Abstract
Orexin (or hypocretin) has been implicated in mediating drug addiction and reward. Here, we investigated orexin's contribution to morphine-induced behavioral sensitization and place preference. Orexin -/- (OKO) mice and littermate wild-type (WT) controls (n= 56) and C57BL/6J mice (n=67) were tested for chronic morphine-induced locomotor sensitization or for conditioned place preference (CPP) for a morphine- or a cocaine-paired environment. C57BL/6J mice received the orexin receptor 1 (Ox1r) antagonist, SB-334867, prior to test sessions. OKO mice did not significantly differ from WT controls in locomotor activity following acute- or chronic-morphine treatments. Similarly, mice treated with the Ox1r antagonist did not differ from vehicle controls in locomotor activity following acute- or chronic-morphine treatments. In contrast, while OKO mice did not differ from WT controls in preference for a morphine-paired environment, the Ox1r antagonist significantly attenuated place preference for a morphine-, but not a cocaine-paired, environment. These data suggest that orexin action is not required for locomotor responses to acute and chronic morphine, but Ox1r signaling can influence morphine-seeking in WT animals.
Keywords: Orexin, hypocretin, morphine, opiate, cocaine, sensitization, conditioned place preference
1. Introduction
Orexin- (or hypocretin-) containing neurons in the lateral hypothalamus (LH) project widely throughout the brain (Date et al., 1999; Nambu et al., 1999; Peyron et al., 1998). The orexin receptors, Ox1r and Ox2r, are located in multiple brain regions, including the ventral tegmental area (VTA) and nucleus accumbens (Acb) (Marcus et al., 2001), that are known to regulate drug addiction and reward processes. Behavioral analysis also supports a role for orexin in different components of drug addiction. We have shown that a mutation of the orexin gene (Georgescu et al., 2003) and blockade of Ox1r (Sharf et al., 2008) result in attenuated somatic withdrawal symptoms in morphine-dependent mice. Evidence suggests that orexin is also involved in drug reward and reinstatement. Preference for an environment previously paired with morphine, cocaine, or food is positively correlated with activation of orexin-producing neurons in the LH (Harris et al., 2005). Intra-VTA blockade of Ox1r dose-dependently reduced morphine-induced conditioned place preference (CPP) in rats and genetic deletion of orexin resulted in an attenuation of morphine-induced CPP (Narita et al., 2006). Orexin is also important for stress-mediated reinstatement of cocaine seeking (Boutrel et al., 2005). These results suggest a role for orexin in mediating dependence and behavioral plasticity that occurs in the absence of the actual drugs (i.e. during states of withdrawal or seeking).
Studies of locomotor activity also implicate orexin in response to both acute and chronic drug exposure. Orexin -/- (OKO) mice have been reported to display reduced locomotor activity in response to acute morphine (Narita et al., 2006) and intra-VTA Ox1r blockade attenuated the development, but not the expression, of cocaine sensitization (Borgland et al., 2006). It is, however, not known if orexin mediates sensitization to other drugs of abuse, such as morphine.
Here, we report that OKO animals display normal morphine sensitization, and in contrast to previous reports (Narita et al., 2006), normal locomotor response and CPP to morphine. The Ox1r antagonist also failed to block locomotor responses to morphine, but did attenuate morphine, but not cocaine, CPP. Together these data demonstrate that orexin may not be necessary for morphine-induced hyperlocomotion, but that altered orexin signaling can nonetheless modify drug-context associations.
2. Results
Orexin -/- mice display normal acute locomotor and sensitization responses to morphine
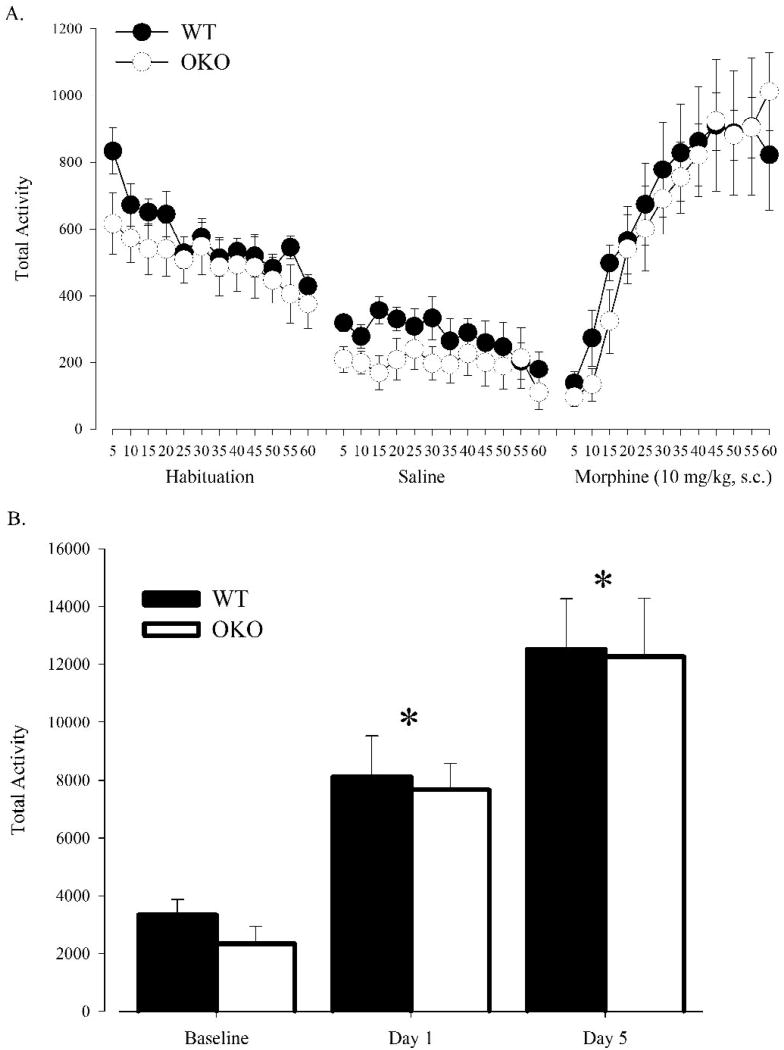
Locomotor activity was assessed during a one hour habituation session, during which naïve OKO mice and WT controls were first expose to the locomotor chambers. After habituation, animals received a saline injection and locomotor activity was measured for one hour. Following the saline session, animals received a morphine injection (10 mg/kg, s.c.) and locomotor activity was again assessed for one hour. Surprisingly, OKO mice did not differ from WT controls in locomotor activity during any of these phases (Fig. 1a). Repeated morphine treatments and locomotor tests were then conducted to determine if orexin was required for sensitization. Locomotor activity following the fifth morphine injection was significantly greater than that following the first morphine injection (Session effect, F(2, 28) = 33.57, P<0.0001; Day 1 vs. Day 5, P<0.002 by Tukey's LSD), suggesting the development of locomotor sensitization (Fig. 1b). However, there was no significant effect of genotype nor a significant interaction between genotype and session indicating normal sensitization in orexin mutant mice.
Figure 1. Orexin -/- mice (n=8) display normal acute locomotor and sensitization responses to morphine compared to WT controls (n=8).
(A) Activity measured in 5 minute intervals during habituation, saline, and morphine treatment sessions. (B) Activity following saline, first morphine, and fifth morphine treatment session. Vertical lines represent the standard error of the mean (SEM). * indicates significant differences from all other sessions.
Pharmacological blockade of Ox1r fails to affect acute locomotor and sensitization responses to morphine
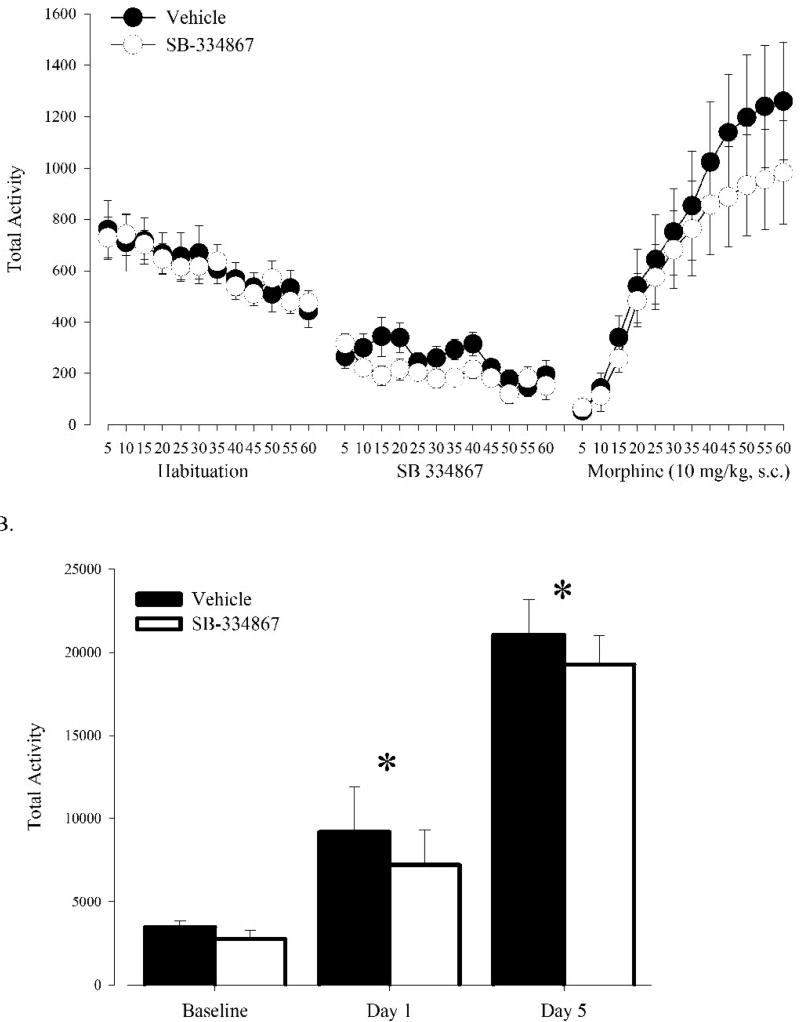
To complement the mutant studies, the Ox1r antagonist SB-334867 was administered to naïve C57BL/6J mice prior to each 10 mg/kg morphine treatment. Overall, total activity counts for OKO mice and their littermate WT controls were lower when compared to the C57BL/6J mice used the pharmacological experiment. This reduced general locomotion may be the result of age differences among the cohorts as it has previously been reported that age can contribute to locomotor activity differences (Ingram et al., 1981). SB-334867 did not affect locomotor activity compared to saline and had no affect on acute hyperlocomotion to morphine (Fig 2a), as confirmed by statistical analysis between SB-334867 and saline treated animals (F(1,11) = 0.34, P=0.57). While sensitization was clear (Session effect, F(2, 22) = 47.78, P<0.0001; Day 1 vs. Day 5, P< 0.0001 by Tukey's LSD), there were no significant SB-334867 or interaction effects (Fig 2b). This suggests that, in normal animals, Ox1r blockade during morphine exposure does not influence motor response or the development of sensitization.
Figure 2. Pharmacological blockade of Ox1r in mice treated with SB-334867 (n=7) fails to affect acute locomotor and sensitization responses to morphine (10 mg/kg, s.c.) compared to mice treated with vehicle (n=6).
(A) Activity measured in 5 minute intervals during habituation, saline, and morphine treatment sessions. (B) Activity following saline, first morphine, and fifth morphine treatment session. Vertical lines represent the standard error of the mean (SEM). * indicates significant differences from all other sessions.
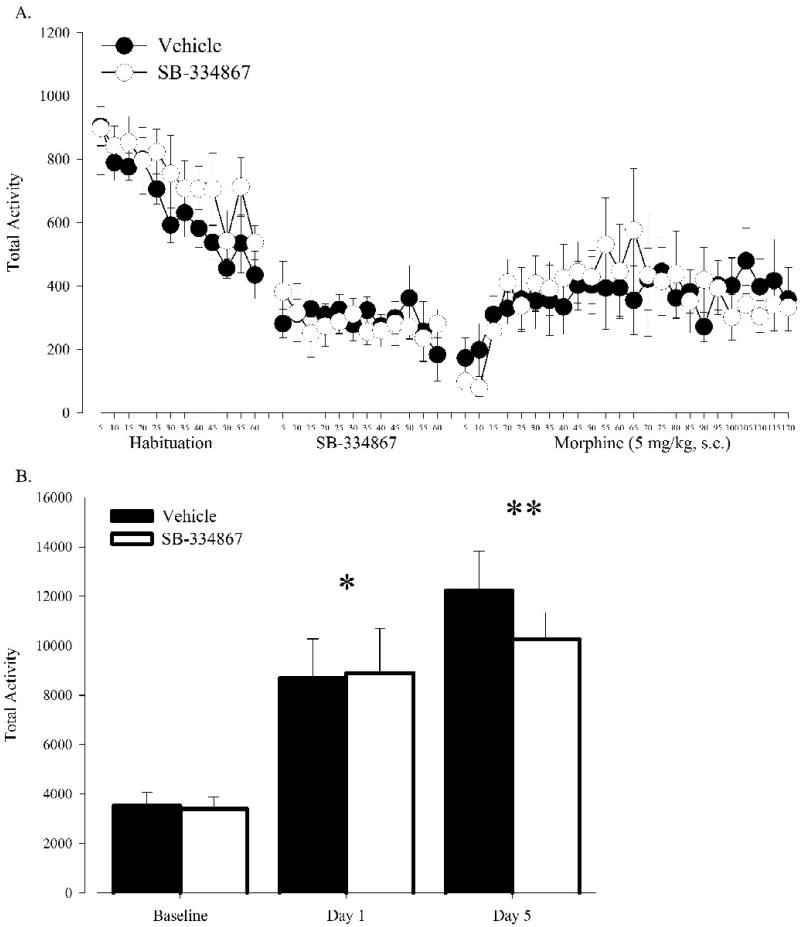
To further ascertain that orexin does not contribute to morphine-induced locomotor responses, a separate group of C57BL/6J mice underwent locomotor analysis with 5mg/kg morphine injections and morphine-induced locomotor responses were recorded for two hours. As seen with the higher morphine dose, SB-334867 failed to effect acute morphine induced hyperlocomotion (Fig 3a). Although morphine significantly enhanced locomotion on day 1 (F(2,20)=23.44, P<0.000), locomotor activity on day 5 was not significantly different than on day 1 (P<.09), suggesting that animals did not sensitize to the lower morphine dose (Fig 3b). However, no significant interactions were found, thus further supporting that orexin is not required for morphine-induced locomotion at this lower dose.
Figure 3. Pharmacological blockade of Ox1r in mice treated with SB-334867 (n=6) fails to affect acute locomotor and sensitization responses to morphine (5 mg/kg, s.c.) compared to mice treated with vehicle (n=6).
(A) Activity measured in 5 minute intervals during habituation, saline, and morphine treatment sessions. (B) Activity following saline, first morphine, and fifth morphine treatment session. Vertical lines represent the standard error of the mean (SEM). * indicates significant differences from all other sessions. ** indicates significance from baseline only.
Pharmacological blockade of Ox1r, but not genetic mutation of the orexin gene, attenuates morphine CPP
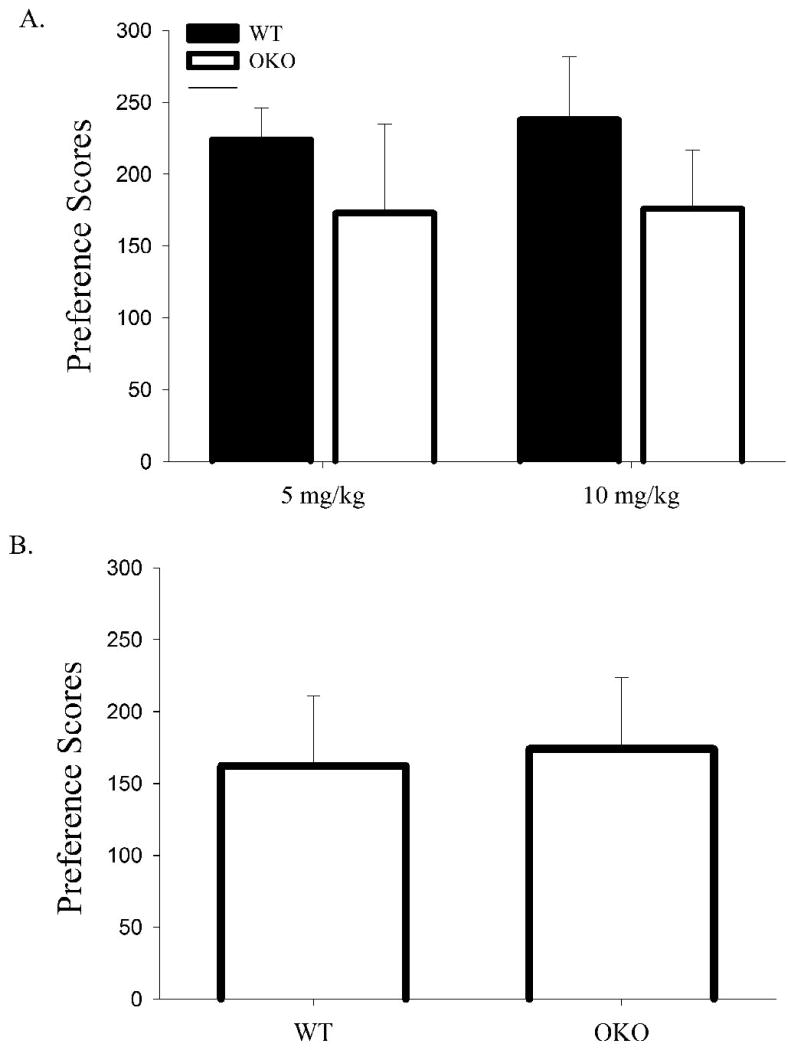
Based on previous reports on the role of orexin in morphine responsiveness (Narita et al., 2006), the minimal effects of genetic and pharmacological treatments on locomotor responses were unexpected. To explore further orexin-morphine interactions, Pavlovian reward responses to morphine were assessed using the conditioned place preference (CPP) paradigm. Here, animals were placed in an enclosed environment divided into two chambers, each containing distinctive environmental cues. During the habituation session, animals were allowed to freely explore both chambers. On days 1, 3 and 5 animals received an injection of saline or morphine (5 or 10 mg/kg, s.c.) and were confined to one of the chambers. On days 2, 4, and 6, mice received the alternate drug treatment and were confined to the other chamber. On the test day, animals were once again allowed to freely roam among the chambers and the time spent in each was recorded. Male OKO and WT mice showed statistically comparable preferences for an environment previously paired with either a 5 mg/kg or 10 mg/kg dose of morphine (Fig. 4a, Table 1). We also tested female OKO mice and their WT controls for the expression of morphine CPP to the higher morphine dose and again found no behavioral differences based on genotype (Fig. 4b). Furthermore, no significant gender differences were observed (Fig. 4a-b).
Figure 4. Genetic mutation of the orexin gene fails to affect preference for an environment previously paired with morphine.
Difference in time spent in the drug-paired environment compared with time spent in the unpaired environment (paired minus unpaired) for (A) male OKO mice (n=14) and WT controls (n=14) and (B) female OKO mice (n=6) and WT controls (n=6) conditioned with morphine. Vertical lines represent the standard error of the mean (SEM).
Table 1.
Time spent in drug-paired and saline-paired chambers and final preference scores during conditioned place preference test session. Scores are divided by experimental groups.
| Group | Treatment | Paired | Unpaired | Preference |
|---|---|---|---|---|
| WT (males) | Morphine (5 mg/kg) | 440.0 (±12.9) | 215.8 (±10.7) | 224.2 (±21.8) |
| WT (males) | Morphine (10 mg/kg) | 445.9 (±24.5) | 207.8 (±19.5) | 238.5 (±43.8) |
| OKO (males) | Morphine (5 mg/kg) | 424.2 (±39.3) | 220.2 (±33.5) | 204.0 (±67.8) |
| OKO (males) | Morphine (10 mg/kg) | 423.6 (±22.1) | 247.5 (±22.9) | 176.2 (±40.4) |
| WT (females) | Morphine (10 mg/kg) | 405.0 (±37.8) | 243.0 (±13.9) | 162.0 (±48.9) |
| OKO (females) | Morphine (10 mg/kg) | 365.9 (±35.7) | 191.9 (±24.4) | 174.0 (±49.5) |
| C57BL/6J (Veh) | Morphine (10 mg/kg) | 404.0 (±22.9) | 202.6 (±18.4) | 201.4 (±31.5) |
| C57BL/6J (SB) | Morphine (10 mg/kg) | 343.0 (±18.4) | 226.6 (±15.3) | 116.4 (±19.0) |
| C57BL/6J (Veh) | Cocaine (15 mg/kg) | 419.4 (±31.8) | 202.5 (±12.4) | 216.9 (±35.4) |
| C57BL/6J (SB) | Cocaine (15 mg/kg) | 443.8 (±36.7) | 198.7 (±35.2) | 245.2 (±68.1) |
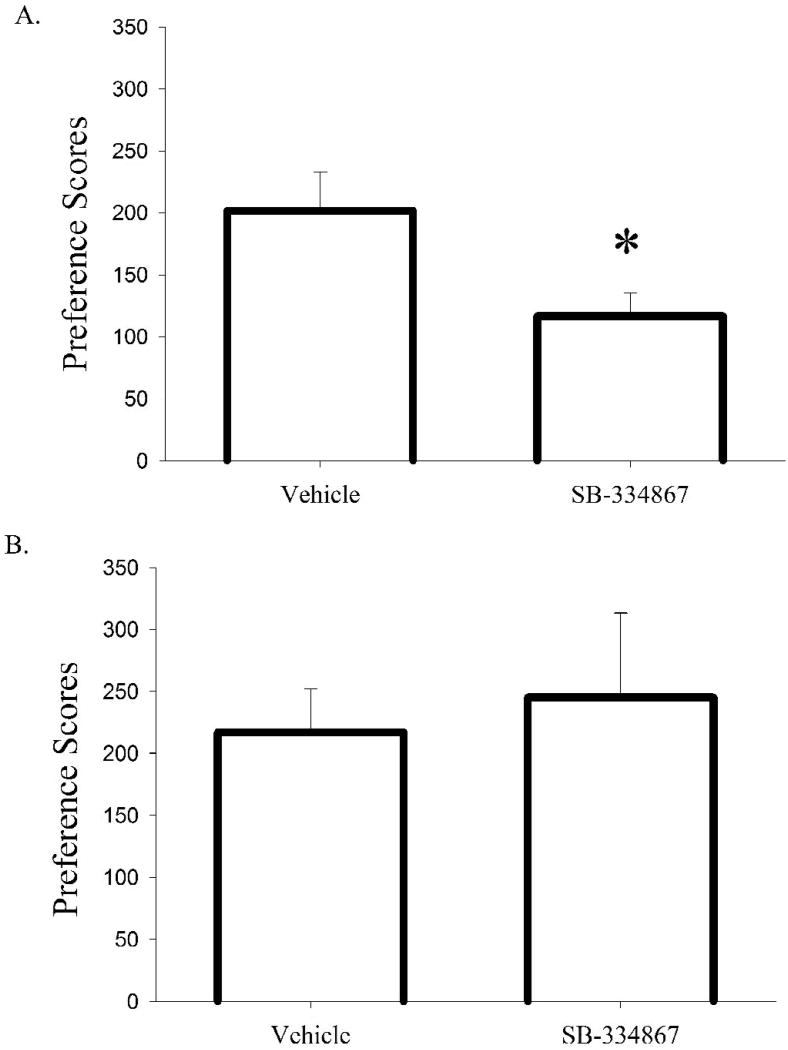
In contrast, C57BL/6J mice treated with SB-334867 demonstrated significantly reduced preference for a chamber previously paired with morphine (t(24) = 2.30, P<0.03; Fig. 5a). However, SB-334867 failed to alter CPP to cocaine (Fig. 5b), suggesting that SB-334867's effects are restricted to Pavlovian responses to opiates, and may not play a role in conditioned responses to psychostimulants.
Figure 5. Pharmacological blockade of Ox1r attenuates preference for an environment previously paired with morphine, but not cocaine.
Difference in time spent in the drug-paired environment compared with time spent in the unpaired environment (paired minus unpaired) for (A) SB-334867 treated mice (n=13) and vehicle treated controls (n=13) conditioned with morphine, and (B) SB-334867 treated mice (n=8) and vehicle treated controls (n=8) conditioned with cocaine. Vertical lines represent the standard error of the mean (SEM). * represents a significant SB-334867 effect on preference for drug-paired side (P<0.03).
3. Discussion
The present study investigates orexin's contribution to morphine locomotor sensitization and CPP. Surprisingly, we found that in response to acute morphine, OKO mice do not differ from WT controls in locomotor activity. Similarly, both genotypes displayed normal sensitization in response to chronic morphine administration. Pharmacological blockade of Ox1r also failed to affect acute morphine-induced locomotor enhancement and locomotor sensitization. Together, these data suggest that orexin does not play a role in locomotor responses to morphine, in contrast to previously published data (Narita et al., 2006).
As previous reports suggest a role for orexin in morphine locomotor enhancement (Narita et al., 2006) and Pavlovian responses (Harris et al., 2005; Harris et al., 2007; Narita et al., 2006), we also tested for the expression of CPP in OKO mice and mice treated with an Ox1r antagonist. While OKO mice again demonstrated a morphine-induced CPP comparable to WT controls, pharmacological blockade of Ox1r significantly attenuated preference for an environment previously paired with morphine. These data are consistent with previous reports that Ox1r blockade attenuates the expression of place preference for morphine (Harris et al., 2005), and that coincident unilateral lesions of LH orexin neurons and contralateral intra-VTA Ox1r pharmacological blockade block the development of morphine-induced CPP (Harris et al., 2007).
The current data suggest that the Ox1r is not involved in expression of place preference to a cocaine-paired environment. While Ox1r has been implicated in cocaine sensitization (Borgland et al., 2006), the role of Ox1r in cocaine CPP has not yet been reported. It has been demonstrated that preference for an environment previously paired with cocaine is correlated with enhanced c-Fos expression in the LH (Harris et al., 2005), and it might have been predicted that SB-334867 would affect the expression of cocaine CPP. It is possible that Ox1r plays a more important role during conditioning. Moreover, since SB-334867 has a higher binding affinity and ∼50-fold selectivity for the Ox1r over the Ox2r (Duxon et al., 2001), it is also possible that orexin action via the Ox2r contributes to cocaine CPP. Chronic cocaine has been associated with increased Ox2r in the Acb (Zhang et al., 2007) whereas such a change following chronic morphine administration has yet to be investigated. Further investigation into the role of Ox2r in cocaine CPP is warranted and is dependent upon the use of selective Ox2r pharmacological agents. A possible mechanism of action may be that activation of Ox2r at the level of VTA enhances glutamatergic transmission (Borgland et al., 2008).
Orexin's role in the expression of morphine-, but not cocaine, CPP is notable and may be explained by the different mode of action of the drugs. While both increase dopamine (DA) in the Acb (Carboni et al., 1989; Di Chiara and Imperato, 1988; Imperato and Di Chiara, 1986), cocaine acts by blocking the DA re-uptake transporter (Ritz et al., 1987) whereas morphine enhances DA transmission by inhibiting VTA GABAergic interneurons that would normally inhibit DAergic neurons (Gysling and Wang, 1983). This differential primary mode of action of the two drugs likely produces distinct cellular and molecular mechanisms of long-term plasticity. While both drugs are known to engage the VTA, the current CPP data suggest that orexin in more critical for morphine's effects in the drug-free state. Additionally, while orexin cells may be activated during expression of cocaine CPP, as previously reported by c-Fos analysis (Harris et al., 2005), but the functionality of such activation may not be directly related to the expression of cocaine place preference. In contrast to the CPP data, the current work suggests a more minimal role for Ox1r in the development of morphine sensitization, whereas Ox1r has been implicated in cocaine sensitization (Borgland et al., 2006). This could be due to the models used (rat vs. mouse) in the studies or may simply reflect a more direct role in cocaine sensitization processes. Again, while the VTA plays an integral role in sensitization to both psychostimulants (Henry et al., 1989) and opiates (Kalivas and Duffy, 1987), the differential mode of action of cocaine and morphine may be relevant. Orexin excites both DA- and GABA-containing neurons in the VTA (Korotkova et al., 2003; Nakamura et al., 2000), raising the possibility of orexin's interactions with morphine take place in both dopamine and non-dopamine neurons.
The differences between data reported here and data previously published (Narita et al., 2006) are difficult to explain. While both studies use male mice, we also evaluated female OKO animals and found no differences in CPP or locomotor response to morphine. In addition to genotyping, the orexin knockout in our cohorts was confirmed via immunodetection and no orexin was detected in OKO mice (data not shown). There are, however, some differences in procedures where our conditioning sessions are for 30 minutes whereas Narita et al. used 60 minute pairings. It is also unclear whether previous experiments were conducted during the light cycle. It has been previously demonstrated that OKO mice display bouts of behavioral arrests during the dark (Chemelli et al., 1999), which would contribute to decreased activity and altered behavioral responses.
The contrasting data obtained from the OKO and the Ox1r antagonist treated mice suggests that some developmental compensatory mechanism may exist in OKO mice, such that the relevant function served by Ox1r stimulation is acquired by another neural and/or molecular mechanism in mice lacking orexin. This is often seen in knockout animals and these data are not the first to suggest behavioral differences due to Ox1r blockade and genetic manipulations of the orexin system. We have previously reported that in response to morphine withdrawal both OKO mice and SB-334867 treated mice display attenuated somatic withdrawal symptoms; however, different symptoms were affected. For instance, OKO mice, but not SB-334867 treated animals, displayed significant reductions in jumps whereas SB-334867 treated animals displayed significant reductions in wet dog shakes, which were normal in OKO mice (Georgescu et al., 2003; Sharf et al., 2008). Furthermore, ongoing experiments in our laboratory suggest that food reward and food-related learning is regulated differently in OKO mice and SB-334867 treated mice (Sharf et al., Submitted).
Together, these data suggest that while morphine-induced hyperlocomotion or sensitization can occur in the absence of orexin, Ox1r can modulate morphine-seeking in WT animals when the drug is no longer available (e.g. during the CPP test). A role for orexin, and Ox1r, in the expression of somatic morphine withdrawal symptoms has been demonstrated (Georgescu et al., 2003; Sharf et al., 2008; Zhou et al., 2006). Moreover, previous work with Ox1r blockade (Harris et al., 2005) and the current CPP data suggest a role for Ox1r in drug-abstinent states. Here, we suggest that orexin may contribute to opiate-induced neural plasticity, which becomes evident following cessation of drug-treatment, such as during withdrawal or drug-seeking. Orexin function may not directly affect morphine-induced locomotor responses, but once morphine is no longer present, morphine withdrawal and place preference depend on orexin action on Ox1r. This may be consistent with a general role for orexin in activating or coordinating behavior (Aston-Jones et al., 2009; Sharf et al., 2009; Sutcliffe and De Lecea, 2002; Willie et al., 2001), which includes withdrawal or drug-seeking behaviors. More recent evidence further suggests that orexin signaling via the Ox1r contributes to attentional performance (Boschen et al., 2009), which could include attentive responses to environmental cues previously associated with morphine.
4. Experimental Procedure
Subjects
Subjects were male C57BL/6J mice (n=67), between 10-12 weeks of age, and male (n=44) and female (n=12) pre-pro-orexin knock out (OKO) mice and wild-type (WT) controls, between 16-24 weeks of age, that have been backcrossed to C57BL/6J for six generations(Chemelli et al., 1999). OKO mice and their littermate WT controls were bred and housed in groups of three to five. C57BL/6J mice were obtained from Jackson Laboratories and housed in groups of five. Female OKO mice were tested only for morphine-induced place preference. All mice were maintained on a 12:12 hour light:dark cycle (7am-7pm). Experimental sessions occurred during the light phase as OKO mice exhibit behavioral arrests similar to narcoleptic episodes during the dark phase, but fail to display any overt abnormalities during the light cycle (Chemelli et al., 1999). All animal procedures were approved by the Yale Animal Care and Use Committee.
Locomotor Activity
Locomotor activity was monitored in standard cages (17″× 8.5″× 8″) in chambers equipped with infrared photocells. Locomotor activity was measured by consecutive beam breaks in 5 minute intervals using MicroPro software (Columbus, OH, USA). To habituate animals, mice were placed in the locomotor chambers for one hour. Following habituation, OKO mice were injected with saline and C57BL/6J mice were injected with SB-334867 or vehicle and returned to the locomotor chambers for one hour. Locomotor activity during this hour served as baseline. Mice were then injected with morphine and returned to the locomotor chambers for one hour.
To assess morphine sensitization, mice received five morphine injections, one per day, prior to placement in the locomotor chambers for one hour sessions. C57BL/6J mice received SB-334867 or vehicle one hour prior to each morphine injection.
Conditioned Place preference
Conditioned place preference (CPP) was performed using Med Associates (Georgia, VT) place conditioning boxes consisting of two chambers with retractable doors. One chamber consisted of a wire mesh floor with vertical black and white stripes on the wall. The other consisted of a grid floor with gray marble and diagonal black stripes on the wall. The conditioning chambers were separated by a gray neutral chamber. Time spent in each chamber was calculated using Med-PC IV software by photocell beam breaks.
During habituation, mice were placed in the neutral chamber for 15 minutes with both doors retracted so that mice could freely explore both chambers. During the conditioning phase, mice were treated with either morphine or cocaine immediately prior to placement into the CPP apparatus. On days 1, 3, and 5, mice received either a saline or drug injection and were confined to one of the conditioning chambers for 30 minutes. On days 2, 4, and 6, mice received the opposite treatment and were confined to the opposite chamber. On day 7, mice were placed in the neutral chamber and were allowed free access to both chambers for 15 minutes. C57BL/6J mice received SB-334867 or vehicle 20 minutes prior to the test session.
Drugs
Morphine sulfate and cocaine hydrochloride (NIDA, Bethesda, MD, USA) were dissolved in saline. Morphine was administered at a dose of 10 mg/kg via s.c. injections. Cocaine was administrated at a dose of 15 mg/kg via s.c. injections. SB-334867 (1-(2-methylbenzoxazol-6-yl)-3-[1,5]naphthyridin-4-yl urea hydrochloride) (Tocris, Ellisville, Missouri) was dissolved in 10% (w/v) (2-hyroxypropyl)-β-cyclodextrin/10% dimethyl sulfoxide (DMSO) in sterile water and was administrated at a dose of 20 mg/kg via i.p. injections. This dose of SB-334867 is consistent with other recent studies (Harris et al., 2005; Lawrence et al., 2006), and is not associated with locomotor deficits often seen when higher doses are administered (Rodgers et al., 2001). SB-334867 was administrated one hour prior to each locomotor activity session and 20 minutes prior to each CPP test session. SB-334867 has been found to reach peak plasma and brain concentrations at 30 minutes post-dosing and to maintain good exposure over four hours post-dosing (Ishii et al., 2005).
Data Analysis
For sensitization data, mixed design analyses of variance (ANOVAs) were conducted with either genotype or dose of SB-334867 as the between-subjects factor and session as the within-subjects factor. Significant results (p<0.05) were followed by Tukey's LSD post hoc analyses. For the CPP data, preference scores were measured as the difference in time spent in the paired chamber compared to the unpaired chamber (paired minus unpaired) and separate independent samples t-tests were conducted to analyze preference scores.
Acknowledgments
We thank Masashi Yanagisawa for supplying the orexin knockout mice. This work was supported by National Institutes of Health grants RO1DA017676 (R.J.D), F32DA023739 (R.S.), UL1-DE19586 (R.J.D. and J.R.T.), and RL1AA017537 (R.J.D. and J.R.T.) and the State of Connecticut Department of Mental Health and Addiction Services (R.J.D. and J.R.T.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56:112–21. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Storm E, Bonci A. Orexin B/hypocretin 2 increases glutamatergic transmission to ventral tegmental area neurons. Eur J Neurosci. 2008;28:1545–56. doi: 10.1111/j.1460-9568.2008.06397.x. [DOI] [PubMed] [Google Scholar]
- Boschen KE, Fadel JR, Burk JA. Systemic and intrabasalis administration of the orexin-1 receptor antagonist, SB-334867, disrupts attentional performance in rats. Psychopharmacology (Berl) 2009;206:205–13. doi: 10.1007/s00213-009-1596-2. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, De Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–73. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E, Imperato A, Perezzani L, Di Chiara G. Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience. 1989;28:653–61. doi: 10.1016/0306-4522(89)90012-2. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A. 1999;96:748–53. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duxon MS, Stretton J, Starr K, Jones DN, Holland V, Riley G, Jerman J, Brough S, Smart D, Johns A, Chan W, Porter RA, Upton N. Evidence that orexin-A-evoked grooming in the rat is mediated by orexin-1 (OX1) receptors, with downstream 5-HT2C receptor involvement. Psychopharmacology (Berl) 2001;153:203–9. doi: 10.1007/s002130000550. [DOI] [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23:3106–11. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gysling K, Wang RY. Morphine-induced activation of A10 dopamine neurons in the rat. Brain Res. 1983;277:119–27. doi: 10.1016/0006-8993(83)90913-7. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–9. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behavioural Brain Research. 2007;183:43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry DJ, Greene MA, White FJ. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: repeated administration. J Pharmacol Exp Ther. 1989;251:833–9. [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–28. [PubMed] [Google Scholar]
- Ingram DK, London ED, Reynolds MA, Waller SB, Goodrick CL. Differential effects of age on motor performance in two mouse strains. Neurobiol Aging. 1981;2:221–7. doi: 10.1016/0197-4580(81)90025-7. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Blundell JE, Halford JC, Upton N, Porter RA, Johns A, Jeffrey P, Summerfield S, Rodgers RJ. Anorexia and weight loss in male rats 24 h following single dose treatment with orexin-1 receptor antagonist SB-334867. Behav Brain Res. 2005;157:331–41. doi: 10.1016/j.bbr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Sensitization to repeated morphine injection in the rat: possible involvement of A10 dopamine neurons. J Pharmacol Exp Ther. 1987;241:204–12. [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–9. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Uramura K, Nambu T, Yada T, Goto K, Yanagisawa M, Sakurai T. Orexin-induced hyperlocomotion and stereotypy are mediated by the dopaminergic system. Brain Res. 2000;873:181–7. doi: 10.1016/s0006-8993(00)02555-5. [DOI] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–60. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake T, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–495. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–23. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Halford JC, Nunes de Souza RL, Canto de Souza AL, Piper DC, Arch JR, Upton N, Porter RA, Johns A, Blundell JE. SB-334867, a selective orexin-1 receptor antagonist, enhances behavioural satiety and blocks the hyperphagic effect of orexin-A in rats. Eur J Neurosci. 2001;13:1444–52. doi: 10.1046/j.0953-816x.2001.01518.x. [DOI] [PubMed] [Google Scholar]
- Sharf R, Sarhan M, DiLeone RJ. Orexin mediates the expression of precipitated morphine withdrawal and concurrent activation of the nucleus accumbens shell. Biol Psychiatry. 2008;64:175–83. doi: 10.1016/j.biopsych.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharf R, Sarhan M, DiLeone RJ. Role of orexin/hypocretin in dependence and addiction. Brain Res. 2009 doi: 10.1016/j.brainres.2009.08.028. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JG, De Lecea L. The hypocretins: setting the arousal threshold. Nat Rev Neurosci. 2002;3:339–49. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429–58. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- Zhang GC, Mao LM, Liu XY, Wang JQ. Long-lasting up-regulation of orexin receptor type 2 protein levels in the rat nucleus accumbens after chronic cocaine administration. J Neurochem. 2007;103:400–7. doi: 10.1111/j.1471-4159.2007.04748.x. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Bendor J, Hofmann L, Randesi M, Ho A, Kreek MJ. Mu opioid receptor and orexin/hypocretin mRNA levels in the lateral hypothalamus and striatum are enhanced by morphine withdrawal. J Endocrinol. 2006;191:137–45. doi: 10.1677/joe.1.06960. [DOI] [PubMed] [Google Scholar]