Abstract
In a previously developed infant macaque model mimicking HIV infection by breast feeding, we demonstrated that intramuscular immunization with recombinant poxvirus vaccines expressing simian immunodeficiency virus (SIV) structural proteins provided partial protection against infection following oral inoculation with virulent SIV. In an attempt to further increase systemic but also local antiviral immune responses at the site of viral entry, we tested the immunogenicity of different orally administered, replicating vaccines. One group of newborn macaques received an oral prime immunization with a recombinant vesicular stomatitis virus expressing SIVmac239 gag, pol and env (VSV-SIVgpe), followed 2 weeks later by an intramuscular boost immunization with MVA-SIV. Another group received two immunizations with live-attenuated SIVmac1A11, administered each time both orally and intravenously. Control animals received mock immunizations or non-SIV VSV and MVA control vectors. Analysis of SIV-specific immune responses in blood and lymphoid tissues at 4 weeks of age demonstrated that both vaccine regimens induced systemic antibody responses and both systemic and local cell-mediated immune responses. The safety and immunogenicity of the VSV-SIVgpe +MVA-SIV immunization regimen described in this report provide the scientific incentive to explore the efficacy of this vaccine regimen against virulent SIV exposure in the infant macaque model.
Keywords: pediatric, SIV, HIV, oral, vaccine
INTRODUCTION
Despite the progress in reducing intra-partum transmission using short-term antiretroviral regimens, breast-feeding continues to be a considerable risk factor for postnatal mother-to-child transmission of HIV in developing countries [1–3]. Breastfeeding constitutes a big dilemma for many HIV-infected women, because breast milk can transmit HIV, but in many low-resource areas is usually the best way to provide the nursing infant with much-needed nutrition and protection against other serious infectious diseases [4]. While prolonged administration of antiviral drugs to nursing infants has the potential to reduce HIV transmission [5], their cost, risk of toxicity and need for regular administration are limiting factors in resource-poor areas. Ideally, a vaccine regimen should be developed that, when administered to the infant shortly after birth, could protect against HIV transmission via breastfeeding (see review [6]). Because breast-feeding starts early after delivery and a significant portion of HIV transmission occurs during the first months of breast-feeding [1, 3], such neonatal vaccine regimen has the daunting task that it needs to induce protective immune responses rather rapidly.
Because of its many similarities in host physiology, immunology and disease pathogenesis, simian immunodeficiency virus (SIV) infection of infant macaques is a highly relevant animal model of pediatric HIV infection, and has been used to test drug strategies and pediatric HIV vaccine candidates [7–10]. In particular, for developing a neonatal vaccine against HIV breastmilk transmission, it is essential to evaluate safety, immunogenicity and efficacy of candidate vaccine strategies in nonhuman primate infants whose immune system development and vaccine responses most accurately reflect those of human infants (reviewed in [11–13]. Using this infant macaque model, we have previously demonstrated that intramuscular administration of attenuated poxvirus-based SIV vaccines (ALVAC-SIV and modified vaccine virus Ankara [MVA]-SIV) to infant macaques during the first 4 weeks after birth was immunogenic and partially protective against infection when animals were exposed repeatedly at 4 weeks of age to low doses of virulent SIVmac251 [14]; this partial resistance to infection was still evident when uninfected immunized animals were rechallenged orally with virulent SIV 16 months later. In addition, we demonstrated that in unimmunized infant macaques, the early immune responses at mucosal entry sites after oral SIVmac251 challenge were dominated by the induction of proinflammatory cytokines that likely promoted high virus replication [15]. Based on our hypothesis that an effective vaccine needs to elicit fast and potent antiviral immune responses at the oral mucosal sites, we investigated the ability of replicating SIV vaccines to induce such immune responses after oral administration.
Recombinant vesicular stomatitis virus (VSV) is an attractive viral vaccine vector candidate because of its very low VSV seroprevalence in humans, its ability to infect and robustly express foreign antigens in a broad range of cells, and its ability to infect after mucosal inoculation [16, 17]. In addition, recombinant VSV expressing SIV proteins (VSV-SIV) was demonstrated to be safe, immunogenic and effective in reducing viremia after challenge in juvenile macaques, particularly when boosted with MVA-SIV [18, 19]. There have been no prior reports of testing VSV-SIV in infant macaques.
We demonstrated previously that immunizing infant macaques with SIVmac1A11, which induces transient low-level viremia, was safe, immunogenic and effective in preventing infection or reducing viremia following subsequent oral inoculation with two high doses of SIVmac251 [10, 20, 21]. Based on these results, and because live-attenuated SIV s have generally been the most effective vaccines due to the induction of a broad spectrum of immune responses [22–24], we compared the immunogenicity of a VSV-SIV plus MVA-SIV vaccine strategy with that of live-attenuated SIVmac1A11 in infant macaques. We demonstrate that a vaccine regimen consisting of oral VSV-SIV followed by intramuscular MVA-SIV administration is safe and elicits mucosal and systemic humoral and cell-mediated immune responses, that are similar in magnitude and breadth to those induced by the SIVmac1A11 vaccination regimen.
METHODS
Animals
Newborn rhesus macaques (Macaca mulatta) negative for HIV-2, SIV, type D retrovirus, and simian T-cell lymphotropic virus type 1 were hand-reared in a primate nursery at the California National Primate Research Center (CNPRC). Animals were housed in accordance with American Association for Accreditation of Laboratory Animal Care standards. We adhered to the “Guide for Care and Use of Laboratory Animals” [25]. We and others have previously established that nursery-raised newborn macaques have normal growth and development, clinical parameters, and immune responses to routine colony vaccinations (e.g. tetanus and measles virus), and are useful to establish specific pathogen-free breeding colonies as well as for experimental research projects [21, 26, 27]. Animals were randomly assigned at birth to study groups and were between 1 and 6 days of age at the start of the first immunization (“time zero”). The animal protocols for these studies were reviewed and approved by the University of California, Davis Institutional Animal Care and Use Committee prior to implementation.
Vaccine and immunization regimens
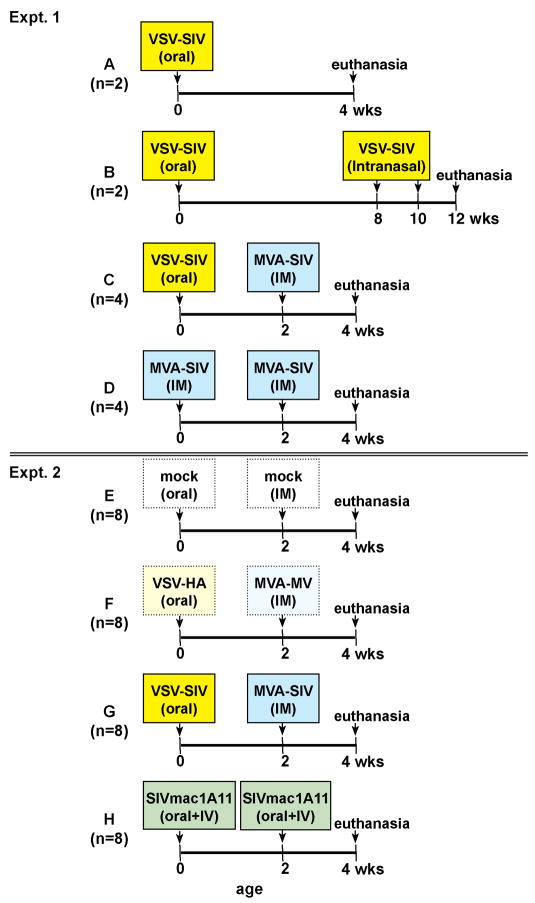
All immunizations were performed under ketamine anesthesia. Two sets of experiments were performed during 2 consecutive years. The data obtained during the pilot study (Experiment 1, Groups A-D) determined the experimental design of the larger study (Experiment 2, Groups EH). The various SIV vaccines were administered as summarized in Fig. 1. The VSV-SIV vaccine was administered orally in a total of 1 ml containing107 pfu of each recombinant VSV construct. In Experiment 1, a combination of 4 constructs was used: VSV-SIVgag, -SIV pol, -SIVenvG-1 and VSV-SIVRTNV (containing rev-tat-nef-vif). In Experiment 2, the VSV-SIVRTNV construct was no longer included because it had no detectable effect on SIV-specific immune responses (J. Rose, unpublished data). To control for a possible vector effect, a recombinant VSV expressing influenza hemagglutinin was used (VSV-HA) [28].
Figure 1. Summary of infant vaccine groups.
As described in the materials and methods, in two sets of experiments newborn macaques were immunized shortly after birth via the oral route with replicating SIV vaccines, either VSV-SIV (expressing SIV gag, pol, env) or live-attenuated SIVmac1A11. Booster immunizations on some groups consisted of intramuscular MVA-SIV. The 2nd set of experiments had also a group that received control vectors expressing non-SIV antigens (VSV-HA, expressing influenza hemagglutinine, and MVA-MV expressing measles virus).
The MVA-SIV vaccine consisted of vJH4 (= MVA/SIV239gagpol) and MVA/SIV239env, the construction of which has been described previously [14, 29]. Each MVA-SIV immunization consisted of 1 ml containing 1 × 108 infectious units (IU) of each recombinant construct per ml, administered intramuscularly (divided as 250 μl in 4 injection sites). As non-SIV MVA control vector, a previously described MVA-measles virus (MVA-MV) construct expressing measles hemagglutinin (H) and fusion (F) proteins [30] was used at the same dose (1 ml of 1 × 108 IU, intramuscularly, divided over 4 injection sites).
The SIVmac1A11 stock was propagated on CEMx174 cells and had a titer of ~105 50% tissue culture infectious doses (TCID50) per ml. Each SIVmac1A11 immunization consisted of a combined administration of 1 ml by the intravenous route and 1 ml via the oral route.
To monitor general immunocompetence, all infants in each vaccine group were also immunized intramuscularly with 0.5 ml of commercial pediatric/adolescent Hepatitis B vaccine (Recombivax HB®) at 0 and 2 weeks of age.
Collection and processing of specimens
For blood collections and immunizations, animals were immobilized with 10 mg/kg intramuscular ketamine-HCL (Parke-Davis, Morris Plains, NJ, USA). EDTA-anticoagulated blood samples were collected regularly for monitoring immunologic and virologic parameters. Complete blood cell counts were measured using an automated electronic cell counter (Baker 9000; Serono Baker Diagnostics); differential counts were determined manually. Lymphocyte phenotypic analysis of lymphocyte subsets in blood was performed using 4-color flow cytometry techniques as described previously [31].
Whole saliva were collected by inserting two Weck-Cel cellulose sponges (Medtronic, Jacksonville, Florida) in the cheek pouches of the anesthetized animals for at least 5 minutes before euthanasia. Sponges were then stored in 12×75 mm tubes (Fisher Scientific, Pittsburgh PA) at −80°C until analysis. Secretions were extracted from sponges, as previously described [32], by centrifugation in the presence of PBS containing protease inhibitors and 0.5% Igepal detergent (Sigma, St. Louis, MO).
Lymphoid tissues collected at euthanasia were processed to obtain cell suspensions by dissecting them with scalpels in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; Gemini BioProducts, Calabasas, CA) (complete RPMI) and passing the cell homogenate through a cell strainer (Fisher, Pittsburgh, PA). Mononuclear cells were isolated from the splenic cell suspensions and the blood by density gradient centrifugation with Lymphocyte Separation Medium from MP Biomedicals (Aurora, OH), followed by two washes with RPMI 1640.
Cell isolation from intestinal tissues was performed according to previously described methods [33]. Briefly, ~2 inch pieces of the ileum and colon were rinsed with PBS and then minced using sterile scalpels. The tissue suspensions were placed in a shaking waterbath in RPMI 1640 containing 7.5% FBS and collagenase type II (0.5 mg/ml) for 30 minutes at 37°C. After the digestion, the single cell suspension was passed through a 100 μm filter, spun down and resuspended in 10% FBS in RPMI 1640. The remaining undigested tissue was resuspended in collagenase-media and the digestion step was repeated a total of 3 times. Mucosal lymphocytes were then isolated from the obtained single cell suspension by performing a 35%/60% Percoll (Sigma) gradient centrifugation. Intestinal lymphocytes were collected from the 35%/60% interface and washed twice with PBS before being resuspended in 10% FBS in RPMI 1640.
Virological detection of replication of SIV vaccines
In the SIVmac1A11-immunized animals, plasma SIV RNA was quantified using a branched DNA (bDNA) signal amplification assay specific for SIV, version 4.0, which has a lower quantitation limit of 125 copies per sample [34]; due to the limited available volumes of plasma, the quantitation limit was 500 copies per ml plasma. Similarly, VSV RNA was measured in tissue samples by real-time polymerase chain reaction (RT-PCR) with a detection limit of 100 copies of genomic VSV RNA [35].
Measurement of antiviral antibodies and total IgA
ELISA was used to measure antibodies to the SIV envelope protein or the SIV gag, pol proteins as previously described [36]. Briefly, microtiter plates were coated with either recombinant SIVmac251 gp130 envelope protein (ImmunoDiagnostics, Woburn, MA) or SIVmac251 viral lysate (Advanced Biotechnologies Inc, Columbia, MD). The lysate lacks detectable envelope protein at the 1/400 coating dilution used. Serial dilutions of samples and previously described macaque serum standards [36] were reacted overnight at 4°C with coated/blocked plates. Plates were developed by treatment with biotinylated polyclonal goat anti-human IgG (SouthernBiotech, Birmingham, AL) or –monkey IgA (Open Biosystems, Huntsville, AL), followed by avidin peroxidase, and tetramethylbenzidine (Sigma). Total IgA was measured by ELISA as previously described [37]. Concentrations of SIV env- or SIV gag, pol -specific IgA were divided by the concentration of total IgA to obtain the specific activity (ng IgA antibody per μg total IgA). The specific activity was considered significant if it was greater than or equal to the mean specific activity + 3 SD obtained using negative control macaque saliva.
IgG antibodies to whole SIV were measured by ELISA, in which Costar EIA/RIA plates (Fisher Scientific) were coated with SIVmac251 (Advanced Biotechnologies Inc) at 500 ng of total protein per well and then developed as described previously [38]. Hepatitis B-specific IgG antibodies were determined by performing a similar ELISA in which the ELISA plate wells were coated with hepatitis B surface antigen (Fitzgerald Industries International, Concord MA, USA) at a concentration of 5.0 μg/ml.
ELISPOT for IFN-γ secreting cells
In experiment 1, to estimate the number of SIV-specific interferon-gamma (IFN-γ) producing T cells in cryopreserved PBMC, an ELISPOT assay using a pool of 20-mer peptides of the entire p27 gag region of SIVmac239 was used according to methods described previously [10, 39]; values are reported after subtraction of the values of the medium-control wells.
Intracellular cytokine staining
In experiment 2, fresh PBMC and lymphoid cells obtained at euthanasia were stained for intracellular interferon-gamma (IFN-γ), tumor necrosis factor alpha (TNF-α) and interleukin-2 after stimulation with 300 ng/ml aldrithiol-2 (AT-2)-inactivated whole SIVmac239 (provided by Dr. J. Lifson, NCI), or with of a pool of overlapping 15mer peptides (provided by the NIH Reference and Reagent Program) spanning the SIVgag p27 protein. The final concentration of each peptide within the peptide pool used for T cell stimulation was 5 μg/ml. Cells were stimulated in the presence of CD49d and CD28 antibodies (0.5 μg/ml each). Positive control cultures were stimulated with 50 ng/ml PMA (SIGMA) and 1 μg/ml ionomycin (SIGMA). Negative control cells were cultured in media only. The intracellular cytokine assays were performed according to standard protocols using 1×106 cells, and incubation at 37°C in 5% CO2 for 6 hours. Brefeldin A (10 μg) was added 1 hour after the start of the incubation [15]. Data were acquired (300,000 lymphocyte events) on a FACS ARIA (Beckton-Dickinson) and analyzed using FlowJo software version 8.1 (TreeStar, Ashland, OR), and are reported as frequencies (percent) of cytokine positive cells per CD4+ or CD8+T cells. Frequency values were considered positive if they were at least 0.04% and >2.5-fold greater than that of the medium-only cultures.
Statistical analysis
Statistical analyses were performed using Prism Version 4.0 for Mac, and Instat 3 (GraphPad Software Inc. San Diego, CA). Mann-Whitney test and Kruskal Wallis were used to compare the groups. P values < 0.05 were considered statistically significant.
RESULTS
Pilot study on safety and immunogenicity of VSV-SIV vaccine
To investigate the safety and immunogenicity of oral VSV-SIV administration, and the effect of an intramuscular booster immunization with MVA-SIV, a pilot experiment was performed, as summarized in Figure 1 and Table 1. As a control antigen (to verify neonatal immunocompetence), animals were immunized with hepatitis B vaccine at 0 and 2 weeks. All animals had antibodies against hepatitis B 4 weeks after the first immunization (median titer 1:1,600; range 1:400-1:25,600); the titers against hepatitis B were indistinguishable among the animal groups (data not shown).
Table 1.
Pilot study on immunogenicity of VSV-SIV with our without MVA booster.
| Group | Immunization | Time of euthanasia (age) | Animal number | Vector-specific immunity1 |
SIV-specific immunity1 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VSV neutr. antibody titer2 | MVA- binding antibody titer2 | Whole SIV-specific antibody titer2 | SIV-p27gag-specific IFN-γ secreting SFC/million cells3 |
|||||||||
| PBMC | Retro LN | Cerv LN | Subm LN | Mes LN | Spleen | |||||||
| A | VSV-SIV oral at 0 wks | 4 wks | 36229 | - | nd | - | na | - | na | na | - | - |
| 36231 | - | nd | - | na | - | na | na | - | - | |||
| B | VSV-SIV oral at 0, 8 and 10 wks | 12 wks | 36232 | 10,240 | nd | - | - | - | - | - | - | - |
| 36245 | 10,240 | nd | - | - | - | - | - | - | - | |||
| C | VSV-SIV oral at 0; MVA-SIV IM at 2 wks | 4 wks | 36552 | 1,280 | 6,400 | 400 | 100 | 125 | - | - | - | 65 |
| 36554 | - | 19,200 | 100 | - | - | - | - | - | - | |||
| 36558 | 160 | 12,800 | 100 | 55 | - | - | - | - | 85 | |||
| 36568 | 640 | 19,200 | 100 | 155 | 150 | 165 | - | 60 | 150 | |||
| D | MVA-SIV IM at 0 and at 2 wks | 4 wks | 36659 | nd | 200,000 | 400 | - | - | - | - | - | - |
| 36672 | nd | 100,000 | 400 | - | - | - | - | - | - | |||
| 36680 | nd | 150,000 | 400 | 75 | - | - | - | - | - | |||
| 36688 | nd | 76,000 | 400 | - | - | - | na | - | - | |||
Immune responses measured at time of euthanasia (with exception for group B, for which VSV neutralizing antibodies were measured at 10 weeks of age).
Antibody titers are expressed as the reciprocal of the highest dilution that gave values above the cut-off of the whole SIV ELISA assay.
SIV-p27gag specific spot-forming cells (SFC) were measured by interferon-gamma ELISPOT assay with 20-mer peptides of the SIV gag region. nd: not done; na: not available; minus sign indicates undetectable (below cut-off value of the respective assay).
In a first experiment, two animals (Group A) were given a single oral administration of VSV-SIV. However, when animals were euthanized 4 weeks later, no immune responses against VSV (as determined by neutralizing antibody titers) or against SIV (as determined by SIV-specific antibody ELISA and ELISPOT assays) were detectable, suggesting insufficient VSV-SIV replication in these 2 animals.
In contrast, two additional animals receiving a single oral administration of VSV-SIV (Group B: 36232 and 36245), developed VSV-neutralizing antibody titers by 4 weeks (animal 36232, 1:160; animal 36245, 1:1,280), although no SIV-specific antibodies were detectable. Therefore, these animals were given 2 booster doses of VSV-SIV at week 8 and 10 of age. To promote mucosal immunity, booster immunizations were given intranasally. Although VSV-neutralizing antibody titers further increased in both animals by week 10 (titer of 1:10,240), only one animal (36245) showed low levels of SIV-specific antibodies in plasma, and only at a single time point (titer 1:100, i.e. at the cut-off value of the assay). At the time of euthanasia at 12 weeks of age, SIV-specific antibodies were not detectable in plasma, and there was no evidence of SIV gag-specific cell-mediated immune responses (measured by ELISPOT) in PBMC and tissues (Table 1). Thus, the results of Group A and B indicate that even though mucosal administration of the replicating VSV-SIV vaccine induced VSV-specific immune responses in some animals, it was insufficient to induce SIV-specific immune responses reliably.
Therefore, we tested the immunogenicity of the VSV-SIV vaccine as part of a mucosal prime- systemic boost regimen. Four newborn macaques (Group C) were immunized orally at birth with VSV-SIV and boosted two weeks later intramuscularly with MVA-SIV (Fig. 1). Based on our previous studies with poxvirus vaccines, 4 additional animals (Group D) received intramuscular immunizations with MVA-SIV at birth and 2 weeks of age. Animals of both Groups C and D were euthanized at 4 weeks of age.
Three out of 4 animals in Group C had detectable VSV-neutralizing antibodies, and 4 out of 4 animals developed high vaccinia-specific antibody titers (> 1: 6,400) two weeks after the MVA-SIV immunization. Although SIV-specific antibody titers were low at 4 weeks, the 3 animals with VSV-specific antibodies had also detectable SIV-specific cell-mediated immune responses in PBMC and in at least one other lymphoid tissue (Table 1). In contrast, the one animal without detectable anti-VSV antibodies had no SIV-specific cell-mediated immune responses (Table 1, Group C, animal 36554), suggesting insufficient replication of the VSV-SIV vector in this animal. While the MVA-SIV prime/MVA-SIV boost regimen induced higher SIV-specific antibody titers in plasma compared to the animals receiving the VSV-SIV/MVA-SIV regimen, only 1 animal had detectable SIV-specific cell-mediated immune responses in PBMC (Table 1, Group D, animal 36680).
None of the VSV-SIV immunized animals in Groups A-D exhibited any clinical signs or had any pathological lesions at the time of euthanasia that suggested adverse effects of the immunization regimen with these attenuated VSV s.
In conclusion, this pilot experiment demonstrated that an oral VSV-SIV prime immunization followed by an intramuscular MVA-SIV booster immunization was safe and induced stronger cell-mediated immune responses in local lymph nodes draining the oral cavity than two systemic immunizations with MVA-SIV. However, because animal groups were relatively small, a more detailed immunological study was subsequently performed with larger animal groups to confirm these initial results.
Immunogenicity of a VSV-SIV prime/MVA-SIV booster regimen compared to live-attenuated SIVmac1A11
Based on the results of the pilot study described above, a 2nd set of experiments was performed. A group of eight additional newborn macaques was vaccinated with the VSV-SIV vaccine orally at birth and received an intramuscular MVA-SIV vaccine at 2 weeks of age (Fig. 1, Group G). Although immune correlates of protection against virulent SIV are not known, we previously demonstrated that intravenous and mucosal SIVmac1A11 infection reduces viremia and prolongs survival after oral challenge with a high dose of SIVmac251 [10]. Thus, as comparison, we infected 8 newborn macaques (Figure 1, Group H) via both the oral and intravenous route with SIVmac1A11. In addition, 8 control animals (Figure 1, Group E) received mock immunizations (RPMI-1640 medium only) at week 0 (oral) and at week 2 (i.m). To control for vector-induced responses, the animals in Group F (Figure 1) received the respective non-SIV vectors, i.e. VSV-HA orally at birth, and MVA-MV intramuscularly at week 2. All animals were assessed for immune responses in blood and tissues at 4 weeks of age.
To monitor the general immunocompetence of the animals to mount antibody responses, all animals received hepatitis B immunization at 0 and 2 weeks of age. All animals had moderate to high levels of antibodies against hepatitis B surface antigen at 4 weeks of age (titers ranging from 1:1600 to 1:102,400; median 1:6400) with no significant difference among the 4 immunization arms (Kruskal Wallis: p=0.22).
Detection of vaccine virus and vector-specific immune responses
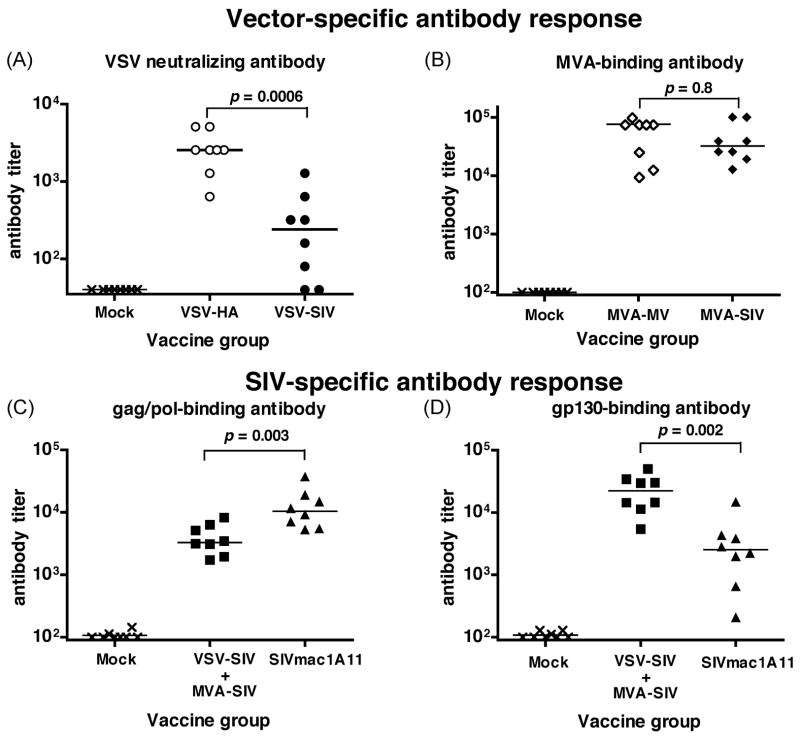
To attempt to confirm the live viral “vaccine-take”, animals in Group G were tested for the presence of VSV RNA in tissues, and animals in Group H for SIV RNA in plasma. All animals were tested for the development of antibody responses. For VSV-RNA testing, only two tissues, the cervical and submandibular lymph node, were chosen because these drain the oral site of inoculation. Although we did not detect VSV-RNA in any of the 8 VSV-SIV inoculated infant macaques (Group G) 4 weeks after immunization, all 8 VSV-HA immunized animals and 6 out of 8 VSV-SIV immunized animals had detectable VSV-neutralizing antibodies in their plasma at 4 weeks of age (Table 2; Figure 2A). Interestingly though, titers in the VSV-HA immunized animals (median 1:2,560) were significantly higher than the titers in the VSV-SIV animals (median 1:240; p=0.0006, Mann-Whitney test) suggesting that although similar infectious amounts (pfu) of virus were given, the VSV-SIV constructs may have replicated less efficiently after oral inoculation than the VSV-HA constructs. In contrast, no difference was observed in vaccinia-specific antibody responses by 4 weeks when animals in Group G (MVA-SIV) and F (MVA-MV) were compared (Table 2; Figure 2B; median titers of 32,200 and 76,000 respectively; p=0.8, Mann-Whitney test).
Table 2.
Experiment 2: summary of humoral immune responses at 4 weeks.
| Group (see Fig. 1) | Immunization | Animal number | Vector-specific immunity |
SIV-specific antibody in plasma |
SIV-specific antibody in saliva |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| VSV neutral. antibody titer1 | MVA- binding antibody titer1 | whole SIV IgG titer1 | gag/pol IgG (μg/ml)2 | gp130 IgG (μg/ml)2 | gag/pol IgA (ng/ml)2 | gp130 IgA (ng/ml)2 | Saliva gag/pol IgG (ng/ml)2 | Saliva gp130 IgG (ng/ml)2 | |||
| E. | Mock at 0 and 2 wks | 37093 | - | nd | - | - | - | - | - | - | - |
| 37106 | - | nd | - | - | - | - | - | - | - | ||
| 37147 | - | nd | - | - | - | - | - | - | - | ||
| 37151 | - | nd | - | - | - | - | - | - | - | ||
| 37303 | - | nd | - | - | - | - | - | - | - | ||
| 37304 | - | nd | - | - | - | - | - | - | - | ||
| 37371 | - | nd | - | - | - | - | - | - | - | ||
| 37375 | - | - | - | - | - | - | - | - | - | ||
| F. | VSV-HA oral at 0, MVA-MV IM at 2 wks | 37113 | 2,560 | 76,000 | - | - | - | - | - | - | - |
| 37117 | 1,280 | 100,000 | - | - | - | - | - | - | - | ||
| 37141 | 2,560 | 9,600 | - | - | - | - | - | - | - | ||
| 37142 | 2,560 | 12,800 | - | - | - | - | - | - | - | ||
| 37187 | 5,120 | 76,000 | - | - | - | - | - | - | - | ||
| 37188 | 640 | 25,600 | - | - | - | - | - | - | - | ||
| 37233 | 2,560 | 76,000 | - | - | - | - | - | - | - | ||
| 37239 | 5,120 | 76,000 | - | - | - | - | - | - | - | ||
| G. | VSV-SIV oral at 0; MVA-SIV IM at 2 wks | 37086 | 320 | 100,000 | 100 | 1.21 | 31.16 | - | 21.06 | - | 6.94 |
| 37089 | 160 | 12,800 | 100 | 1.94 | 18.68 | - | 27.33 | 0.47 | 3.66 | ||
| 37126 | 1,280 | 25,600 | 100 | 3.95 | 9.05 | 23.69 | - | 2.09 | 1.81 | ||
| 37128 | 80 | 38,800 | 100 | 3.20 | 18.63 | - | 17.12 | 0.18 | 2.63 | ||
| 37196 | - | 25,600 | - | 1.08 | 3.39 | - | - | 0.13 | 0.56 | ||
| 37198 | 320 | 19,200 | 100 | 5.16 | 21.38 | - | 11.89 | 0.43 | 1.16 | ||
| 37315 | 640 | 38,800 | 100 | 2.16 | 7.04 | - | 2.66 | na | na | ||
| 37320 | - | 100,000 | - | 1.97 | 9.02 | - | - | - | 1.45 | ||
| H. | SIVmac1A11 (oral + IV) at 0 and at 2 wks | 37055 | - | nd | 400 | 9.32 | 1.24 | - | - | 4.26 | - |
| 37062 | - | nd | 100 | 3.45 | 0.41 | - | - | 2.49 | 0.38 | ||
| 37075 | - | nd | 1,600 | 7.24 | 1.40 | - | - | 6.75 | - | ||
| 37076 | - | nd | 1,600 | 5.80 | 2.39 | - | - | 1.40 | 1.79 | ||
| 37254 | - | - | 1,600 | 4.44 | 0.13 | - | - | 1.05 | - | ||
| 37261 | - | nd | 1,600 | 23.54 | 9.28 | - | - | 8.73 | 1.05 | ||
| 37331 | - | nd | 400 | 3.31 | 1.77 | - | - | 1.30 | 0.53 | ||
| 37356 | - | nd | 1,600 | 11.94 | 2.71 | - | - | 1.96 | - | ||
Antibody titers are expressed as the reciprocal of the highest dilution that gave values above the cutoff of the ELISA assay.
Concentrations of antigen-specific IgA and IgG concentrations were interpolated from standard curves as described in materials and methods.
Minus sign indicates undetectable; na: not available.
Figure 2. Humoral immune responses.
Vector-specific antibody responses in the immunized groups (see Figure 1) were evaluated by measuring VSV neutralizing antibodies (graph A) or MVA-binding antibodies by ELISA (graph B). SIV-specific IgG was measured via SIV gag/pol or SIV gp130 env-specific ELISA s. All titers are endpoint titers (i.e. highest dilution above cut-off value of the respective assays). For each graph, p values refer to comparison of 2 groups via the Mann-Whitney test
Consistent with our observations in previous studies (i.e., peak viremia ≤ 7 days after high-dose intravenous SIVmac1A11 inoculation [10, 40, 41]), only 4 out of the 8 SIVmac1A11 infected animals had plasma SIV RNA levels above cut-off value (500/ml). Plasma viremia was detectable at 2 weeks post-infection in animals 37076 and 37356 (4324 and 1268 copies/ml, respectively) and at week 4 in 3 animals (37062, 37254, and 37356: 1310, 6796 and 1228 copies per ml, respectively). The detection of this SIVmac1A11 plasma viremia did not correlate with levels of SIV-specific antibodies or cell-mediated immune responses at 4 weeks of age (see below).
SIV-specific antibody responses in plasma and saliva
Plasma obtained at 4 weeks of age was tested by whole-virus antibody ELISA. Although 6 out of 8 VSV-SIV+MVA-SIV immunized animals had detectable SIV-specific antibodies, titers were at threshold value (1:100; Table 2). In contrast, SIVmac1A11-immunized animals had statistically significantly higher SIVmac251 binding antibody levels (median 1:1,600; p=0.001, Mann-Whitney test; Table 1). Subsequently, more sensitive ELISA s to measure antibodies against SIV gag/pol or gp130 envelope were applied. At 4 weeks of age, all VSV-SIV+MVA-SIV and all SIVmac1A11-immunized animals had mounted detectable antibody responses to gag/pol and gp130 in plasma (Fig. 2C-D). However, VSV-SIV+MVA-SIV immunized animals had significantly higher gp130-specific IgG concentrations, but significantly lower gag/pol-specific IgG concentrations in plasma than the SIVmac1A11-immunized group (Mann-Whitney test, two-tailed p=0.002 and p=0.003, respectively). It should be pointed out that there was no significant correlation between the plasma antibody titers against any of the vectors (MVA and VSV) and the concentration of SIV-specific anti-gag/pol and anti-env IgG in the animals of Group G (Pearson correlation test, all two-tailed p values > 0.26). Gag/pol and env-specific IgG in the saliva were low and reflected the patterns observed in the plasma, i.e. the VSV-SIV+MVA-SIV immunized animals had more env-specific IgG and less gag/pol-specific IgG than the SIVmac1A11 immunized animals (Mann Whitney test, p=0.004; table 2).
Low amounts of gag/pol- and gp130-specific IgA were detected in the plasma of 1 out of 8 and 5 out of 8 VSV-SIV+MVA-SIV immunized animals, respectively (Table 2); these 6 animals with gag/pol- or gp130-specific IgA in plasma were also the same 6 animals that had detectable VSV-neutralizing antibodies. In contrast, none of the SIVmac1A11-infected animals had detectable SIV-specific IgA in plasma. Thus, systemic IgA induction was dependent on the specific vaccine regimen. However, no SIV gag/pol- or gp130-specific IgA was detected in the saliva of any animal in Groups G or H (data not shown).
SIV-specific cell-mediated immune responses in VSV-SIV+MVA-SIV vaccinated animals
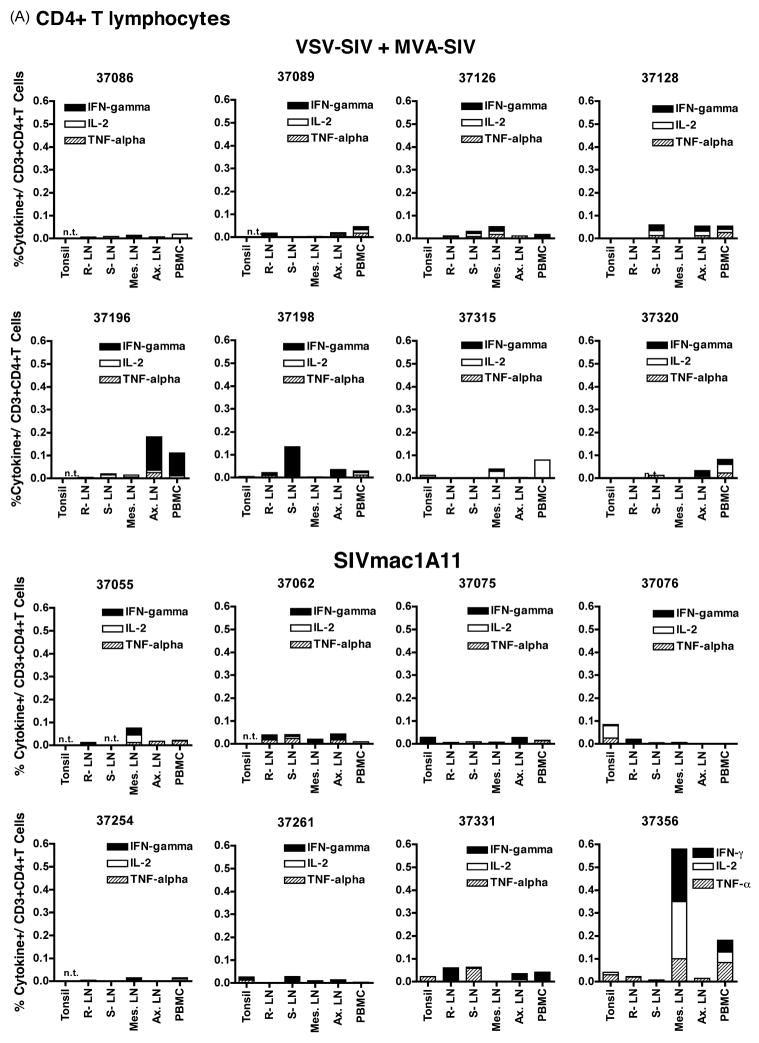
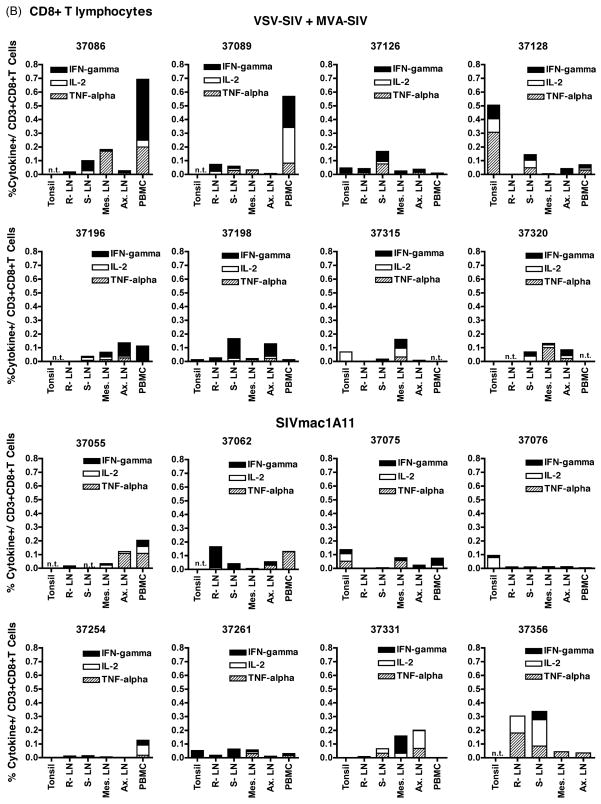
SIV-specific cell-mediated immune responses in PBMC and lymphoid tissues, collected at the time of euthanasia at 4 weeks of age, were measured by intracellular cytokine flow cytometry assays. SIV-specific cell-mediated immune responses were induced by both SIV vaccine regimens (VSV-SIV+ MVA-SIV; SIVmac1A11) in all animals. In general, SIV-specific immune responses were more pronounced in CD8+ T cells than in CD4+ T cells. There was no statistically significant difference in CD4+ or CD8+ T cell responses between the two SIV vaccine groups for the overall magnitude, quality, tissue location or proportion of animals with detectable T cell responses (Fig. 3–4). Furthermore, among animals of the same vaccine groups, there was no consistent pattern of a tissue-specific response pattern with regard to magnitude or cytokine profile (Fig. 3–4).
Figure 3. SIV-specific cell-mediated immune responses in CD4+ T and CD8+ lymphocytes.
In experiment 2, SIV specific immune responses in PBMC and lymphoid tissues were measured for the two SIV vaccine arms (groups G and H) via intra-cellular cytokine flow cytometry for interferon-γ, TNF-α and IL-2. Values are expressed as percentages of cytokine-expressing cells per CD4+CD3+ T lymphocytes (panel A) or CD8+CD3+ T lymphocytes (panel B) for the 2 vaccine groups. Abbreviations: LN: lymphnode; R.: retropharyngeal; S.: submandibular; Mes.: mesenteric; Ax.: axillary; n.t. indicates not tested.
Figure 4. Proportion of animals with SIV-specific cell-mediated immune responses.
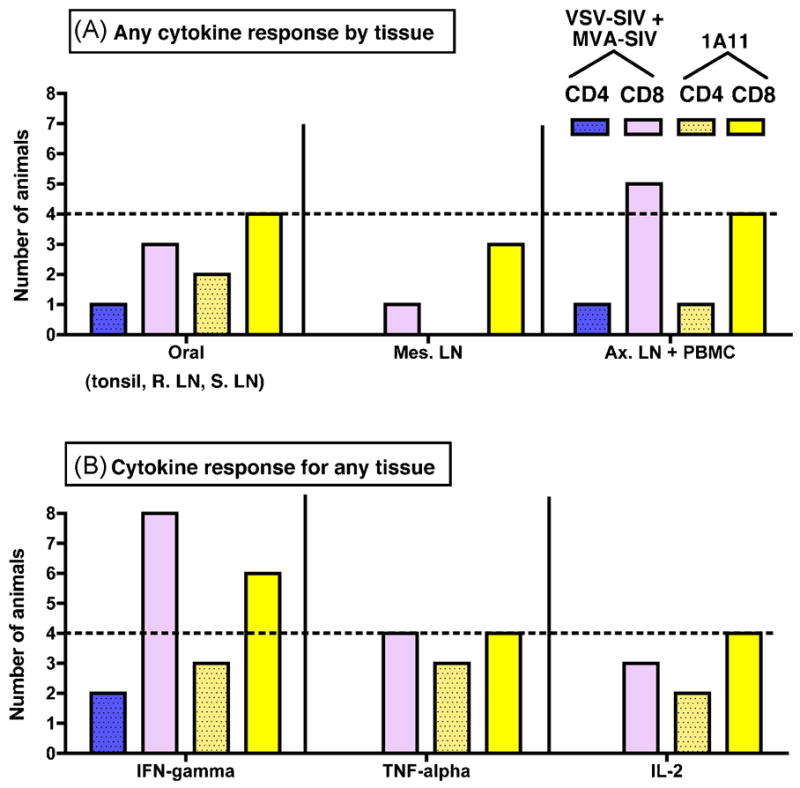
In graph A, using the data presented in Figure 3, the number of animals (out of a total of 8 animals for each of the 2 vaccine groups) with a detectable SIV-specific cell-mediated immune response for any of the 3 cytokines measured (IFN-γ, TNF-α and IL-2) was tabulated for 3 groups of tissues: the oral lymphoid tissues (tonsil, retropharyngeal lymph node (R. LN) and submandibular lymph node (S. LN)); Mesenteric LN; and the peripheral lymphoid system consisting of axillary lymph node (Ax. LN) and PBMC. In graph B, the number of animals with a detectable SIV-specific cell-mediated immune response in any of the tissues was tabulated for each of the 3 cytokines that were measured.
Still, the 3 animals with the strongest CD8+ cell-mediated responses to SIV gag antigens all belonged to the VSV-SIV+MVA-SIV vaccine group (animals 37086, 37089 and 37128; Fig. 4). It is further noteworthy that, although there was no apparent correlation between these cell-mediated immune response to SIV gag and their antibody response to gag/pol, these 3 animals were among the ones with the highest anti-gp130 IgG and IgA titers in plasma and saliva (Table 2). There were no other obvious correlations between the SIV-specific CD4+ and CD8+ T cell-mediated immune responses and antibody responses.
DISCUSSION
The experiments described in this report build on our previous observations in the infant macaque model to explore vaccine strategies that, if administered to the infant shortly after birth, would reduce the infant s risk of acquiring HIV infection from breast-feeding. The infant macaque model is especially appropriate for such studies because of its many similarities to HIV infection of human infants, including transmission, pathogenesis and immunology (reviewed in [11]). Previously we demonstrated that intramuscular administration of attenuated poxvirus-based SIV vaccines during the first 4 weeks after birth was partially effective when animals were exposed repeatedly at 4 weeks of age to low doses of virulent SIVmac251, because fewer animals became infected, and those that became infected had longer survival than unimmunized SIV-infected infant macaques [14]. These results, while already predictive of and promising for human vaccine trials with poxvirus-based HIV vaccines (e.g., RV 144 in adults and HPTN 027 in infants, respectively) underscore the need to explore strategies aimed at even higher efficacy [42].
Studies in infant macaques have demonstrated that following oral inoculation, SIV rapidly disseminates systemically [15, 43, 44]. Early immune responses at mucosal entry sites were dominated by the induction of proinflammatory cytokines, and low or no effective antiviral responses [15]. Accordingly, we hypothesized that an HIV vaccine to prevent infection after oral exposure will only be most effective if it elicits local antiviral immune responses strong enough to halt virus replication at the site of viral entry and in the locally draining lymphoid organs, thus preventing it from reaching the systemic circulation. We also hypothesized that induction of such local immune responses may be more feasible through immunization with replicating SIV vaccines that can be administered orally and have a good safety profile, such as recombinant VSV. However, as has been demonstrated in some SIV vaccine efficacy studies in older macaques [22, 45], it is also possible that SIV vaccines could promote proinflammatory immune responses in infant macaques that might diminish or abrogate vaccine-mediated protection.
The vaccines that were used in the current study, VSV-SIV, MVA-SIV and SIVmac1A11, have previously also been tested in juvenile or adult macaques in a variety of regimens, and were found to be immunogenic in regimens that consisted generally of a prime immunization followed 4 to 8 weeks later by a booster immunization [19, 24, 46–52]. However, any comparison of the immunogenicity data of the current infant study with those observed in older animals has to be done with the caveat that (i) we used an accelerated vaccine regimen (i.e., prime with 2 weeks later booster immunization) in an attempt to induce much-needed protection early after birth, and (ii), different assays (tetramer assessment, Elispot, multicolor flow cytometry) with sometimes different in vitro stimuli (antigens expressed by recombinant vaccinia virus versus SIV peptide pools) were used to assess SIV-specific T-cell responses. To our knowledge, there have been no SIV vaccine studies in juvenile or adult macaques comparing SIV-specific immune responses elicited by typical and accelerated immunization schedules.
In the current report, the first experiment demonstrated that an oral immunization regimen of VSV-SIV alone had low SIV-specific immunogenicity, even in animals in which the detection of VSV-specific immune responses indicated sufficient replication of the vector. However, an oral VSV-SIV prime immunization followed by an intramuscular MVA-SIV booster immunization gave higher humoral and particularly cell-mediated SIV-specific immune responses at 4 weeks of age than either vaccine candidate by itself. These results are consistent with VSV-SIV studies in juvenile and adult macaques, where the combination of VSV-SIV with other SIV vaccines (e.g., plasmid DNA, MVA) showed higher immunogenicity and antiviral efficacy after SHIV89.6P challenge than VSV-SIV alone [18, 19, 53]. But in contrast to older animals, infant macaques immunized with VSV-SIV + MVA-SIV regimens, developed lower levels of SIV-specific T cell responses [19].
In the second set of experiments, more sensitive immune assays that can utilize the small sample volumes of newborn macaques were used to measure humoral and cell-mediated immune responses in 4-weeks old infant macaques that had received a VSV-SIV prime and MVA-SIV booster immunization shortly after birth and 2 weeks of age, respectively. Immune responses were compared with those in infant macaques that had received SIVmac1A11, because SIVmac1A11 had proven vaccine efficacy in the infant macaque model [10, 20], and because live-attenuated vaccines induce a broader range of immune responses that most likely include the beneficial immune responses needed to control virulent infections.
Both vaccine regimens induced SIV-specific humoral immune responses, but there were qualitative differences in antigen specificity. SIVmac1A11 induced more gag/pol-binding antibodies, while the VSV-SIV +MVA-SIV regimen induced more envelope-binding antibodies. Without more data on the functionality of these vaccine-induced antibodies, it is unclear which pattern is expected to be most beneficial in vivo. The available knowledge indicates that the desired profile may also depend on whether the goal is prevention of infection or delay of disease progression in already infected individuals. Previous studies in SIV-infected macaques and HIV-infected humans found that higher titers and the persistence of gag-specific antibodies correlated better with slower disease progression than envelope-specific antibodies [54–57]. However, the potential value of envelope-specific antibodies has been demonstrated in studies where some envelope-specific monoclonal antibodies protected infant macaques against oral SHIV infection [58].
In the current study, because antibody binding titers were still relatively low at the time of the early euthanasia, we did not assess the in vitro antiviral functions of the antibodies. In previous studies of SIVmac1A11 and MVA-SIV vaccines, we have shown that immunized infant rhesus macaques do not develop neutralizing antibodies against the pathogenic challenge isolate, SIVmac251 before oral exposure to this virus [59]. In studies of unimmunized infant macaques that were passively immunized with SIV-specific antibodies (elicited by vaccinating older macaques), we found that only high titer SIV-binding antibodies exhibited detectable ADCVI (antibody dependent cellular virus inhibition) activity [60] or ADCC activity [61].
In the current study, both vaccine regimens also induced detectable SIV-specific IgG in the saliva; however, as their concentrations were much lower than in plasma and had a similar pattern of antigen-specificity, it is unclear whether this detection of IgG represents transudation from the plasma or local synthesis of antibodies. As postulated previously in passive immunization studies, SIV-specific IgG in the saliva, even if merely a transudate, may play a role in protecting against oral infection [62]. Antiviral IgA antibodies were not detected in the saliva of the infant macaques; however, this observation is not surprising because total IgA levels in saliva of infant macaques are very low during the first months of life (these authors, unpublished data).
Both vaccine regimens also induced SIV-specific cell-mediated immune responses in the local lymphoid tissues that drain the oral cavity as well as in systemic lymphoid tissues. This is the first study to demonstrate vaccine-mediated SIV-specific T cell responses in such tissues as well as in peripheral blood of infant primates within the first weeks of life. While all animals had at least one detectable cell-mediated response in one local or systemic tissue, there was much individual variability in frequency, cytokine-specificity and tissue distribution. As we have reported for other vaccines [14], all VSV-SIV + MVA-SIV -vaccinated infant macaques in this study had lower SIV-specific T cell responses than older macaques given the same vaccines [19]. Although we administered the vaccines orally at birth, there was no preferential induction of SIV-specific immune responses in the lymphoid tissues that drain the oral cavity in comparison to the peripheral tissues. This observation is consistent with the relative low replicative capacity of both orally administered vaccines, SIVmac1A11 and VSV-SIV, in macaques [10, 18, 19]. However, because the currently available in vitro immunological assays have so far not identified clear correlates of protection against virus infection or immunodeficiency disease in SIV and HIV vaccine studies, we cannot extrapolate reliably from the immunological data observed in the current study to predicted efficacy against oral infection with virulent SIV.
In conclusion, the safety and immunogenicity data from the accelerated VSV-SIV + MVA-SIV immunization regimen described in this report provide the scientific incentive to proceed to the next essential step: exploring the efficacy of this vaccine regimen against virulent SIV exposure in the infant macaque model.
Acknowledgments
We thank the Colony Services, Pathology, Veterinary, and Clinical Laboratory staff of the California National Primate Research Center for expert technical assistance; Jeffrey Americo for preparation and characterization of recombinant MVA s and for performing vaccine virus ELISA s; the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH for providing the SIV p55 gag protein and the 15mer and 20mer peptides of the p27 gag region; Dr. R. Desrosiers (New England Regional Primate Research Center) for the plasmid containing the SIVmac239 env gene; This work was supported by grants R01-AI062518 (MLM) and grant RR-00169 from the National Center for Research Resources (NCRR; a component of the National Institutes of Health (NIH)) to the California National Primate Research Center. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.John-Stewart G, Mbori-Ngacha D, Ekpini R, Janoff EN, Nkengasong J, Read JS, et al. Breast-feeding and Transmission of HIV-1. J Acquir Immune Defic Syndr. 2004;35(2):196–202. doi: 10.1097/00126334-200402010-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson JB, Musoke P, Fleming T, Guay LA, Bagenda D, Allen M, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003;362(9387):859–68. doi: 10.1016/S0140-6736(03)14341-3. [DOI] [PubMed] [Google Scholar]
- 3.The Breastfeeding and HIV International Transmission Study (BHITS) Group. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis. 2004;189:2154–66. doi: 10.1086/420834. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn L, Aldrovandi GM, Sinkala M, Kankasa C, Semrau K, Mwiya M, et al. Effects of early, abrupt weaning on HIV-free survival of children in Zambia. N Engl J Med. 2008;359(2):130–41. doi: 10.1056/NEJMoa073788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shetty AK, Coovadia HM, Mirochnick MM, Maldonado Y, Mofenson LM, Eshleman SH, et al. Safety and trough concentrations of nevirapine prophylaxis given daily, twice weekly, or weekly in breast-feeding infants from birth to 6 months. J Acquired Immune Defic Syndr. 2003;34(5):482–90. doi: 10.1097/00126334-200312150-00006. [DOI] [PubMed] [Google Scholar]
- 6.Safrit JT, Ruprecht R, Ferrantelli F, Xu W, Kitabwalla M, Van Rompay K, et al. Immunoprophylaxis to Prevent Mother to Child Transmission of HIV-1. J Acquired Immune Defic Syndr. 2004;35(2):169–77. doi: 10.1097/00126334-200402010-00012. [DOI] [PubMed] [Google Scholar]
- 7.Van Rompay KK. Evaluation of antiretrovirals in animal models of HIV infection. Antiviral Res. 2009 Jul 19; doi: 10.1016/j.antiviral.2009.07.008. In press. [DOI] [PubMed] [Google Scholar]
- 8.Van Rompay KKA, Cherrington JM, Marthas ML, Berardi CJ, Mulato AS, Spinner A, et al. 9-[2-(Phosphonomethoxy)propyl]adenine therapy of established simian immunodeficiency virus infection in infant rhesus macaques. Antimicrob Agents Chemother. 1996;40(11):2586–91. doi: 10.1128/aac.40.11.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Rompay KKA, McChesney MB, Aguirre NL, Schmidt KA, Bischofberger N, Marthas ML. Two low doses of tenofovir protect newborn macaques against oral simian immunodeficiency virus infection. J Infect Dis. 2001;184(4):429–38. doi: 10.1086/322781. [DOI] [PubMed] [Google Scholar]
- 10.Van Rompay KKA, Greenier JL, Cole KS, Earl P, Moss B, Steckbeck JD, et al. Immunization of newborn rhesus macaques with simian immunodeficiency virus (SIV) vaccines prolongs survival after oral challenge with virulent SIVmac251. J Virol. 2003;77:179–90. doi: 10.1128/JVI.77.1.179-190.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abel K. The rhesus macaque pediatric SIV infection model - a valuable tool in understanding infant HIV-1 pathogenesis and for designing pediatric HIV-1 prevention strategies. Curr HIV Res. 2009;7(1):2–11. doi: 10.2174/157016209787048528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaspan HB, Lawn SD, Safrit JT, Bekker LG. The maturing immune system: implications for development and testing HIV-1 vaccines for children and adolescents. Aids. 2006;20(4):483–94. doi: 10.1097/01.aids.0000210602.40267.60. [DOI] [PubMed] [Google Scholar]
- 13.Jaspan HB, Hanekom WA. Immunology of infants through adolescents: responses to emulate for HIV vaccines. Current opinion in HIV and AIDS. 2007;2(5):391–8. doi: 10.1097/COH.0b013e3282e1c633. [DOI] [PubMed] [Google Scholar]
- 14.Van Rompay KKA, Abel K, Lawson JR, Singh RP, Schmidt KA, Evans T, et al. Attenuated poxvirus-based SIV vaccines given in infancy partially protect infant and juvenile macaques against repeated oral challenge with virulent SIV. J Acquired Immune Defic Syndr. 2005;38(2):124–34. doi: 10.1097/00126334-200502010-00002. [DOI] [PubMed] [Google Scholar]
- 15.Abel K, Pahar B, Van Rompay KK, Fritts L, Sin C, Schmidt K, et al. Rapid virus dissemination in infant macaques after oral simian immunodeficiency virus exposure in the presence of local innate immune responses. J Virol. 2006;80(13):6357–67. doi: 10.1128/JVI.02240-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke DK, Cooper D, Egan MA, Hendry RM, Parks CL, Udem SA. Recombinant vesicular stomatitis virus as an HIV-1 vaccine vector. Springer Semin Immunopathol. 2006;28(3):239–53. doi: 10.1007/s00281-006-0042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egan MA, Chong SY, Rose NF, Megati S, Lopez KJ, Schadeck EB, et al. Immunogenicity of attenuated vesicular stomatitis virus vectors expressing HIV type 1 Env and SIV Gag proteins: comparison of intranasal and intramuscular vaccination routes. AIDS Res Hum Retroviruses. 2004;20(9):989–1004. doi: 10.1089/aid.2004.20.989. [DOI] [PubMed] [Google Scholar]
- 18.Rose NF, Marx PA, Luckay A, Nixon DF, Moretto WJ, Donahoe SM, et al. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell. 2001;106(5):539–49. doi: 10.1016/s0092-8674(01)00482-2. [DOI] [PubMed] [Google Scholar]
- 19.Ramsburg E, Rose NF, Marx PA, Mefford M, Nixon DF, Moretto WJ, et al. Highly effective control of an AIDS virus challenge in macaques by using vesicular stomatitis virus and modified vaccinia virus Ankara vaccine vectors in a single-boost protocol. J Virol. 2004;78(8):3930–40. doi: 10.1128/JVI.78.8.3930-3940.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otsyula MG, Miller CJ, Tarantal AF, Marthas ML, Greene TP, Collins JR, et al. Fetal or neonatal infection with attenuated simian immunodeficiency virus results in protective immunity against oral challenge with pathogenic SIVmac251. Virology. 1996;222:275–8. doi: 10.1006/viro.1996.0420. [DOI] [PubMed] [Google Scholar]
- 21.Marthas ML, Van Rompay KKA, Otsyula M, Miller CJ, Canfield D, Pedersen NC, et al. Viral factors determine progression to AIDS in simian immunodeficiency virus-infected newborn rhesus macaques. J Virol. 1995;69(7):4198–205. doi: 10.1128/jvi.69.7.4198-4205.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abel K, Compton L, Rourke T, Montefiori D, Lu D, Rothaeusler K, et al. Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses. J Virol. 2003;77(5):3099–118. doi: 10.1128/JVI.77.5.3099-3118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson RP, Lifson JD, Czajak SC, Cole KS, Manson KH, Glickman R, et al. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: inverse relationship of degree of protection with level of attenuation. J Virol. 1999;73(6):4952–61. doi: 10.1128/jvi.73.6.4952-4961.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lohman BL, McChesney MB, Miller CJ, McGowan E, Joye SM, Van Rompay KKA, et al. A partially attenuated simian immunodeficiency virus induces host immunity that correlates with resistance to pathogenic virus challenge. J Virol. 1994;68(11):7021–9. doi: 10.1128/jvi.68.11.7021-7029.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Research Council. Guide for the care and use of laboratory animals. Washington, D. C: National Academy Press; 1996. [Google Scholar]
- 26.Sackett GP, Ruppenthal GC, Davis AE. Survival, growth, health, and reproduction following nursery rearing compared with mother rearing in pigtailed monkeys (Macaca nemestrina) Am J Primatol. 2002;56(3):165–83. doi: 10.1002/ajp.1072. [DOI] [PubMed] [Google Scholar]
- 27.Van Rompay KKA, Brignolo LL, Meyer DJ, Jerome C, Tarara R, Spinner A, et al. Biological effects of short-term and prolonged administration of 9-[2-(phosphonomethoxy)propyl]adenine (PMPA; tenofovir) to newborn and infant rhesus macaques. Antimicrob Agents Chemother. 2004;48:1469–87. doi: 10.1128/AAC.48.5.1469-1487.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts A, Kretzschmar E, Perkins AS, Forman J, Price R, Buonocore L, et al. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J Virol. 1998;72(6):4704–11. doi: 10.1128/jvi.72.6.4704-4711.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Earl PL, Wyatt LS, Montefiori DC, Bilska M, Woodward R, Markham PD, et al. Comparison of vaccine strategies using recombinant env-gag-pol MVA with or without an oligomeric env protein boost in the SHIV rhesus macaque model. Virology. 2002;294:270–81. doi: 10.1006/viro.2001.1345. [DOI] [PubMed] [Google Scholar]
- 30.Zhu Y, Rota P, Wyatt L, Tamin A, Rozenblatt S, Lerche N, et al. Evaluation of recombinant vaccinia virus-measles vaccines in infant rhesus macaques with preexisting measles antibody. Virology. 2000;276:202–13. doi: 10.1006/viro.2000.0564. [DOI] [PubMed] [Google Scholar]
- 31.Van Rompay KK, Johnson JA, Blackwood EJ, Singh RP, Lipscomb J, Matthews TB, et al. Sequential emergence and clinical implications of viral mutants with K70E and K65R mutation in reverse transcriptase during prolonged tenofovir monotherapy in rhesus macaques with chronic RT-SHIV infection. Retrovirology. 2007;4(1):25. doi: 10.1186/1742-4690-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozlowski PA, Lynch RM, Patterson RR, Cu-Uvin S, Flanigan TP, Neutra MR. Modified wick method using Weck-Cel sponges for collection of human rectal secretions and analysis of mucosal HIV antibody. J Acquir Immune Defic Syndr. 2000;24(4):297–309. doi: 10.1097/00126334-200008010-00001. [DOI] [PubMed] [Google Scholar]
- 33.Shacklett BL, Cox CA, Sandberg JK, Stollman NH, Jacobson MA, Nixon DF. Trafficking of human immunodeficiency virus type 1-specific CD8+ T cells to gut-associated lymphoid tissue during chronic infection. J Virol. 2003;77(10):5621–31. doi: 10.1128/JVI.77.10.5621-5631.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Rompay KKA, Singh RP, Pahar B, Sodora DL, Wingfield C, Lawson JR, et al. CD8+ cell-mediated suppression of virulent simian immunodeficiency virus during tenofovir treatment. J Virol. 2004;78:5324–37. doi: 10.1128/JVI.78.10.5324-5337.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon ID, Publicover J, Rose JK. Replication and propagation of attenuated vesicular stomatitis virus vectors in vivo: vector spread correlates with induction of immune responses and persistence of genomic RNA. J Virol. 2007;81(4):2078–82. doi: 10.1128/JVI.02525-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manrique M, Kozlowski PA, Wang S-W, Wilson RL, Micewicz E, Montefiori DC, et al. Nasal DNA-MVA SIV vaccination provides more significant protection from progression to AIDS than a similar intramuscular vaccination. Mucosal Immunology. 2009;2(6):536–50. doi: 10.1038/mi.2009.103. [DOI] [PubMed] [Google Scholar]
- 37.Wang SW, Kozlowski PA, Schmelz G, Manson K, Wyand MS, Glickman R, et al. Effective induction of simian immunodeficiency virus-specific systemic and mucosal immune responses in primates by vaccination with proviral DNA producing intact but noninfectious virions. J Virol. 2000;74(22):10514–22. doi: 10.1128/jvi.74.22.10514-10522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Rompay KKA, Marthas ML, Lifson JD, Berardi CJ, Vasquez GM, Agatep E, et al. Administration of 9-[2-(phosphonomethoxy)propyl]adenine (PMPA) for prevention of perinatal simian immunodeficiency virus infection in rhesus macaques. AIDS Res Hum Retroviruses. 1998;14(9):761–73. doi: 10.1089/aid.1998.14.761. [DOI] [PubMed] [Google Scholar]
- 39.Pahar B, Li J, Rourke T, Miller CJ, McChesney MB. Detection of antigen-specific T cell interferon-γ expression by ELISPOT and cytokine flow cytometry assays in rhesus macaques. J Immunol Methods. 2003;282:103–15. doi: 10.1016/j.jim.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Miller CJ, Marthas M, Greenier J, Lu D, Dailey P, Lu Y. In vivo replication capacity rather than in vitro macrophage tropism predicts efficiency of vaginal transmission of simian immunodeficiency virus or simian/human immunodeficiency virus in rhesus macaques. J Virol. 1998;72(4):3248–58. doi: 10.1128/jvi.72.4.3248-3258.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Rompay KK, Blackwood EJ, Landucci G, Forthal D, Marthas ML. Role of CD8+ Cells in controlling replication of nonpathogenic simian immunodeficiency virus SIVmac1A11. Virol J. 2006;3(1):22. doi: 10.1186/1743-422X-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N Engl J Med. 2009 Oct 20; doi: 10.1056/NEJMoa0908492. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43.Van Rompay KKA, Dailey PJ, Tarara RP, Canfield DR, Aguirre NL, Cherrington JM, et al. Early short-term 9-[2-(phosphonomethoxy)propyl]adenine (PMPA) treatment favorably alters subsequent disease course in simian immunodeficiency virus-infected newborn rhesus macaques. J Virol. 1999;73(4):2947–55. doi: 10.1128/jvi.73.4.2947-2955.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Rompay KKA, Singh RP, Heneine W, Johnson JA, Montefiori DC, Bischofberger N, et al. Structured treatment interruptions with tenofovir monotherapy for simian immunodeficiency virus-infected newborn macaques. J Virol. 2006;80(13):6399–410. doi: 10.1128/JVI.02308-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abel K, La Franco-Scheuch L, Rourke T, Ma ZM, De Silva V, Fallert B, et al. Gamma interferon-mediated inflammation is associated with lack of protection from intravaginal simian immunodeficiency virus SIVmac239 challenge in simian-human immunodeficiency virus 89.6-immunized rhesus macaques. J Virol. 2004;78(2):841–54. doi: 10.1128/JVI.78.2.841-854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marthas ML, Sutjipto S, Higgins J, Lohman B, Torten J, Luciw PA, et al. Immunization with a live, attenuated simian immunodeficiency virus (SIV) prevents early disease but not infection in rhesus macaques challenged with pathogenic SIV. J Virol. 1990;64(8):3694–700. doi: 10.1128/jvi.64.8.3694-3700.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marthas ML, Miller CJ, Sutjipto S, Higgins J, Torten J, Lohman BL, Unger RE, Ramos RA, Kiyono H, McGhee JR, Marx PA, Pedersen NC. Efficacy of live-attenuated and whole-inactivated simian immunodeficiency virus vaccines against vaginal challenge with virulent SIV. J Med Primatol. 1992;21:99–107. [PubMed] [Google Scholar]
- 48.Marthas ML, Ramos RA, Lohman BL, Van Rompay KKA, Unger RE, Miller CJ, et al. Viral determinants of simian immunodeficiency virus (SIV) virulence in rhesus macaques assessed by using attenuated and pathogenic molecular clones of SIVmac. J Virol. 1993;67(10):6047–55. doi: 10.1128/jvi.67.10.6047-6055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirsch VM, Fuerst TR, Sutter G, Carroll MW, Yang LC, Goldstein S, et al. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: Effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J Virol. 1996;70(6):3741–52. doi: 10.1128/jvi.70.6.3741-3752.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seth A, Ourmanov I, Schmitz JE, Kuroda MJ, Lifton MA, Nickerson CE, et al. Immunization with a modified vaccinia virus expressing simian immunodeficiency virus (SIV) gag-pol primes for an anamnestic gag-specific cytotoxic T-lymphocyte response and is associated with reduction of viremia after SIV challenge. J Virol. 2000;74:2502–9. doi: 10.1128/jvi.74.6.2502-2509.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shiver JW, Fu T-M, Chen L, Casimiro DR, Davies M-E, Evans RK, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency virus immunity. Nature. 2002;415:331–5. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 52.Robinson HL, Amara RR. T cell vaccines for microbial infections. Nat Med. 2005;11(4 Suppl):S25–32. doi: 10.1038/nm1212. [DOI] [PubMed] [Google Scholar]
- 53.Egan MA, Chong SY, Megati S, Montefiori DC, Rose NF, Boyer JD, et al. Priming with plasmid DNAs expressing interleukin-12 and simian immunodeficiency virus gag enhances the immunogenicity and efficacy of an experimental AIDS vaccine based on recombinant vesicular stomatitis virus. AIDS Res Hum Retroviruses. 2005;21(7):629–43. doi: 10.1089/aid.2005.21.629. [DOI] [PubMed] [Google Scholar]
- 54.Zhang JY, Martin LN, Watson EA, Montelaro RC, West M, Epstein L, et al. Simian immunodeficiency virus/delta-induced immunodeficiency disease in rhesus monkeys: relation of antibody response and antigenemia. J Infect Dis. 1988;158(6):1277–86. doi: 10.1093/infdis/158.6.1277. [DOI] [PubMed] [Google Scholar]
- 55.Hogervorst E, Jurriaans S, de Wolf F, van Wijk A, Wiersma A, Valk M, et al. Predictors for non- and slow progression in human immunodeficiency virus (HIV) type 1 infection: low viral RNA copy numbers in serum and maintenance of high HIV-1 p24-specific but not V3-specific antibody levels. J Infect Dis. 1995;171(4):811–21. doi: 10.1093/infdis/171.4.811. [DOI] [PubMed] [Google Scholar]
- 56.Cheingsong-Popov R, Panagiotidi C, Bowcock S, Aronstam A, Wadsworth J, Weber J. Relation between humoral responses to HIV gag and env proteins at seroconversion and clinical outcome of HIV infection. Br Med J. 1991;302(6767):23–6. doi: 10.1136/bmj.302.6767.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Binley JM, Klasse PJ, Cao Y, Jones I, Markowitz M, Ho DD, et al. Differential regulation of the antibody response to gag and env proteins of human immunodeficiency virus type 1. J Virol. 1997;71(4):2799–809. doi: 10.1128/jvi.71.4.2799-2809.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferrantelli F, Rasmussen RA, Buckley KA, Li PL, Wang T, Montefiori DC, et al. Complete protection of neonatal rhesus macaques against oral exposure to pathogenic simian-human immunodeficiency virus by human anti-HIV monoclonal antibodies. J Infect Dis. 2004;189(12):2167–73. doi: 10.1086/420833. [DOI] [PubMed] [Google Scholar]
- 59.Greenier JL, Van Rompay KKA, Montefiori D, Earl P, Moss B, Marthas ML. Simian immunodeficiency virus (SIV) envelope quasispecies transmission and evolution in infant rhesus macaques after oral challenge with uncloned SIVmac251: increased diversity is associated with neutralizing antibodies and improved survival in previously immunized animals. Virology Journal. 2005;2:11. doi: 10.1186/1743-422X-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Forthal DN, Landucci G, Cole KS, Marthas M, Becerra JC, Van Rompay K. Rhesus macaque polyclonal and monoclonal antibodies inhibit simian immunodeficiency virus in the presence of human or autologous rhesus effector cells. J Virol. 2006;80(18):9217–25. doi: 10.1128/JVI.02746-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Florese RH, Van Rompay KK, Aldrich K, Forthal DN, Landucci G, Mahalanabis M, et al. Evaluation of passively transferred, nonneutralizing antibody-dependent cellular cytotoxicity-mediating IgG in protection of neonatal rhesus macaques against oral SIVmac251 challenge. J Immunol. 2006;177(6):4028–36. doi: 10.4049/jimmunol.177.6.4028. [DOI] [PubMed] [Google Scholar]
- 62.Van Rompay KKA, Berardi CJ, Dillard-Telm S, Tarara RP, Canfield DR, Valverde CR, et al. Passive immunization of newborn rhesus macaques prevents oral simian immunodeficiency virus infection. J Infect Dis. 1998;177(5):1247–59. doi: 10.1086/515270. [DOI] [PubMed] [Google Scholar]