Abstract
Lithium naphthalocyanine (LiNc) is a microcrystalline EPR oximetry probe with high sensitivity to oxygen (Pandian et al. J. Mater. Chem., 19, 4138, 2009). However, direct implantation of the crystals in the tissue for in vivo oxygen measurements may be hindered by concerns associated with their direct contact with the tissue/cells and loss of EPR signal due to particle migration in the tissue. In order to address these concerns, we have developed encapsulations (chips) of LiNc microcrystals in polydimethyl siloxane (PDMS), an oxygen-permeable, bioinert polymer. Oximetry evaluation of the fabricated chips revealed that the oxygen sensitivity of the crystals was unaffected by encapsulation in PDMS. Chips were stable against sterilization procedures or treatment with common biological oxidoreductants. In vivo oxygen measurements established the ability of the chips to provide reliable and repeated measurements of tissue oxygenation. This study establishes PDMS-encapsulated LiNc as a potential probe for long-term and repeated measurements of tissue oxygenation.
Keywords: Oxygen sensor, high-sensitivity probe, polymer coating, biocompatible EPR probe
INTRODUCTION
Electron paramagnetic resonance (EPR) oximetry is a minimally invasive technique that can provide reliable and repeated measurements of absolute tissue pO2 [1; 2], which is vital for the diagnosis and treatment of a number of disease conditions [3; 4; 5; 6; 7]. Many of these disease states, such as ischemic disease, cancer, and peripheral vascular disease, are characterized by a relatively lower level of tissue oxygenation (1–4 mmHg). Measurement of such low concentrations of oxygen using EPR will be rendered more accurate and reliable with the use of a probe that possesses high sensitivity to oxygen. Lithium naphthalocyanine (LiNc) is a particulate EPR oximetry probe that possesses significantly higher oxygen sensitivity (of the order of 30 mG/mmHg) [8] than other crystalline probes, such as lithium phthalocyanine (~ 6–7 mG/mmHg) [9; 10] and lithium octa-n-butoxynaphthalocyanine (~8–9 mG/mmHg) [11]. LiNc can be used in its neat crystalline form to measure oxygen. However, the direct implantation and in vivo application of LiNc may be limited by particle migration within tissue leading to loss of EPR signal intensity over time, and potential biocompatibility concerns due to direct exposure of crystals to tissue. Further, exposure to an in vivo environment may lead to the degradation of the oximetry properties of the implanted crystals.
Encapsulation or coating of the crystalline probes in biocompatible polymer matrices is a strategy, employed in the past, to overcome the limitations associated with direct implantation of these probes, and to enhance their clinical applicability [12; 13; 14; 15; 16]. In this study, we report the development of the implantable encapsulation of LiNc in polydimethyl siloxane (PDMS), a well-studied, biocompatible polymer with high oxygen permeability [17; 18]. We refer to the encapsulation of LiNc in PDMS as the LiNc:PDMS chip. We evaluated the in vitro oximetry properties of this new material in response to sterilization and oxidoreductant treatment, with a specific emphasis on the high oxygen-sensitivity of the LiNc crystals. Further, we tested the in vivo oxygen-sensing performance of a LiNc:PDMS chip to evaluate its applicability for high-resolution, in vivo, EPR oximetry. The study demonstrated that the encapsulation of LiNc microcrystals in PDMS does not affect the high oxygen-sensitivity of LiNc, while enhancing the biocompatibility and suitability of the probe for direct in vivo application.
MATERIALS AND METHODS
Fabrication of LiNc:PDMS chips
LiNc was synthesized as a microcrystalline powder as reported [8]. Polydimethyl siloxane (PDMS) was obtained as medical grade Silastic (MDX4-4210) from Dow Corning (Midland, MI). LiNc:PDMS chips were fabricated by cast-molding and polymerization as described [15].
Oxygen calibration and spin density evaluation
The calibration of the oxygen response and evaluation of the spin density of the LiNc:PDMS chip were performed, using an X-band (9.8 GHz) EPR spectrometer, as reported [15]. The spin density of LiNc:PDMS chips was calculated using a standard of known spin density, triarylmethyl radical (TAM) [19].
Autoclaving and UV-sterilization
Autoclaving was performed using a bench-top analog sterilizer (Tuttnauer® by Brinkmann Instruments, New York) at the following settings: 121°C for 1 hour at 1 atm. pressure (wet cycle using steam), followed by exhaust-drying for 15–20 minutes. UV-sterilization was carried out by exposing the chips to UV light, for 1 hour, in a laboratory cell-culture hood. Separate unsterilized control chips were used for comparison with sterilized chips.
Oxidoreductant treatment
LiNc:PDMS chips were treated with the following oxidants and reductants: nitric oxide, hydrogen peroxide, and glutathione. Nitric oxide was produced using a 5-mM solution of S-nitroso-N-acetyl penicillamine (SNAP) in distilled water. Hydrogen peroxide was diluted to a final concentration of 1 mM. Glutathione (5 mM) was prepared in distilled water. All chemicals were obtained from Sigma-Aldrich (St. Louis, MO), except SNAP which was obtained from Invitrogen (Eugene, OR). LiNc:PDMS chips were immersed in solutions of the oxidoreductants for 30 minutes, and the chips were dried in air for 60 minutes. Subsequently, EPR measurements of the oxygen response and spin density of the treated chips were made. LiNc:PDMS chips that were not treated with any of the oxidoreductants were used as controls. Separate untreated controls were used for each treatment.
Animal procurement and handling
Female C3H mice, used in this study, were obtained from the Frederick Cancer Research Center, Animal Production Unit (Frederick, MD, USA). The mice were housed, fed/maintained, and used for experiments, as per established guidelines, as reported [20].
Anesthesia
Animals were anesthetized by intraperitoneal administration of ketamine and xylazine (200 mg/kg body weight and 4 mg/kg body weight, respectively) during implantation of LiNc:PDMS chips, as well as, in vivo oxygen measurements.
Implantation of LiNc:PDMS chips in muscle
LiNc:PDMS chips were implanted subcutaneously in the gastrocnemius muscle of the right hind limb of the mice. A small flap of skin was cut and a LiNc:PDMS chip (~2 mm × 2 mm) was inserted into the muscle tissue, before the flap was sutured back in place. The sutured skin was allowed to heal for at least 24 hours prior to making EPR measurements.
In vivo EPR measurements
Muscle pO2 measurements were performed using an L-band (1.2 GHz) spectrometer (Magnettech, Berlin, Germany), as described [11]. Post-implantation pO2 measurements in muscle were made every week, for up to 30 days. In order to verify that the implanted LiNc:PDMS chip was responsive to changes in tissue oxygenation, the muscle tissue was subjected to constriction of blood flow, thereby a reduction in oxygen supply, to the hind limb. This was achieved by gently tying an elastic band on the limb above the location of chip implant. The constriction was maintained in place until EPR measurements were made (usually less than 5 min) and then removed.
Statistical analysis
Data were expressed as mean±SD and compared using a Student’s t-test with the level of significance (p) set at 0.05.
RESULTS AND DISCUSSION
Fabrication of the LiNc:PDMS chip
Encapsulation of LiNc in PDMS was carried out by a simple and convenient cast molding procedure, which did not require the use of any special equipment. Photographic images (Figure 1) showed the successful fabrication of the LiNc:PDMS chips, and also pure PDMS film, lacking LiNc crystals, to be used for comparison with the LiNc:PDMS chips. The images further revealed that the distribution of LiNc microcrystals in the PDMS matrix was fairly uniform. The uniformity of the particle distribution in the polymer was dependent on the efficiency of hand-mixing of crystals into the uncured polymer, since the uncured polymer (base-catalyst mixture) was highly viscous.
FIGURE 1. Encapsulation of LiNc in PDMS.
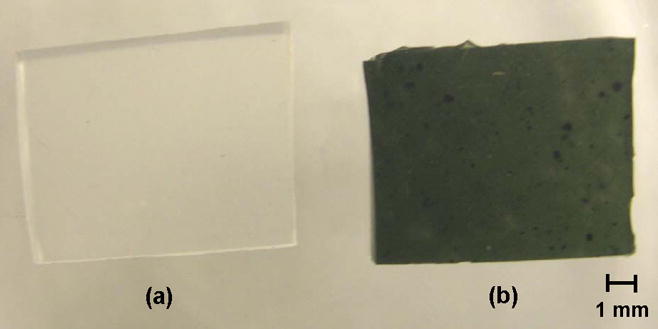
LiNc:PDMS chips were made by cast-molding and polymerization of the uncured polymer base-catalyst mixture, in which LiNc microcrystals were thoroughly dispersed. Control (pure) PDMS films, lacking LiNc crystals, were made using the same procedure, but without adding any particulates. (a) Pure PDMS film without particulates (b) A LiNc:PDMS chip. Photographs show fairly even distribution of LiNc particles in the PDMS matrix.
That the crystal structure of LiNc is intact is imperative to maintain its oxygen-sensing ability. Hence, dissolution of the crystals in the polymer would lead to a loss of oxygen sensitivity. However, the cast molding process, using PDMS, did not cause any dissolution of the embedded LiNc, ensuring that the oximetry properties of embedded crystals were intact after encapsulation (Figure 2). This is a distinct advantage offered by the cast-molding method compared to our previously reported solvent-evaporation method, which was limited by the choice of solvent that could be employed without dissolving the crystals [13]. Further, the cast-molding procedure enabled the fabrication of mechanically robust chips, unlike the solvent-evaporation methods that led to the formation of thin and brittle oximetry films [13].
FIGURE 2. Oxygen calibration of LiNc:PDMS chips.
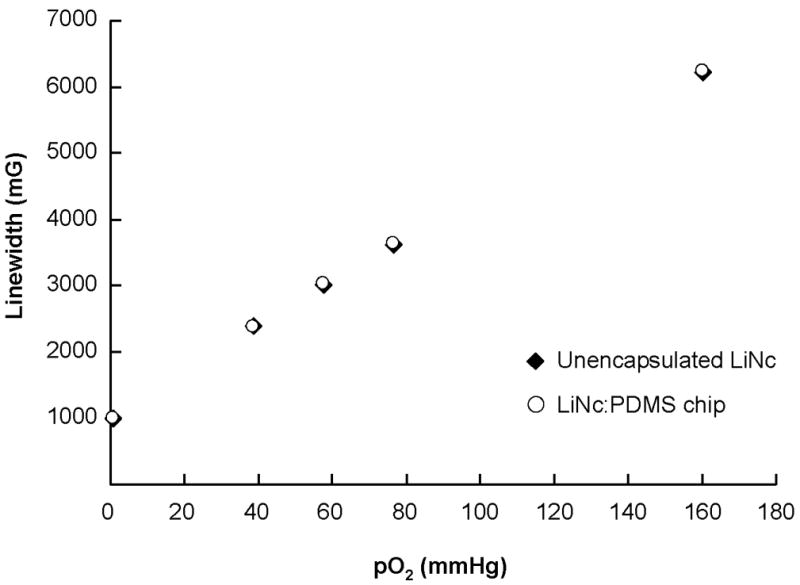
LiNc:PDMS chips (n=3) were exposed to different oxygen partial pressures (0–160 mmHg) and first-harmonic EPR spectra were recorded, using a X-band (9.8 GHz) EPR spectrometer, for the construction of calibration curves. Oxygen response of LiNc:PDMS chips was linear and reproducible with an oxygen sensitivity (slope of the calibration curve) of 32.6±0.4 mG/mmHg. Data are represented as mean±SD. The chip response was not significantly different from unencapsulated LiNc, which exhibited a sensitivity of 32.3±0.1 mG/mmHg. Data demonstrate that the encapsulation of LiNc in PDMS did not adversely affect the oxygen-sensing properties of LiNc:PDMS chips.
Oxygen calibration of LiNc:PDMS chips
In order to evaluate the effect of encapsulation in PDMS on the oxygen sensitivity of LiNc, we recorded the oxygen response of a LiNc:PDMS chip and an unencapsulated (neat) LiNc (control), at different oxygen partial pressures (pO2), to construct calibration curves (Figure 2). Since the motivation for encapsulating LiNc in PDMS was to enable high-resolution oxygen measurements, while also enhancing the biocompatibility and surgical removability of the probe, ensuring that the high oxygen sensitivity of embedded LiNc was retained after encapsulation was of paramount importance. The oxygen response of the chip was linear and reproducible, with slope identical to that of neat LiNc crystals, thus confirming that PDMS-encapsulation did not have any adverse effect on the high oxygen-sensitivity of LiNc. In addition, the fact that the oxygen sensitivities of the LiNc:PDMS chip and neat LiNc crystals were not significantly different indicates that the PDMS matrix was not saturated with oxygen, within the range of pO2 (0–160 mmHg) employed. Saturation of PDMS with oxygen could potentially lead to the embedded LiNc crystals experiencing lower oxygen content than the actual oxygen concentration in the surrounding environment. However, normal physiological pO2 in tissues is unlikely to exceed 160 mmHg, which serves to validate the adequacy of our testing conditions. Further, the encapsulation of LiNc in PDMS did not compromise its ability to make accurate and reliable measurements of in vivo pO2.
Effect of sterilization and oxidoreductant treatment on LiNc:PDMS chips
In order to evaluate the durability and biostability of LiNc:PDMS chips, we subjected them to sterilization by autoclaving or UV-treatment, or exposed it to biological oxidizing and reducing agents, respectively.
Sterilization of any biomaterial intended for implantation is essential to ensure that its in vivo performance is not hindered by the adverse effects of an infection, which could lead to the rejection of the implanted material. As a result, we evaluated the effects of autoclaving and UV-treatment on the oximetry properties of LiNc:PDMS chips. Figure 3A shows that sterilization by autoclaving or UV-treatment did not have any significant effect on the linearity of the oxygen calibration, oxygen sensitivity, and the spin density of LiNc:PDMS chips. PDMS is known for its resistance to high temperatures (degradation temperature ~ 300–350°C [21]) and UV-radiation [18]. Therefore, the high temperature of the autoclave (121°C) or exposure to UV-rays did not cause any degradation or additional crosslinking of the polymer, thereby precluding any loss of polymer-oxygen permeability and a resultant loss in the oxygen sensitivity of the embedded crystals. In addition, unencapsulated (neat) LiNc crystals are also known for their high temperature- and radiation-stability [8].
FIGURE 3. Effect of sterilization and oxidoreductant treatment on LiNc:PDMS chips.
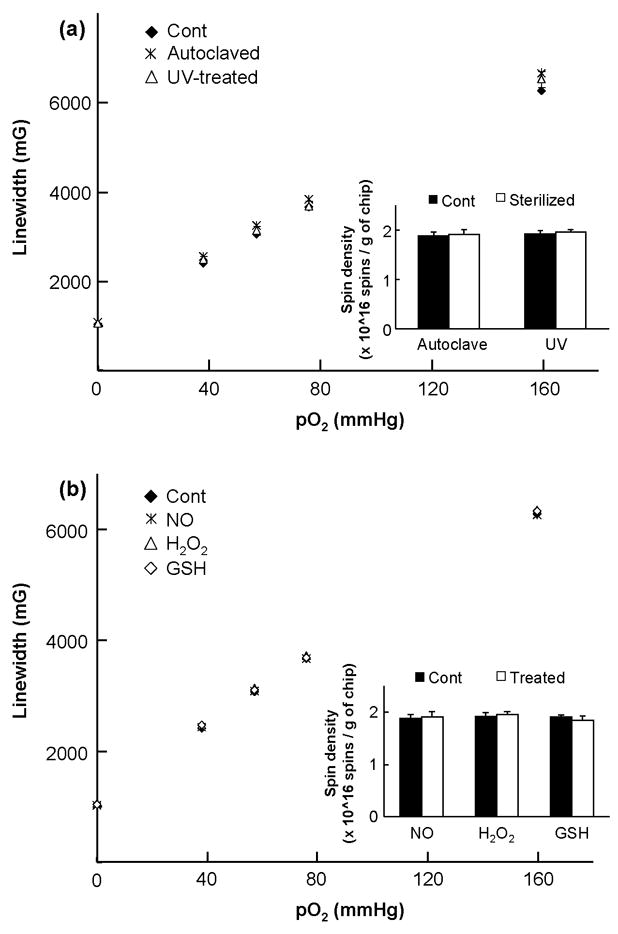
X-band EPR spectroscopy was used to evaluate the effect of sterilization and oxidoreductant treatment on LiNc:PDMS chips (a) Effect of sterilization on the oxygen calibration and spin density of LiNc:PDMS. Oxygen response of LiNc:PDMS chips (n=3, mean±SD), sterilized by autoclaving and UV-treatment, was not significantly different from the response of unsterilized control (Cont), with comparable oxygen sensitivities (autoclaving: 33.6±0.9 mG/mmHg; UV-treatment: 33.9±1.1 mG/mmHg; unsterilized control: 32.6±0.4 mG/mmHg). Inset shows that the spin densities of autoclaved or UV-treated LiNc:PDMS chips (n=6, mean±SD), calculated using a standard of known spin density, were not significantly different from the spin density of unsterilized controls. (b) Effect of oxidoreductant treatment on the oxygen calibration and spin density of LiNc:PDMS chips. Chips were treated with nitric oxide (NO), 1-mM hydrogen peroxide (H2O2), and 5-mM glutathione (GSH) for 30 min. After air-drying, the oxygen responses of treated chips and untreated controls (Cont) were evaluated using X-band EPR spectroscopy. Data (n=3, mean±SD) show that the response was linear with increase in oxygen partial pressure after treatment with all three agents. Oxygen sensitivity of the chips treated with NO (32.6±1.3 mG/mmHg), H2O2 (32.8±0.1 mG/mmHg), or GSH (32.8±0.4 mG/mmHg) was not significantly different from the sensitivity of untreated control (32.6±0.4 mG/mmHg). Inset shows that treatment with biological oxidoreductants did not significantly affect the spin density of LiNc:PDMS chips (n=6, mean±SD). Collectively, results indicate that LiNc:PDMS chips were durable and biostable.
Subsequently, we studied the effect of treatment with biological oxidizing and reducing agents on LiNc:PDMS chips. Interaction of chips, upon implantation, with highly potent oxidoreductants that are characteristic of the in vivo environment, could have an adverse effect on their oxygen-sensing properties. Hence, in order to verify the biostability of LiNc:PDMS chips, we exposed them to biological oxidoreductants, namely nitric oxide (NO), hydrogen peroxide (H2O2), and glutathione (GSH). Figure 3B shows the effect of in vitro oxidoreductant exposure on the oxygen calibration and spin density of LiNc:PDMS chips. Results demonstrate that the linearity and slope (sensitivity) of the oxygen calibration, as well as, the spin density of LiNc:PDMS chips remained intact (not significantly different from the untreated controls) after treatment with these agents.
In vivo oxygen-sensing response of LiNc:PDMS chips
Having established that LiNc:PDMS chip were unaffected by sterilization procedures and were biostable after oxidoreductant exposure, we evaluated their applicability for in vivo oximetry by implanting a chip in the gastrocnemius muscle tissue of mice and monitoring in vivo oxygenation for up to 30 days using L-band EPR spectroscopy. Normal muscle pO2 values (filled symbols in Figure 4), reported by an implanted LiNc:PDMS chip, demonstrated the ability of the chip to provide repeated measurements of in vivo tissue oxygenation over 30 days. Further, in order to test the ability of the implanted chip to respond to changes in tissue pO2, we temporarily constricted blood flow to the muscle using an elastic band. Constricted-state pO2 values (open symbols in Figure 4) showed that the implanted chip was capable of sensing the changes in pO2. In addition, subsequent measurements from the same animals showed that the muscle pO2 returned to normal levels when the constriction was removed (data not shown). The average muscle pO2 measured by implanted LiNc:PDMS chips was 21.4±1.3 mmHg, which was within range of the gastrocnemius muscle pO2 values reported previously [11; 13; 16; 22; 23]. Further, oxygen calibration of each LiNc:PDMS chip that was surgically removed from the animals showed no significant difference when compared to an unimplanted control (data not shown), indicating that LiNc:PDMS chips exhibited excellent in vivo biodurability.
FIGURE 4. In vivo oxygen measurements using LiNc:PDMS chips.
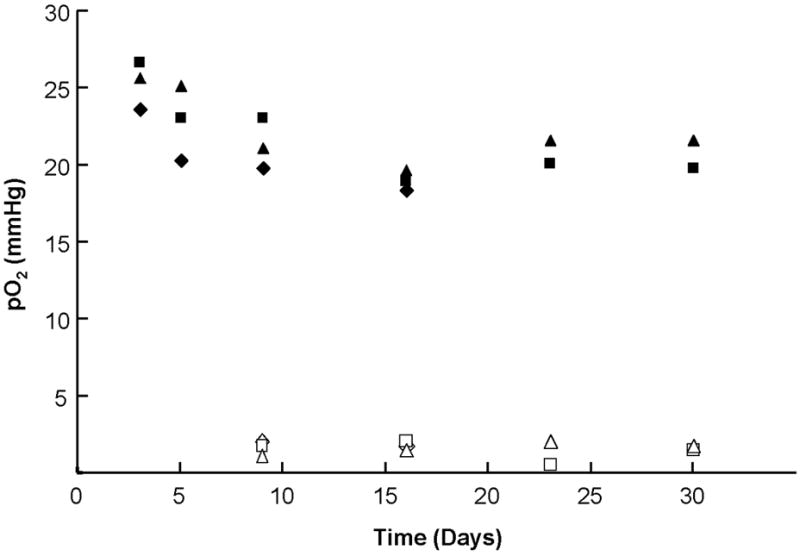
LiNc:PDMS chips were implanted in the gastrocnemius muscle tissue of mice, and in vivo oxygen measurements were performed using an L-band EPR spectrometer for up to 30 days. Muscle pO2 from three mice, in the normal (filled symbols) and constricted (open symbols) condition of the muscle, demonstrate that implanted LiNc:PDMS chips were capable of reliable measurements of in vivo tissue pO2, and were also responsive to changes in pO2.
SUMMARY AND CONCLUSIONS
We have demonstrated the fabrication, biostability evaluation, and in vitro/in vivo oxygen-sensing performance of an implantable, high-sensitivity EPR oximetry probe, the LiNc:PDMS chip, made by the encapsulation of LiNc microcrystals in PDMS. Fabrication of the chip was carried out by a simple cast molding and polymerization method. The encapsulation procedure did not have any adverse effect on the oxygen-sensing properties of the embedded crystals, while providing a means to render them biocompatible and surgically retrievable. The chips were biostable, and unaffected by sterilization procedures or treatment with biological oxidoreductants. The in vivo oxygen-sensing performance of LiNc:PDMS chips and their sensitivity to changes in tissue pO2 were confirmed by implanting chips in murine muscle tissue and monitoring oxygenation for up to 30 days. Overall, we have shown that LiNc:PDMS chips are a promising choice of probes for high-resolution in vivo EPR oximetry.
Acknowledgments
This study was supported by NIH grant EB004031. We thank Nancy Trigg for her critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khan N, Williams BB, Hou H, Li H, Swartz HM. Repetitive tissue pO2 measurements by electron paramagnetic resonance oximetry: Current status and future potential for experimental and clinical studies. Antioxid Redox Signal. 2007;9:1169–1182. doi: 10.1089/ars.2007.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swartz HM. Using EPR to measure a critical but often unmeasured component of oxidative damage: Oxygen. Antioxid Redox Signal. 2004;6:677–686. doi: 10.1089/152308604773934440. [DOI] [PubMed] [Google Scholar]
- 3.Hopf HW, Rollins MD. Wounds: An overview of the role of oxygen. Antioxid Redox Signal. 2007;9:1183–1192. doi: 10.1089/ars.2007.1641. [DOI] [PubMed] [Google Scholar]
- 4.Kulkarni AC, Kuppusamy P, Parinandi N. Oxygen, the lead actor in the pathophysiologic drama: Enactment of the trinity of normoxia, hypoxia, and hyperoxia in disease and therapy. Antioxid Redox Signal. 2007;9:1717–1730. doi: 10.1089/ars.2007.1724. [DOI] [PubMed] [Google Scholar]
- 5.Kutala VK, Khan M, Angelos MG, Kuppusamy P. Role of oxygen in postischemic myocardial injury. Antioxid Redox Signal. 2007;9:1193–1206. doi: 10.1089/ars.2007.1636. [DOI] [PubMed] [Google Scholar]
- 6.Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006;47:921–929. doi: 10.1016/j.jacc.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 7.Vaupel P, Hockel M, Mayer A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid Redox Signal. 2007;9:1221–35. doi: 10.1089/ars.2007.1628. [DOI] [PubMed] [Google Scholar]
- 8.Pandian RP, Dolgos M, Marginean C, Woodward PM, Hammel PC, Manoharan PT, Kuppusamy P. Molecular packing and magnetic properties of lithium naphthalocyanine crystal: Hollow channels enabling permeability and paramagnetic sensitivity to molecular oxygen. J Mater Chem. 2009;19:4138–4147. doi: 10.1039/b901886g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ilangovan G, Zweier J, Kuppusamy P. Electrochemical preparation and EPR studies of lithium phthalocyanine. Part 2: Particle-size-dependent line broadening by molecular oxygen and its implications as an oximetry probe. J Phys Chem B. 2000;104:9404–9410. [Google Scholar]
- 10.Liu KJ, Gast P, Moussavi M, Norby SW, Vahidi N, Walczak T, Wu M, Swartz HM. Lithium Phthalocyanine - a Probe for Electron-Paramagnetic-Resonance Oximetry in Viable Biological-Systems. Proc Natl Acad Sci USA. 1993;90:5438–5442. doi: 10.1073/pnas.90.12.5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandian RP, Parinandi NL, Ilangovan G, Zweier JL, Kuppusamy P. Novel particulate spin probe for targeted determination of oxygen in cells and tissues. Free Radic Biol Med. 2003;35:1138–1148. doi: 10.1016/s0891-5849(03)00496-9. [DOI] [PubMed] [Google Scholar]
- 12.Dinguizli A, Jeumont S, Beghein N, He J, Walczak T, Lesniewski PN, Hou H, Grinberg OY, Sucheta A, Swartz HM, Gallez B. Development and evaluation of biocompatible films of polytetrafluoroethylene polymers holding lithium phthalocyanine crystals for their use in EPR oximetry. Biosens Bioelectron. 2006;21:1015–1022. doi: 10.1016/j.bios.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Eteshola E, Pandian RP, Lee SC, Kuppusamy P. Polymer coating of paramagnetic particulates for in vivo oxygen-sensing applications. Biomed Microdevices. 2009;11:379–387. doi: 10.1007/s10544-008-9244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallez B, Mader K. Accurate and sensitive measurements of pO2 in vivo using low frequency EPR spectroscopy: how to confer biocompatibility to the oxygen sensors. Free Radic Biol Med. 2000;29:1078–84. doi: 10.1016/s0891-5849(00)00405-6. [DOI] [PubMed] [Google Scholar]
- 15.Meenakshisundaram G, Eteshola E, Pandian RP, Bratasz A, Lee SC, Kuppusamy P. Fabrication and physical evaluation of a polymer-encapsulated paramagnetic probe for biomedical oximetry. Biomed Microdevices. 2009;11:773–782. doi: 10.1007/s10544-009-9292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meenakshisundaram G, Eteshola E, Pandian RP, Bratasz A, Selvendiran K, Lee SC, Krishna MC, Swartz HM, Kuppusamy P. Oxygen sensitivity and biocompatibility of an implantable paramagnetic probe for repeated measurements of tissue oxygenation. Biomed Microdevices. 2009;11:817–826. doi: 10.1007/s10544-009-9298-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abbasi F, Mirzadeh H, Katbab AA. Modification of polysiloxane polymers for biomedical applications: a review. Polym Int. 2001;50:1279–1287. [Google Scholar]
- 18.Mata A, Fleischman AJ, Roy S. Characterization of polydimethylsiloxane (PDMS) properties for biomedical micro/nanosystems. Biomed Microdevices. 2005;7:281–293. doi: 10.1007/s10544-005-6070-2. [DOI] [PubMed] [Google Scholar]
- 19.Kutala VK, Parinandi NL, Pandian RP, Kuppusamy P. Simultaneous measurement of oxygenation in intracellular and extracellular compartments of lung microvascular endothelial cells. Antioxid Redox Signal. 2004;6:597–603. doi: 10.1089/152308604773934350. [DOI] [PubMed] [Google Scholar]
- 20.Bratasz A, Pandian RP, Deng Y, Petryakov S, Grecula JC, Gupta N, Kuppusamy P. In vivo imaging of changes in tumor oxygenation during growth and after treatment. Magn Reson Med. 2007;57:950–9. doi: 10.1002/mrm.21212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dvornic PR. Thermal Properties of Polysiloxanes at Low Temperatures. In: Jones RG, Ando W, Chojnowski J, editors. Silicon-Containing Polymers - The Science and Technology of Their Synthesis and Applications. Kluwer Academic Publishers; Norwell, MA: 2000. pp. 185–212. [Google Scholar]
- 22.Ilangovan G, Bratasz A, Li H, Schmalbrock P, Zweier JL, Kuppusamy P. In vivo measurement and imaging of tumor oxygenation using coembedded paramagnetic particulates. Magn Reson Med. 2004;52:650–7. doi: 10.1002/mrm.20188. [DOI] [PubMed] [Google Scholar]
- 23.Ilangovan G, Manivannan A, Li HQ, Yanagi H, Zweier JL, Kuppusamy P. A naphthalocyanine-based EPR probe for localized measurements of tissue oxygenation. Free Radic Biol Med. 2002;32:139–147. doi: 10.1016/s0891-5849(01)00784-5. [DOI] [PubMed] [Google Scholar]


