Abstract
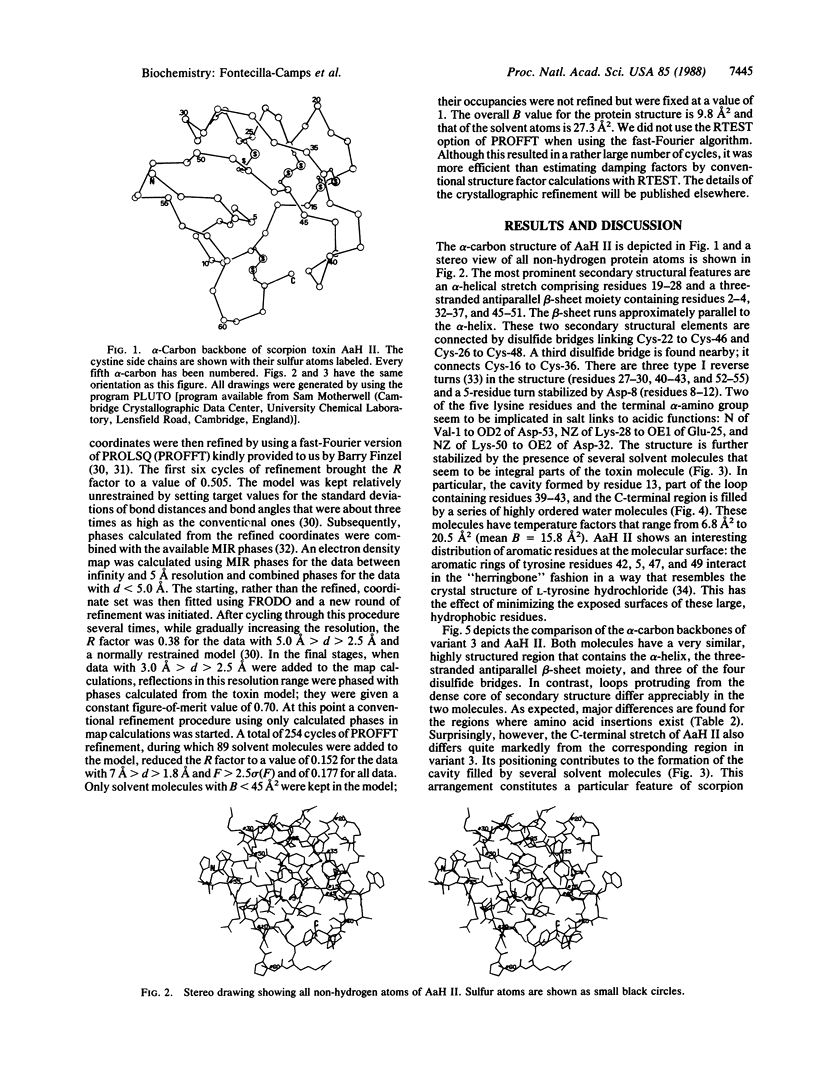
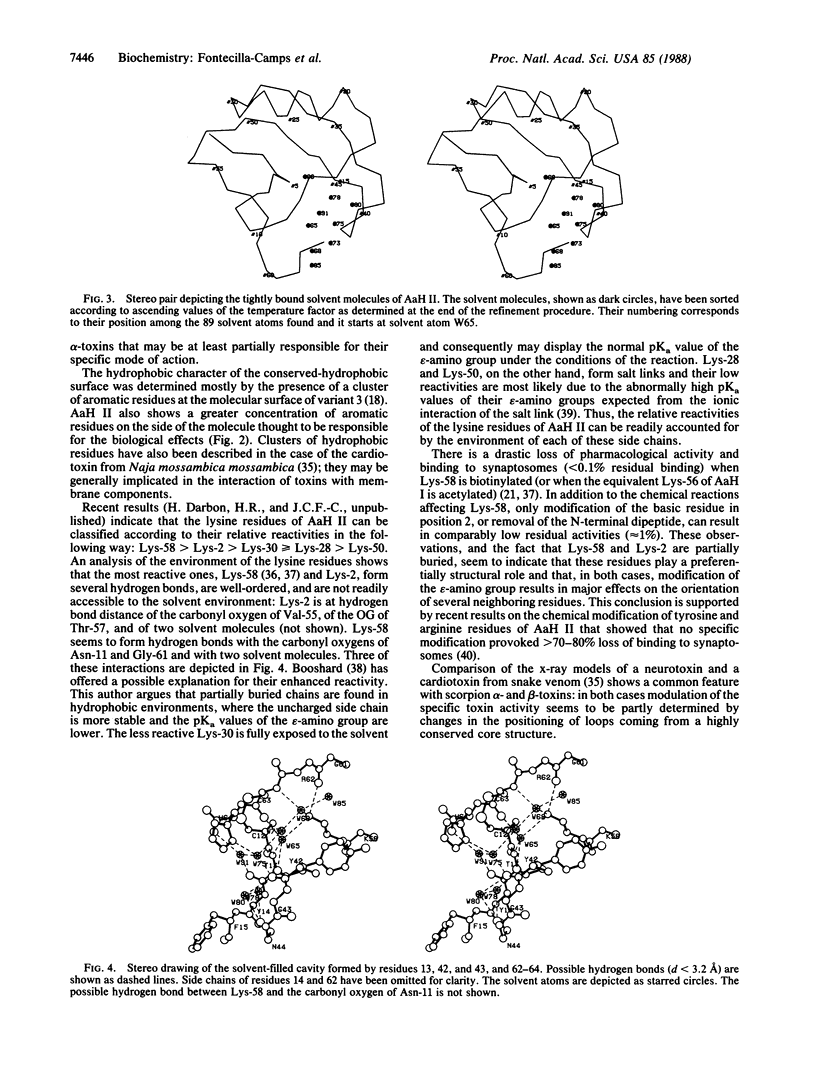
Orthorhombic crystals (space group P212121, a = 45.94 A, b = 40.68 A, c = 29.93 A) of the potent scorpion alpha-toxin II from Androctonus australis Hector were grown using sterile techniques. The structure was solved by a combination of heavy-atom and model phasing. Subsequently, it was refined at 1.8 A resolution by a fast-Fourier restrained least-squares procedure. The crystallographic R factor is 0.152 for data with 7.0 A greater than d greater than 1.8 A and F greater than 2.5 sigma (F) and 0.177 when all data are considered. Eighty-nine solvent molecules have been incorporated into the model. The dense core formed by the alpha-helical and antiparallel beta-sheet moieties and three of the four disulfide bridges is similar in variant 3, a toxin purified from the North American scorpion Centruroides sculpturatus, and in toxin II. However, the two molecules differ markedly in the orientation of loops protruding from the core. Toxin II seems to contain several highly ordered solvent molecules. Eight of them occupy a cavity consisting of the C-terminal region and a loop found only in scorpion alpha-toxins. The highly reactive and pharmacologically important Lys-58 is found at one of the extremes of this cavity, where it establishes a series of hydrogen bonds with protein and solvent atoms. The reactivities of the five lysine residues of toxin II are highly correlated with the formation of hydrogen bonds, hydrophobic interactions, and salt links.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almassy R. J., Fontecilla-Camps J. C., Suddath F. L., Bugg C. E. Structure of variant-3 scorpion neurotoxin from Centruroides sculpturatus Ewing, refined at 1.8 A resolution. J Mol Biol. 1983 Oct 25;170(2):497–527. doi: 10.1016/s0022-2836(83)80159-4. [DOI] [PubMed] [Google Scholar]
- Angelides K. J., Nutter T. J. Molecular and cellular mapping of the voltage-dependent na channel. Biophys J. 1984 Jan;45(1):31–34. doi: 10.1016/S0006-3495(84)84096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babin D. R., Watt D. D., Goos S. M., Mlejnek R. V. Amino acid sequence of neurotoxin I from Centruroides sculpturatus Ewing. Arch Biochem Biophys. 1975 Jan;166(1):125–134. doi: 10.1016/0003-9861(75)90371-9. [DOI] [PubMed] [Google Scholar]
- Barhanin J., Schmid A., Lombet A., Wheeler K. P., Lazdunski M., Ellory J. C. Molecular size of different neurotoxin receptors on the voltage-sensitive Na+ channel. J Biol Chem. 1983 Jan 25;258(2):700–702. [PubMed] [Google Scholar]
- Beneski D. A., Catterall W. A. Covalent labeling of protein components of the sodium channel with a photoactivable derivative of scorpion toxin. Proc Natl Acad Sci U S A. 1980 Jan;77(1):639–643. doi: 10.1073/pnas.77.1.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosshard H. R. Mapping of contact areas in protein-nucleic acid and protein-protein complexes by differential chemical modification. Methods Biochem Anal. 1979;25:273–301. doi: 10.1002/9780470110454.ch4. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Membrane potential-dependent binding of scorpion toxin to the action potential Na+ ionophore. Studies with a toxin derivative prepared by lactoperoxidase-catalyzed iodination. J Biol Chem. 1977 Dec 10;252(23):8660–8668. [PubMed] [Google Scholar]
- Couraud F., Jover E., Dubois J. M., Rochat H. Two types of scorpion receptor sites, one related to the activation, the other to the inactivation of the action potential sodium channel. Toxicon. 1982;20(1):9–16. doi: 10.1016/0041-0101(82)90138-6. [DOI] [PubMed] [Google Scholar]
- Darbon H., Angelides K. J. Structural mapping of the voltage-dependent sodium channel. Distance between the tetrodotoxin and Centruroides suffusus suffusus II beta-scorpion toxin receptors. J Biol Chem. 1984 May 25;259(10):6074–6084. [PubMed] [Google Scholar]
- Darbon H., Jover E., Couraud F., Rochat H. Photoaffinity labeling of alpha- and beta- scorpion toxin receptors associated with rat brain sodium channel. Biochem Biophys Res Commun. 1983 Sep 15;115(2):415–422. doi: 10.1016/s0006-291x(83)80160-0. [DOI] [PubMed] [Google Scholar]
- Darbon H., Zlotkin E., Kopeyan C., van Rietschoten J., Rochat H. Covalent structure of the insect toxin of the North African scorpion Androctonus australis Hector. Int J Pept Protein Res. 1982 Oct;20(4):320–330. doi: 10.1111/j.1399-3011.1982.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Fontecilla-Camps J. C., Almassy R. J., Suddath F. L., Watt D. D., Bugg C. E. Three-dimensional structure of a protein from scorpion venom: a new structural class of neurotoxins. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6496–6500. doi: 10.1073/pnas.77.11.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habersetzer-Rochat C., Sampieri F. Structure-function relationships of scorpion neurotoxins. Biochemistry. 1976 Jun 1;15(11):2254–2261. doi: 10.1021/bi00656a002. [DOI] [PubMed] [Google Scholar]
- Hendrickson W. A. Stereochemically restrained refinement of macromolecular structures. Methods Enzymol. 1985;115:252–270. doi: 10.1016/0076-6879(85)15021-4. [DOI] [PubMed] [Google Scholar]
- Jover E., Bablito J., Couraud F. Binding of beta-scorpion toxin: a physicochemical study. Biochemistry. 1984 Mar 13;23(6):1147–1152. doi: 10.1021/bi00301a018. [DOI] [PubMed] [Google Scholar]
- Jover E., Martin-Moutot N., Couraud F., Rochat H. Binding of scorpion toxins to rat brain synaptosomal fraction. Effects of membrane potential, ions, and other neurotoxins. Biochemistry. 1980 Feb 5;19(3):463–467. doi: 10.1021/bi00544a010. [DOI] [PubMed] [Google Scholar]
- Kaplan H., Stevenson K. J., Hartley B. S. Competitive labelling, a method for determining the reactivity of individual groups in proteins. The amino groups of porcine elastase. Biochem J. 1971 Sep;124(2):289–299. doi: 10.1042/bj1240289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda F., Kupeyan C., Rochat H., Rochat C., Lissitzky S. Purification of animal neurotoxins. Isolation and characterization of eleven neurotoxins from the venoms of the scorpions Androctonus australis hector, Buthus occitanus tunetanus and Leiurus quinquestriatus quinquestriatus. Eur J Biochem. 1970 Nov;16(3):514–523. doi: 10.1111/j.1432-1033.1970.tb01111.x. [DOI] [PubMed] [Google Scholar]
- Rees B., Samama J. P., Thierry J. C., Gilibert M., Fischer J., Schweitz H., Lazdunski M., Moras D. Crystal structure of a snake venom cardiotoxin. Proc Natl Acad Sci U S A. 1987 May;84(10):3132–3136. doi: 10.1073/pnas.84.10.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. S. The anatomy and taxonomy of protein structure. Adv Protein Chem. 1981;34:167–339. doi: 10.1016/s0065-3233(08)60520-3. [DOI] [PubMed] [Google Scholar]
- Sampieri F., Habersetzer-Rochat C., Astier J. P., Frey M., Haser R. Preliminary x-ray diffraction studies on a scorpion neurotoxin: toxin II of Androctonus australis Hector. J Mol Biol. 1978 Dec 5;126(2):289–291. doi: 10.1016/0022-2836(78)90364-9. [DOI] [PubMed] [Google Scholar]
- Sampieri F., Habersetzer-Rochat C. Structure-function relationships in scorpion neurotoxins. Identification of the supperreactive lysine residue in toxin I of Androctonus australis Hector. Biochim Biophys Acta. 1978 Jul 21;535(1):100–109. doi: 10.1016/0005-2795(78)90037-5. [DOI] [PubMed] [Google Scholar]
- Wang G. K., Strichartz G. Simultaneous modifications of sodium channel gating by two scorpion toxins. Biophys J. 1982 Nov;40(2):175–179. doi: 10.1016/S0006-3495(82)84473-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler K. P., Watt D. D., Lazdunski M. Classification of Na channel receptors specific for various scorpion toxins. Pflugers Arch. 1983 Apr;397(2):164–165. doi: 10.1007/BF00582058. [DOI] [PubMed] [Google Scholar]
- Zlotkin E., Rochat H., Kopeyan, Miranda F., Lissitzky S. Purification and properties of the insect toxin from the venom of the scorpion Androctonus australis Hector. Biochimie. 1971;53(10):1073–1078. doi: 10.1016/s0300-9084(71)80195-5. [DOI] [PubMed] [Google Scholar]
- el Ayeb M., Bahraoui E. M., Granier C., Rochat H. Use of antibodies specific to defined regions of scorpion alpha-toxin to study its interaction with its receptor site on the sodium channel. Biochemistry. 1986 Oct 21;25(21):6671–6678. doi: 10.1021/bi00369a052. [DOI] [PubMed] [Google Scholar]
- el Ayeb M., Darbon H., Bahraoui E. M., Vargas O., Rochat H. Differential effects of defined chemical modifications on antigenic and pharmacological activities of scorpion alpha and beta toxins. Eur J Biochem. 1986 Mar 3;155(2):289–294. doi: 10.1111/j.1432-1033.1986.tb09488.x. [DOI] [PubMed] [Google Scholar]


