Abstract
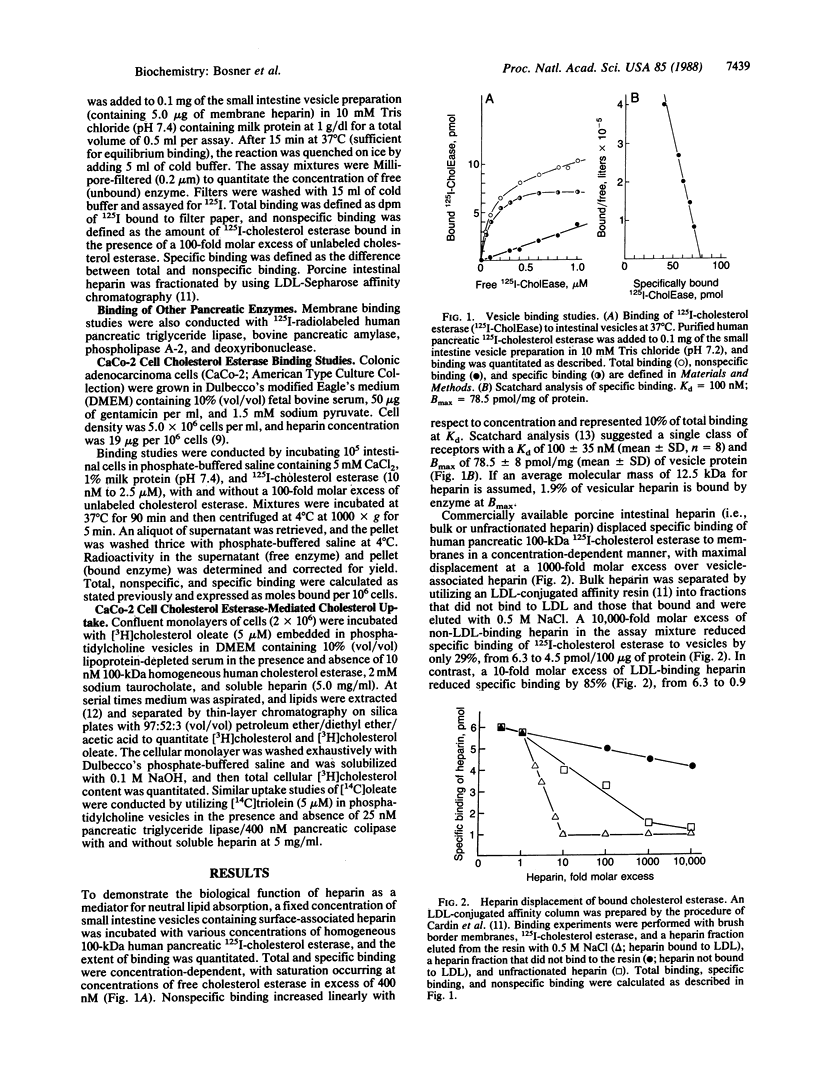
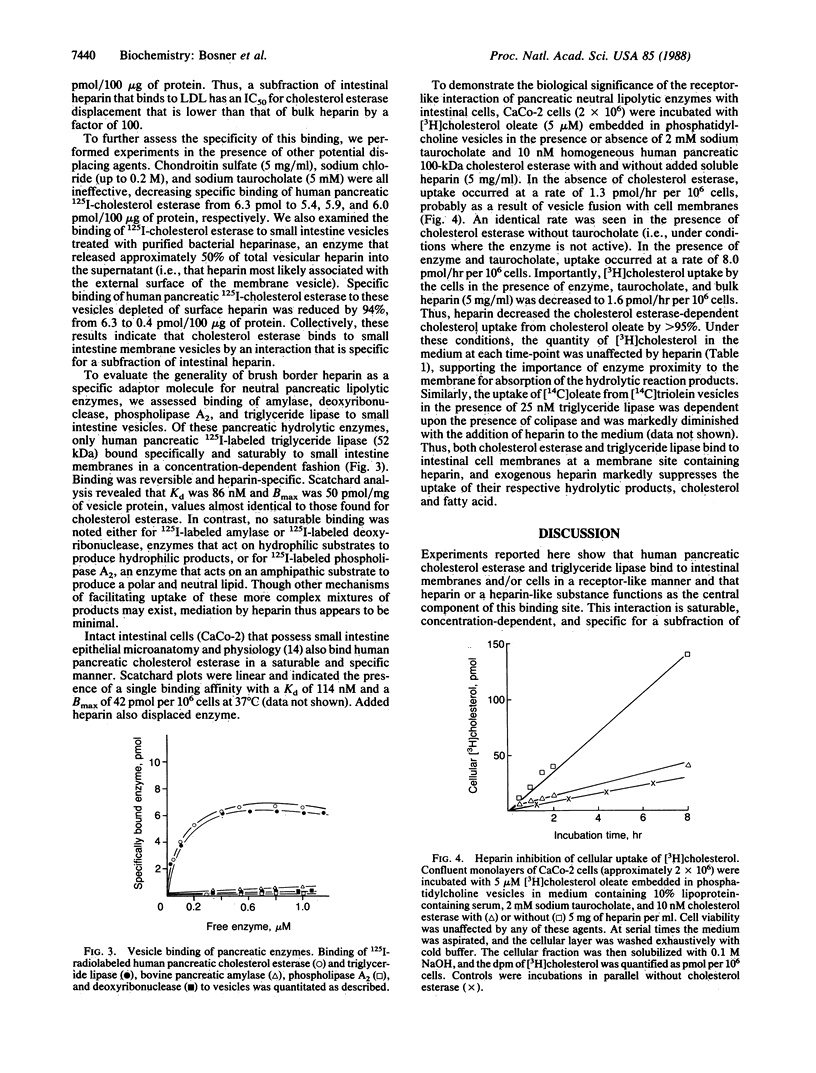
Molecular mechanisms regulating the binding, amphipathic stabilization, and metabolism of the major neutral lipids (e.g., cholesteryl esters, triglycerides, and fatty acids) are well studied, but the details of their movement from a binding compartment to a metabolic compartment deserve further attention. Since all neutral lipids must cross hydrophilic segments of plasma membranes during such movement, we postulate that a critical receptor-like site exists on the plasma membrane to mediate a step between binding and metabolism and that membrane-associated heparin is a key part of this mediator. For example, intestinal brush border membranes containing heparin bind homogeneous human pancreatic 125I-labeled cholesterol esterase (100 kDa) and 125I-labeled triglyceride lipase (52 kDa). This interaction is enzyme concentration-dependent, specific, and saturable and is reversed upon addition of soluble heparin. Scatchard analysis demonstrates a single class of receptors with a Kd of 100 nM and a Bmax of approximately 50-60 pmol per mg of vesicle protein. In contrast, enzymes associated with the hydrolysis of hydrophilic compounds such as amylase, phospholipase A2, and deoxyribonuclease do not bind to intestinal membranes in this manner. Human pancreatic cholesterol esterase also binds specifically and saturably to cultured intestinal epithelial cells (CaCo-2), and soluble heparin significantly diminishes the cellular uptake of the resultant hydrophobic reaction products (cholesterol and free fatty acids). We conclude that a physiological role for intestinal heparin is that of a mediator to bind neutral lipolytic enzymes at the brush border and thus promote absorption of the subsequent hydrolyzed nutrients in the intestine. This mechanism may be a generalizable pathway for transport of neutral lipids into endothelial and other cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Cardin A. D., Barnhart R. L., Witt K. R., Jackson R. L. Reactivity of heparin with the human plasma heparin-binding proteins thrombin, antithrombin III, and apolipoproteins E and B-100. Thromb Res. 1984 Jun 15;34(6):541–550. doi: 10.1016/0049-3848(84)90258-5. [DOI] [PubMed] [Google Scholar]
- DAHLQVIST A. METHOD FOR ASSAY OF INTESTINAL DISACCHARIDASES. Anal Biochem. 1964 Jan;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Hauser H., Howell K., Dawson R. M., Bowyer D. E. Rabbit small intestinal brush border membrane preparation and lipid composition. Biochim Biophys Acta. 1980 Nov 18;602(3):567–577. doi: 10.1016/0005-2736(80)90335-1. [DOI] [PubMed] [Google Scholar]
- Jackson R. L., McLean L. R., Ponce E., Rechtin A., Demel R. A. Mechanism of action of lipoprotein lipase and hepatic triglyceride lipase. Adv Exp Med Biol. 1987;210:73–77. doi: 10.1007/978-1-4684-1268-0_11. [DOI] [PubMed] [Google Scholar]
- Jackson R. L., Socorro L., Fletcher G. M., Cardin A. D. Heparin binding to lipoprotein lipase and low density lipoproteins. FEBS Lett. 1985 Oct 14;190(2):297–300. doi: 10.1016/0014-5793(85)81304-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H., Innerarity T. L. Interaction of plasma lipoproteins containing apolipoproteins B and E with heparin and cell surface receptors. Biochim Biophys Acta. 1979 Oct 26;575(1):81–91. doi: 10.1016/0005-2760(79)90133-4. [DOI] [PubMed] [Google Scholar]
- Olivecrona T., Bengtsson G., Marklund S. E., Lindahl U., Hök M. Heparin-lipoprotein lipase interactions. Fed Proc. 1977 Jan;36(1):60–65. [PubMed] [Google Scholar]
- Steele R. H., Wagner W. D., Rowe H. A., Edwards I. J. Artery wall derived proteoglycan-plasma lipoprotein interaction: lipoprotein binding properties of extracted proteoglycans. Atherosclerosis. 1987 May;65(1-2):51–62. doi: 10.1016/0021-9150(87)90007-4. [DOI] [PubMed] [Google Scholar]
- VAHOUNY G. V., TREADWELL C. R. ABSOLUTE REQUIREMENT FOR FREE STEROL FOR ABSORPTION BY RAT INTESTINAL MUCOSA. Proc Soc Exp Biol Med. 1964 Jun;116:496–498. doi: 10.3181/00379727-116-29289. [DOI] [PubMed] [Google Scholar]
- ZLATKIS A., ZAK B., BOYLE A. J. A new method for the direct determination of serum cholesterol. J Lab Clin Med. 1953 Mar;41(3):486–492. [PubMed] [Google Scholar]


