet-specific T cells (25–27). To determine if this was the case, we assessed the ability of T cell receptor transgenic T cells of known reactivity to beta-islet cell antigens to induce diabetes in the cathepsin-deficient NOD mice. Activated CD8+ and CD4+ diabetogenic T cell clones (2×106 insulin-reactive G9C8 or 10×106 GAD65-specific BDC2.5, respectively) (28,29) were transferred into sublethally irradiated Ctss−/−, Ctsb−/− and Ctsl−/− NOD mice, and blood glucose levels in these mice were monitored. Mice in each group developed diabetes with similar kinetics within 4–6 days after transfer (data not shown), implying that the pancreatic tissues in these mice are not necessarily resilient to infiltration and destruction by pathogenic T cells. This experiment also indicates that presentation of specific beta-islet cell antigens occurs in all three cathepsin deficient mice, and is sufficient to induce diabetes.
Cathepsin L-deficient NOD mice exhibit a defect in CD4+ T cell selection and an increase in the relative size of the Foxp3+ regulatory T cell subset
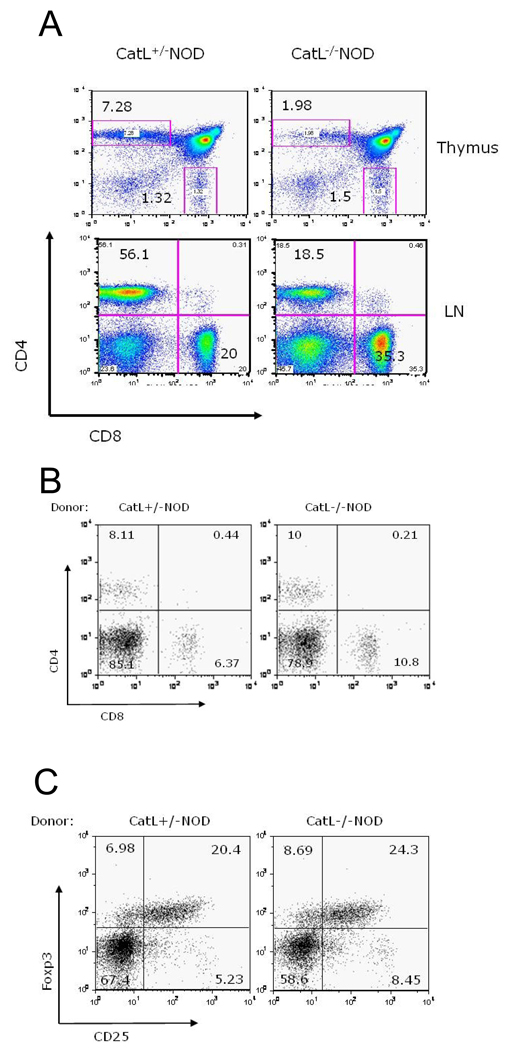
It is known that cathepsin L deficiency causes a profound defect in CD4+ T cell development which is MHC class II haplotype independent (1, 11). In addition, for another colony of Ctsl−/− NOD mice (11), disease resistance was shown to be associated with both the smaller CD4+ T cell population, a reduced CD4:CD8 ratio and an increased ratio of regulatory CD4+ T cells as compared to normal CD4+ T cells. We confirmed these finding in our new colony of Ctsl−/− NOD mice. We found that CD4+ T cells were 3-fold reduced in thymus and lymph nodes of Ctsl-deficient versus Ctsl-sufficient NOD mice (Figure 3A). We also showed that the reduced CD4:CD8 ratio offered protection from diabetes by looking for diabetes development in lymphopenic NOD (NOD.scid) mice that received 15×106 splenocytes from pre-diabetic Ctsl-heterozygote NOD mice. The CD4:CD8 ratio of the Ctsl-heterozygote NOD splenocytes was adjusted to resemble that seen in Ctsl−/− NOD mice (Figure 3A) by the addition of CD8+ T cells isolated from Catl-heterozygote NOD mice. NOD.scid recipients of Ctsl-sufficient or Ctsl-deficient NOD splenocytes were then monitored for hyperglycemia. Up to 75 days post-transfer, neither group developed diabetes. This result raised the possibility that a skewed CD4:CD8 ratio is responsible for protection against diabetes. However, flow cytometric analysis revealed that CD4:CD8 ratios in both groups had approached a ratio normally seen in wild-type mice (Figure 3B) showing that diabetes was not dependent upon a skewed CD4:CD8 ratio. Upon further examination using flow cytometry, we found an enrichment in Foxp3-expressing cells in splenocytes in recipients of T cells from Ctsl-heterozygote and Ctsl−/− NOD mice (Figure 3C). These results suggested that an expanded regulatory T cell population prevented diabetes progression in both groups.
Figure 3. Characterization of T-cells in cathepsin L-deficient (Ctsl−/−) mice.
(A) Ctsl−/− NOD mice exhibit a defect in CD4+ T-cell selection as seen by comparing ratios of CD4/CD8 in the thymus and lymph nodes of Ctsl-sufficient and Ctsl-deficient NOD mice. Thymocytes and lymphocytes from Catl+/− and Catl−/− mice were stained with PerCP conjugated anti-CD4 and APC-conjugated anti-CD8 antibodies (BD Pharmingen), and CD4/CD8 ratios were determined using flow cytometry. (B) CD4:CD8 ratio is adjusted in Ctsl−/− congenic splenocytes to resemble that of Ctsl+/− splenocytes for the adoptive transfer study. Peripheral blood was taken for from NOD.scid recipients for flow cytometry 48 days after the splenocytes were transferred into NOD.scid recipients. (C) Lymphopenic conditions in NOD.scid hosts induce Foxp3+ regulatory T cell expansion, regardless of the genotype of the donor cells. We show Foxp3 profiles of splenocytes from NOD.scid recipients 75 days post-transfer. Cells were gated on the CD4+ T cell population. N=11–13 in each group and representative scans are shown here.
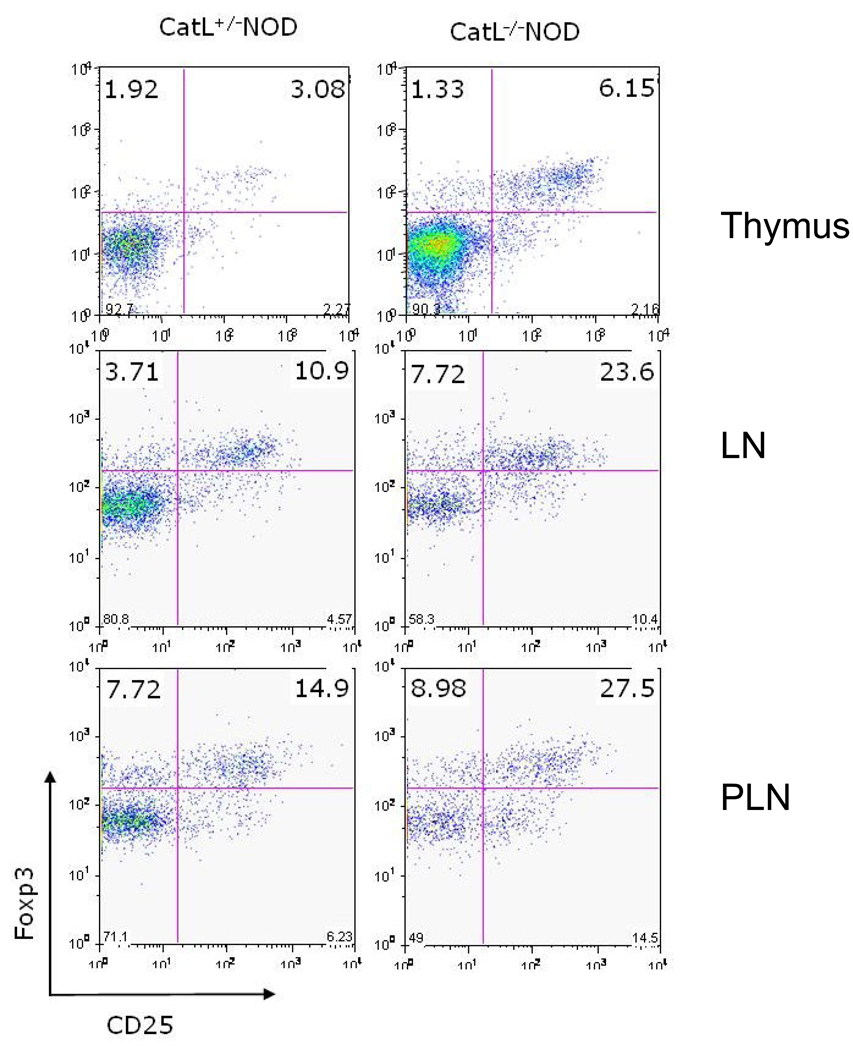
Consistent with the hypothesis that the regulatory T (Tr) cell population is offering protection against diabetes in Ctsl−/− NOD mice, we found a 2-fold higher frequency of Foxp3-expressing CD4+ T cells in unmanipulated Ctsl−/− NOD mice compared to heterozygote controls (Figure 4). We also observed an increased proportion of Foxp3-expressing T cells in Catl-deficient C57BL/6 mice, leading us to believe that the increase in the proportion of Tr cells in the absence of Cathepsin L is not a strain-dependent phenomenon (data not shown). Therefore, in an autoimmune disease-susceptible strain, it is highly likely that an imbalance in the proportion of regulatory to autoreactive effector T cells, in favor of regulatory T cells, led to a completely penetrant form of protection against disease development.
Figure 4. Foxp3+ regulatory T-cells are present at a higher proportion in Ctsl−/− NOD mice in comparison to littermate controls.
Foxp3 profiles of lymphocytes from thymus, lymph nodes (LN), and pancreatic lymph nodes (PLN) from 6 month-old Ctsl-sufficient and Ctsl-deficient NOD mice. Cells were gated on the CD4+ T cell population. Regulatory T cell frequency was assessed using a rabbit polyclonal antibody against Foxp3. N=11–13 in each group and representative scans are shown here.
Diabetogenic T cells develop in Cathepsin L-deficient NOD mice
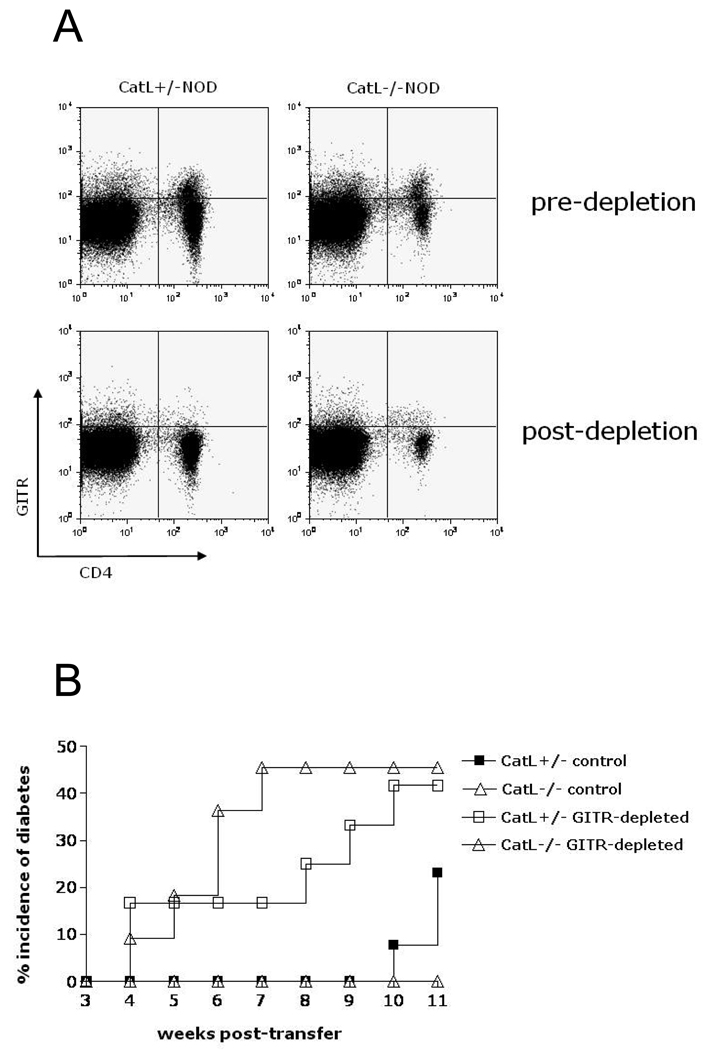
Islet –reactive T cells were shown to exist in mice of the Ctsl−/− NOD colony established by Maehr et al. (11). Here we confirm this occurs in our colony and show the extent of diabetogenic potential of these cells. To determine whether pathogenic T cells develop in our Ctsl−/− NOD mice, we examined the pathogenic potential of their splenocytes. Because we observe an enrichment of Tr cells in Ctsl−/− NOD mice, and our previous experiments in NOD.SCID mice also suggest that the presence of Tr cells may delay the onset of diabetes, we wanted to determine whether Tr-depleted splenocytes from Ctsl−/− NOD mice could induce diabetes. Using GITR antibody-mediated MACS depletion, we depleted GITRhi T cells from splenocytes before transferring them into NOD.scid mice (Figure 5A). GITR, a member of the tumor necrosis receptor family, is highly expressed on Tr cells and its expression – in comparison to CD25 – correlates better with Foxp3 expression (30). 10–15×106 GITR-depleted or control splenocytes from 8–9 week-old Ctsl-heterozygous NOD or Ctsl−/− NOD mice were then adoptively transferred into 5–6 week-old NOD.scid hosts. The incidence of diabetes in each group was determined over the course of 11 weeks. Recipients of control splenocytes from Ctsl-heterozygous NOD mice had a diabetes incidence of 23.07 %, while none of the recipients of control Ctsl−/− NOD splenocytes developed diabetes (Figure 5B). However, recipients of GITR-depleted splenocytes from Ctsl-heterozygous NOD and Ctsl−/− NOD mice developed diabetes at a similar rate and frequency (41.7% and 45.5%, respectively) (Figure 5B). These data show that diabetogenic T cells develop in Ctsl−/− NOD mice, and suggest that splenocytes from these mice are just as capable of inducing diabetes as splenocytes from Ctls-heterozygous NOD mice.
Figure 5. Anti-GITR depletion of splenocytes from Ctsl-sufficient and Ctsl-deficient NOD mice.
(A) Depletion with DTA-1 monoclonal antibody results in the exclusion of GITRhi cells, but not GITRlo cells. (B) Diabetogenic T-cells develop in Ctsl−/− NOD mice and are pathogenic. Transfer of GITR-depleted splenocytes from Ctsl-deficient and Ctsl-sufficient mice induced diabetes in NOD.scid mice with similar kinetics. NOD.scid recipient mice received 10–15×106 GITR-depleted or control splenocytes from Ctsl-deficient and Ctsl-sufficient mice by i.v. injection and their blood glucose levels were monitored for 11 weeks. There were n=11–13 mice in each group; p<0.00001 between control and GITR-depleted groups through 10 weeks post-transfer; p<0.001 between Catl heterozygous and homozygous groups between 7 to 9 weeks post transfer.
Through these adoptive cell transfer studies in NOD.SCID mice, we also rule out effects of the lack of Ctsl expression on non-hematopoietic cells, a finding consistent with that of Maehr et al. (11). Together, our results suggest that the presence of an expanded Tr cell population in Ctsl−/− NOD mice prevents the activation of diabetogenic T cells and mononuclear cell infiltration of beta-islet cells.
DISCUSSION
We have shown that in a spontaneous model of autoimmune diabetes, deficiency in Cathepsin S, Cathepsin B, or Cathepsin L offers protection from type I diabetes mellitus development on the NOD background. Deficiency in Cathepsin S or Cathepsin B led to a decrease in diabetes incidence, whereas deficiency in Cathepsin L provided complete resistance to the disease.
Incomplete penetrance in Ctls−/− or Ctsb−/− NOD mice is surprising given the important roles of Cathepsin S and B in antigen processing and presentation by MHC class II molecules to CD4 cells. At this point it is not known if incomplete penetrance is due to reduced generation of autoreactive T cells or to other non-specific inflammatory activities initiated or perpetuated by these enzymes. The reduced penetrance could be attributed to their ascribed functions outside of MHC class II antigen processing and presentation. Some support for this idea is provided by the observation that in NOD spenocytes, Ctss is not required for removal of Ii remnants from class II MHC molecules, as demonstrated using a Ctss-specific inhibitor (11). Additionally, Ctss has been recently shown to participate in leukocyte infiltration, medial elastic lamina degradation, endothelial cell invasion, and neovascularization in atherosclerosis-prone mice (31). Ctsb may contribute to the autoimmune processes through pathways involving inflammation and apoptosis. The absence of Ctsb results in decreased liver injury as a result of mitigated TNF alpha-induced apoptosis (13). Ctsb has also been show to participate in the export of TNFalpha from macrophages (32). In addition, Ctsb regulates matrix degradation and the invasiveness of human glioblastoma cell lines, and therefore could also regulate the accessibility of autoreactive T cells to the pancreas (33). Because even diabetic Ctss- or Cstb-deficient NOD mice displayed decreased peri-insulits and islet cell infiltration, it is possible that pathogenic T cells were not optimally activated to program tissue infiltration. It is possible that if Ctss- and Ctsb-deficient mice were allowed to age beyond six months of age, diabetes incidence would increase. Some evidence for this is suggested by the similarity in insulitis score between diabetic and non-diabetic Ctsb heterozygous mice (Figure 2). As more autoreactive T cells are activated by the increased presentation of pancreatic antigens and the threshold for autoreactivity is reached, diabetes would likely ensue.
Unlike Ctss- or Cssb-deficient NOD mice, Ctsl-deficient NOD mice exhibit complete disease resistance. We have ruled out the possibility that diabetogenic T cells do not develop in Ctsl deficient mice, because Tr cell-depleted splenocytes from Ctsl-deficient NOD mice were equally capable of inducing diabetes in NOD.SCID mice, compared to splenocytes from Ctsl-sufficient NOD mice. These findings are different from those of Maehr et al., who also examined the phenotype of Ctsl-deficiency in NOD mice and found that the pathogenicity of splenocytes from Ctsl-deficient NOD mice was reduced (11). We also found that Ctsl-deficient NOD mice were not more resistant to T cell-dependent tissue damage, as transferred diabetogenic T cell clones were perfectly capable of inducing disease in these mice. Instead, an increase in the frequency of Tr cells appears to be primarily responsible for disease resistance. Earlier studies by Salomon et al. demonstrated that the transfer of Tr cells inhibits the progression of diabetes in NOD mice lacking Tr cells (34). In contrast to observations by Chen and colleagues (35), we find that protection mediated by Tr cells may also occur prior to islet infiltration – not only in the pancreatic tissue itself – as suggested by the complete lack of islet cell infiltration observed in Ctsl-deficient NOD mice.
While our studies provided a likely explanation for disease resistance in Ctsl-deficient NOD mice, the roles of Ctsb and Ctss in autoimmune diabetes deserve further examination. Manipulation of Cathepsin activity may offer new insights to clinical applications in autoimmune diabetes as well as other autoimmune diseases.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by grants from NIH (RO1 DK063159 [RCL] and AI34206 [AYR] and a Pilot and Feasibility grant awarded by the Diabetes Endocrinology Research Center (NIH P30 DK017047) [AYR]. We would like to acknowledge Dr. Alexander Chervonsky (Department of Pathology, University of Chicago, Chicago, IL) for help with data collection and review of study proposal.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Honey K, Rudensky AY. Lysosomal cysteine proteases regulate antigen presentation. Nat Rev Immunol. 2003;3:472–482. doi: 10.1038/nri1110. [DOI] [PubMed] [Google Scholar]
- 2.Beers C, Burich A, Kleijmeer MJ, Griffith JM, Wong P, Rudensky AY. Cathepsin S controls MHC class II-mediated antigen presentation by epithelial cells in vivo. J Immunology. 2005;174:1205–1212. doi: 10.4049/jimmunol.174.3.1205. [DOI] [PubMed] [Google Scholar]
- 3.Aoki T, Kataoka H, Ishibashi R, Nozaki K, Hashimoto N. Cathepsin B, K, and S are expressed incerebral aneurysms and promote the progression of cerebral aneurysms. Stroke. 2008;39:603–2610. doi: 10.1161/STROKEAHA.107.513648. [DOI] [PubMed] [Google Scholar]
- 4.Sukhova GK, Zhang Y, Pan JH, Wada Y, Yamamoto T, Naito M, Kodama T, Tsimikas S, Witztum JL, Lu ML, Sakara Y, Chin MT, Libby P, Shi GP. Deficiency of cathepsin S reduced atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;111:897–906. doi: 10.1172/JCI14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burden RE, Snoddy P, Buick RJ, Johnston JA, Walker B, Scott CJ. Recombinant cathepsin S propeptide attenuates cell invasion by inhibition of cathepsin L-like proteases in tumor microenvironment. Mol Cancer Ther. 2008;7:538–547. doi: 10.1158/1535-7163.MCT-07-0528. [DOI] [PubMed] [Google Scholar]
- 6.Sever S, Altintas MM, Nankoe SR, Möller CC, Ko D, Wei C, Henderson J, de Re EC, Hsing L, Erickson A, Cohen CD, Kretzler M, Kerjaschki D, Rudensky A, Nikolic B, Reiser J. Proteolytic processing of dynamin by cytoplasmic cathepsin L is a mechanism for proteinuric kidney disease. J Clin Invest. 2007;117:2095–2104. doi: 10.1172/JCI32022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitamoto S, Sukhova GK, Sun J, Yang M, Libby P, Love V, Duramad P, Sun C, Zhang Y, Yang X, Peters C, Shi GP. Cathepsin L deficiency reduces diet-induced atherosclerosis in low-density lipoprotein receptor-knockout mice. Circulation. 2007;115:2065–2075. doi: 10.1161/CIRCULATIONAHA.107.688523. [DOI] [PubMed] [Google Scholar]
- 8.Baici A, Lang A, Zwicky R, Müntener K. Cathepsin B in osteoarthritis:uncontrolled proteolysis in the wrong place. Semin Arthritis Rheum. 2005;34:24–28. doi: 10.1016/j.semarthrit.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Taleb S, Clément K. Emerging role of cathepsin S in obesity and its associated diseases. Clin Chem Lab Med. 2007;45:328–332. doi: 10.1515/CCLM.2007.083. [DOI] [PubMed] [Google Scholar]
- 10.Hsing LC, Rudensky AY. The lysosomal cysteine proteases in MHC class II antigen presentation. Immunol Rev. 2005;207:229–241. doi: 10.1111/j.0105-2896.2005.00310.x. [DOI] [PubMed] [Google Scholar]
- 11.Maehr R, Mintern JD, Herman AE, Lennon-Duménil A-M, Mathis D, Benoist C, Ploegh HL. Cathepsin L is essential for onset of autoimmune diabetes in NOD mice. J Clin Invest. 2005;115:2934–2943. doi: 10.1172/JCI25485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakagawa TY, Brissette WH, Lira PD, Griffiths RJ, Petrushova N, Stock J, McNeish JD, Eastman SE, Howard ED, Clarke SR, Rosloniec EF, Elliott EA, Rudensky AY. Impaired invariant chain degradation and antigen presentation and diminished collagen-induced arthritis in cathepsin S null mice. Immunity. 1999;10:207–217. doi: 10.1016/s1074-7613(00)80021-7. [DOI] [PubMed] [Google Scholar]
- 13.Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C, Kaufmann SH, Gores GJ. Cathepsin B contributes to TNF-α-mediated hepatocytes apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest. 2000;106:1127–1137. doi: 10.1172/JCI9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halangk W, Lerch MM, Brandt-Nedelev B, Roth W, Ruthenbuerger M, Reinheckel T, Domschke W, Lippert H, Peters C, Deussing J. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J Clin Invest. 2000;106:773–781. doi: 10.1172/JCI9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roth W, Deussing J, Botchkarev VA, Pauly-Evers M, Saftig P, Hafner A, Schmidt P, Schmahl W, Scherer J, Anton-Lamprecht I, Von Figura K, Paus R, Peters C. Cathepsin L deficiency as molecular defect of furless: hyperproliferation of keratinocytes and perturbation of hair follicle cycling. FASEB J. 2000;14:2075–2086. doi: 10.1096/fj.99-0970com. [DOI] [PubMed] [Google Scholar]
- 16.Shi G-P, Villadangos JA, Dranoff G, Small C, Gu L, Haley KJ, Riese R, Ploegh HL, Chapman HA. Cathepsin S required for normal MHC Class II peptide loading and germinal center development. Immunity. 1999;10:197–206. doi: 10.1016/s1074-7613(00)80020-5. [DOI] [PubMed] [Google Scholar]
- 17.Halangk W, Lerch MM, Brandt-Nedelev B, Roth W, Rughenbuerger M, Reinheckel T, Domschke W, Lippert H, Peters C, Deussing J. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J Clin Invest. 2000;106:773–781. doi: 10.1172/JCI9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deeg MA, Bowen RF, Williams MD, Olson LK, Kirk EA, LeBoeuf RC. Increased expression of GPI-specific phospholipase D in mouse models of type 1 diabetes. Am J Physiol Endocrinol Metab. 2001;281:E147–E154. doi: 10.1152/ajpendo.2001.281.1.E147. [DOI] [PubMed] [Google Scholar]
- 19.Debussche X, Lormeau B, Boitard C, Toublanc M, Assan R. Course of pancreatic beta cell destruction in prediabetic NOD mice: a histomorphometric evaluation. Diabetes Metab. 1994;20:282–290. [PubMed] [Google Scholar]
- 20.Yamano S, Atkinson JC, Baum BJ, Fox PC. Salivary gland cytokine expression in NOD and normal BALB/c mice. Clin Immunol. 1999;92:265–275. doi: 10.1006/clim.1999.4759. [DOI] [PubMed] [Google Scholar]
- 21.Serreze DV, Leiter EH. Genetic and pathogenic basis of autoimmune diabetes in NOD mice. Curr Opin Immunol. 1994;6:900–906. doi: 10.1016/0952-7915(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 22.Leiter EH, Serreze DV. Antigen presenting cells and the immunogenetics of autoimmune diabetes in NOD mice. Reg Immunol. 1992;4:263–273. [PubMed] [Google Scholar]
- 23.Yoon J-W, Jun H-S. Autoimmune destruction of pancreatic β cells. Amer J Therap. 2005;12:580–591. doi: 10.1097/01.mjt.0000178767.67857.63. [DOI] [PubMed] [Google Scholar]
- 24.Prochazka M, Serreze DV, Frankel WN, Leiter EH. NOR/Lt mice: MHC-matched diabetes-resistant control strain for NOD mice. Diabetes. 1992;41:98–106. doi: 10.2337/diab.41.1.98. [DOI] [PubMed] [Google Scholar]
- 25.Lakka SS, Gondi CS, Yanamandra N, Olivero WC, Dinh DH, Gujrati M, Rao JS. Inhibition of cathepsin B and MMP-9 gene expression in glioblastoma cell line via RNA interference reduces tumor cell invasion, tumor growth and angiogenesis. Oncogene. 2004;23:4681–4689. doi: 10.1038/sj.onc.1207616. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Sukhova GK, Sun JS, Xu WH, Libby P, Shi GP. Lysosomal cysteine proteases in atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:1359–1366. doi: 10.1161/01.ATV.0000134530.27208.41. [DOI] [PubMed] [Google Scholar]
- 27.Woods CC, Sundar K, Tessler C, Lebsack TW, Grainger L, Nielsen A, Bleich D, DeLuca D. Tissue inhibitor of metalloproteinase-2 inhibits T-cell infiltration and preserves pancreatic beta-cell function in an in vitro type 1 diabetes mellitus model. J Autoimmun. 2006;27:28–37. doi: 10.1016/j.jaut.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 29.Wong FS, Karttunen J, Dumont C, Wen L, Visintin I, Pilip IM, Shastri N, Pamer EG, Janeway CA., Jr Identification of an MHC class I-restricted autoantigen in type 1 diabetes by screening an organ-specific cDNA library. Nat Med. 1999;5:1026–1031. doi: 10.1038/12465. [DOI] [PubMed] [Google Scholar]
- 30.Ono M, Shimizu J, Miyachi Y, Sakaguchi S. Control of autoimmune myocarditis and multiorgan inflammation by glucocorticoid-induced TNF receptor family-related protein(high), Foxp3-expressing CD25+ and CD25− regulatory T cells. J Immunol. 2006;176:4748–4756. doi: 10.4049/jimmunol.176.8.4748. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Sukhova GK, Sun JS, Xu WH, Libby P, Shi GP. Lysosomal cysteine proteases in atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:1359–1366. doi: 10.1161/01.ATV.0000134530.27208.41. [DOI] [PubMed] [Google Scholar]
- 33.Woods CC, Sundar K, Tessler C, Lebsack TW, Grainger L, Nielsen A, Bleich D, DeLuca D. Tissue inhibitor of metalloproteinase-2 inhibits T-cell infiltration and preserves pancreatic beta-cell function in an in vitro type 1 diabetes mellitus model. J Autoimmun. 2006;27:28–37. doi: 10.1016/j.jaut.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 35.Chen Z, Herman AE, Matos M, Mathis D, Benoist C. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J Exp Med. 2005;202:1387–1397. doi: 10.1084/jem.20051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.