Abstract
The Forkhead/Fox transcription factor Foxc2 is a critical regulator of vascular development. However, the role of Foxc2 in pathological angiogenesis in cancer remains unknown. Here we show that FoxC2 is highly expressed in human breast and colonic tumors and in the tumor endothelium in human and mouse melanomas. Using the B16 melanoma tumor model, we investigated the function of Foxc2 in tumor angiogenesis. After subcutaneous injection of B16 melanoma cells, primary tumor growth as well as neovascularization was markedly reduced in mice lacking one copy of the Foxc2 gene (Foxc2+/-). Consistently, expression levels of several angiogenic factors, including vascular endothelial growth factor (Vegf), matrix metallopeptidase 2 (Mmp2), and platelet-derived growth factor-B (Pdgfb), were significantly decreased in B16 tumors grown in Foxc2+/- mice, and tumor blood vessels formed in Foxc2+/- mice showed reduced coverage of mural cells and endothelial cell apoptosis. In addition, the tumor tissue in Foxc2+/- mice had an accumulation of necrotic cells. Taken together, these findings demonstrate that haplodeficiency of Foxc2 results in impaired formation of tumor blood vessels as well as reduced tumor growth and thereby provide evidence that Foxc2 is critical for tumor development and angiogenesis.
Keywords: Tumor angiogenesis, VEGF, Dll4, PDGF, Forkhead, endothelial cell
Introduction
Tumor initiation and progression involve complex interactions between tumor cells and their microenvironment [1]. One of the critical processes for tumor progression is angiogenesis (neovascularization) in the tumor microenvironment [2, 3, 4]. The tumor vasculature supplies oxygen to the tumor tissue and facilitates cancer cell invasion and metastasis, whereas the formation of tumor blood vessels is regulated by multiple angiogenic factors secreted from tumor cells themselves as wells as various cells in the tumor microenvironment. Tumor blood vessels are usually disorganized and have excessive branching. Perivascular mural cells (vascular smooth muscle cells and pericytes) are loosely associated with the tumor endothelium. Although the roles of angiogenic factors such as vascular endothelial growth factor (VEGF) in promoting tumor angiogenesis have been extensively studied [2, 3, 4], little is known about transcription factors that are involved in tumor neovascularization and growth.
Recent evidence demonstrates that several Forkhead box (Fox) transcription factors, including FoxO and FoxM, are critical for cancer development and progression [5]. For example, FoxM1 in glioma and pancreatic cancer cells promotes angiogenesis [6, 7]. Whereas recent studies show that human FOXC2 is strongly expressed in highly aggressive basal-like breast cancers and is responsible for invasion and metastasis [8], murine Foxc2 is expressed in blood vessels during embryonic development and is required for formation of the aortic arch, remodeling of primitive blood vessels into a vascular network, and arterial-venous specification during embryonic development [9, 10, 11, 12]. Our laboratory has recently shown that Foxc2 is crucial for endothelial cell migration and microvessel formation in vitro [13]. These data indicate that Foxc2 plays important roles in vascular formation [14]; however, its functions in tumor blood vessels need to be revealed.
In this study, we show that the expression of human and mouse FoxC2 is detected in the tumor endothelium. We demonstrate by subcutaneous implantation of B16 melanoma that Foxc2 heterozygous mutant mice exhibit diminished tumor growth, accompanied with reduced formation of tumor blood vessels. Expression of several angiogenic factors, including Vegf, Mmp2 and Pdgfb, is greatly decreased in B16 tumors grown in Foxc2 heterozygous mutant mice, and these tumor blood vessels show impaired smooth muscle coverage and apoptosis of endothelial cells. These findings indicate an essential role of Foxc2 in tumor angiogenesis.
Materials and Methods
Cell culture
Murine B16-F10 melanoma cells, provided by Dr. Jin Chen of Vanderbilt University Medical Center, were cultured at 37°C in a 5 % CO2 incubator in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10 % fetal bovine serum, 50 IU/mL penicillin, and 50 μg/mL streptomycin sulfate.
In vivo tumor development
Foxc2 heterozygous mutant (Foxc2+/-) mice were maintained and genotyped as previously described [10]. Foxc2+/- mice were backcrossed for seven generations onto the C57BL/6J genetic background, and wild-type (WT) and Foxc2+/- littermates (seven- to eight-weeks-old) from backcrossing were used in this study. B16 melanoma cells (5 × 105) in 100 μl of PBS were implanted subcutaneously into the flank of WT or Foxc2+/- mice. Each group consisted of five mice, and tumor growth was monitored periodically. Eleven days later, the mice were sacrificed, and the tumors were analyzed.
Immunohistochemistry
Human frozen sections were obtained from BioChain Institute, Inc. All of mouse sections were frozen in the OCT compound and cut between 7-8 μm. The primary antibodies used were as follows: goat anti-FOXC2 (Abcam), anti-mouse smooth muscle alpha actin (Sigma-Aldrich), and anti-mouse PECAM-1 (BD Pharmingen). Secondary antibodies used: donkey anti-goat biotin-SP conjugated (Jackson ImmunoResearch) and alexa fluor 594 donkey anti-rat conjugate (Invitrogen). Further details are given in Supplementary Material.
RNA extraction and quantitative real-time RT-PCR
Isolation of total RNA from tumor tissues and cDNA synthesis were performed using RNeasy Mini Kit (Qiagen) and iScript (Bio-Rad), respectively. Real-time RT-PCR analysis was carried out using the SYBR GREEN PCR Master Mix (ABI) or Absolute SYBR Fluorescein Mix (Thermo Scientific) and i-Cycler (Bio-Rad) according to the manufacturers' instructions. The primers used are shown in Supplementary Table. Each data was normalized by the expression level of peptidylprolyl isomerase A (Ppia), as an internal control.
TUNEL assay
Terminal deoxy-nucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) assays were carried out on cryosections using ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (Chemicon International), according to the manufacturer's instruction. The sections were counterstained with 0.4% methyl green for optical microscopic analysis. Further details are given in Supplementary Material.
Statistical analysis
All data are presented as the mean ± SD. Statistical analysis was performed using the standard 2-tailed Student's t test on Microsoft Excel. A probability value of less than 0.05 was considered statistically significant.
Results
Human and mouse FoxC2 expression in the tumor endothelium and breast and colonic adenocarcinomas
Although it has recently been shown that FOXC2 is expressed in human invasive breast carcinomas [8], its expression remains less characterized in other types of tumors. We therefore tested FOXC2 expression in various human tumors using T-MTA-6A tissue array (NCI/NIH). FOXC2 immunoreactivity was observed in majority of breast adenocarcinomas including lobular and ductal adenocarcinoma and about half of colonic adenocarcinoma (Fig. 1A), whereas lung and ovarian tumor tissues did not demonstrate definitive immunoreactivity (data not shown). Evaluation of tissues at higher power demonstrated predominant nuclear and perinuclear staining in cancer cells (Fig. 1A, arrowheads), however cytosolic localization was also observed in few cases. Thus, overexpression of FOXC2 protein in the majority of human breast cancer and large proportion of colonic cancer suggest that it may play a role in the pathogenesis and/or progression of cancer.
Fig. 1.
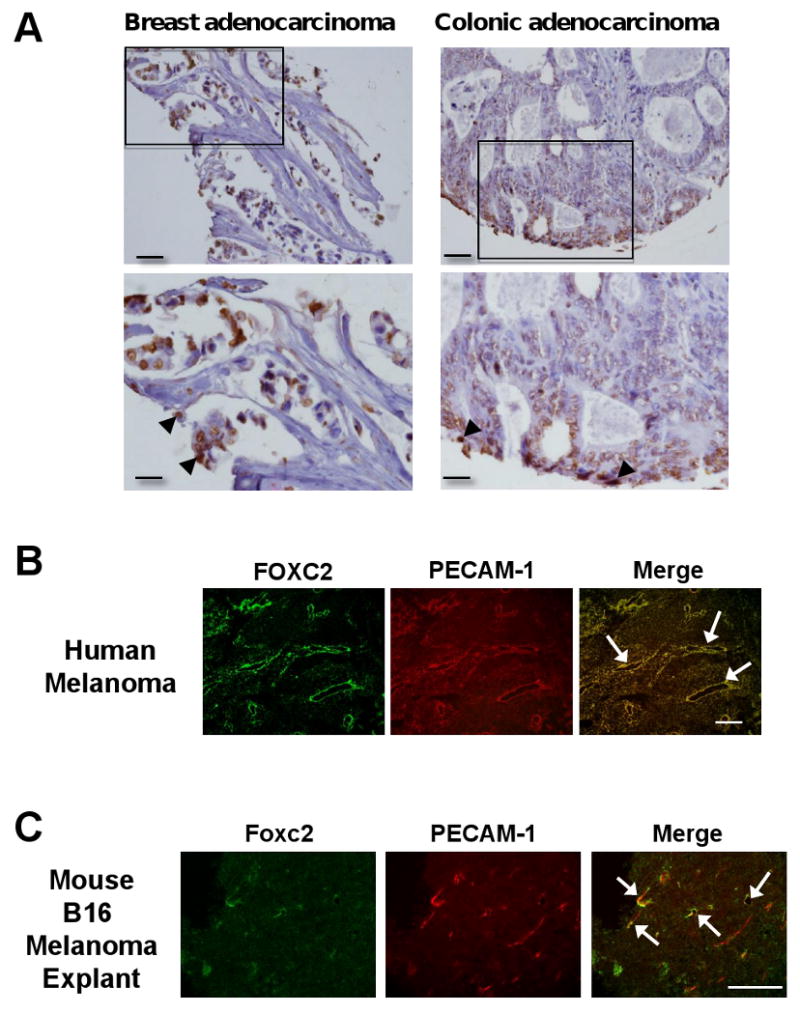
Expression of human and mouse FoxC2. (A) FOXC2 expression in the human breast and colon tumors. FOXC2 expression in lobular adenocarcinoma of breast and colonic adenocarcinoma at 20× (upper panels) and 40× (lower panels) magnifications; the areas presented at higher magnification are indicated by square. Note that FOXC2 immunostaining is primarily limited to cancer cells in tumor tissue. Scale bars, 500 μm and 100 μm for upper and lower, respectively. (B) Expression of FOXC2 is detected in tumor endothelial cells of human malignant melanoma. Frozen sections of malignant melanoma in the human skin were co-immunostained with anti-FOXC2 and anti-PECAM-1 antibodies. FOXC2 protein (green) was detected in tumor endothelial cells positive for PECAM-1 (red). Arrows indicate FOXC2-positive human endothelial cells. Scale bar, 250 μm. (C) Expression of Foxc2 is detected in endothelial cells of mouse B16 melanoma. Co-immunostaining with anti-FOXC2 and anti-PECAM-1 antibodies in mouse B16 tumors grown in C57BL/6 mice for 11 days after subcutaneous injection. Arrows indicate overlapping expression of Foxc2 (red) and PECAM-1 (green) in tumor endothelial cells. Scale bar, 250 μm.
Since murine Foxc2 is expressed in the developing blood vessels [10, 11], we next examined expression patterns of human FOXC2 during tumor angiogenesis using sections from human malignant melanoma (67 years old male; skin). By co-immunostaining with anti-FOXC2 antibody and anti-PECAM-1 antibody as an endothelial cell marker, FOXC2 protein was detected in tumor endothelial cells positive for PECAM-1 in human melanoma tissues (Fig. 1B). Foxc2 protein was also detected in endothelial cells of microvessels in B16-F10 murine melanoma tumors subcutaneously implanted into C57BL/6 mice (Fig. 1C). These results indicate that FoxC2 is expressed in tumor blood vessels and is likely to play a role in angiogenesis during tumor development.
Reduced tumor growth and angiogenesis in Foxc2 heterozygous mutant mice
We next tested the effects of Foxc2 on B16 melanoma tumor growth in mice after subcutaneous implantation of B16 cells. Because Foxc2 homozygous null mutants die pre- and perinatally, Foxc2 heterozygous mutant (Foxc2+/-) mice backcrossed to the C57BL/6 genetic background were used to monitor tumor development in vivo (Fig. 2). Remarkably, the growth of subcutaneous B16 tumors in Foxc2+/- mice was significantly decreased compared to that in WT mice (0.59 ± 0.16 g versus 0.32 ± 0.12 g; P = 0.02) (Fig. 2A and B). We also found that Foxc2+/- mice had sparse cells with a possible accumulation of necrotic cells in the tumor (Supplementary Fig. 1).
Fig. 2.
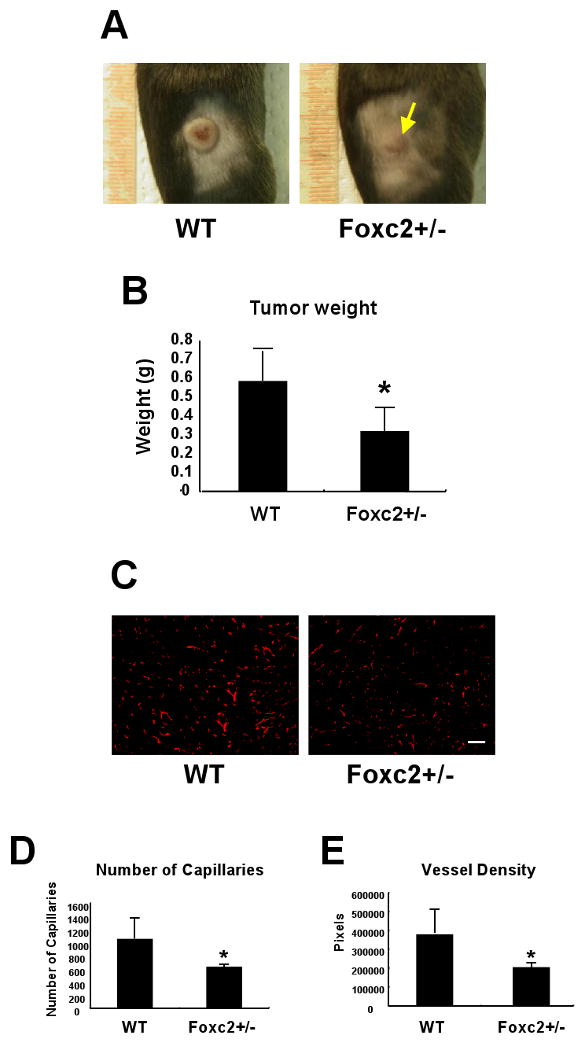
Tumor development and angiogenesis is impaired in Foxc2+/- mice. (A) Subcutaneous growth of B16 melanoma cells inoculated in WT and Foxc2+/- mice at day 11 after injection. Yellow arrow indicates reduced tumor growth in Foxc2+/- mouse. (B) The difference in tumor weight between WT and Foxc2+/- mice was examined 11 days after injection of B16 melanoma cells. The data reflect five independent experiments. Data are presented as mean ± SD (n = 5). Statistical significance was determined by Student's t-tests. *, P < 0.05 versus WT. (C-E) Tumor angiogenesis is reduced in Foxc2+/- mice. (C) Cryosections from WT and Foxc2+/- mice 11 days after injection of B16 melanoma cells were immunostained with anti-PECAM-1 antibody. Scale bar, 250 μm. (D) The total number of capillaries was calculated by counting PECAM-1 positive endothelial cells in B16 tumors. Results are presented as mean ± SD from four or five independent experiments in which nine microscopic fields (total magnification ×200) were analyzed by the ImageJ program. Statistical significance was determined by Student's t-tests (*p<0.05 versus WT). (E) Total vessel density was calculated by counting PECAM-1 positive vessel area. Results are presented as mean ± SD from four or five independent experiments in which nine fields of each specimen (total magnification ×200) were analyzed by the ImageJ program. The Y axis represents the number of pixels. Statistical significance was determined by Student's t-tests. *, P < 0.05 versus WT.
To define the role of Foxc2 in tumor angiogenesis, we further analyzed the formation of blood vessels in B16 tumors grown in WT and Foxc2+/- mice by PECAM-1 immunostaining. Compared with WT mice, Foxc2+/- mice exhibited the reduced number of PECAM-1 positive endothelial cells (Fig. 2C). Quantitative analyses revealed that Foxc2+/- mice had a remarkable decrease in the number of capillaries (Foxc2+/- versus WT; 633.5 ± 61.5 versus 1061.3 ± 320.7, P = 0.040) (Fig. 2D) and total vessel density (Foxc2+/- versus WT; 206220 ± 31007 versus 380409 ± 123940, P = 0.034) (Fig. 2E). This considerable decrease in neovascularization in B16 tumors correlates with impaired tumor growth in Foxc2+/- mice (Fig. 2A and B), suggesting that Foxc2 is crucial for tumor angiogenesis.
Differential expression of angiogenic factors in B16 tumors grown in Foxc2+/- mice
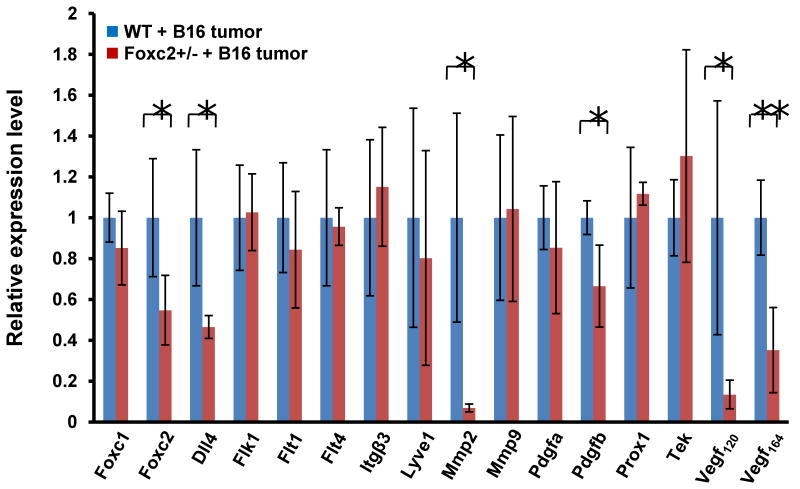
Because tumor growth is dependent on angiogenesis, we therefore analyzed expression levels of genes associated with angiogenesis in B16 tumors grown subcutaneously in WT and Foxc2+/- mice 11days after implantation (Fig. 3). With respect to the expression of Foxc2 and a related Foxc gene, Foxc1 [10, 11], expression of Foxc2, but not Foxc1, was reduced by 45.3% in B16 tumors of Foxc2+/- mice, compared with WT mice. Given the low levels of Foxc2 expression in B16 melanoma cells (data not shown), its reduced expression is likely attributable to host-derived cells. Of interest, transcriptional levels of VEGF-A (Vegf120 and Vegf164) and matrix metallopeptidase 2 (Mmp2) were significantly decreased in Foxc2+/- mice. Platelet-derived growth factor (PDGF) signaling has been implicated in cancer, including tumor angiogenesis [15], and Pdgfb expression was indeed reduced in Foxc2+/- mice compared with WT mice. Whereas Foxc2 regulates Delta-like 4 (Dll4) and integrin β3 (Itgβ3) in endothelial cells [11, 13, 16], expression of Dll4 in tumors was notably decreased in Foxc2+/- mice, but Itgβ3 expression was detected at similar levels in WT and Foxc2+/- mice. Whereas tumor-associated lymphangiogenesis is critical for the promotion of metastasis [17], expression levels of lymphatic endothelial markers, Prox1 and Lyve1, were similarly expressed in B16 tumors of WT and Foxc2+/- mice. Thus, gene expression profiling reveals that several angiogenic factors such as Vegf, Mmp2, and Pdgfb are reduced in B16 tumors grown in Foxc2+/- mice.
Fig. 3.
Differential expression of (lymph)angiogenesis-related genes in B16 tumors grown subcutaneously in Foxc2+/- mice for 11 days. Fold change in mRNA levels of Foxc genes as well as of genes related to angiogenesis and lymphangiogenesis was measured by real-time RT-PCR. Note a significant reduction in expression of Vegf120, Vegf164, Dll4, Mmp2, and Pdgfb in B16 tumors grown in Foxc2+/- mice. Results are presented as mean ± SD (n = 4 or 5). Statistical significance was determined by Student's t-tests. *, P < 0.05; **, P < 0.01 versus WT.
Tumor blood vessels formed in Foxc2+/- mice show reduced coverage by mural cells
Recruitment of mural cells into the tumor endothelium (i.e., endothelial-mural cell association) is an important process in tumor neovascularization and is regulated, at least in part, by the PDGF signaling pathway [15]. In B16 tumors grown in WT mice 11 days after implantation, smooth muscle α-actin (αSMA) was detected in mural cells associated with endothelial cells (Fig. 4A, white arrows), as well as tumor-associated fibroblasts critical for cancer progression and secret various growth factors such as VEGF (Fig. 4A, yellow arrows). In contrast, Foxc2+/- mice had little or almost no mural cell layer surrounding the tumor endothelium (Fig. 4A, arrowheads) and fewer αSMA-positive fibroblasts, compared with B16 tumors grown in WT mice. These findings, together with reduced Pdgfb expression (Fig. 3), indicate that the tumor blood vessels in Foxc2+/- mice show impairment in mural cell coverage.
Fig. 4.
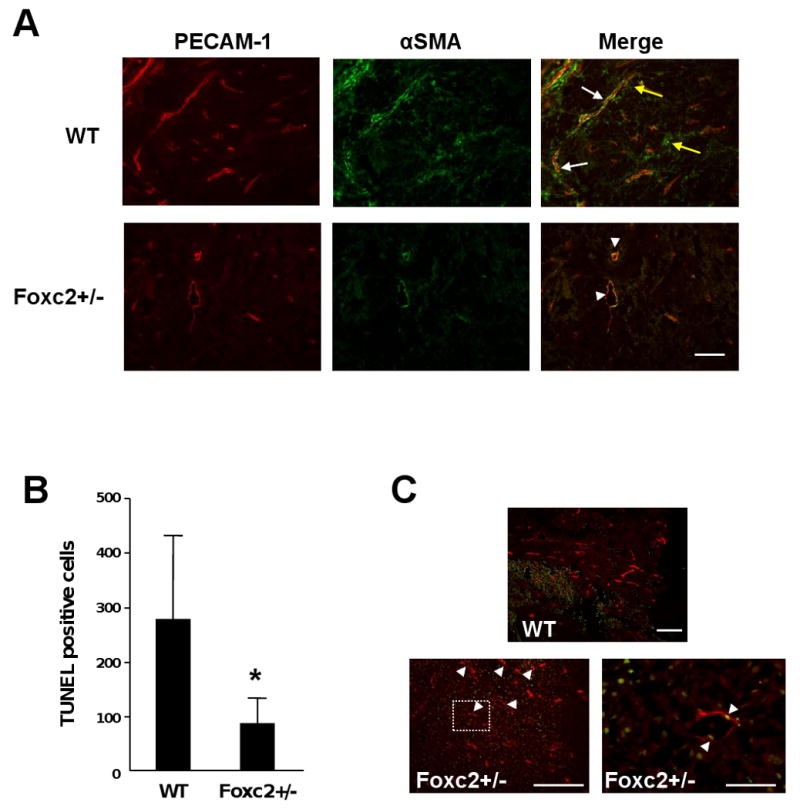
Tumor blood vessels in Foxc2+/- mice show reduced coverage of mural cells and endothelial cell apoptosis. (A) Tumor tissues isolated from WT and Foxc2+/- mice 11 days after implantation of B16 melanoma cells were co-immunostained with PECAM-1 (red) and smooth muscle alpha-actin (αSMA) antibodies. The merged images show the coverage of mural cells in tumor blood vessels. While the tumor endothelium was surrounded by the smooth muscle layer (white arrows) in WT mouse, endothelial cells in tumors of Foxc2+/- mouse exhibited almost no coverage of smooth muscle cells (arrowheads). Tumor-associated fibroblasts expressing αSMA (yellow arrows) were barely detected in Foxc2+/- mouse. Scale bar, 250 μm. (B and C) Analysis of apoptotic cells in B16 tumors by TUNEL staining. (B) Cell counts of positive cells from TUNEL assay on B16 tumor tissue taken from WT and Foxc2+/- mice. Statistical significance was determined by Student's t-tests. *, P < 0.05 versus WT. (C) In contrast with WT mouse, tumor endothelial cells in Foxc2+/- mouse underwent apoptosis detected by TUNEL staining (green) and immunostaining with anti-PECAM-1 antibody (red). Arrowheads indicate TUNEL-positive endothelial cells (yellow). Scale bar, 250 μm. The right panel for Foxc2+/- mouse represents a high magnification (scale bar, 50 μm) of the boxed area in the left panel.
Apoptotic endothelial cells of tumor vessels formed in Foxc2+/- mice
VEGF signaling is essential for endothelial cell survival [2, 3], whereas inhibition of the PDGFR-β pathway causes apoptosis in vascular endothelial cells [18, 19]. We therefore examined endothelial cell survival in B16 tumors by TUNEL assay (Fig. 4B and C). While overall apoptosis in B16 tumors grown in Foxc2+/- mice was significantly less than in those grown in WT mice (Fig. 4B), it is important to note that tumor blood vessels formed in Foxc2+/- mice significantly showed endothelial cell apoptosis (Fig. 4C, arrowheads). These results suggest that haplodeficiency of Foxc2 results in dysfunctional tumor vessels, thereby suppressing tumor growth.
Discussion
Since the late Judah Folkman proposed the original hypothesis that the formation of new blood vessels (angiogenesis) is critical for tumor growth [20], significant progress has been made in our understanding of tumor angiogenesis. Indeed, numerous clinical trials are underway to test the efficacy of antiangiogenic drugs that block critical signaling pathways such as VEGF, and a couple of inhibitors of angiogenesis have recently been approved by the U.S. Food and Drug Administration [21]. Yet the roles of transcription factors in tumor neovascularization remain largely unknown. We show in this paper that Foxc2 has a pivotal role in tumor angiogenesis. To our knowledge, this is the first report on the contribution of Foxc2 to pathological angiogenesis.
Expression of both human and mouse FoxC2 is highly detected in tumor endothelial cells (Fig. 1B and C), and the formation of tumor blood vessels in Foxc2+/- mice is markedly diminished compared with WT mice (Fig. 2). Given that endothelial cells isolated from Foxc2+/- mice show decreased migration and microvessel outgrowth in vitro [13], this vascular phenotype is most likely to contribute to reduced tumor growth in Foxc2+/- mice. Interestingly, FOXC1 expression is also detected in vascular endothelial cells in human melanomas, whereas we found normal formation of B16 tumors grown subcutaneously in Foxc1+/- mice (Supplementary Fig. 2). Although Foxc1 and Foxc2 have overlapping roles in vascular development [10, 11], reasons for the discrepancy between Foxc1 and Foxc2 mutant mice in tumor formation remain unclear.
We found that Foxc2+/- mice bearing B16 tumors had reduced expression of pro-angiogenic factors (Fig. 3). An intriguing phenotype is a significant reduction in expression of VEGF, an essential regulator of tumor angiogenesis. Tumor cells express and secrete VEGF, and various host cells in the tumor microenvironment themselves also express VEGF, including tumor-associated fibroblasts [22]. In addition, autocrine VEGF signaling promotes endothelial cell survival [23]. Thus, primary source(s) of reduced VEGF expression in B16 tumors grown in Foxc2+/- mice remain unclear, but insufficient VEGF signaling is highly likely to lead to reduced tumor angiogenesis in these mutant mice. Decreased expression of Mmp2 in B16 tumors grown in Foxc2+/- mice is consistent with the previous findings that tumor growth and angiogenesis is reduced in Mmp2-/- mice [24] and that stroma-derived MMP2 stimulates tumor growth [25]. The importance of our observation is also reinforced by the fact that MMP2 can release biologically active VEGF [26]. Dll4 is a direct target of Foxc2 in endothelial cells [11, 16], and Foxc2+/- mice bearing B16 tumors showed decreased expression of Dll4. However, Dll4 plays a critical role in suppressing angiogenic sprouting as a negative feedback loop of VEGF signaling, and lack of Dll4 function inhibits tumor development by inducing excessive, nonproductive angiogenesis [27]. Because the formation of tumor blood vessels was impaired in Foxc2+/- mice, it is therefore possible that the drastic reduction of VEGF signaling attenuates the influence of the partial decrease of Dll4 on neovascularization in Foxc2+/- mice.
PDGF signaling is integral to the maturation and stabilization of blood vessels, by regulating the interaction between endothelial and mural cells. Tumor blood vessels within B16 tumors grown in Foxc2+/- mice showed reduced mural cell coverage and endothelial cell apoptosis, indicating vessel destabilization and regression (Fig. 4). Although the precise mechanisms by which PDGF signaling functions in tumor angiogenesis still remain elusive [15], progressive regression of tumor vessels can be attributed to loss of mural cells after PDGF-B inhibition [28]. Combined blockage of VEGF and PDGFR-β signaling in tumor blood vessels by kinase inhibitors suppresses tumor neovascularization [29, 30], whereas a recent study shows that VEGF inhibition alone can indirectly prevent pericyte recruitment by reducing PDGF-B expression [31]. Thus, the disruption of mural cell coverage in tumor blood vessels of Foxc2+/- mice may primarily be attributable to impaired VEGF signaling in endothelial cells. Alternatively, given that there is the reciprocal paracrine cross talk between tumor endothelium and pericytes [32], reduced signaling of both VEGF and PDGF-B may diminish tumor angiogenesis in Foxc2+/- mice. This idea is supported in part by the finding that lymphatic endothelial cells of Foxc2-/- mice are phenotypically converted into blood endothelial-like cells by increasing the expressing PDGF-B [33].
In conclusion, our work in this paper provides evidence that Foxc2 is important for primary tumor formation associated with angiogenesis.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH (HL74121 to T.K.; CA100562 to M.M.D.), a grant from the March of Dimes Foundation to T.K., and Vanderbilt-Ingram Cancer Center Support Grant to M.M.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 4.McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat Med. 2003;9:713–725. doi: 10.1038/nm0603-713. [DOI] [PubMed] [Google Scholar]
- 5.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–859. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z, Banerjee S, Kong D, Li Y, Sarkar FH. Down-regulation of Forkhead Box M1 transcription factor leads to the inhibition of invasion and angiogenesis of pancreatic cancer cells. Cancer Res. 2007;67:8293–8300. doi: 10.1158/0008-5472.CAN-07-1265. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Zhang N, Dai B, Liu M, Sawaya R, Xie K, Huang S. FoxM1B transcriptionally regulates vascular endothelial growth factor expression and promotes the angiogenesis and growth of glioma cells. Cancer Res. 2008;68:8733–8742. doi: 10.1158/0008-5472.CAN-08-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mani SA, Yang J, Brooks M, Schwaninger G, Zhou A, Miura N, Kutok JL, Hartwell K, Richardson AL, Weinberg RA. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci U S A. 2007;104:10069–10074. doi: 10.1073/pnas.0703900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iida K, Koseki H, Kakinuma H, Kato N, Mizutani-Koseki Y, Ohuchi H, Yoshioka H, Noji S, Kawamura K, Kataoka Y, Ueno F, Taniguchi M, Yoshida N, Sugiyama T, Miura N. Essential roles of the winged helix transcription factor MFH-1 in aortic arch patterning and skeletogenesis. Development. 1997;124:4627–4638. doi: 10.1242/dev.124.22.4627. [DOI] [PubMed] [Google Scholar]
- 10.Kume T, Jiang H, Topczewska JM, Hogan BL. The murine winged helix transcription factors, Foxc1 and Foxc2, are both required for cardiovascular development and somitogenesis. Genes Dev. 2001;15:2470–2482. doi: 10.1101/gad.907301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seo S, Fujita H, Nakano A, Kang M, Duarte A, Kume T. The forkhead transcription factors, Foxc1 and Foxc2, are required for arterial specification and lymphatic sprouting during vascular development. Dev Biol. 2006;294:458–470. doi: 10.1016/j.ydbio.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 12.Winnier GE, Kume T, Deng K, Rogers R, Bundy J, Raines C, Walter MA, Hogan BL, Conway SJ. Roles for the winged helix transcription factors MF1 and MFH1 in cardiovascular development revealed by nonallelic noncomplementation of null alleles. Dev Biol. 1999;213:418–431. doi: 10.1006/dbio.1999.9382. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi H, Sano H, Seo S, Kume T. The Foxc2 transcription factor regulates angiogenesis via induction of integrin beta3 expression. J Biol Chem. 2008;283:23791–23800. doi: 10.1074/jbc.M800190200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kume T. Foxc2 transcription factor: a newly described regulator of angiogenesis. Trends Cardiovasc Med. 2008;18:224–228. doi: 10.1016/j.tcm.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi H, Kume T. Foxc transcription factors directly regulate Dll4 and Hey2 expression by interacting with the VEGF-Notch signaling pathways in endothelial cells. PLoS One. 2008;3:e2401. doi: 10.1371/journal.pone.0002401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Achen MG, Stacker SA. Molecular control of lymphatic metastasis. Ann N Y Acad Sci. 2008;1131:225–234. doi: 10.1196/annals.1413.020. [DOI] [PubMed] [Google Scholar]
- 18.Sano H, Ueda Y, Takakura N, Takemura G, Doi T, Kataoka H, Murayama T, Xu Y, Sudo T, Nishikawa S, Nishikawa S, Fujiwara H, Kita T, Yokode M. Blockade of platelet-derived growth factor receptor-beta pathway induces apoptosis of vascular endothelial cells and disrupts glomerular capillary formation in neonatal mice. Am J Pathol. 2002;161:135–143. doi: 10.1016/s0002-9440(10)64165-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 21.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 22.Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK, Seed B. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 23.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- 25.Taniwaki K, Fukamachi H, Komori K, Ohtake Y, Nonaka T, Sakamoto T, Shiomi T, Okada Y, Itoh T, Itohara S, Seiki M, Yana I. Stroma-derived matrix metalloproteinase (MMP)-2 promotes membrane type 1-MMP-dependent tumor growth in mice. Cancer Res. 2007;67:4311–4319. doi: 10.1158/0008-5472.CAN-06-4761. [DOI] [PubMed] [Google Scholar]
- 26.Belotti D, Paganoni P, Manenti L, Garofalo A, Marchini S, Taraboletti G, Giavazzi R. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: implications for ascites formation. Cancer Res. 2003;63:5224–5229. [PubMed] [Google Scholar]
- 27.Thurston G, Noguera-Troise I, Yancopoulos GD. The Delta paradox: DLL4 blockade leads to more tumour vessels but less tumour growth. Nat Rev Cancer. 2007;7:327–331. doi: 10.1038/nrc2130. [DOI] [PubMed] [Google Scholar]
- 28.Sennino B, Falcon BL, McCauley D, Le T, McCauley T, Kurz JC, Haskell A, Epstein DM, McDonald DM. Sequential loss of tumor vessel pericytes and endothelial cells after inhibition of platelet-derived growth factor B by selective aptamer AX102. Cancer Res. 2007;67:7358–7367. doi: 10.1158/0008-5472.CAN-07-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erber R, Thurnher A, Katsen AD, Groth G, Kerger H, Hammes HP, Menger MD, Ullrich A, Vajkoczy P. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004;18:338–340. doi: 10.1096/fj.03-0271fje. [DOI] [PubMed] [Google Scholar]
- 30.Potapova O, Laird AD, Nannini MA, Barone A, Li G, Moss KG, Cherrington JM, Mendel DB. Contribution of individual targets to the antitumor efficacy of the multitargeted receptor tyrosine kinase inhibitor SU11248. Mol Cancer Ther. 2006;5:1280–1289. doi: 10.1158/1535-7163.MCT-03-0156. [DOI] [PubMed] [Google Scholar]
- 31.Kuhnert F, Tam BY, Sennino B, Gray JT, Yuan J, Jocson A, Nayak NR, Mulligan RC, McDonald DM, Kuo CJ. Soluble receptor-mediated selective inhibition of VEGFR and PDGFRbeta signaling during physiologic and tumor angiogenesis. Proc Natl Acad Sci U S A. 2008;105:10185–10190. doi: 10.1073/pnas.0803194105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo P, Hu B, Gu W, Xu L, Wang D, Huang HJ, Cavenee WK, Cheng SY. Platelet-derived growth factor-B enhances glioma angiogenesis by stimulating vascular endothelial growth factor expression in tumor endothelia and by promoting pericyte recruitment. Am J Pathol. 2003;162:1083–1093. doi: 10.1016/S0002-9440(10)63905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrova TV, Karpanen T, Norrmen C, Mellor R, Tamakoshi T, Finegold D, Ferrell R, Kerjaschki D, Mortimer P, Yla-Herttuala S, Miura N, Alitalo K. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med. 2004;10:974–981. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.