Abstract
The song system of oscine songbirds mediates multiple complex perceptive and productive behaviors. These discrete behaviors are modulated according to external variables such as social context, directed attention and other forms of experience. In addition, sleep has been implicated in song learning and song maintenance. Changes in behavioral state are associated with complex changes in auditory responsiveness and tonic/bursting properties of song system neurons. Cholinergic input, principally from the basal forebrain has been implicated in some of these state dependent properties. Cholinergic modulation may affect numerous song system nuclei, with in vivo and in vitro studies indicating that a major target of cholinergic input is the forebrain nucleus HVC. Within HVC, a muscarinic cholinergic system has strong regulatory effects on most neurons, and may serve to couple and uncouple circuitry within HVC projecting along the premotor pathway with circuitry within HVC projecting along the cortico-basal ganglia pathway. These observations begin to describe how neuromodulatory regulation in the song system may contribute to learning phenomena.
Keywords: Acetylcholine; cholinergic regulation; bird song system, HVC
Introduction
The brain uses a finite number of neural elements and circuits to produce a wide array of behaviors with almost limitless variability. The reconfiguration of neural circuits with resulting changes in functional properties is an important mechanism for producing such variability. Even a comparatively small neural network such as the crustacean stomatogastric ganglion, which regulates chewing and digestion, has been studied for decades in efforts to understand its ability to respond to modulatory signals by adjusting network dynamics for the production of discretely and continuously variable motor patterns (Marder and Bucher, 2007). Modulatory regulation is well established in vertebrates, however its relation to more complex vertebrate behaviors is not as well understood. Here we explore the control of more complex behaviors such as social communication and sensorimotor learning, which occur through a balanced allocation of frequently overlapping neural resources.
The song system of oscine songbirds is one example of a discrete neural system that mediates multiple complex perceptive and productive behaviors through the same circuitry. Auditory feedback is processed through the same sets of pathways also involved in regulating motor output (e.g., Brainard and Doupe, 2000; Andalman and Fee, 2009); auditory memories of song involved in song learning likely impinge on these pathways (e.g. Bolhuis and Gahr, 2006); and these pathways have been implicated in conspecific song recognition (e.g. Brenowtiz, 1991). These discrete behaviors are furthermore varied or modulated according to external variables such as social context, directed attention and other forms of experience. Consistent with this observation, many song system regions are points for integration of a large number of identified and likely yet unidentified neuromodulatory and hormonal signals. These inputs potentially carry information on a range of timescales about the bird's behavioral state and current behavioral demands (Ball and Balthazart, 2009, this issue). For this reason, the song system affords a rare opportunity to study the neurophysiological effects of modulatory signals and relate them to natural behaviors and imitative learning.
The Song System
Learned vocal behavior in songbirds is mediated by a discrete set of interconnected forebrain and brainstem nuclei, commonly collectively referred to as the `song system'. Ball and Balthazart (2009) provide an excellent overview of the functional neuroanatomy of this system in the first review of this special issue. For our review, we will focus on the forebrain sensorimotor nucleus HVC and the two major anatomical pathways it elaborates.
HVC was discovered to be essential for normal song production in adult songbirds by Nottebohm et al. (1976), revealing a critical premotor function. Given that song is a behavior guided by auditory feedback (Konishi, 1965), it was intriguing that this nucleus was also discovered to exhibit auditory properties (Katz and Gurney, 1981) and that its auditory responses were tuned to learned features of the bird's own song (BOS) (e.g. Margoliash, 1983, 1986; Margoliash and Fortune, 1992). However, responses to conspecific song in HVC and downstream in a basal ganglia circuit suggested that HVC may also serve an important perceptual role, possibly by comparing conspecific vocalizations with vocal motor corollary discharge (Prather et al., 2008). There are still many outstanding questions about how sensory responses and motor commands are enmeshed in HVC, and how their interaction is modulated to facilitate different behaviors. Indeed, recordings in awake birds lead to the conclusion that auditory and motor activity in the song motor circuit are not at all clearly delineated from one another (Dave and Margoliash, 2000). For example, one particularly interesting subpopulation of HVC neurons appears to rapidly toggle between mutually exclusive sensitivity to either sensory or motor events, while apparently encoding the structure of those events identically (Prather et al., 2008).
HVC receives input from several areas that are candidates for conveying these auditory signals. The Field L complex is the primary thalamorecipient auditory area in songbirds, and several subregions of Field L project axons to HVC or its immediate surround (Kelley and Nottebohm, 1979; Fortune and Margoliash, 1995; Vates et al., 1996). In addition, the interfacial nucleus (NIf) provides a rich innervation that pervades HVC (Nottebohm et al., 1982). Activity in NIf is highly correlated with HVC spontaneous and auditory-driven bursting activity (Janata and Margoliash, 1999), and acute inactivation of NIf abolishes both of these patterns (Cardin and Schmidt, 2004b; Coleman and Mooney, 2004). Additional auditory input comes to HVC directly and indirectly from the caudal mesopallium (CM), a broad area containing cells that exhibit selectivity for BOS (Bauer et al., 2008) as well as conspecific songs (Gentner and Margoliash, 2003). CM projects to HVC and NIf, and inactivation of CM suppresses song auditory responses in NIf as well as auditory responses that emerge in HVC neurons following complete chronic lesions of NIf (Bauer et al., 2008). Finally, there is a substantial input to HVC (and NIf) from the uvaeform nucleus of the thalamus (Uva), which does encode auditory information unselectively, however inactivation of Uva does not affect HVC auditory responses or spontaneous activity. Nonetheless, brief high frequency electrical stimulus trains in Uva dramatically modulate HVC BOS responses on relatively short timescales (Coleman et al., 2007).
HVC consists of several classes of excitatory projection neurons and inhibitory interneurons that have no extrinsic projection. Some of the projection neurons innervate the premotor robust nucleus of the arcopallium (RA), which in turn projects to brainstem motor neurons controlling song, thus forming an obligatory motor pathway for song control (Nottebohm et al., 1976; McCasland and Konishi, 1981; Vu et al., 1994; Yu and Margoliash, 1996; Hahnloser et al., 2002). A separate population of HVC projection neurons innervates the basal ganglia, projecting to a structure known as Area X. This so called `anterior forebrain pathway' (AFP) continues through the medial dorsolateral nucleus of the thalamus (DLM) and the lateral magnocellular nucleus (lMAN) to form an alternative route for information to propagate from HVC to RA (Kimpo et al., 2003). The AFP is not strictly obligatory for song in adults, however it is essential for juveniles to develop song normally (Bottjer et al., 1984), and for adults to modify song in response to feedback perturbations (Williams and Mehta, 1999; Brainard and Doupe, 2000). This latter effect may stem from lMAN's capacity to regulate the variability of motor commands in RA (Kao et al., 2005; Olveczky et al., 2005). While this capacity has not yet been causally linked to imitative learning, a recent study showed that lMAN is necessary for adaptive, instructed changes to song in adults (Andalman and Fee, 2009).
In addition to their important roles in song production, learning, and maintenance, HVC and the AFP have been repeatedly implicated in the perception of conspecific and other acoustic stimuli by lesion studies (Brenowitz, 1991; Del Negro et al., 1998; Scharff et al., 1998; Hamilton et al, 1997; Burt et al., 2000; Gentner et al., 2000). While much interest has been placed in BOS selectivity in HVC, there are still substantial responses to non-BOS stimuli at many sites. Indeed, BOS responses observed in HVC of awake zebra finches during passive listening are considerably less selective than those seen during sleep or under anesthesia (Cardin and Schmidt, 2003; Rauske et al., 2003). Stronger auditory responses than observed in zebra finches are observed in awake birds in the HVC of some other species, for example white-crowned sparrows (Margoliash, 1986). Together, these findings suggest that the dynamic coding range of HVC neurons is broadened in a species-specific and state-dependent manner to accommodate perception of a larger stimulus set when birds are awake and not singing. Some particularly compelling data with regard to HVC and song perception come from recordings of X-projecting HVC neurons in awake swamp sparrows. These neurons activate in conjunction with specific acoustic features either when detected in playback or emitted as song (Prather et al., 2008). This functional mapping between acoustic features and premotor activity patterns is also observed in RA in sleeping zebra finches, suggesting it is a global feature of song system physiology shared across many species (Dave and Margoliash, 2000). Furthermore, in swamp sparrows the selectivity of HVC neurons for acoustic features has been shown to map onto the perceptual selectivity of natural territorial behavior elicited by these stimuli in field tests (Prather et al., 2009). While still correlative, these data suggest that song motor system circuitry in HVC is used to evaluate the information content and significance of conspecific vocalizations. Given that HVC likely plays a central role in at least two broad aspects of song-related behavior (production and perception), it is worthwhile to examine and compare patterns of HVC activity observed in different states and behavioral conditions.
Behavioral state modulation of song system activity
The neurophysiology of the song system is known to be modulated by behavioral state and social context in a number of respects. One well-studied example is the modulation of activity in the AFP by social context. When a male zebra finch is singing to a female (directed singing), song-driven gene expression is suppressed (Jarvis et al., 1998) and neuronal firing is weaker and less variable in the AFP nucleus lMAN (Hessler and Doupe, 1999; Kao et al., 2008) when compared with singing alone (undirected singing). This phenomenon is mirrored by context-dependent changes in singing behavior: the spectral and temporal features of song are less variable during directed singing (Sossinka and Bohner, 1980; Kao et al., 2008; Woolley and Doupe, 2008). Reversible lesions and microstimulation have causally linked lMAN activity to the expression of this and other forms of song variability in adults and juveniles (Kao et al., 2005; Olveczky et al., 2005; Kao and Brainard, 2006). It has been speculated that lMAN modulates its activity according to social context and other factors to adjust the level of variability injected into the song motor system via its projection to RA (Kao et al., 2005; Olveczky et al., 2005). This could allow the male bird to decrease the degree of vocal improvisation for maximizing the attractiveness of the song, or to increase motor exploration for accelerating reinforcement learning.
Song system physiology is also dramatically regulated by circadian patterns and arousal level. Chronic recordings from zebra finches in HVC (Schmidt and Konishi, 1998) and RA (Dave et al., 1998) revealed dramatic differences in the magnitude of expression of auditory responses between awake birds and birds that were either anesthetized or asleep; these responses were absent or dramatically weaker in the awake, passive listening state. Initially, the term `gating' was used to describe this difference (Schmidt and Konishi, 1998), which may be taken to imply a binary admission or exclusion of auditory input into the song motor system. Other studies reported at least some substantial auditory responses in HVC of awake birds (McCasland and Konishi, 1981; Margoliash, 1986), however those studies did not make direct comparisons across states, and may reflect some species differences (Nealen and Schmidt, 2006; Prather et al., 2008). It is possible that the relatively dramatic changes in HVC physiology with behavioral state seen in zebra finches represents an extreme case when compared with other species. If so, it is worth considering how these species differences at the neural level might relate to how the species differ in their song behavior and in how they use audition for vocal performance feedback and/or social communication. Nonetheless, the particularly robust relationship of state and neural activity seen in zebra finches actually make them a most advantageous model for examining this relationship.
Subsequent detailed studies of the activity of individual HVC neurons across behavioral state transitions revealed a more complex modulation of auditory properties. Virtually all sites in HVC are robustly auditory during sleep or sedation, however auditory responses in some cells are dramatically suppressed during wakefulness while others retain substantial auditory activity upon waking. Those responses that are observed in the awake zebra finch are considerably less BOS-selective than responses measured during sleep, with the functional implication that the encoding of acoustic stimuli and not merely the strength of input is altered (Cardin and Schmidt, 2003; Rauske et al., 2003). The dramatic decrease in the strength and selectivity of BOS-driven activity during wakefulness raises questions about how sensory responses to self-generated vocalizations are useful during sleep. One suggestion has been that sleep replay of daytime sensorimotor experiences contributes to feedback-dependent song modification (Dave and Margoliash, 2000). Indeed, the developmental appearance of sleep bursting activity in the song motor pathway is conspicuously correlated with exposure to a model song, and first appears on the night before the initiation of imitative learning (Shank and Margoliash, 2009). This framework for interpreting sleep activity raises the likelihood that sleep BOS responses are not sensory in the conventional sense, but that the network is operating in an unprotected state that allows ongoing patterns to be brought into resonance or registry with a matching external event.
It is also observed that over time within the awake state, the magnitude of HVC auditory responses fluctuates, which suggests that auditory processing is regulated on finer timescales than that of the sleep/wake cycle (Cardin and Schmidt, 2003). Possibly related to this finding, even in cells that express auditory responses in the awake quiescent bird, arousal by a startling stimulus virtually abolishes song-driven firing (Cardin and Schmidt, 2003, 2004a). Similar phenomena have also been reported in awake and anesthetized white-crowned sparrows, where strong auditory responses in HVC are suppressed just prior to and during brief periods of arousal (Margoliash, 1986). It is unclear when in the natural behavior of an awake, unrestrained bird this latter mechanism is engaged. However, taken together these findings point to a model in which auditory activity in HVC is expressed in several discrete levels or even a smooth continuum with respect to magnitude and extent (as opposed to binarily and uniformly) and is reconfigured with respect to stimulus selectivity as behavioral state shifts. Such a model is not well described by gating and instead is likely to involve a more nuanced regulation of circuit dynamics. This feature of sensory regulation is likely to be common across songbird species.
The foregoing studies effectively demonstrate that sensory responses are strongly but slowly regulated by circadian rhythms and periods of alertness, but they only assessed auditory sensitivity during non-singing periods. This leaves open the possibility of finer modulation of auditory activity on the time scale of song bouts. For example, cells in HVC or elsewhere in the song system may become acutely sensitive to changes in auditory feedback during a narrow window around song, consistent with ability to detect errors in song performance as they occur. Nonetheless, attempts to observe changes in activity in the AFP (Leonardo, 2004) or AFP-projecting HVC neurons (Kozhevnikov and Fee, 2007; Prather et al., 2008) during perturbations of song sensory feedback detected no difference. On the other hand, at least in Bengalese finches, small differences in neuronal activity were reported for HVC interneurons when comparing perturbed and unperturbed trials (Sakata and Brainard, 2008). In swamp sparrows, Prather et al. (2008) saw an apparent switch between sensitivity to song playback during non-singing periods and a disappearance of that sensitivity when singing commenced. These authors and others have observed increases in population firing in HVC at this transition, further suggesting some transition in neural dynamics (McCasland and Konishi, 1981; Yu and Margoliash, 1996; Prather et al., 2008). In any case, many of these results point to widespread changes in HVC activity patterns as the bird transitions to singing.
Initial evidence linking state-dependent modulation of song system activity to neuromodulatory signaling in HVC came from microinjections of noradrenaline during recordings from HVC and RA of anesthetized birds (Dave et al., 1998). In that study, injections of noradrenaline (NA) into HVC abolished RA auditory activity, while leaving at least some response in HVC multiunit records. Injections of NA into RA increased RA spontaneous firing rates, but did not suppress responses to BOS. Taken together, these injections therefore captured several important features of the changes in physiology upon waking. This observation was interpreted as a potential mechanism by which arousal could alter activity in the HVC-RA pathway. While we independently confirmed this result in later experiments, we were unable to attribute it to a specific NA receptor type (Shea and Margoliash, unpublished data). Subsequent experiments that involved injection of noradrenergic drugs into NIf revealed that nucleus is also influenced by NA, however the effects were complex and inverted their sign with increasing dose (Cardin and Schmidt, 2004b).
While noradrenaline has a strong link to arousal level and is therefore a plausible candidate for participating in state-dependent modulation of song system activity, it is likely that other neurotransmitter systems play a role as well. One particularly likely contributor is acetylcholine (ACh). As mentioned, the physiology in HVC and RA strongly correlates with sleep/wake cycles, and ACh is closely associated with circadian rhythms, promoting the desynchronized states of wakefulness and REM sleep. There is also evidence that behavioral state influences could mediate a balance in the song system between attention to self-generated feedback and perception of conspecific vocalizations. ACh is also believed to be a key player in the allocation of attention (e.g. Parikh and Sarter, 2008 and see below).
Cholinergic systems and attention and arousal
The majority of cholinergic input to the mammalian cortex originates in the basal forebrain nucleus basalis (NB) of the substantia innominata (Mesulam et al., 1983). This brain area also contains a substantial population of non-cholinergic, mostly GABAergic, neurons that are also cortically-projecting (Gritti et al., 1997). Brainstem cholinergic regions play a role in arousal-related changes in the activity of subcortical structures such as the thalamus, but sensory activity is also shaped across behavioral states by way of a direct projection from BF to the cortex (Buzsaki et al., 1988). NB stimulation evokes cortical desynchronization, which is a hallmark of both wakefulness and REM sleep, and this effect can be blocked by cortical application of atropine (a muscarinic cholinergic antagonist) (Metherate et al., 1992). In early studies of cells not known to be cholinergic or GABAergic, cortically-projecting NB neurons showed elevated firing rates in relation to periods of desynchrony (Detari and Vanderwolf, 1987; Szymusiak and McGinty, 1989). Subsequent detailed studies that allowed identification of the neurotransmitter associated with each cell revealed considerable heterogeneity (Manns et al., 2000a, b, 2003; Lee et al., 2004). In general however, cholinergic NB neurons showed elevated firing during periods of wakefulness and REM, with little to no activity during slow wave sleep. GABAergic neurons in NB typically exhibit a complementary pattern, preferentially firing during slow wave sleep.
In parallel with its function for circadian control of cortical activity, NB also seems to participate in the allocation of attention to important stimuli. Many NB neurons exhibit transiently elevated firing within the awake state in response to rewards or reward-predicting stimuli, but also to stimuli associated with negative outcomes or punishment (Richardson and DeLong, 1990; Wilson and Rolls, 1990; Whalen et al., 1994; Lin and Nicolelis, 2008). This property of elevated firing irrespective of outcome valence has been attributed to NB encoding of motivational salience or behavioral significance, and is shared by neurons that are both GABAergic and cholinergic (Lin and Nicolelis, 2008). A large literature of behavioral studies in which cholinergic NB neurons were specifically immunolesioned is generally consistent with deficits in the ability to attend to behaviorally-relevant stimuli (reviewed in Wenk, 1997). Cholinergic basal forebrain lesions are also implicated in recovery of motor function following brain injury (Conner et al., 2005). It is not yet clear whether this effect is sensorimotor in nature or whether ACh acts directly on motor cortical areas, however ACh seems to be specifically necessary for plasticity that requires behavioral learning (Ramanathan et al., 2009).
The increase in cortical ACh release that presumably results from these responses to salient stimuli is likely to enhance concomitant sensory cortical responses. Cortical iontophoresis of ACh increases firing in response to sensory stimuli (Sillito and Kemp, 1983; Donoghue and Carroll, 1987; McKenna et al., 1988) and application of a muscarinic cholinergic antagonist blocks attentional enhancement of V1 neuronal responses during a visual task (Herrero et al., 2008). Stimulation of NB also acutely enhances sensory cortical responses (Tremblay et al., 1990; Metherate and Ashe, 1991; Hars et al., 1993; Howard and Simons, 1994) through cortical release of ACh (Metherate et al., 1992). Short-term ACh-dependent changes in cortical responses can be converted into longer-term sculpting of neuronal receptive fields and population sensory maps through associational plasticity engaged when pairing NB activation with repeated sensory stimulation (Bakin and Weinberger, 1996; Kilgard and Merzenich, 1998). This lasting effect may be initially triggered by a persistent disinhibition of the cortical circuit (Froemke et al., 2007).
Anatomy of forebrain cholinergic systems in songbirds
A range of markers have been used to delienate the anatomy of cholinergic systems in avian species including: acetylcholinesterase (AChE), an ACh degradation enzyme present at cholinergic synapses; choline acetyltransferase (ChAT), which labels somata and fibers of cholinergic neurons; and labeling of postsynaptic cholinergic receptors on neurons that receive cholinergic input. An exhaustive survey of the distribution of ChAT in pigeon brain led Medina and Reiner (1994) to conclude that the organization of subcortical cholinergic systems was quite similar in birds and mammals. This similarity extends to a basal forebrain enrichment of large ChAT-positive cell bodies that is intermingled with a substantial population of GABAergic neurons (Veenman and Reiner, 1994). Based on these and other properties, including a pallial projection from the ChAT neurons, the Avian Brain Nomenclature Forum concluded that this cell field (previously known as part ventral pallidum generally) was the avian homolog of the mammalian nucleus basalis magnocellularis (NBM) (Reiner et al., 2004). Because the methods we have used for activating these neurons (Shea and Margoliash, 2003; see also below) engaged a swath of the basal forebrain enriched for ChAT positive neurons that may not have strictly respected the bounds of NBM, here we choose the term cholinergic basal forebrain (CBF).
Cholinergic fibers are found throughout the telencephalon, striatum, and thalamus in birds, however some histological evidence indicates that the HVC in particular receives a prominent cholinergic input. AChE is enriched in HVC (Ryan and Arnold, 1981; Zuschratter and Scheich, 1990), as are ⟨-bungarotoxin sensitive nicotinic cholinergic receptors (Watson et al., 1988). HVC also shows binding of radiolabeled ligands for muscarinic cholinergic receptors, although to varying degrees with different ligands (Ryan and Arnold, 1981; Ball et al., 1990), as well as some limited ChAT-labeled fibers (Zuschratter and Scheich, 1990). In contrast, while RA is enriched for AChE (Ryan and Arnold, 1981; Zuschratter and Scheich, 1990), it exhibits little binding specific for either major cholinergic receptor class (Ryan and Arnold, 1981; Watson et al., 1988; Ball et al., 1990). Cholinergic input to the song system may also be developmentally regulated, such that, at least in RA, there is heightened production of ACh during the critical period (Sakaguchi and Saito, 1991).
Li and Sakaguchi (1997) used double-labeling from injections into song nuclei and ChAT immunoreactivity to reveal a population of cholinergic neurons that project to HVC and RA. This finding however has not been confirmed with anterograde labeling of HVC or RA following CBF injections. Separately the same group also reported that the CBF receives input from the auditory thalamic nucleus ovoidalis (Ov) and the posterior dorsomedial nucleus (DMP), another thalamic nucleus with connections to the song system (Vates et al., 1997). Recently, another connection to the CBF region was discovered to arise from Area X (Gale et al., 2008). Together these CBF afferents may provide both auditory input and song-related reafference, however it is not known which if any of these inputs access cholinergic circuitry. Additionally, an independent cholinergic input to the song system reaches Uva from the medial habenula (Akutagawa and Konishi, 2005). Finally, a point of caution is that for several of the sources of cholinergic input to the song system, the connection has not been confirmed in both directions.
Physiological effects of cholinergic input in song system
To explore a possible role for CBF input in state-dependent modulation of the song system or other aspects of song learning and maintenance, the physiological effects of cholinergic manipulations were assessed in HVC both in vivo (Shea and Margoliash, 2003) and in vitro (Shea SD, Margoliash D (2003) Basal forebrain control of HVc: cellular mechanisms and behavioral consequences. Soc Neurosci Abstr 29:942.12). There are a number of examples of anatomical and neurochemical studies that correlate neuromodulator levels including those of ACh to the song learning critical period (e.g. Sakaguchi and Saito, 1991; Soha et al, 1996; Harding et al, 1998), however for the initial examination of the physiological effects of ACh, experiments were limited to adult birds.
First, injections of cholinergic agonists were made directly into HVC of anesthetized adult male zebra finches (Shea and Margoliash, 2003). During these injections song-evoked and spontaneous activity in HVC and RA were monitored. Under urethane anesthesia, the activity in the song motor pathway resembles that seen during sleep in that recordings from both nuclei exhibit strong bursts that occur spontaneously and are also evoked by song. These bursting patterns are weaker in HVC and are not evident in RA of awake birds (Dave et al., 1998; Schmidt and Konishi, 1998). HVC injections of the cholinergic agonist carbachol or the muscarinic cholinergic agonist muscarine both abolished this pattern in recordings from both HVC and RA. In contrast, injection of nicotine into HVC disrupted auditory responses in HVC, but not RA, suggesting a different mechanism of action. However, the variance in baseline and response happened to be higher for the RA recordings in the nicotine experiments, so this conclusion is not secure.
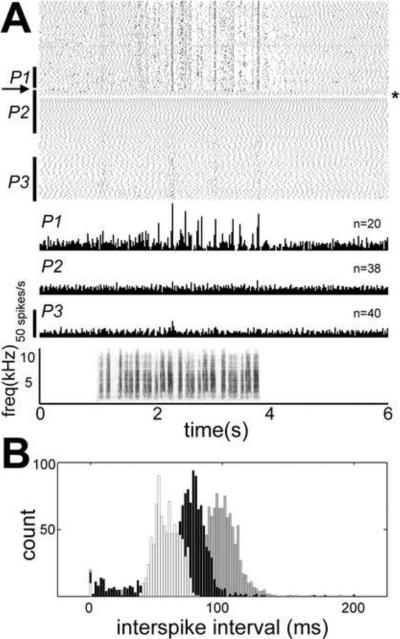
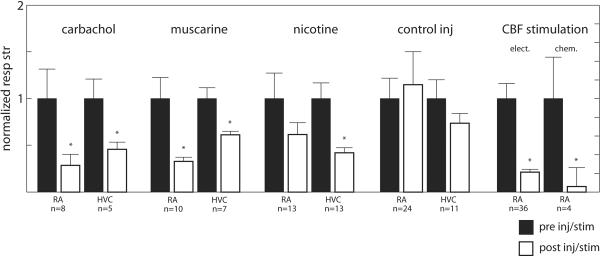
Subsequently, the effects of endogenous release of ACh on HVC and RA physiology in anesthetized birds were tested (Shea and Margoliash, 2003). Activation of CBF mimicked the key features of muscarine and carbachol injections, with some additional effects. Auditory-driven and spontaneous bursting in HVC and RA were abolished for minutes after CBF stimulation, and RA firing rates also increased, thus song motor pathway physiology became more like a wakeful pattern. Figure 1 shows an example of this effect from Shea and Margoliash (2003). In Figure 1A, a raster plot of 180 sequential trials of song playback and peristimulus time histograms from three epochs (P1 – P3) show the changes in response to a single electrical stimulus (one 500 ms burst, 50 μA @ 400 Hz) applied to CBF at the arrow. There is a reduction in auditory response strength and an elevation of firing rate for minutes after the stimulation. Figure 1B depicts interspike interval (ISI) histograms corresponding to the three epochs reflecting the changes in spontaneous discharge. CBF stimulation effects also commonly included an accelerating high frequency burst and a brief (< 1 min) complete cessation of all firing in HVC and RA (as evident in Figure 1A), suggesting some additional likely network effects not evoked by bulk injections. All effects of CBF stimulation were moreover prevented by prior injection of HVC with either a nicotinic or muscarinic antagonist, specifically implicating ACh release local to HVC in the changes seen in both RA and HVC. A summary of all in vivo stimulation and injection effects on auditory responses in HVC and RA is depicted in the bar graph in Figure 2.
Figure 1.
CBF stimulation elicits changes in RA physiology. (A) An electrical stimulus (one 500 ms burst, 50 μA @ 400 Hz) was applied at the arrow. A loss of auditory responses and increased firing rate can be seen prior to a brief complete cessation of firing (at asterisk). Following stimulation, there was no response (unpaired t test, p < 0.001). Later, there was significant recovery (unpaired t test, p < 0.001). Data represent 180 repetitions of BOS (2370 s). (B) Interspike interval (ISI) histograms (bin size = 2 ms) of ongoing discharge, corresponding to the three epochs (P1, P2, P3) in part A. There was much greater variation in ISI distribution before (black bars) than after (white bars) stimulation, as well as significant changes in means (unpaired t test, p < 0.001). Later (gray bars), ongoing rates slowed significantly (p < 0.001). (From Shea and Margoliash (2003), used with permission.)
Figure 2.
Cholinergic manipulations suppress auditory responses in RA and HVC. Shown are a summary of effects of cholinergic manipulations on RA and HVC auditory responses, representing injections of various agents in all cases except for the electrical stimulation of CBF. Normalized pre (black bars) and post manipulation (white bars) response strength is plotted for each nucleus and each manipulation.
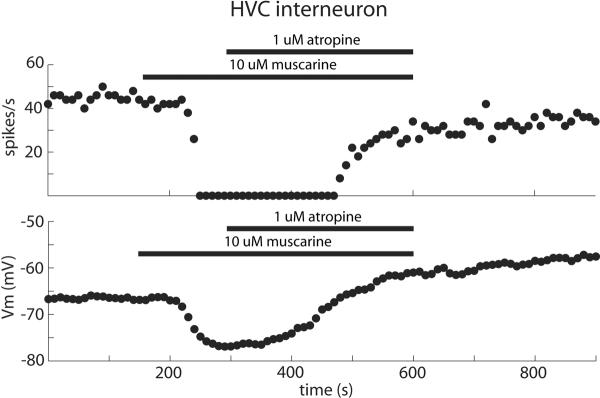
We have further investigated over a number of years the effects of cholinergic input on the HVC circuit using in vitro slice recordings and observed a mixture of inhibitory and excitatory effects depending on cell type (e.g. Shea SD, Margoliash D (2003) Basal forebrain control of HVc: cellular mechanisms and behavioral consequences. Soc Neurosci Abstr 29:942.12). Of the projection neurons that we recorded, one type of HVC-RAn was uniformly excited by muscarine, as were most HVC-Xn whereas a subset of HVC-Xn were clearly inhibited by muscarine. The other physiologically distinct class of HVC-RAn gave inconsistent results. Finally, all of the interneurons that we recorded were inhibited by muscarine. An example of one such recording is shown in Figure 3. Spike rate evoked by somatic current injection and membrane voltage are measured and plotted every 10 s. Application of muscarine elicited inhibition of the cell by both measures and these effects were nearly completely reversed by addition of the muscarinic antagonist atropine. The effect of muscarine on HVC interneurons is reminiscent of the effects we observed in vivo with muscarine injections into HVC (see above). The multiunit recordings we obtained in vivo were likely dominated by HVC interneurons.
Figure 3.
HVC neurons in vitro are inhibited by muscarine. A typical example of the effects of muscarine and atropine on an HVC interneuron recorded in vitro. Current was injected (500 ms pulse, 150 pA) every 10 s. At the time indicated by the bars, 10 μM muscarine and 1 μM atropine were applied to the slice. Muscarine abruptly caused a pronounced inhibition of spiking and membrane hyperpolarization, and these effects were nearly completely reversed by atropine.
For all cell classes, the effects of muscarinic agonists were reversed by atropine. These data provide the most direct evidence that HVC neurons are highly sensitive to ACh. They are also inconsistent with uniform, binary gating of auditory responses by ACh, and they instead suggest that the HVC network is regulated in a more complex fashion. One speculation is that firing relationship between the two projection neuron classes, which is mediated primarily disynaptically via the HVC-In (Mooney, 2000; Mooney and Prather, 2005; Rosen and Mooney, 2006), may be dynamically affected by ACh. Thus, the motor commands in HVC-RAn could be uncoupled from firing of HVCXn, a pathway that may send a motor efference copy to the AFP to drive song variability or plasticity. This occurs in tandem with an apparent enhancement of activity in each class of projection neuron.
Data on the behavioral significance of the cholinergic input to the song system are scant. We have analyzed in adult birds the effects of lesions of the basal forebrain field containing the cholinergic neurons that project to the song system (Levin T, Shea SD, Margoliash D (2007) Contribution of the cholinergic basal forebrain in a novel songbird model of aphasia. Soc. Neurosci. Abstr 33:430.18.). Electrolytic lesions that included nucleus basalis resulted in deterioration of the spectrotemporal qualities of song. The time course of these effects was complex. In some birds, immediate effects on the morphology of some syllables were observed. These included changes in the structure of the syllables and in some cases loss of those syllables. Other birds also showed effects on the morphology of some syllables, but the onset of these effects was delayed by days. In these birds, the changes in syllable structure were much more modest in nature. Interestingly, birds with the more severe, rapid onset effects recovered normal song whereas birds with delayed effects did not recover normal song. These data are consistent with the hypothesis that ACh release in the song motor pathway interacts with maintenance of the adult song. The variety of effects on syllable morphology and correlated changes in time course hints at multiple mechanisms for cholinergic regulation.
Multiple sites of modulatory action
At least one other cholinergic connection to the song system has recently been revealed. The medial habenula contains cholinergic neurons that project to the thalamic uvaeform nucleus (Uva) which is immediately presynaptic to NIf and HVC (Akutagawa and Konishi, 2005). This finding motivated a re-examination of whether there are multiple sources of cholinergic input into the song system. Akutagawa and Konishi (2005) raised the possibility that activation of fibers from Uva passing near the CBF, and not cells in the CBF itself, gave rise to the modulatory effects in HVC seen by Shea and Margoliash (2003). This explanation is difficult to reconcile with the observation that glutamate injected in the CBF mimicked the effects of electrical stimulation (Shea and Margoliash, 2003). To address this issue, Akutagawa and Konishi (2005) further postulated that diffusion of glutamate into the thalamic reticular nucleus, which projects to Uva, indirectly activated the same Uva-dependent HVC modulation. The neurochemistry of the UVA-projecting reticular neurons was not identified, however the thalamic reticular nucleus has an inhibitory coupling to the thalamus in mammals (Huguenard and McCormick, 2007). It is also difficult to discount a direct cholinergic effect of CBF on HVC given that the effects of CBF stimulation on HVC and RA auditory activity in anesthetized birds were blocked by cholinergic antagonists in HVC (Shea and Margoliash, 2003). If this was somehow mediated by Uva it would also require that Uva is cholinergic, for which there is no evidence.
Subsequent in vitro recordings from the isolated HVC network have demonstrated that many HVC neurons are directly sensitive to cholinergic stimulation (see above). This provides a physiological substrate for cholinergic input to HVC as demonstrated by a host of anatomical studies. Much work remains to be done to determine when and how the modulatory capacity of CBF is exercised in behaving birds. Nonetheless, forebrain cholinergic systems are widely implicated in fluctuations of arousal state, and the effects on the song motor pathway following CBF stimulation in many respects resemble the changes seen across behavioral state transitions. Therefore, it is a reasonable hypothesis that ACh release in HVC is a key participant in those transitions.
Cholinergic systems are likely to act in parallel or interactively with other modulatory systems to regulate song system physiology during different behavioral states. The different neuromodulatory systems may act on overlapping but distinct clusters of nuclei. For example, Cardin and Schmidt (2004a) reported compelling evidence that under certain circumstances, arousal can modulate auditory activity in NIf, which is presynaptic to HVC. These changes are moreover propagated into HVC, which covaries with NIf under the conditions of these experiments. Noradrenergic processes in NIf are implicated in these effects (Cardin and Schmidt, 2004b). State-dependent modulation in this circuit is not binary, rather it is multi-tiered or possibly expresses a continuum of effects. Nested within the broad timescale modulation of HVC auditory responses across sleep and wake cycles (Cardin and Schmidt, 2003; Rauske et al., 2003; Cardin and Schmidt, 2004a), there is also finer timescale modulation of the strength and selectivity of wakeful responses that fluctuates spontaneously (Cardin and Schmidt, 2003). Additionally, the weaker responses seen during undisturbed wakefulness can be briefly completely abolished by a startling tactile stimulus (Cardin and Schmidt, 2003). Further complexity is revealed in the details of the noradrenergic pharmacology in NIf. High doses of noradrenaline and agonists block auditory responses in NIf and HVC, possibly linking arousal to song system physiology. However, lower doses of these drugs actually substantially enhanced auditory responses in this circuit (Cardin and Schmidt, 2004b). Biphasic effects of noradrenergic receptor activation are common in a variety of systems (e.g. Sullivan et al., 1989; Devilbiss and Waterhouse, 2004).
Noradrenergic processes in NIf (Cardin and Schmidt, 2004b) and HVC (Dave et al., 1998), along with ACh and other neuromodulators acting at a number of song system stations, may contribute to a more nuanced and multifaceted relationship between behavioral state and song system activity. In particular, the convergence of NA and ACh modulatory effects in HVC raises the possibility of direct interactions between them, whether synergistic or antagonistic (Briand et al, 2007). Further work should be directed at disentangling the roles that circadian rhythms, attention, alertness and startle play in shaping song-related activity patterns, and at discerning their pharmacological substrates. In addition, it may be useful to move beyond studying these systems in isolation, an approach that might help resolve some unexplained results such as the failure to account for the reliable effects of NA in HVC (Dave et al. 1998) with any more specific drugs (Shea and Margoliash, 2003).
Future directions
The most fundamental outstanding question that bears on our understanding of cholinergic participation on song is how do the cholinergic modulation phenomena we observed in vivo and in vitro relate to processes that occur in the behaving bird? When and how does cholinergic tone fluctuate in the song motor pathway during productive and perceptive behavior? What are the synaptic mechanisms of cholinergic modulation in HVC and how do they affect network dynamics? Is: is activity of the CBF in behaving birds necessary and/or sufficient for song maintenance or modification? The last question could be particularly profitable to raise in the context of juvenile song learning; to date, most of the work had been conducted in adult animals.
Questions about the functional behavioral relevance of CBF for singing behavior could be partly answered by manipulations of the CBF in behaving animals. Initial results based on lesions suggest an essential role for the CBF in song (see above). Another broad approach would be to inject cholinergic drugs (agonists and antagonists) into song system nuclei, specifically HVC. This could allow some spatial specificity to the outcome, and with further refinement of the technique may also allow low resolution temporal specificity as well. For instance, it could be informative to manipulate cholinergic activity during different phases of the sleep wake cycle to assess an interaction with circadian processes. More refined temporal control of CBF activation (e.g. electrical stimulation triggered by specific components of song as a bird sings) could investigate fine timescale modulation of cholinergic inputs during singing. Chronic electrical stimulation could moreover be coupled with neurophysiology to ask whether the effects of stimulation on song system physiology differ when applied during sleep or wakefulness and during singing or non-singing periods.
Whatever the approach to CBF manipulation, it must be interpreted with caution and properly controlled to determine whether any observed behavioral effects are due to altered sensorimotor activity in HVC or plasticity lower in the auditory system (Bakin and Weinberger, 1996; Kilgard and Merzenich, 1998). A related speculative question is whether CBF activation favors or obstructs purely perceptual behavior, and if so is that effect mediated through either or both of the song control and auditory systems?
Direct observation of changes in cholinergic activity in freely behaving birds would also be of great value. One could address whether rapid fluctuations occur that are temporally locked to song or whether cholinergic release is only regulated more slowly according to arousal. Also, is the signal relatively binary, that is only fully on or off, or is it finely graded along a continuum, and if the latter, what regulates the degree of cholinergic activation? It would be helpful in such experiments to demonstrate whether the recorded cells are projection neurons, which cells are cholinergic and which are GABAergic, and whether they project to HVC. This is a challenging experiment, but it would connect the firing patterns of CBF cells to singing behavior more directly. Possibly a more practical or perhaps complementary approach would be to directly measure ACh release in behaving birds with voltammetric neurochemical techniques. Newly developed ACh sensitive electrodes show some promise for use in this kind of experiment (Giuliano et al., 2008). This would allow observation of an integrated signal of overall cholinergic tone seen at a given song system nucleus on a time scale that is still appropriate for relatively fine-grained analysis (i.e. song-related activity).
Finally, many questions remain regarding the synaptic effects of cholinergic modulation in HVC. In vitro approaches, while reduced, are nonetheless useful for dissecting network interactions in some detail. This would be a particularly attractive preparation to explore the potential role of nicotine in modulating activity in HVC. Ideally one might also be able to develop an in vitro approach that preserves network activity, and yet still readily allows pharmacological manipulations. For example, a living ex vivo preparation of the entire HVC (R. Mooney, personal communication) may satisfy this condition. In this experimental context, paired intracellular recordings or single neuron recordings paired with field potential records may reveal how ACh can modify pairwise synaptic interactions or the relationship of single neurons to large scale network dynamics. These studies could be extended by in vivo experiments using intracellular recordings to identify HVC projection neurons during CBF stimulation and observe subthreshold processes.
Neuromodulatory processes including cholinergic processes are intricate and likely have a complex relationship to behavior. Researchers in mammalian systems have made great strides in revealing the connections between the basal forebrain cholinergic system and arousal and attention. What remains substantially unresolved is the relationship of cholinergic activity to the regulation of behavior and particularly sensorimotor integration. Work in a system such as songbirds in which ACh and other transmitters modulate discrete circuits with defined roles for behavior has great promise to help clarify these relationships.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akutagawa E, Konishi M. Connections of thalamic modulatory centers to the vocal control system of the zebra finch. Proc Natl Acad Sci U S A. 2005;102:14086–14091. doi: 10.1073/pnas.0506774102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andalman AS, Fee MS. A basal ganglia-forebrain circuit in the songbird biases output to avoid vocal errors. Proc Natl Acad Sci U S A. 2009;106:12518–12523. doi: 10.1073/pnas.0903214106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Natl Acad Sci U S A. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball GF, Nock B, Wingfield JC, McEwen BS, Balthazart J. Muscarinic cholinergic receptors in the songbird and quail brain: a quantitative autoradiographic study. J Comp Neurol. 1990;298:431–442. doi: 10.1002/cne.902980405. [DOI] [PubMed] [Google Scholar]
- Bauer EE, Coleman MJ, Roberts TF, Roy A, Prather JF, Mooney R. A synaptic basis for auditory-vocal integration in the songbird. J Neurosci. 2008;28:1509–1522. doi: 10.1523/JNEUROSCI.3838-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhuis JJ, Gahr M. Neural mechanisms of birdsong memory. Nat Rev Neurosci. 2006;7:347–357. doi: 10.1038/nrn1904. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature. 2000;404:762–766. doi: 10.1038/35008083. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA. Altered perception of species-specific song by female birds after lesions of a forebrain nucleus. Science. 1991;251:303–305. doi: 10.1126/science.1987645. [DOI] [PubMed] [Google Scholar]
- Briand LA, Gritton H, Howe WM, Young DA, Sarter M. Modulators in concert for cognition: modulator interactions in the prefrontal cortex. Prog Neurobiol. 2007;83:69–91. doi: 10.1016/j.pneurobio.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt JM, Lent KL, Beecher MD, Brenowitz EA. Lesions of the anterior forebrain song control pathway in female canaries affect song perception in an operant task. J Neurobiol. 2000;42:487. doi: 10.1002/(sici)1097-4695(200003)42:4<487::aid-neu9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci. 1988;8:4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Schmidt MF. Song system auditory responses are stable and highly tuned during sedation, rapidly modulated and unselective during wakefulness, and suppressed by arousal. J Neurophysiol. 2003;90:2884–2899. doi: 10.1152/jn.00391.2003. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Schmidt MF. Auditory responses in multiple sensorimotor song system nuclei are co-modulated by behavioral state. J Neurophysiol. 2004a;91:2148–2163. doi: 10.1152/jn.00918.2003. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Schmidt MF. Noradrenergic inputs mediate state dependence of auditory responses in the avian song system. J Neurosci. 2004b;24:7745–7753. doi: 10.1523/JNEUROSCI.1951-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MJ, Mooney R. Synaptic transformations underlying highly selective auditory representations of learned birdsong. J Neurosci. 2004;24:7251–7265. doi: 10.1523/JNEUROSCI.0947-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MJ, Roy A, Wild JM, Mooney R. Thalamic gating of auditory responses in telencephalic song control nuclei. J Neurosci. 2007;27:10024–10036. doi: 10.1523/JNEUROSCI.2215-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Chiba AA, Tuszynski MH. The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron. 2005;46:173–179. doi: 10.1016/j.neuron.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Dave AS, Margoliash D. Song replay during sleep and computational rules for sensorimotor vocal learning. Science. 2000;290:812–816. doi: 10.1126/science.290.5492.812. [DOI] [PubMed] [Google Scholar]
- Dave AS, Yu AC, Margoliash D. Behavioral state modulation of auditory activity in a vocal motor system. Science. 1998;282:2250–2254. doi: 10.1126/science.282.5397.2250. [DOI] [PubMed] [Google Scholar]
- Del Negro C, Gahr M, Leboucher G, Kreutzer M. The selectivity of sexual responses to song displays: effects of partial chemical lesion of the HVC in female canaries. Behav Brain Res. 1998;96:151–159. doi: 10.1016/s0166-4328(98)00009-6. [DOI] [PubMed] [Google Scholar]
- Detari L, Vanderwolf CH. Activity of identified cortically projecting and other basal forebrain neurones during large slow waves and cortical activation in anaesthetized rats. Brain Res. 1987;437:1–8. doi: 10.1016/0006-8993(87)91521-6. [DOI] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD. The effects of tonic locus ceruleus output on sensory-evoked responses of ventral posterior medial thalamic and barrel field cortical neurons in the awake rat. J Neurosci. 2004;24:10773–10785. doi: 10.1523/JNEUROSCI.1573-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue JP, Carroll KL. Cholinergic modulation of sensory responses in rat primary somatic sensory cortex. Brain Res. 1987;408:367–371. doi: 10.1016/0006-8993(87)90407-0. [DOI] [PubMed] [Google Scholar]
- Fortune ES, Margoliash D. Parallel pathways and convergence onto HVc and adjacent neostriatum of adult zebra finches (Taeniopygia guttata) J Comp Neurol. 1995;360:413–441. doi: 10.1002/cne.903600305. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nature. 2007;450:425–429. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- Gale SD, Person AL, Perkel DJ. A novel basal ganglia pathway forms a loop linking a vocal learning circuit with its dopaminergic input. J Comp Neurol. 2008;508:824–839. doi: 10.1002/cne.21700. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Margoliash D. Neuronal populations and single cells representing learned auditory objects. Nature. 2003;424:669–674. doi: 10.1038/nature01731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH, Bentley GE, Ball GF. Individual vocal recognition and the effect of partial lesions to HVc on discrimination, learning, and categorization of conspecific song in adult songbirds. J Neurobiol. 2000;42:117–133. doi: 10.1002/(sici)1097-4695(200001)42:1<117::aid-neu11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Giuliano C, Parikh V, Ward JR, Chiamulera C, Sarter M. Increases in cholinergic neurotransmission measured by using choline-sensitive microelectrodes: enhanced detection by hydrolysis of acetylcholine on recording sites? Neurochem Int. 2008;52:1343–1350. doi: 10.1016/j.neuint.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritti I, Mainville L, Mancia M, Jones BE. GABAergic and other noncholinergic basal forebrain neurons, together with cholinergic neurons, project to the mesocortex and isocortex in the rat. J Comp Neurol. 1997;383:163–177. [PubMed] [Google Scholar]
- Hahnloser RH, Kozhevnikov AA, Fee MS. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature. 2002;419:65–70. doi: 10.1038/nature00974. [DOI] [PubMed] [Google Scholar]
- Hamilton KS, King AP, Sengelaub DR, West MJ. A brain of her own: a neural correlate of song assessment in a female songbird. Neurobiol Learn Mem. 1997;68:325–332. doi: 10.1006/nlme.1997.3781. [DOI] [PubMed] [Google Scholar]
- Harding CF, Barclay SR, Waterman SA. Changes in catecholamine levels and turnover rates in hypothalamic, vocal control, and auditory nuclei in male zebra finches during development. J Neurobiol. 1998;34:329–356. [PubMed] [Google Scholar]
- Hars B, Maho C, Edeline JM, Hennevin E. Basal forebrain stimulation facilitates tone-evoked responses in the auditory cortex of awake rat. Neuroscience. 1993;56:61–74. doi: 10.1016/0306-4522(93)90562-t. [DOI] [PubMed] [Google Scholar]
- Herrero JL, Roberts MJ, Delicato LS, Gieselmann MA, Dayan P, Thiele A. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454:1110–1114. doi: 10.1038/nature07141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler NA, Doupe AJ. Social context modulates singing-related neural activity in the songbird forebrain. Nat Neurosci. 1999;2:209–211. doi: 10.1038/6306. [DOI] [PubMed] [Google Scholar]
- Howard MA, 3rd, Simons DJ. Physiologic effects of nucleus basalis magnocellularis stimulation on rat barrel cortex neurons. Exp Brain Res. 1994;102:21–33. doi: 10.1007/BF00232435. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, McCormick DA. Thalamic synchrony and dynamic regulation of global forebrain oscillations. Trends Neurosci. 2007;30:350–356. doi: 10.1016/j.tins.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Janata P, Margoliash D. Gradual emergence of song selectivity in sensorimotor structures of the male zebra finch song system. J Neurosci. 1999;19:5108–5118. doi: 10.1523/JNEUROSCI.19-12-05108.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Scharff C, Grossman MR, Ramos JA, Nottebohm F. For whom the bird sings: context-dependent gene expression. Neuron. 1998;21:775–788. doi: 10.1016/s0896-6273(00)80594-2. [DOI] [PubMed] [Google Scholar]
- Kao MH, Brainard MS. Lesions of an avian basal ganglia circuit prevent context-dependent changes to song variability. J Neurophysiol. 2006;96:1441–1455. doi: 10.1152/jn.01138.2005. [DOI] [PubMed] [Google Scholar]
- Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal gangliaforebrain circuit to real-time modulation of song. Nature. 2005;433:638–643. doi: 10.1038/nature03127. [DOI] [PubMed] [Google Scholar]
- Kao MH, Wright BD, Doupe AJ. Neurons in a forebrain nucleus required for vocal plasticity rapidly switch between precise firing and variable bursting depending on social context. J Neurosci. 2008;28:13232–13247. doi: 10.1523/JNEUROSCI.2250-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Gurney ME. Auditory responses in the zebra finch's motor system for song. Brain Res. 1981;221:192–197. doi: 10.1016/0006-8993(81)91073-8. [DOI] [PubMed] [Google Scholar]
- Kelley DB, Nottebohm F. Projections of a telencephalic auditory nucleus-field Lin the canary. J Comp Neurol. 1979;183:455–469. doi: 10.1002/cne.901830302. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kimpo RR, Theunissen FE, Doupe AJ. Propagation of correlated activity through multiple stages of a neural circuit. J Neurosci. 2003;23:5750–5761. doi: 10.1523/JNEUROSCI.23-13-05750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M. The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Z Tierpsychol. 1965;22:770–783. [PubMed] [Google Scholar]
- Kozhevnikov AA, Fee MS. Singing-related activity of identified HVC neurons in the zebra finch. J Neurophysiol. 2007;97:4271–4283. doi: 10.1152/jn.00952.2006. [DOI] [PubMed] [Google Scholar]
- Lee MG, Manns ID, Alonso A, Jones BE. Sleep-wake related discharge properties of basal forebrain neurons recorded with micropipettes in head-fixed rats. J Neurophysiol. 2004;92:1182–1198. doi: 10.1152/jn.01003.2003. [DOI] [PubMed] [Google Scholar]
- Leonardo A. Experimental test of the birdsong error-correction model. Proc Natl Acad Sci U S A. 2004;101:16935–16940. doi: 10.1073/pnas.0407870101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Sakaguchi H. Cholinergic innervation of the song control nuclei by the ventral paleostriatum in the zebra finch: a double-labeling study with retrograde fluorescent tracers and choline acetyltransferase immunohistochemistry. Brain Res. 1997;763:239–246. doi: 10.1016/s0006-8993(97)00417-4. [DOI] [PubMed] [Google Scholar]
- Lin SC, Nicolelis MA. Neuronal ensemble bursting in the basal forebrain encodes salience irrespective of valence. Neuron. 2008;59:138–149. doi: 10.1016/j.neuron.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns ID, Alonso A, Jones BE. Discharge profiles of juxtacellularly labeled and immunohistochemically identified GABAergic basal forebrain neurons recorded in association with the electroencephalogram in anesthetized rats. J Neurosci. 2000a;20:9252–9263. doi: 10.1523/JNEUROSCI.20-24-09252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns ID, Alonso A, Jones BE. Discharge properties of juxtacellularly labeled and immunohistochemically identified cholinergic basal forebrain neurons recorded in association with the electroencephalogram in anesthetized rats. J Neurosci. 2000b;20:1505–1518. doi: 10.1523/JNEUROSCI.20-04-01505.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns ID, Alonso A, Jones BE. Rhythmically discharging basal forebrain units comprise cholinergic, GABAergic, and putative glutamatergic cells. J Neurophysiol. 2003;89:1057–1066. doi: 10.1152/jn.00938.2002. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- Margoliash D. Acoustic parameters underlying the responses of song-specific neurons in the white-crowned sparrow. J Neurosci. 1983;3:1039–1057. doi: 10.1523/JNEUROSCI.03-05-01039.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margoliash D. Preference for autogenous song by auditory neurons in a song system nucleus of the white-crowned sparrow. J Neurosci. 1986;6:1643–1661. doi: 10.1523/JNEUROSCI.06-06-01643.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margoliash D, Fortune ES. Temporal and harmonic combination-sensitive neurons in the zebra finch's HVc. J Neurosci. 1992;12:4309–4326. doi: 10.1523/JNEUROSCI.12-11-04309.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCasland JS, Konishi M. Interaction between auditory and motor activities in an avian song control nucleus. Proc Natl Acad Sci U S A. 1981;78:7815–7819. doi: 10.1073/pnas.78.12.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna TM, Ashe JH, Hui GK, Weinberger NM. Muscarinic agonists modulate spontaneous and evoked unit discharge in auditory cortex of cat. Synapse. 1988;2:54–68. doi: 10.1002/syn.890020109. [DOI] [PubMed] [Google Scholar]
- Medina L, Reiner A. Distribution of choline acetyltransferase immunoreactivity in the pigeon brain. J Comp Neurol. 1994;342:497–537. doi: 10.1002/cne.903420403. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1–Ch6) Neuroscience. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Basal forebrain stimulation modifies auditory cortex responsiveness by an action at muscarinic receptors. Brain Res. 1991;559:163–167. doi: 10.1016/0006-8993(91)90301-b. [DOI] [PubMed] [Google Scholar]
- Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci. 1992;12:4701–4711. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R. Different subthreshold mechanisms underlie song selectivity in identified HVc neurons of the zebra finch. J Neurosci. 2000;20:5420–5436. doi: 10.1523/JNEUROSCI.20-14-05420.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R, Prather JF. The HVC microcircuit: the synaptic basis for interactions between song motor and vocal plasticity pathways. J Neurosci. 2005;25:1952–1964. doi: 10.1523/JNEUROSCI.3726-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealen PM, Schmidt MF. Distributed and selective auditory representation of song repertoires in the avian song system. J Neurophysiol. 2006;96:3433–3447. doi: 10.1152/jn.01130.2005. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Kelley DB, Paton JA. Connections of vocal control nuclei in the canary telencephalon. J Comp Neurol. 1982;207:344–357. doi: 10.1002/cne.902070406. [DOI] [PubMed] [Google Scholar]
- Olveczky BP, Andalman AS, Fee MS. Vocal experimentation in the juvenile songbird requires a basal ganglia circuit. PLoS Biol. 2005;3:e153. doi: 10.1371/journal.pbio.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Sarter M. Cholinergic mediation of attention: contributions of phasic and tonic increases in prefrontal cholinergic activity. Ann N Y Acad Sci. 2008;1129:225–235. doi: 10.1196/annals.1417.021. [DOI] [PubMed] [Google Scholar]
- Prather JF, Peters S, Nowicki S, Mooney R. Precise auditory-vocal mirroring in neurons for learned vocal communication. Nature. 2008;451:305–310. doi: 10.1038/nature06492. [DOI] [PubMed] [Google Scholar]
- Prather JF, Nowicki S, Anderson RC, Peters S, Mooney R. Neural correlates of categorical perception in learned vocal communication. Nat Neurosci. 2009;12:221–228. doi: 10.1038/nn.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan D, Tuszynski MH, Conner JM. The basal forebrain cholinergic system is required specifically for behaviorally mediated cortical map plasticity. J Neurosci. 2009;29:5992–6000. doi: 10.1523/JNEUROSCI.0230-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauske PL, Shea SD, Margoliash D. State and neuronal class-dependent reconfiguration in the avian song system. J Neurophysiol. 2003;89:1688–1701. doi: 10.1152/jn.00655.2002. [DOI] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Gunturkun O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RT, DeLong MR. Context-dependent responses of primate nucleus basalis neurons in a go/no-go task. J Neurosci. 1990;10:2528–2540. doi: 10.1523/JNEUROSCI.10-08-02528.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MJ, Mooney R. Synaptic interactions underlying song-selectivity in the avian nucleus HVC revealed by dual intracellular recordings. J Neurophysiol. 2006;95:1158–1175. doi: 10.1152/jn.00100.2005. [DOI] [PubMed] [Google Scholar]
- Ryan SM, Arnold AP. Evidence for cholinergic participation in the control of bird song; acetylcholinesterase distribution and muscarinic receptor autoradiography in the zebra finch brain. J Comp Neurol. 1981;202:211–219. doi: 10.1002/cne.902020207. [DOI] [PubMed] [Google Scholar]
- Sakaguchi H, Saito N. Developmental change of cholinergic activity in the forebrain of the zebra finch during song learning. Brain Res Dev Brain Res. 1991;62:223–228. doi: 10.1016/0165-3806(91)90169-j. [DOI] [PubMed] [Google Scholar]
- Sakata JT, Brainard MS. Online contributions of auditory feedback to neural activity in avian song control circuitry. J Neurosci. 2008;28:11378–11390. doi: 10.1523/JNEUROSCI.3254-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F, Cynx J. Conspecific and heterospecific song discrimination in male zebra finches with lesions in the anterior forebrain pathway. J Neurobiol. 1998;36:81–90. [PubMed] [Google Scholar]
- Schmidt MF, Konishi M. Gating of auditory responses in the vocal control system of awake songbirds. Nat Neurosci. 1998;1:513–518. doi: 10.1038/2232. [DOI] [PubMed] [Google Scholar]
- Shank SS, Margoliash D. Sleep and sensorimotor integration during early vocal learning in a songbird. Nature. 2009;458:73–77. doi: 10.1038/nature07615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea SD, Margoliash D. Basal forebrain cholinergic modulation of auditory activity in the zebra finch song system. Neuron. 2003;40:1213–1226. doi: 10.1016/s0896-6273(03)00723-2. [DOI] [PubMed] [Google Scholar]
- Sillito AM, Kemp JA. Cholinergic modulation of the functional organization of the cat visual cortex. Brain Res. 1983;289:143–155. doi: 10.1016/0006-8993(83)90015-x. [DOI] [PubMed] [Google Scholar]
- Soha JA, Shimizu T, Doupe AJ. Development of the catecholaminergic innervation of the song system of the male zebra finch. J Neurobiol. 1996;29:473–489. doi: 10.1002/(SICI)1097-4695(199604)29:4<473::AID-NEU5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Sossinka R, Bohner J. Song types in the zebra finch (Poephila guttata castanotis) Z Tierpsychol. 1980;53:123–132. [Google Scholar]
- Sullivan RM, Wilson DA, Leon M. Norepinephrine and learning-induced plasticity in infant rat olfactory system. J Neurosci. 1989;9:3998–4006. doi: 10.1523/JNEUROSCI.09-11-03998.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymusiak R, McGinty D. Sleep-waking discharge of basal forebrain projection neurons in cats. Brain Res Bull. 1989;22:423–430. doi: 10.1016/0361-9230(89)90069-5. [DOI] [PubMed] [Google Scholar]
- Tremblay N, Warren RA, Dykes RW. Electrophysiological studies of acetylcholine and the role of the basal forebrain in the somatosensory cortex of the cat. II. Cortical neurons excited by somatic stimuli. J Neurophysiol. 1990;64:1212–1222. doi: 10.1152/jn.1990.64.4.1212. [DOI] [PubMed] [Google Scholar]
- Vates GE, Vicario DS, Nottebohm F. Reafferent thalamo- “cortical” loops in the song system of oscine songbirds. J Comp Neurol. 1997;380:275–290. [PubMed] [Google Scholar]
- Vates GE, Broome BM, Mello CV, Nottebohm F. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches. J Comp Neurol. 1996;366:613–642. doi: 10.1002/(SICI)1096-9861(19960318)366:4<613::AID-CNE5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Veenman CL, Reiner A. The distribution of GABA-containing perikarya, fibers, and terminals in the forebrain and midbrain of pigeons, with particular reference to the basal ganglia and its projection targets. J Comp Neurol. 1994;339:209–250. doi: 10.1002/cne.903390205. [DOI] [PubMed] [Google Scholar]
- Vu ET, Mazurek ME, Kuo YC. Identification of a forebrain motor programming network for the learned song of zebra finches. J Neurosci. 1994;14:6924–6934. doi: 10.1523/JNEUROSCI.14-11-06924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JT, Adkins-Regan E, Whiting P, Lindstrom JM, Podleski TR. Autoradiographic localization of nicotinic acetylcholine receptors in the brain of the zebra finch (Poephila guttata) J Comp Neurol. 1988;274:255–264. doi: 10.1002/cne.902740209. [DOI] [PubMed] [Google Scholar]
- Wenk GL. The nucleus basalis magnocellularis cholinergic system: one hundred years of progress. Neurobiol Learn Mem. 1997;67:85–95. doi: 10.1006/nlme.1996.3757. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Kapp BS, Pascoe JP. Neuronal activity within the nucleus basalis and conditioned neocortical electroencephalographic activation. J Neurosci. 1994;14:1623–1633. doi: 10.1523/JNEUROSCI.14-03-01623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams H, Mehta N. Changes in adult zebra finch song require a forebrain nucleus that is not necessary for song production. J Neurobiol. 1999;39:14–28. [PubMed] [Google Scholar]
- Wilson FA, Rolls ET. Neuronal responses related to reinforcement in the primate basal forebrain. Brain Res. 1990;509:213–231. doi: 10.1016/0006-8993(90)90546-n. [DOI] [PubMed] [Google Scholar]
- Woolley SC, Doupe AJ. Social context-induced song variation affects female behavior and gene expression. PLoS Biol. 2008;6:e62. doi: 10.1371/journal.pbio.0060062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AC, Margoliash D. Temporal hierarchical control of singing in birds. Science. 1996;273:1871–1875. doi: 10.1126/science.273.5283.1871. [DOI] [PubMed] [Google Scholar]
- Zuschratter W, Scheich H. Distribution of choline acetyltransferase and acetylcholinesterase in the vocal motor system of zebra finches. Brain Res. 1990;513:193–201. doi: 10.1016/0006-8993(90)90457-m. [DOI] [PubMed] [Google Scholar]