Abstract
Bacterial genomes vary in size over two orders of magnitude. The Mycoplasma genitalium genome has traditionally defined the extreme small end of this spectrum, and has therefore heavily informed theoretical and experimental work aimed at determining the minimal gene content necessary to support cellular life. Recent genomic data from insect symbionts has revealed bacterial genomes that are incredibly small—two to four times smaller than M. genitalium—and these tiny genomes have raised questions about the limits of genome reduction and have blurred the once-clear distinction between autonomous cellular life and highly integrated organelle. New data from various systems with symbiotic bacterial or archaeal partners have begun to shed light on how these bacteria may function with such small gene sets, but major mechanistic questions remain.
Introduction
In most bacterial genomes, genes are tightly packed and uniformly distributed at about one gene per kilobase (kb) [1], so that in most cases genome reduction implies gene loss. Bacteria that have close associations with animals often show reduced genomes compared to free-living relatives [2–4], and for decades the smallest cellular genome observed in nature was from the human pathogen M. genitalium [5,6]. As the ancestors of both mitochondria and chloroplasts were free-living bacteria [7,8], they can be considered the most extreme examples of bacterial genome reduction. Despite their bacterial origins, however, mitochondria and chloroplasts are defined as cellular organelles, not as autonomous bacteria. This distinction is based on lifestyle and gene content: M. genitalium can be grown in the lab, while organelles are highly genetically integrated with the nucleus and are completely dependent on being in the host environment [7,8]; M. genitalium has 524 genes in a 580 kb genome [6], while the largest mitochondrial genome has 97 genes in a 69 kb genome [9], and the most gene-rich chloroplast genome has 253 genes in a 191 kb genome [10]. A long-standing empirical limit for genome reduction in autonomous bacteria was therefore established by the mycoplasma, however they remain clearly distinct from organelles by almost any measure except their shared bacterial ancestry.
This clean differentiation between organelle and independent bacteria has been muddied in the last few years by data from genome sequencing projects targeting uncultured intracellular symbionts of insects. This review will briefly describe these tiny symbiont genomes and discuss them in the context of the minimal genome concept, compare their gene content with that of organelles, and summarize recent experiments that give the first clues as to how these organisms might survive with such small gene sets.
Bacterial endosymbionts of insects
Like all animals, insects form associations with diverse bacterial lineages [4]. These symbioses vary by type, falling anywhere on the parasitic-commensal-mutualistic continuum. Once established, these relationships are not necessarily static, sometimes rapidly switching between association type (e.g., from parasite to mutualist [11**]). The intimacy of the interactions can also vary, as symbionts can be horizontally transferred among unrelated insects and/or strictly vertically transmitted in a species-specific manner, and are found in a wide range of tissues, from the extracellular space of the gut to the cytoplasm of specialized host cells. A well-known example of an intracellular parasite that can be either horizontally or vertically transferred is the reproductive manipulator Wolbachia, an α-Proteobacteria which skews the sex ratios of offspring in infected mothers [12*]. Many insects with restricted or specialized diets (e.g., plant sap, animal blood) have one or more intracellular bacterial mutualist, which provision the insect with nutrients that are missing in their diet [13,14]. These associations are usually extremely stable—in some cases cospeciating for hundreds of millions of years—by virtue of strict transovarial transmission of the symbionts through insect generations [15,16]. Most of these associations are thought to be reciprocally obligate, i.e., neither the insect nor its symbiotic bacteria can survive without the other [14,17]. These symbionts also tend to have highly reduced genomes compared to their free-living relatives [4].
The first several insect nutritional symbionts to have their genomes sequenced—all γ-Proteobacteria—included three strains of the aphid symbiont Buchnera aphidicola [15,18,19], the tsetse fly symbiont Wigglesworthia glossinidia [20], and two strains of the carpenter ant symbiont Blochmannia [21,22]. While all of these symbionts showed significant levels of genome reduction (616 – 792 kb) and their limited gene sets indicated they could not (easily) live outside of the host cell environment, their genome sizes were above the minimal size threshold established by M. genitalium (although physical mapping of various Buchnera strains indicated that some had smaller genomes, in the range of 450 kb [23]).
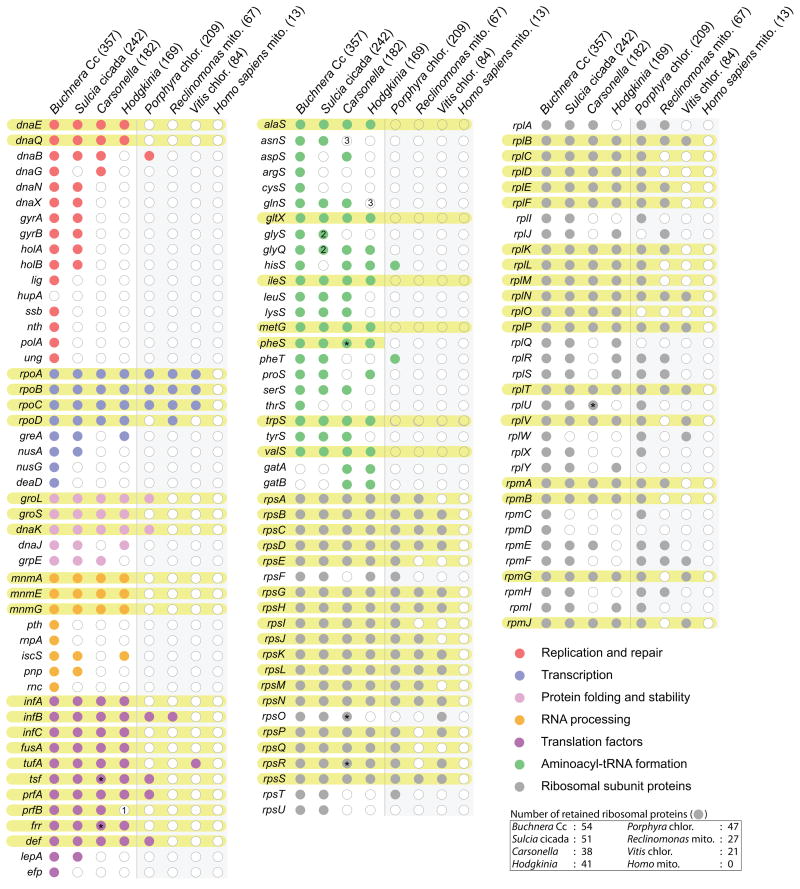
Recent results from genome sequencing of diverse bacterial symbionts of sap-feeding insects have begun to blur the clear distinction between independent bacterial life and organelle, crashing through the 500 kb genome barrier established by M. genitalium in dramatic fashion. In 2006, the 422 kb genome from Buchnera aphidicola Cc [24] and the 160 kb genome from Carsonella ruddii [25], a γ-Proteobacterial symbiont of psyllid, were reported. The next year, a Bacteroidetes called Sulcia muelleri, which is symbiotic with the glassy-winged sharpshooter, was reported to have a genome of 245 kb [26]. Finally, in 2009, the genome for an α-Proteobacterial symbiont of singing cicadas, Hodgkinia cicadicola, was shown to have a genome of only 144 kb, encoding a paltry 188 genes [27*]. (Carsonella is the sole symbiont in the species of psyllid studied, but Buchnera Cc [28], Sulcia [16,26,29], and Hodgkinia [30] all have co-symbionts inhabiting the same insect tissue; Sulcia and Hodgkinia are partners in cicada.) Amazingly, Carsonella and Hodgkinia have smaller genomes and fewer protein-coding genes than some chloroplasts (Fig. 1), and questions as to whether or not these organisms can still be considered autonomous bacteria have arisen [31*,32].
Figure 1. Gene content of the smallest cellular genomes and some organelles.
Genes present in the four smallest bacterial genomes [24,25,27*,30] together with large [9,10] and more typical [56,57] mitochondrial and chloroplast genomes are shown as colored circles, missing genes as open circles. The number of protein coding genes is shown in parentheses after the organism name. Abbreviations: mitochondria (mito.) and chloroplast (chlor). Rows for genes present in all four symbiont genomes are highlighted in yellow. Asterisks represent genes that are highly divergent from typical sequences. Numbered positions indicate: (1) translational release factor 2 (prfB) is not needed in the Hodgkinia genome because the stop codon UGA has been recoded as tryptophan [27*]; (2) Sulcia uses the single subunit version of glycyl-tRNA synthetase; and (3) these aminoacyl-tRNA synthetases are not necessary due to the presence of proteins (GatAB) that catalyze a tRNA-dependent amidotransferase activity [58]. The numbers of retained ribosomal genes are shown in the table at the bottom right of the figure. The list of genes in this figure are a subset of genes listed in the smallest minimal genome set [37].
Metabolic vs. genetic integration and the minimal genome concept
The small genome of Mycoplasma genitalium has made it a central player in the “minimal genome concept,” which can be defined as the experimental and computational search for the minimal gene content required for independent life, given the richest possible growth environment [33–39]. Predictions of the minimal genome, based on either comparative genomics [33,37] or global transposition mutagenesis of M. genitalium [38], range from about 200 to 400 genes.
The organism(s) that would fulfill the minimal genome concept are usually, but not always [39], assumed to be both genetically and metabolically independent. That is, these organisms would be capable of replicating their genome, transcribing RNA, and translating protein (genetic independence); and would be able to obtain energy from simple metabolites to make nucleotides, amino acids, lipids, and cofactors (metabolic independence). Gene content analysis of Buchnera Cc, Sulcia, Carsonella, and Hodgkinia reveal that these organisms are not metabolically independent, as they cannot make fatty acids (except Buchnera), phospholipids, nucleotides, pyridines, and in the case of Buchnera Cc and Hodgkinia, have lost their F1F0 ATP synthase. This loss of metabolic independence is typical of both intracellular [2] and extracellular [40] symbionts. It is assumed that the required compounds are somehow derived from the host (or possibly a co-symbiont, in some systems), but the mechanisms are not well understood. Therefore, the remainder of this discussion will focus on the potential genetic independence of the most highly reduced symbiont genomes.
The gene contents of symbionts and organelles are different
While the number of genes predicted in the smallest symbiont genomes rival that of some organelles, gene content analysis reveals a clear difference in retained activities (Fig. 1). Insect symbionts have retained genes involved in the core enzymatic activities involved in chromosome replication, transcription, and translation, while in organellar genomes many of these functions have been lost, with some exceptions (Fig. 1). For example, all of the bacterial symbionts contain a homolog of the core replicative DNA polymerase (dnaE), the protein responsible for the 5′ to 3′ polymerization activity of the replication holoenzyme, but lack homologs for many of the accessory components involved in increasing processivity, initiation, and error correction (Fig. 1). These patterns suggest, not surprisingly [8], that the forces governing gene loss in symbionts and organelles are different. Although it is not at all clear how the genes present in symbiont genomes could work to form a fully functional replicating unit, they do suggest a stronger bacterial identity for nutritional symbionts than for organelles.
There are a number of possible ways these symbionts could cope with such small gene sets, such as: i) the transfer some genes to the nucleus for subsequent reimportation, similar to what is observed in organelles; ii) the importation host (or co-symbiont) proteins or RNAs that complement the lost activities; or, perhaps most interestingly, iii) the evolution of unexpected coadaptations to the loss of various genes, resulting in mechanisms for cellular processes that are difficult to predict. While some data exist concerning the host’s role in the symbiosis [41], there is no information presently available concerning the import of proteins or RNA into these symbionts, so this point will not be discussed further.
Is gene transfer the answer?
Given the extremely small gene sets of these insect endosymbionts, it is tempting to speculate whether some of the lost genes have been transferred to the host nucleus for subsequent expression and protein reimportation to the symbiont [25,42], as this process has occurred with some frequency in organelles, and in fact has been shown to be ongoing in some cases [8]. This idea might be considered particularly seductive given the apparent ease with which Wolbachia species—another transovarially transmitted intracellular bacterial symbiont found in insects and other invertebrates—have been shown to exchange DNA with the host nucleus [43–49]. Remarkably, some of these Wolbachia-to-host transfers include DNA fragments approaching the size of entire Wolbachia genomes (about 1 Mb) [44**]. Early evidence suggested that the majority of these transferred genes were non-functional, as they typically are not expressed at high levels and contain mutations that would result in nonfunctional proteins if expressed in the recipient host cell [44**,49,50]. However, recent experiments from various systems have shown that some transferred genes might be functional, in that they contain no premature stop codons, are undergoing purifying selection, and in some cases are expressed at high levels in the appropriate tissues [45–47].
Of particular relevance here is the report of transferred bacterial genes in the pea aphid Acyrthosiphon pisum [47], as the pea aphid is host to Buchnera aphidicola, a long-term coevolving bacterial symbiont with a reduced genome. While Buchnera from the pea aphid does not show as much genome reduction as Hodgkinia, Carsonella, or Sulcia, at 641 kb it is still a small bacterial genome [18], and its publication has fueled speculation that some lost genes might have been transferred to the host nucleus [42]. By analyzing an mRNA expression library made from aphid tissues for genes that looked bacterial in nature, two potential transfers were identified: ldcA (LD-carboxypeptidase) and rplA (rare lipoprotein A) [47]. Phylogenetic analysis indicated that ldcA was derived from a Wolbachia-like α-Proteobacteria, while the classification of rplA was less clear [47]. Importantly, both genes were preferentially expressed in the tissue type containing bacterial symbionts [47]. These results suggest two interesting possibilities: i) the maintenance of some symbioses may be aided by genes transferred to the host from unrelated bacterial lineages, and ii) lost Buchnera genes could be complemented by genes transferred to the host nucleus from an unrelated symbiotic bacterium such as Wolbachia. Although these data are preliminary, they also hint at the possibility that the large amount of genome reduction seen in insect symbionts may not have been accompanied by gene transfer to the host nucleus, as no clear case of gene transfer from Buchnera was observed in this study. It should be noted that firm results on the number of potentially transferred Buchnera genes will soon be available upon completion and analysis of the pea aphid genome [NCBI Aphid Genome Resources; URL: www.ncbi.nlm.nih.gov/projects/genome/guide/aphid/].
It is important to note that although both Wolbachia and insect nutritional symbionts are transferred via a transovarial route, the timing and cell biology of these transfers are different. In the fruit fly, Wolbachia is intimately associated with germ line cells throughout the development of an infected insect, including cytoplasmic localization in the germ line stem cells and physical association with oocyte nuclei at later points in oogenesis (e.g., see [51]). By contrast, in aphid development (the best studied system for insect nutritional symbionts, though the rough outlines seem similar in other sap-feeding insects [13]), Buchnera cells are not transferred to the oocyte until later in oogenesis, where the bacteria are held in a matrix of filamentous actin at the posterior end of the egg until being cellularized by the developing embryo (e.g., see [52]). If further work continues to show a dearth of gene transfer between nutritional symbionts and their hosts compared with Wolbachia, the close association with the germ line in the latter may account for the difference.
Unexpected coadaptations to gene loss
The concept of an “essential” gene is difficult to precisely define. Some genes are required only in certain metabolic contexts, and other genes found to be required experimentally in one bacterial lineage are completely missing in other lineages [36,53,54]. Furthermore, there are only about 60 universally conserved proteins derived from the analysis of genome projects, this list being dominated by translation related functions [36]. Clearly, though, there are a core of highly conserved genes that seem to have essential activities for which it is difficult to imagine how the cell survives without. One possible solution to the problem of “essential” gene loss that is rarely mentioned is the emergence of novel coadaptations elsewhere in the genome to accommodate the lost activity [54]. The main problem with this solution is that mechanisms are difficult to imagine in many cases, and concrete examples have been rare until recently.
The most compelling example of coadaptation to the loss of an “essential” gene comes from the smallest Archaeal genome, Nanoarchaeum equitans, the extracellular symbiont of Ignicoccus hospitalis (itself an archaeon) [40]. Nanoarchaeum—as well as Sulcia, Carsonella, and Hodgkinia—lacks the ribonucleoprotein RNase P, the enzyme involved in processing 5′ leader sequence from tRNAs. RNase P is a (nearly) ubiquitous enzyme, and therefore is included in even the smallest proposed minimal genome [37]. The absence of RNase P in Nanoarchaeum prompted Söll and colleagues to look at this system more closely, where they found that unlike most organisms, Nanoarchaeum tRNAs have transcriptional promoters placed at uniform distances upstream of the first base of the tRNA [55**]. This precise promoter positioning allows for leaderless tRNAs; if transcription always starts at the first base of tRNA, RNase P is no longer needed. This result shows how the cell can cope with the loss of an “essential” and nearly universal gene in a novel and unexpected way, and serves as a warning not to expect cellular processes, even highly conserved and seemingly essential ones, to proceed by standard mechanisms in highly reduced symbiont genomes.
Conclusions
Continued sequencing of symbiont genomes, whether from insects or elsewhere, will likely continue to uncover organisms with even smaller gene sets than the ones discussed here. These genomes will continue to contribute to our understanding of the breadth and depth of bacterial symbioses with animals, but will likely not advance the field in terms of understanding how these organisms survive with such limited gene sets. It seems reasonable that the answer lies in a complex combination of metabolite, protein, and/or RNA importation combined with both small incremental and large unexpected coadaptations to the loss of genes. Untangling this web will not be easy, as none of these insect systems containing the smallest symbiont genomes are currently genetically tractable or even easily cultured in the lab. Progress will have to come from creative biochemical and cell biological experiments that complement the intriguing genomic data described here.
Acknowledgments
J.P.M. was funded by the Center for Insect Science at the University of Arizona through National Institutes of Health Training Grant # 1K12 GM000708.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ochman H, Davalos LM. The nature and dynamics of bacterial genomes. Science. 2006;311:1730–1733. doi: 10.1126/science.1119966. [DOI] [PubMed] [Google Scholar]
- 2.Klasson L, Andersson SG. Evolution of minimal-gene-sets in host-dependent bacteria. Trends Microbiol. 2004;12:37–43. doi: 10.1016/j.tim.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Moya A, Pereto J, Gil R, Latorre A. Learning how to live together: genomic insights into prokaryote-animal symbioses. Nat Rev Genet. 2008;9:218–229. doi: 10.1038/nrg2319. [DOI] [PubMed] [Google Scholar]
- 4.Moran NA, McCutcheon JP, Nakabachi A. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet. 2008;42:165–190. doi: 10.1146/annurev.genet.41.110306.130119. [DOI] [PubMed] [Google Scholar]
- 5.Wallace DC, Morowitz HJ. Genome size and evolution. Chromosoma. 1973;40:121–126. doi: 10.1007/BF00321457. [DOI] [PubMed] [Google Scholar]
- 6.Fraser CM, Gocayne JD, White O, Adams MD, Clayton RA, Fleischmann RD, Bult CJ, Kerlavage AR, Sutton G, Kelley JM, et al. The minimal gene complement of Mycoplasma genitalium. Science. 1995;270:397–403. doi: 10.1126/science.270.5235.397. [DOI] [PubMed] [Google Scholar]
- 7.Adams KL, Palmer JD. Evolution of mitochondrial gene content: gene loss and transfer to the nucleus. Mol Phylogenet Evol. 2003;29:380–395. doi: 10.1016/s1055-7903(03)00194-5. [DOI] [PubMed] [Google Scholar]
- 8.Timmis JN, Ayliffe MA, Huang CY, Martin W. Endosymbiotic gene transfer: organelle genomes forge eukaryotic chromosomes. Nature Rev Genet. 2004;5:123–135. doi: 10.1038/nrg1271. [DOI] [PubMed] [Google Scholar]
- 9.Lang BF, Burger G, O’Kelly CJ, Cedergren R, Golding GB, Lemieux C, Sankoff D, Turmel M, Gray MW. An ancestral mitochondrial DNA resembling a eubacterial genome in miniature. Nature. 1997;387:493–497. doi: 10.1038/387493a0. [DOI] [PubMed] [Google Scholar]
- 10.Reith M, Munholland J. Complete nucleotide sequence of the Porphyra purpurea chloroplast genome. Plant Mol Biol Rep. 1995;13:333–335. [Google Scholar]
- 11**.Weeks AR, Turelli M, Harcombe WR, Reynolds KT, Hoffmann AA. From parasite to mutualist: rapid evolution of Wolbachia in natural populations of Drosophila. PLoS Biology. 2007;5:e114. doi: 10.1371/journal.pbio.0050114. This paper documents an amazing case of Wolbachia populations shifting from a parasitic interaction with its host to a more mutualistic one over a 20 year time period. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12*.Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. A detailed and interesting overview of Wolbachia biology. [DOI] [PubMed] [Google Scholar]
- 13.Buchner P. Endosymbiosis of animals with plant microorganisms. New York, NY: Interscience; 1965. [Google Scholar]
- 14.Douglas AE. Mycetocyte symbiosis in insects. Biol Rev Camb Philos Soc. 1989;64:409–434. doi: 10.1111/j.1469-185x.1989.tb00682.x. [DOI] [PubMed] [Google Scholar]
- 15.Tamas I, Klasson L, Canback B, Naslund AK, Eriksson AS, Wernegreen JJ, Sandstrom JP, Moran NA, Andersson SG. 50 million years of genomic stasis in endosymbiotic bacteria. Science. 2002;296:2376–2379. doi: 10.1126/science.1071278. [DOI] [PubMed] [Google Scholar]
- 16.Moran NA, Tran P, Gerardo NM. Symbiosis and insect diversification: an ancient symbiont of sap-feeding insects from the Bacterial phylum Bacteroidetes. Appl Environ Microbiol. 2005;71:8802–8810. doi: 10.1128/AEM.71.12.8802-8810.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakabachi A, Ishikawa H. Provision of riboflavin to the host aphid, Acyrthosiphon pisum, by endosymbiotic bacteria, Buchnera. Journal of Insect Physiology. 1999;45:1–6. doi: 10.1016/s0022-1910(98)00104-8. [DOI] [PubMed] [Google Scholar]
- 18.Shigenobu S, Watanabe H, Hattori M, Sakaki Y, Ishikawa H. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature. 2000;407:81–86. doi: 10.1038/35024074. [DOI] [PubMed] [Google Scholar]
- 19.van Ham RC, Kamerbeek J, Palacios C, Rausell C, Abascal F, Bastolla U, Fernandez JM, Jimenez L, Postigo M, Silva FJ, et al. Reductive genome evolution in Buchnera aphidicola. Proc Natl Acad Sci U S A. 2003;100:581–586. doi: 10.1073/pnas.0235981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akman L, Yamashita A, Watanabe H, Oshima K, Shiba T, Hattori M, Aksoy S. Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia. Nature Genetics. 2002;32:402–407. doi: 10.1038/ng986. [DOI] [PubMed] [Google Scholar]
- 21.Gil R, Silva FJ, Zientz E, Delmotte F, Gonzalez-Candelas F, Latorre A, Rausell C, Kamerbeek J, Gadau J, Holldobler B, et al. The genome sequence of Blochmannia floridanus: comparative analysis of reduced genomes. Proc Natl Acad Sci U S A. 2003;100:9388–9393. doi: 10.1073/pnas.1533499100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Degnan PH, Lazarus AB, Wernegreen JJ. Genome sequence of Blochmannia pennsylvanicus indicates parallel evolutionary trends among bacterial mutualists of insects. Genome Res. 2005;15:1023–1033. doi: 10.1101/gr.3771305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gil R, Sabater-Munoz B, Latorre A, Silva FJ, Moya A. Extreme genome reduction in Buchnera spp. : toward the minimal genome needed for symbiotic life. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:4454–4458. doi: 10.1073/pnas.062067299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez-Brocal V, Gil R, Ramos S, Lamelas A, Postigo M, Michelena JM, Silva FJ, Moya A, Latorre A. A small microbial genome: the end of a long symbiotic relationship? Science. 2006;314:312–313. doi: 10.1126/science.1130441. [DOI] [PubMed] [Google Scholar]
- 25.Nakabachi A, Yamashita A, Toh H, Ishikawa H, Dunbar HE, Moran NA, Hattori M. The 160-kilobase genome of the bacterial endosymbiont Carsonella. Science. 2006;314:267. doi: 10.1126/science.1134196. [DOI] [PubMed] [Google Scholar]
- 26.McCutcheon JP, Moran NA. Parallel genomic evolution and metabolic interdependence in an ancient symbiosis. Proc Natl Acad Sci U S A. 2007;104:19392–19397. doi: 10.1073/pnas.0708855104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27*.McCutcheon JP, McDonald BR, Moran NA. Origin of an alternative genetic code in the extremely small and GC-rich genome of a bacterial symbiont. PLoS Genet. 2009;5:e1000565. doi: 10.1371/journal.pgen.1000565. This paper describes a highly unusal bacterial genome, which has the smallest cellular genome known, a high GC content, and a genetic code change of UGA coding for tryptophan instead of stop. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gosalbes MJ, Lamelas A, Moya A, Latorre A. The striking case of tryptophan provision in the cedar aphid Cinara cedri. Journal of Bacteriology. 2008;190:6026–6029. doi: 10.1128/JB.00525-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu D, Daugherty SC, Van Aken SE, Pai GH, Watkins KL, Khouri H, Tallon LJ, Zaborsky JM, Dunbar HE, Tran PL, et al. Metabolic complementarity and genomics of the dual bacterial symbiosis of sharpshooters. PLoS Biol. 2006;4:e188. doi: 10.1371/journal.pbio.0040188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCutcheon JP, McDonald BR, Moran NA. Convergent evolution of metabolic roles in bacterial co-symbionts of insects. Proc Natl Acad Sci U S A. 2009;106:15394–15399. doi: 10.1073/pnas.0906424106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Bhattacharya D, Archibald JM, Weber AP, Reyes-Prieto A. How do endosymbionts become organelles? Understanding early events in plastid evolution. Bioessays. 2007;29:1239–1246. doi: 10.1002/bies.20671. A good overview of some of the issues concerning the definition of an organelle. [DOI] [PubMed] [Google Scholar]
- 32.Tamames J, Gil R, Latorre A, Pereto J, Silva FJ, Moya A. The frontier between cell and organelle: genome analysis of Candidatus Carsonella ruddii. BMC Evol Biol. 2007;7:181. doi: 10.1186/1471-2148-7-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mushegian AR, Koonin EV. A minimal gene set for cellular life derived by comparison of complete bacterial genomes. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:10268–10273. doi: 10.1073/pnas.93.19.10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maniloff J. The minimal cell genome: “on being the right size”. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:10004–10006. doi: 10.1073/pnas.93.19.10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mushegian A. The minimal genome concept. Current Opinion in Genetics and Development. 1999;9:709–714. doi: 10.1016/s0959-437x(99)00023-4. [DOI] [PubMed] [Google Scholar]
- 36.Koonin EV. Comparative genomics, minimal gene-sets and the last universal common ancestor. Nature Rev Microbiol. 2003;1:127–136. doi: 10.1038/nrmicro751. [DOI] [PubMed] [Google Scholar]
- 37.Gil R, Silva FJ, Pereto J, Moya A. Determination of the core of a minimal bacterial gene set. Microbiol Mol Biol Rev. 2004;68:518–537. doi: 10.1128/MMBR.68.3.518-537.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glass JI, Assad-Garcia N, Alperovich N, Yooseph S, Lewis MR, Maruf M, Hutchison CA, 3rd, Smith HO, Venter JC. Essential genes of a minimal bacterium. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:425–430. doi: 10.1073/pnas.0510013103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forster AC, Church GM. Towards synthesis of a minimal cell. Mol Syst Biol. 2006;2:45. doi: 10.1038/msb4100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waters E, Hohn MJ, Ahel I, Graham DE, Adams MD, Barnstead M, Beeson KY, Bibbs L, Bolanos R, Keller M, et al. The genome of Nanoarchaeum equitans: insights into early archaeal evolution and derived parasitism. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12984–12988. doi: 10.1073/pnas.1735403100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakabachi A, Shigenobu S, Sakazume N, Shiraki T, Hayashizaki Y, Carninci P, Ishikawa H, Kudo T, Fukatsu T. Transcriptome analysis of the aphid bacteriocyte, the symbiotic host cell that harbors an endocellular mutualistic bacterium, Buchnera. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5477–5482. doi: 10.1073/pnas.0409034102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andersson JO. Evolutionary genomics: is Buchnera a bacterium or an organelle? Current Biology. 2000;10:R866–868. doi: 10.1016/s0960-9822(00)00816-2. [DOI] [PubMed] [Google Scholar]
- 43.Kondo N, Nikoh N, Ijichi N, Shimada M, Fukatsu T. Genome fragment of Wolbachia endosymbiont transferred to X chromosome of host insect. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:14280–14285. doi: 10.1073/pnas.222228199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44**.Hotopp JC, Clark ME, Oliveira DC, Foster JM, Fischer P, Torres MC, Giebel JD, Kumar N, Ishmael N, Wang S, et al. Widespread lateral gene transfer from intracellular bacteria to multicellular eukaryotes. Science. 2007;317:1753–1756. doi: 10.1126/science.1142490. The authors document multiple cases of gene transfer from Wolbachia to insect and nematode genomes. [DOI] [PubMed] [Google Scholar]
- 45.Woolfit M, Iturbe-Ormaetxe I, McGraw EA, O’Neill SL. An ancient horizontal gene transfer between mosquito and the endosymbiotic bacterium Wolbachia pipientis. Molecular Biology and Evolution. 2009;26:367–374. doi: 10.1093/molbev/msn253. [DOI] [PubMed] [Google Scholar]
- 46.Klasson L, Kambris Z, Cook PE, Walker T, Sinkins SP. Horizontal gene transfer between Wolbachia and the mosquito Aedes aegypti. BMC Genomics. 2009;10:33. doi: 10.1186/1471-2164-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nikoh N, Nakabachi A. Aphids acquired symbiotic genes via lateral gene transfer. BMC Biol. 2009;7:12. doi: 10.1186/1741-7007-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aikawa T, Anbutsu H, Nikoh N, Kikuchi T, Shibata F, Fukatsu T. Longicorn beetle that vectors pinewood nematode carries many Wolbachia genes on an autosome. Proc Biol Sci. 2009;276:3791–3798. doi: 10.1098/rspb.2009.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fenn K, Conlon C, Jones M, Quail MA, Holroyd NE, Parkhill J, Blaxter M. Phylogenetic relationships of the Wolbachia of nematodes and arthropods. PLoS Pathog. 2006;2:e94. doi: 10.1371/journal.ppat.0020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nikoh N, Tanaka K, Shibata F, Kondo N, Hizume M, Shimada M, Fukatsu T. Wolbachia genome integrated in an insect chromosome: evolution and fate of laterally transferred endosymbiont genes. Genome Research. 2008;18:272–280. doi: 10.1101/gr.7144908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferree PM, Frydman HM, Li JM, Cao J, Wieschaus E, Sullivan W. Wolbachia utilizes host microtubules and Dynein for anterior localization in the Drosophila oocyte. PLoS Pathog. 2005;1:e14. doi: 10.1371/journal.ppat.0010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miura T, Braendle C, Shingleton A, Sisk G, Kambhampati S, Stern DL. A comparison of parthenogenetic and sexual embryogenesis of the pea aphid Acyrthosiphon pisum (Hemiptera: Aphidoidea) J Exp Zool B Mol Dev Evol. 2003;295:59–81. doi: 10.1002/jez.b.3. [DOI] [PubMed] [Google Scholar]
- 53.Sassetti CM, Boyd DH, Rubin EJ. Genes required for mycobacterial growth defined by high density mutagenesis. Molecular Microbiology. 2003;48:77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- 54.Moran NA. Tracing the evolution of gene loss in obligate bacterial symbionts. Curr Opin Microbiol. 2003;6:512–518. doi: 10.1016/j.mib.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 55**.Randau L, Schroder I, Soll D. Life without RNase P. Nature. 2008;453:120–123. doi: 10.1038/nature06833. A terrific paper describing how Nanoarcheaum equitans is able to function without the ubiquitous enzymatic activity of RNase P. [DOI] [PubMed] [Google Scholar]
- 56.Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 57.Jansen RK, Kaittanis C, Saski C, Lee SB, Tomkins J, Alverson AJ, Daniell H. Phylogenetic analyses of Vitis (Vitaceae) based on complete chloroplast genome sequences: effects of taxon sampling and phylogenetic methods on resolving relationships among rosids. BMC Evol Biol. 2006;6:32. doi: 10.1186/1471-2148-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheppard K, Yuan J, Hohn MJ, Jester B, Devine KM, Soll D. From one amino acid to another: tRNA-dependent amino acid biosynthesis. Nucleic Acids Res. 2008;36:1813–1825. doi: 10.1093/nar/gkn015. [DOI] [PMC free article] [PubMed] [Google Scholar]