Abstract
LcrV, a protein that resides at the tip of the type III secretion needles of Yersinia pestis, is the single most important plague protective antigen. Earlier work reported monoclonal antibody MAb 7.3, which binds a conformational epitope of LcrV and protects experimental animals against lethal plague challenge. By screening monoclonal antibodies directed against LcrV for their ability to protect immunized mice against bubonic plague challenge, we examined here the possibility of additional protective epitopes. MAb BA5 protected animals against plague, neutralized the Y. pestis type III secretion pathway and promoted opsonophagocytic clearance of bacteria in blood. LcrV residues 196–225 were necessary and sufficient for MAb-BA5 binding. Compared to full length LcrV, a variant lacking its residues 196–225 retained the ability of eliciting plague protection. These results identify LcrV residues 196–225 as a linear epitope that is recognized by the murine immune system to confer plague protection.
1. Introduction
Yersinia pestis, the causative agent of plague [1], is a Gram-negative bacterium harboring a 70-kB pCD1 virulence plasmid that encodes a type III secretion system (T3SS) [2]. Upon contact with the host cell, T3SS components are assembled and effector proteins are secreted across the bacterial membrane to specific locations within the host cytoplasm where they disrupt actin filaments [3], suppress γ-interferon and tumor necrosis factor-α secretion [4], inhibit phagocytosis [5], and produce cytotoxic effects [6, 7]. The sum of all these events precipitates the massive depletion of host immune cells, which is accompanied by Y. pestis replication and often a fatal outcome for plague infected individuals [8]. LcrV, low calcium response V antigen [9], is a 35 kDa protein that resides at the tip of the type III secretion needles [10]. LcrV is absolutely required for Y. pestis virulence [11], as the polypeptide enables the transport of effector proteins into host cells [12, 13]. Along with YopB and YopD [14, 15], LcrV is thought to form a translocation pore through which effector proteins enter the host cell and induce cell death [16].
Y. pestis LcrV was first described as a plague protective antigen by Burrows and co-workers [17–19]. Immunization with LcrV confers robust protection against plague in multiple animal models [20–23]. Several studies highlighted the fact that LcrV based protection is mediated by specific antibodies, as polyclonal serum raised against purified LcrV is protective in passive transfer models [19, 24, 25]. Plague protective monoclonal antibody (MAbs) specific for LcrV have been reported [26]. This work identified MAbs that bind the minor protective epitope (region I, LcrV 2–135) or the major protective epitope (region II, LcrV 135–275) [26, 27]. Passive administration of MAb 7.3, which binds region II, into mice protects animals against up to 12 median-lethal-doses (MLD) of Y. pestis [24, 26] and enabled macrophage phagocytosis of virulent plague bacteria in vitro [28, 29]. MAb 101.3, which binds to region I, conferred no protection against plague challenge [26]. Both region I (132 residues) and region II (140 residues) represent large segments of folded LcrV [30], suggesting that MAbs 101.3 and 7.3 bind conformational epitopes but not linear peptide segments within the folded polypeptide [31].
Purified LcrV has been extensively studied as a subunit vaccine for the development of plague prevention strategies [32, 33]. The rV10 vaccine, which lacks amino acids 271–300, was designed to decrease the negative immune-modulatory properties of full-length LcrV without affecting its properties as a protective antigen [34]. rV10 protective immunity is mediated via humoral responses, as rV10 antibodies are sufficient to confer protection in passive transfer experiments [23]. When immunized mice were examined for their repertoire of antibodies, rV10 immune sera harbored antibodies against epitopes 174–190, and 180–196 [23]. LcrV immune serum, on the other hand, harbored antibodies directed against 1–17, 6–22, 12–28, 48–64 as well as 78–94, 252–266, 258–274, and 264–280 [23]. Previous work left unresolved whether any one of these short, linear LcrV epitopes is alone sufficient to induce protective immunity against plague challenge.
2. Materials and methods
2.1. Bacterial strains and plasmids
Yersinia pestis KIM D27 (KIM 5) [35, 36] was used for in vitro assays. Y. pestis CO92 [37] was used for animal experiments. rLcrVΔ196–225 was synthesized using the QuickChange II Site-Directed Mutagenesis Kit from Stratagene® and expression plasmid pLcrV coding for rLcrV as a template [34] and Forward Primer 5’-AAT CCA TTA ATC TCA TGG ATG GTA CCG TGG ATG GGA GCG AGA AAA AAA TAG TCT CG -3’ and Reverse Primer 5’-CGA GAC TAT TTT TTT CTC GCT CCC ATC CAC GGT ACC ATC CAT GAG ATT AAT GGA TT -3’. Primers carry a KpnI site that results in the introduction of two amino acids, Gly and Thr, in place of amino acid 196 to 225.
2.2. Monoclonal antibodies
On day 0, three 8 week old BALB/c female mice, from Jackson Laboratory (Bar Harbor, ME) were immunized intraperitoneally with 100 µg purified LcrV antigen in phosphate buffered saline emulsified 1:1 with Complete Freund’s Adjuvant (DIFCO). On days 21 and 42, all mice were boosted by intraperitoneal injection with 100 µg purified LcrV antigen emulsified 1:1 with Incomplete Freund’s Adjuvant. On days 31 and 52, all mice were bled and screened by ELISA on LcrV coated Nunc MaxiSorp 96 well flat bottom plates. Seventy-nine days after the initial immunization, mice that showed strong immunoreactivity to antigen were boosted with 25 µg LcrV in PBS. Three days later splenocytes were harvested and fused, according to standard methods, with the mouse myeloma cell line SP2/mIL-6, an interleukin 6 secreting derivative of SP2/0 myeloma cell line. Hybridomas were screened by ELISA and antigen-specific clones were subcloned, by limiting dilution, to produce monoclonal antibody-secreting hybridomas arising from single cells. In addition to conformational epitopes, isolated MAbs recognized different linear epitopes including LcrV residues 1–30, 16–45, 31–60, 76–105, 196–225, 256–285, and 301–326, the results for which are being reported in another study.
2.3. ELISA and peptide array
Serum immunoglobulin G (IgG) levels with specific antigen binding activity were determined by a custom enzyme-linked immunosorbent assay (ELISA) at the GLRCE Animal Research & Immunology Core at The University of Chicago [38]. For the peptide array, 19 amino acid peptide fragments spanning the length of LcrV were synthesized and conjugated to KLH. Shorter, non-conjugated 15 to 17-mer peptides spanning the length of LcrV were provided by BEI resources. Depending on the peptide and HPLC purification reports provided by the manufacturer, lyophilized peptides were solubilized using distilled water, DMSO, guanidine-HCl, acetonitrile, 0.1% ammonium hydroxide, ammonium bicarbonate or acetic acid alone or in any combination. Solubilized peptides were aliquoted and frozen at −80 °C. MaxiSorp 96-well flat-bottom ELISA immunoplates (Nunc) were coated with 100 µL of peptide or unconjugated KLH at 1µg/ml in carbonate buffer and incubated overnight at 4°C. Horseradish peroxidase-conjugated anti-mouse IgG diluted at 1:10,000 (100 µl) was added to each well, followed by washing, and was developed with TMB solution (Pierce) (100 µl). The reactions were stopped by adding 50 µl 1M phosphoric acid, and the plates were read at the optical density of 450 nm.
2.4. Rate of association/dissociation
Kinetic interactions between full-length LcrV and MAb BA5 were measured by Surface Plasmon Resonance (SPR) on BIACORE 3000. BA5 was injected at concentrations ranging from 0.15 to 5 nM at a constant flow rate of 10µL/min over 70 RU of immobilized His-tagged LcrV protein and over a control dextran surface (these values were subtracted from the signal). The association and dissociation phases were monitored for 120 s by following the change in SPR signal, given in resonance unit (RU). The rate of complex formation during sample injection is described by the following equation, dR/dt = kaC(Rmax − R) − kdR (for a 1:1 interaction) where R represents the SPR signal in RU, C is the concentration of analyte, Rmax is the maximum analyte binding capacity in RU, and dR/dt is the rate of change of SPR signal. The binding phase was used to determine the association constant (ka) between BA5 and LcrV. The dissociation phase (kd) was measured using the rate of decline in RU on introduction of free buffer at the end of BA5 injections. Data were fitted by the BIAevaluation (Version 4.1) software program (global fitting algorithm), and the dissociation constant (KD) of the complexes was determined as the ratio kd/ka.
2.5. Cytotoxicity assay
Y. pestis KIM D27 was grown overnight at 26°C. Cultures were diluted 1:20 and grown for 2 hours before being switched to 37°C for 1 hour. HeLa cells, grown in a 12-well tissue culture dish and plated at 2 × 105 cells were then infected at an MOI of 10. At the time of infection the cultures were supplemented with either rV10 polyclonal rabbit sera (100 µl) or 500 µg purified monoclonal antibodies. After 3 h of infection, media were removed and the cells were fixed with 3.7% formaldehyde in PBS for 20 min on a rotary shaker. Fixation was quenched with 0.1 M glycine in PBS for 5 min. Cells were permeabilized with 0.1% Triton X-100 in PBS for 30 min and then blocked for 15 min in PBS containing 5% skim milk and 0.05% Tween 20. Filamentous actin was labeled with 165 nM Texas red-conjugated phalloidin in PBS containing 5% skim milk and 0.05% Tween 20 for 20 min. The labeling solution was removed, and each well was washed four times with 1 ml of PBS. On the last wash, the PBS was left in the wells, and fluorescence signals were visualized with an Olympus AX RL module microscope. Texas red-phalloidin visualization was achieved through excitation (591 nm) and emission (608 nm) with a U-MNG cube. Images were captured with a Hamamatsu Ocra digital camera.
2.6. Opsonophagocytosis
Blood was obtained from human volunteers by vein puncture. One ml samples were anti-coagulated with lepirudin (Refludan®) and infected with 1×105 CFU Y. pestis in the presence or absence of 0.1 ml antibody samples (polyclonal sera or 500 µg purified MAb). For this experiment, Y. pestis was grown as described for the cytotoxity assay. At timed intervals, survival of plague bacteria was enumerated in aliquots spread on HIA plates and incubated for colony formation. Experiments were repeated 3 times using the same blood donor. Experiments with human volunteers involved protocols that were reviewed, approved and performed under regulatory supervision of The University of Chicago’s Institutional Review Board (IRB).
2.7. Animal experiments
Monoclonal antibodies were screened for protective properties against plague using a mouse model of bubonic infection. Mice were injected IP with 200 µg of purified monoclonal antibody, one hour prior to being challenged by subcutaneous injection with 0.1-mL aliquots of Y. pestis CO92. For immunization experiments, Groups of 6- to 8-week-old female BALB/c mice (Charles River Labs, MA) were immunized by intramuscular injection into the hind leg with 0.1-ml aliquots of 50 µg of rLcrV or variant in 25% alhydrogel on day 0 and 21. Plague challenge occurred on day 42. For Bubonic Plague infection, Y. pestis CO92 was grown in HIB at 26°C overnight. Plague bacilli were washed and diluted in sterile PBS to 2 × 102 CFU/mL for low dose (20 CFU) and 1 × 104 CFU/mL for high dose (1,000 CFU). MLD for this route of infection is 1 CFU. Mice were infected by subcutaneous injection with bacterial suspensions (100 µl) and observed for morbidity, mortality and recovery over a course of 14 days. Fisher’s exact test was used to compare mortality between groups. All animal and plague experiments were performed in accordance with institutional guidelines following experimental protocol review and approval by the Institutional Biosafety Committee, Select Agent Committee and the Institutional Animal Care and Use Committee at The University of Chicago.
3. Results
3.1. Generation and screening of LcrV monoclonal antibodies
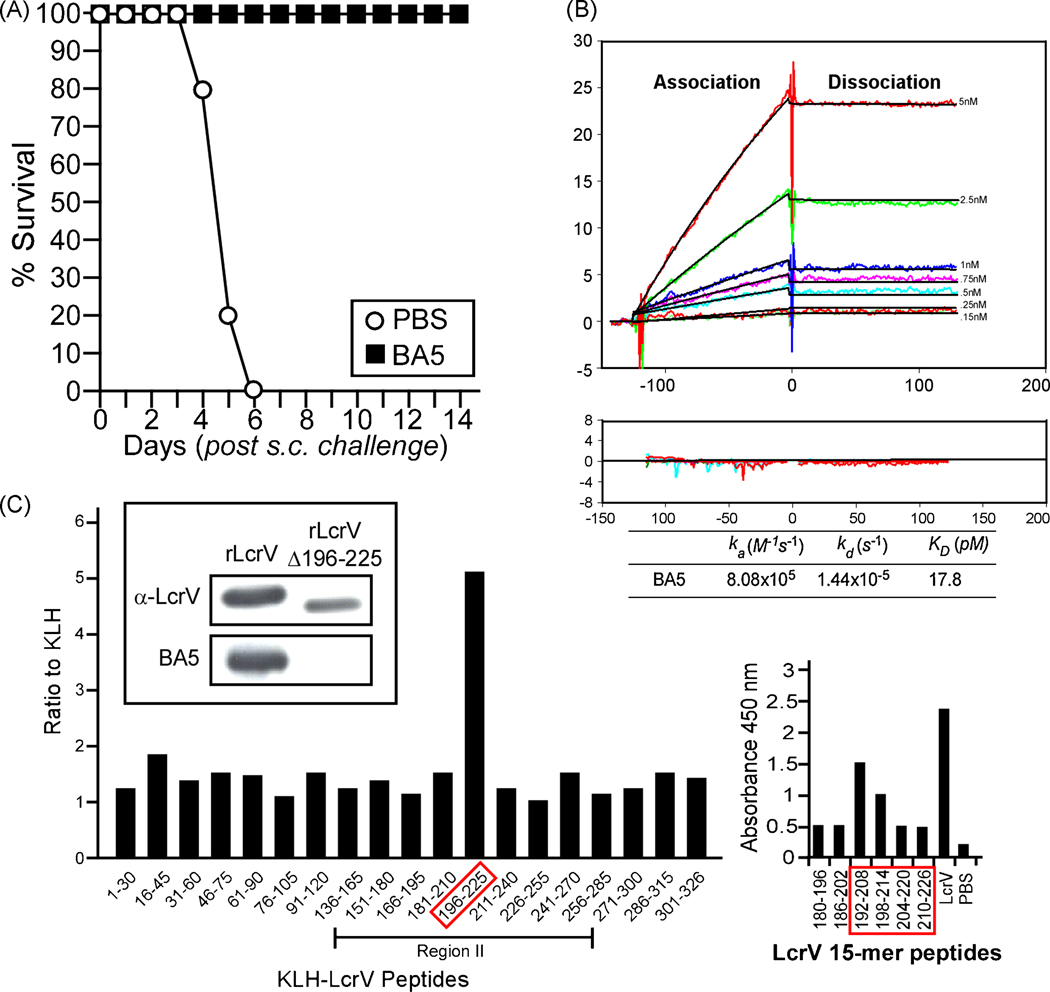
BALB/c mice were immunized with 100 µg rLcrV by intraperitoneal injection, followed by two booster immunizations at 21 days intervals. On day 79, two mice that showed strong immunoreactivity to antigen were boosted intraperitoneally with 25 µg LcrV in phosphate buffered saline and three days later spleens were removed. Single cell derived hybridomas with splenocytes were screened for IgG antibody production and for the ability to bind rLcrV using an end-point ELISA. Forty-seven IgG MAbs with affinity for rLcrV were purified and assayed for their ability to confer protection against bubonic plague. Groups of 10 mice (BALB/c, 6 to 8 weeks old) were injected into the peritoneal cavity with 200 µg of purified MAbs. One hour later, each animal was challenged by subcutaneous injection of 20 MLD of the fully virulent strain Y. pestis CO92 [38]. As expected, control animals that received only PBS succumbed to bubonic plague infection within 6 days (Fig. 1A). Out of 47 monoclonal IgG1 antibodies specific for LcrV, only one, MAb BA5, conferred 100% protection to BALB/c mice when administered at 200 µg (data not shown). As MAb BA5 fulfilled our experimental criteria and conferred complete protection for passively immunized mice, the reagent was subjected to further study (Fig. 1A).
Fig. 1.
Monoclonal antibody (MAb) BA5 binds to rLcrV residues 196 to 225. (A) 8 week old BALB/c mice were injected intraperitoneally with PBS or with 200 µg of LcrV purified monoclonal antibodies one hour prior to subcutaneous challenge with 20 CFU of Yersinia pestis CO92. Mice were monitored for survival for 14 days. (B) Kinetic interaction of full-length LcrV with protective MAb BA5 as visualized by SPR with a BIACORE 3000 machine. BA5 was injected at concentrations ranging from 0.15 to 5 nM (lower to upper curves) at constant flow rate (10µL/min) over 70 RU of immobilized His-tagged LcrV protein or control dextran surface, whose values were subtracted from the signal. The association and dissociation phases were monitored for 120 s by following the change in SPR signal (colored curves) and recording resonance units (RU). Black curves represent the fit of data to a single-site binding model. Kinetic parameters derived from these experiments are listed. Residuals from a single-site binding model indicate excellent fit. (C) Left panel. Peptide fragments (30 residues) spanning the length of LcrV were synthesized and conjugated to KLH. ELISA plates were coated with 100 µL of V peptide fragments or unconjugated KLH (plated at 1µg/mL) and incubated with purified MAb BA5 for 18 hours. Binding was detected with specific secondary antibody conjugated to HRP and read by absorbance at 450 nm. Results are reported as the ratio of binding to the peptide fragment compared to the baseline binding of KLH alone. Region II represents the fragment of LcrV known to contain the epitope for protective MAb 7.3 [26]. Insert: Immunoblots using monoclonal BA5 or rabbit polyclonal antibodies (α-LcrV) to detect recombinant LcrV (rLcrV) or its variant lacking residues 196 to 225 (rLcrVΔ196–225). Right panel. Shorter peptides (15 residues) spanning the length of LcrV ( BEI Resources) were examined for the binding of MAb BA5; immunoreactive peptides, their nearest neighbors in sequence as well as full length and PBS control are shown.
3.2. Protective epitope mapping
To map the epitope recognized by MAb BA5, we assayed an array of 30-mer LcrV peptides by end point ELISA. BA5 bound to a single peptide, LcrV residues 196–225 (Fig. 1C). MAb BA5 also bound to two 17 residue peptides, LcrV amino acids 192–208 and 198–214, but not to 186–202 or 204–220 (Fig. 1C). To examine whether residues 196–225 represent the only binding site for MAb BA5, we deleted this peptide from recombinant LcrV. The immunoblot in Fig. 1C shows that MAb BA5 binds to rLcrV, but not to the variant LcrVΔ196–225. As a control, polyclonal antibodies raised again full length LcrV (α-LcrV) recognized both rLcrV and rLcrVΔ196–225. Interactions between MAb BA5 and purified rLcrV were analyzed by surface plasmon resonance (Fig. 1B). The dissociation constant KD for the binding of MAb BA5 to LcrV was calculated at 17.8 pM. In sum, MAb BA5 binds to a single linear epitope, residues 196–225 of LcrV, and does so with high affinity and specificity.
3.3. MAb BA5 and Yersinia pestis type III secretion
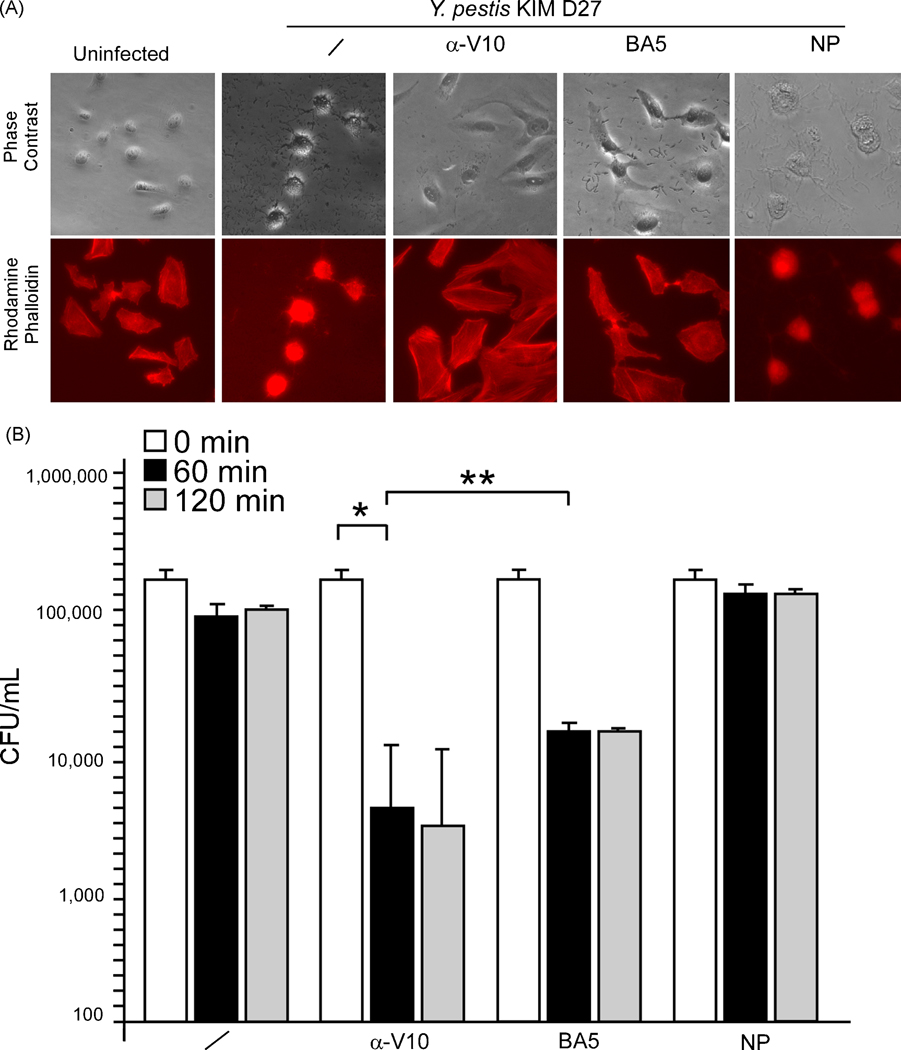
To determine whether MAb BA5 interferes with type III secretion, HeLa cells were infected at an MOI of 10 with Y. pestis strain KIM D27 in the presence or absence of LcrV-specific antibodies. As read-out for Yersinia-transport of type III injection, tissue cultures were stained with Texas red-phalloidin and effector-mediated rearrangement of actin cables in HeLa cells was viewed by fluorescence microscopy [39]. As a positive control, the addition of polyclonal serum directed against rV10 abolished Y. pestis type III mediated-cytotoxicity and actin rearrangements; earlier work demonstrated the plague protective properties of this rV10 serum in passive transfer experiments and bubonic plague challenge in mice [23]. As negative control, non-reactive immune serum (data not shown) or a non-protective (NP) monoclonal antibody from our mouse protection screen above were each unable to block Y. pestis mediated cell rounding and actin filament remodeling (Fig. 2A). Treatment of Y. pestis with MAb BA5 inhibited HeLa cell rounding and actin cable rearrangements. These results suggest that MAb BA5 binding to LcrV blocks Y. pestis type III injection of host cells, which likely provides protective immunity from plague infection.
Fig. 2.
MAb BA5 blocks Y. pestis type III injection of host cells. (A) Y. pestis cytotoxicity for HeLa cells was measured by staining F-actin with Texas red-conjugated phalloidin. Differential interference contrast (DIC) images were captured at a magnification of 100×, and fluorescence was measured at 608 nm emission. Samples were compared with a well that received no bacteria (uninfected). At the time of HeLa cell infection, 100 µl of rV10 rabbit polyclonal sera (α-V10) or 500 µg of monoclonal BA5 (BA5) or 500 µg of non-protective LcrV monoclonal (NP) were added. (B) Human blood, anti-coagulated with lepirudin, was incubated for 60, 120 or 240 minutes with 1 × 105 CFU Y. pestis KIM D27 in the absence (/) or presence of 100 µl of α-V10 rabbit polyclonal sera, 500 µg of monoclonal MAb BA5 or 500 µg of non-protective LcrV monoclonal antibody (NP). Bacterial load (CFU/ml) was recorded by plating aliquots on agar and incubating for colony formation. * P <0.001, ** P = 0.15.
3.4. MAb BA5 promotes blood opsonophagocytosis of Yersinia pestis
In blood of naïve animals or humans, Y. pestis deploys its type III machine to transport effector Yops into immune cells [3], thereby rapidly depleting their hosts of phagocytes and their associated innate immune defenses [8]. When incubated in freshly isolated blood of human volunteers, Y. pestis strain KIM D27 is able to survive for sixty minutes or longer (Fig. 2B). The addition of MAb BA5 to human blood caused a 2 log reduction in viable Y. pestis (P < 0.0001, mock vs. BA5). A comparable level of opsonophagocytosis was achieved by rabbit polyclonal sera against rV10 (P = 0.15, α-V10 vs. BA5). These results suggest that MAb BA5 mediated inhibition of type III secretion enabled the killing of Y. pestis in blood.
3.5. Other LcrV protective epitopes
Epitope mapping of antibodies raised by rV10 vaccine in non-human primates and rats pointed to the existence of non-linear, conformational epitopes [23]. In mice, the rV10 vaccine stimulated the production of antibodies against residues 174–190 and 180–196 [23]. To test whether plague protective immunity is based on epitope 196–225, defined by its reactivity with MAb BA5, we generated an LcrV variant lacking these residues (rLcrVΔ196–225) and tested its protective antigen properties. Groups of ten mice (BALB/c, 6 to 8 weeks) were immunized by intramuscular injection with 50 µg purified rLcrV or rLcrVΔ196–225 adsorbed to 25% alhydrogel twice with 21 day intervals. Twenty-one days following the second immunization, mice were challenged by subcutaneous injection with Y. pestis CO92, using either a low (20 CFU) or high (1,000 CFU) challenge dose. As expected, mock immunized animals, which had been injected with adjuvant alone, succumbed to plague challenge within 7 to 11 days, depending on the dose (Fig. 3). Mice immunized with LcrV were fully protected against bubonic plague regardless of the challenge dose (Fig. 3). Animals immunized with rLcrVΔ196–225 were also protected at the lower (20 CFU) and higher challenge doses (1,000 CFU); in the latter experiment, 20% of rLcrVΔ196–225 immunized mice died of plague infection when challenged with 1,000 CFU Y. pestis CO92, however this did not lead to a statistically significant difference (P = 0.23, rLcrV vs. rLcrVΔ196–225). Thus, removal of the MAb BA5 epitope, 196–225, does not abolish the ability of the rLcrVΔ196–225 variant to act as a plague protective antigen. If so, LcrV must encode at least one additional plague protective epitope.
Fig. 3.
Removal of the BA5 epitope from rLcrV does not abolish its protective antigen attributes. 8 week old BALB/c mice were vaccinated with 50 µg of recombinant LcrV or its variant lacking residues 196 to 225 (rLcrVΔ196–225), adsorbed to 25% Alhydrogel, on day 0 and 21. At day 42 mice were challenged by subcutaneous injection with Y. pestis CO92, using 20 MLD (white symbols) or 1,000 MLD (black symbols). Mice were monitored over 14 days for survival.
4. Discussion
In order to decipher LcrV-based protective immunity against plague, monoclonal antibodies against rLcrV were generated and screened for their ability to protect mice against lethal Y. pestis infection. Out of 47 monoclonal IgG antibodies specific for LcrV, only one, MAb BA5, conferred 100% protection to BALB/c mice. Surface Plasmon resonance demonstrated that MAb BA5 binds LcrV with high affinity and an array of 30-mer peptides derived from LcrV sequence revealed that BA5 binds to a single linear epitope, residues 196–225 (Fig. 4). Specificity of MAb BA5 binding was confirmed with the variant rLcrVΔ196–225, which is recognized by polyclonal antibodies raised against rLcrV but not by MAb BA5 (Fig. 1B, insert). Using a tissue culture assay, we could show that MAb BA5 blocked Y. pestis type III injection of effector Yops into HeLa cells. Further, the addition of MAb BA5 to human blood promoted opsonophagocytic killing of Y. pestis. These results agree with a general model whereby specific antibodies directed against LcrV can block Y. pestis type III injection into host cells [12]. Once their essential virulence mechanism has been disabled, plague bacteria can be phagocytosed and killed by the innate defenses of an infected host. Our results do not preclude the possibility that MAb BA5 may also display opsonizing attributes. Considering currently available reagents, it is difficult to discern this from other possibilities, as Y. pestis lcrV deletion mutants unable to bind MAb BA5 are also defenseless against host phagocytes [40]. If one could identify dominant negative lcrV variants that cannot promote type III injection yet continue to display LcrV at the tip of type III needles, one would be in a position to analyze the opsonizing attributes of LcrV antibodies.
Fig. 4.
Front and back views on the surface of rLcrV as defined by X-ray structural data [30]. Amino acid residues in green represent the region II (LcrV residues 135–275)[26]. Amino acids in blue (258–274) represent a major epitope recognized by LcrV polyclonal sera in mouse, NHP and rats [23]. Amino acid residues in red represent the BA5 epitope (196–225). Image was created with the PyMOL ™ Software.
Our results suggest that more than one LcrV epitope can promote immune responses with protective immunity. It is not clear whether all of these antibodies simply bind to LcrV at the tip of type III secretion needles and whether this physical interaction alone blocks type III injection (Fig. 4). Type III needles are responsive to calcium signals [41]. Encountering the low calcium environment of the host cell cytoplasm is thought to activate type III injection of effector Yops, which subsequently travel along the YscF needle and the translocation pore into the plasma membrane of host cells [42–44]. This pore is thought to be comprised of LcrV, YopB and YopD [45]. An important but unanswered questions is – does LcrV commit to conformational changes and/or physically interact with YopB / YopD as Yersinia type III needles transition from secretion to injection modes? If so, do specific LcrV antibodies block a possible change in translocation pore structure or the physical interactions between LcrV, YopB and YopD? One wonders further whether LcrV at the tip of type III needles may interact with ligands on host cell surfaces. In such a scenario, antibodies against LcrV could block another essential mechanism of the type III pathway [46].
We sought to investigate whether the BA5 epitope of LcrV has a major contribution towards plague protective immunity and observed that immunization with rLcrVΔ196–225 generated plague protection similar to rLcrV, when animals were challenged with 20 or 1,000 MLD. Reduced levels of protection compared to full length LcrV have been reported for three other variants, LcrVΔ180–211, LcrVΔ210–241, and LcrVΔ241–270 [34]. Studies with an array of LcrV peptides suggest that many antibodies raised after immunization with full length rLcrV do not recognize linear peptides but rather interact with conformational epitopes of the folded polypeptide [23]. Such antibodies are difficult to isolate, quantify or map and their contributions to plague immunity are just beginning to be fully appreciated (Fig. 4) [31]. Further, immunization with variant LcrV molecules changes the spectrum of conformational epitopes that can be recognized by the immune system or elicits aberrant immune responses with diminished levels of protection [23, 31]. The reciprocal result can also occur, as is reported for rV10 (LcrVΔ271–300), where the antigen elicits predominantly antibodies to conformational epitopes that provide an equal or even enhanced level of protection [38].
Monoclonal antibodies that protect animals against plague can be valuable experimental tools to analyze human LcrV-specific immune responses as a correlate of protective immunity [25, 28]. Previous work identified MAb 7.3, which interacts with LcrV residues 135–262, i.e. with a conformational epitope of LcrV [26, 31]. Nevertheless, the antibody clearly provides protection against plague. By testing antibodies of human vaccinees for the attribute of blocking interactions between MAbs and LcrV, one may be able to derive correlative information on plague protective immunity [47, 48]. In this regard, it would be most useful to have available several different LcrV-specific monoclonal antibodies that protect experimental animals against plague. MAb BA5 represents a significant advance in this direction.
Acknowledgements
We thank Carol McShan (Fitch Monoclonal Antibody Facility, University of Chicago) for monoclonal antibody expansion and purification. We thank Yating Wang for assistance with crystal structure representation. We thank members of our laboratory for critical comments and discussion and the Animal Research & Immunology Core (ARIC, University of Chicago) for assistance with BSL-3 and animal experiments. The authors acknowledge membership within and support from the Region V “Great Lakes” Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium (NIH Award 1-U54-AI-057153). This work was sponsored in part by the NIH/NIAID Challenge Award U01-AI070559 “LcrV Plague Vaccine with Altered Immune Modulatory Properties”. The following reagent was obtained from the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH: Peptide Array Y. pestis V antigen NR-2867. BA5 antibody was deposited at the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH under reference number NR-3831.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yersin A. La peste bubonique à Hong-Kong. Ann Inst Pasteur. 1894;2:428–430. [Google Scholar]
- 2.Cornelis GR, Boland A, Boyd AP, Geuijen C, Iriarte M, Neyt C, et al. The virulence plasmid of Yersinia, an antihost genome. Microbiol Mol Biol Rev. 1998;62:1315–1352. doi: 10.1128/mmbr.62.4.1315-1352.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosqvist R, Magnusson K-E, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakajima R, Motin VL, Brubaker RR. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect Immun. 1995;63:3021–3029. doi: 10.1128/iai.63.8.3021-3029.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosqvist R, Bolin I, Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: a virulence plasmid-encoded ability involved in the Yop2b protein. Infect Immun. 1998;56:2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee VT, Anderson DM, Schneewind O. Targeting of Yersinia Yop proteins into the cytosol of HeLa cells: one-step translocation of YopE across bacterial and eukaryotic membranes is dependent on SycE chaperone. Mol Microbiol. 1998;28:593–601. doi: 10.1046/j.1365-2958.1998.00822.x. [DOI] [PubMed] [Google Scholar]
- 7.Viboud GI, Bliska JB. A bacterial type III secretion system inhibits actin polymerization to prevent pore formation in host cell membranes. EMBO J. 2001;20:5373–5382. doi: 10.1093/emboj/20.19.5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marketon MM, DePaolo RW, DeBord KL, Jabri B, Schneewind O. Plague bacteria target immune cells during infection. Science. 2005;309:1739–1741. doi: 10.1126/science.1114580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perry RD, Harmon PA, Bowmer WS, Straley SC. A low-Ca2+ response operon encodes the V antigen of Yersinia pestis. Infect Immun. 1986;54:428–434. doi: 10.1128/iai.54.2.428-434.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mueller CA, Broz P, Muller SA, Ringler P, Erne-Brand F, Sorg I, et al. The V-antigen of Yersinia forms a distinct structure at the tip of injectisome needles. Science. 2005;310:674–676. doi: 10.1126/science.1118476. [DOI] [PubMed] [Google Scholar]
- 11.Fields KA, Nilles ML, Cowan C, Straley SC. Virulence role of V antigen of Yersinia pestis at the bacterial surface. Infect Immun. 1999;67:5395–5408. doi: 10.1128/iai.67.10.5395-5408.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petterson J, Holmstrom A, Hill J, Frithz-Lindsten E, von Euler-Matell A, Carlsson E, et al. The V-antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol Microbiol. 1999;32:961–976. doi: 10.1046/j.1365-2958.1999.01408.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee VT, Tam C, Schneewind O, Lcr V. a substrate for Yersinia enterocolitica type III secretion, is required for toxin targeting into the cytosol of HeLa cells. J Biol Chem. 2000;275:36869–36875. doi: 10.1074/jbc.M002467200. [DOI] [PubMed] [Google Scholar]
- 14.Hakansson S, Bergman T, Vanooteghem J-C, Cornelis G, Wolf-Watz H. YopB and YopD constitute a novel class of Yersinia Yop proteins. Infect Immun. 1993;61:71–80. doi: 10.1128/iai.61.1.71-80.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hakansson S, Schesser K, Persson C, Galyov EE, Rosqvist R, Homble F, et al. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J. 1996;15:5812–5823. [PMC free article] [PubMed] [Google Scholar]
- 16.Mueller CA, Broz P, Cornelis GR. The type III secretion system tip complex and translocon. Mol Microbiol. 2008;68:1085–1095. doi: 10.1111/j.1365-2958.2008.06237.x. [DOI] [PubMed] [Google Scholar]
- 17.Burrows TW. An antigen determining virulence in Pasteurella pestis. Nature. 1956;177:426–427. doi: 10.1038/177426b0. [DOI] [PubMed] [Google Scholar]
- 18.Burrows TW. Virulence of Pasteurella pestis. Nature. 1957;179:1246–1247. doi: 10.1038/1791246a0. [DOI] [PubMed] [Google Scholar]
- 19.Burrows TW, Bacon GA. The effect of loss of different virulence determinants on the virulence and immunogenicity of strains of Pasteurella pestis. Br J Exp Pathol. 1958;39:278–291. [PMC free article] [PubMed] [Google Scholar]
- 20.Une T, Brubaker RR. Roles of V antigen in promoting virulence and immunity in yersiniae. J Immunol. 1984;133:2226–2230. [PubMed] [Google Scholar]
- 21.Motin VM, Nedialkov YA, Brubaker RR. V antigen-polyhistidine fusion peptide: binding to LcrH and active immunity against plague. Infect Immun. 1996;64:4313–4318. doi: 10.1128/iai.64.10.4313-4318.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leary SEC, Williamson ED, Griffin KF, Russell P, Eley SM, Titball RW. Active immunization with recombinant V antigen from Yersinia pestis protects mice against plague. Infect Immun. 1995;63:2854–2858. doi: 10.1128/iai.63.8.2854-2858.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cornelius CA, Quenee LE, Elli D, Ciletti NA, Schneewind O. V10 subunit vaccine protects cynomolgus macaques from lethal pneumonic plague. Infect Immun. 2008;76:5588–5597. doi: 10.1128/IAI.00699-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Motin VL, Nakajima R, Smirvov GB, Brubaker RR. Passive immunity to yersiniae mediated by anti-recombinant V antigen and Protein A-V antigen fusion peptide. Infect Immun. 1994;62:4192–4201. doi: 10.1128/iai.62.10.4192-4201.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williamson ED, Flick-Smith HC, Waters E, Miller J, Hodgson I, Le Butt CS, et al. Immunogenicity of the rF1+rV vaccine for plague with identification of potential immune correlates. Microb Pathog. 2007;42:11–21. doi: 10.1016/j.micpath.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Hill J, Leary SEC, Griffin K, Williamson ED, Titball RW. Regions of Yersinia pestis V antigen that contribute to protection against plague identified by passive and active immunization. Infect Immun. 1997;65:4476–4482. doi: 10.1128/iai.65.11.4476-4482.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parent MA, Berggren KN, Mullarky IK, Szaba FM, Kummer LW, Adamovicz JJ, et al. Yersinia pestis V protein epitopes recognized by CD4 T cells. Infect Immun. 2005;73:2197–2204. doi: 10.1128/IAI.73.4.2197-2204.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weeks S, Hill J, Friedlander A, Welkos S. Anti-V antigen antibody protects macrophages from Yersinia pestis-induced cell death and promotes phagocytosis. Microb Pathogen. 2002;32:227–237. doi: 10.1006/mpat.2002.0498. [DOI] [PubMed] [Google Scholar]
- 29.Hill J, Copse C, Leary S, Stagg AJ, Williamson ED, Titball RW. Synergistic protection of mice against plague with monoclonal antibodies specific for the F1 and V antigens of Yersina pestis. Infect Immun. 2003;71:2234–2238. doi: 10.1128/IAI.71.4.2234-2238.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Derewenda U, Mateja A, Devedjiev Y, Routzahn KM, Evdokimov AG, Derewenda ZS, et al. The structure of Yersinia pestis V-antigen, an essential virulence factor and mediator of immunity against plague. Structure. 2004;12:301–306. doi: 10.1016/j.str.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Vernazza C, Lingard B, Flick-Smith HC, Baillie LWJ, Hill J, Atkins HS. Small protective fragments of the Yersinia pestis V antigen. Vaccine. 2009;27:2775–2780. doi: 10.1016/j.vaccine.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Smiley ST. Current challenges in the development of vaccines for pneumonic plague. Expert Rev Vaccines. 2008;7:209–221. doi: 10.1586/14760584.7.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brubaker RR. Interleukin-10 and the inhibition of innate immunity to yersiniae: roles of Yops and LcrV (V antigen) Infect Immun. 2003:3673–3681. doi: 10.1128/IAI.71.7.3673-3681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Overheim KA, Depaolo RW, Debord KL, Morrin EM, Anderson DM, Green NM, et al. LcrV plague vaccine with altered immunomodulatory properties. Infect Immun. 2005;73:5152–5159. doi: 10.1128/IAI.73.8.5152-5159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brubaker RR. Mutation rate to non-pigmentation in Pasteurella pestis. J Bacteriol. 1969;98:1404–1406. doi: 10.1128/jb.98.3.1404-1406.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng W, Burland V, Plunkett Gr, Boutin A, Mayhew GF, Liss P, et al. Genome sequence of Yersinia pestis KIM. J Bacteriol. 2002;184:4601–4611. doi: 10.1128/JB.184.16.4601-4611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parkhill J, Wren BW, Thompson NR, Titball RW, Holden MT, Prentice MB, et al. Genome sequence of Yersinia pestis, the causative agent of plague. Nature. 2001;413:523–527. doi: 10.1038/35097083. [DOI] [PubMed] [Google Scholar]
- 38.DeBord KL, Anderson DM, Marketon MM, Overheim KA, DePaolo RW, Ciletti NA, et al. Immunogenicity and protective immunity against bubonic and pneumonic plague by immunization of mice with the recombinant V10 antigen, a variant of LcrV. Infect Immun. 2006;74:4910–4914. doi: 10.1128/IAI.01860-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorg JA, Miller NC, Marketon MM, Schneewind O. Rejection of impassable substrates by Yersinia type III secretion machines. J Bacteriol. 2005;187:7090–7102. doi: 10.1128/JB.187.20.7090-7102.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quenee L, Cornelius CA, Ciletti NA, Elli D, Schneewind O. Yersinia pestis caf1 (F1) variants and the limits of plague vaccine protection. Infect Immun. 2008;76:2025–2036. doi: 10.1128/IAI.00105-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torruellas J, Jackson MW, Pennock JW, Plano GV. The Yersinia pestis type III secretion needle plays a role in the regulation of Yop secretion. Mol Microbiol. 2005;57:1719–1733. doi: 10.1111/j.1365-2958.2005.04790.x. [DOI] [PubMed] [Google Scholar]
- 42.Ferracci F, Schubot FD, Waugh DS, Plano GV. Selection and characterization of Yersinia pestis YopN mutants that constitutively block Yop secretion. Mol Microbiol. 2005;57:970–987. doi: 10.1111/j.1365-2958.2005.04738.x. [DOI] [PubMed] [Google Scholar]
- 43.Cheng LW, Kay O, Schneewind O. Regulated secretion of YopN by the type III machinery of Yersinia enterocolitica. J Bacteriol. 2001;183:5293–5301. doi: 10.1128/JB.183.18.5293-5301.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee VT, Mazmanian SK, Schneewind O. A program of Yersinia enterocolitica type III secretion reactions is triggered by specific host signals. J Bacteriol. 2001;183:4970–4978. doi: 10.1128/JB.183.17.4970-4978.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Broz P, Mueller CA, Muller SA, Phillipsen A, Sorg I, Engel A, et al. Function and molecular architecture of the Yersinia injectisome tip complex. Mol Microbiol. 2007;65:1311–1320. doi: 10.1111/j.1365-2958.2007.05871.x. [DOI] [PubMed] [Google Scholar]
- 46.DePaolo RW, Tang F, Kim IY, Han M, Levine N, Ciletti NA, et al. TLR6 drives differentiation of tolerogenic dendritic cells and contributes to LcrV-mediated plague pathogenesis. Cell Host Microbe. 2008;4:350–361. doi: 10.1016/j.chom.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bashaw J, Norris S, Weeks S, Trevino S, Adamovicz JJ, Welkos S. Development of in vitro correlate assays of immunity to infection with Yersinia pestis. Clin Vaccine Immunol. 2007;14:605–616. doi: 10.1128/CVI.00398-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williamson ED, Flick-Smith HC, LeButt C, Rowland CA, Jones SM, Waters EL, et al. Human immune response to a plague vaccine comprising recombinant F1 and V antigens. Infect Immun. 2005;73:3598–3608. doi: 10.1128/IAI.73.6.3598-3608.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]