Abstract
Background
Aortic PWV is a measure of arterial stiffness and has proved useful in predicting cardiovascular morbidity and mortality in several populations of patients, including the healthy elderly, hypertensives and those with end stage renal disease receiving hemodialysis. Little data exist characterizing aortic stiffness in patients with chronic kidney disease who are not receiving dialysis, and in particular the effect of reduced kidney function on aortic PWV.
Methods
We performed measurements of aortic PWV in a cross-sectional cohort of participants enrolled in the Chronic Renal Insufficiency Cohort (CRIC) study to determine factors which predict increased aortic PWV in chronic kidney disease.
Results
PWV measurements were obtained in 2564 participants. The tertiles of aortic PWV (adjusted for waist circumference) were < 7.7 m/sec, 7.7–10.2 m/sec and > 10.2 m/sec with an overall mean (± S.D.) value of 9.48 ± 3.03 m/sec [95% CI = 9.35–9.61 m/sec]. Multivariable regression identified significant independent positive associations of age, blood glucose concentrations, race, waist circumference, mean arterial blood pressure, gender, and presence of diabetes with aortic PWV and a significant negative association with the level of kidney function.
Conclusions
The large size of this unique cohort, and the targeted enrollment of chronic kidney disease participants provides an ideal situation to study the role of reduced kidney function as a determinant of arterial stiffness. Arterial stiffness may be a significant component of the enhanced cardiovascular risk associated with kidney failure.
Introduction
The velocity of pulse wave travel in the aorta reflects vascular stiffness and predicts coronary heart disease, stroke and death (1). Although such measurements have been performed in the human aorta since the 1920’s (2) only in the last few decades has it been possible to non-invasively determine aortic PWV (PWV) and apply such knowledge to the epidemiology of cardiovascular disease (CVD) (3). Studies using aortic PWV measures show that it predicts death and CVD as well as, and in some cases better than, systolic blood pressure in the otherwise healthy elderly (4), diabetics (5), those with hypertension (6) and those receiving hemodialysis (7;8). With regard to those on dialysis, a population with exceptionally high CVD morbidity and mortality, determination of aortic PWV predicted death from CVD even when blood pressure is well controlled (7).
Previous studies of aortic PWV indicate that advancing age, increasing blood pressure and the presence of diabetes are typically associated with increased aortic PWV (9). Although aortic PWV has been well studied in End Stage Renal Disease (ESRD) there is a paucity of knowledge regarding the determinants of PWV in chronic kidney disease before the initiation of dialysis and whether the progressive loss of kidney function is independently associated with higher aortic PWV. Some studies suggest that aortic PWV is not directly related to CKD (10) while other studies have found an association (11). The few studies addressing this relationship have usually analyzed the subset with reduced kidney function from a population recruited for another purpose, and are typically analyzing a relatively homogenous ethnic group. In the current study we analyzed aortic PWV data from the Chronic Renal Insufficiency Cohort (CRIC), a multi-ethnic NIH-funded observational study (12) of people recruited by age-stratified estimated glomerular filtration rate (eGFR) to address further the complex interaction between kidney function, other biochemical and demographic factors in determining the spectrum of aortic PWV in CKD.
Methods
Subjects
Aortic PWV measurements were adopted into the CRIC protocol beginning at the 2nd annual follow-up visit and all participants enrolled in the CRIC study were invited to become part of this ancillary protocol. The procedures were approved by the Institutional Review Boards at the 13 enrolling centers and all subjects provided written informed consent.
Procedures
Aortic PWV measurements were performed supine after at least 5 minutes of rest using the right carotid and right femoral arteries. The Sphygmocor PVx System (AtCor Medical, West Ryde, Australia) device was used at each site. All personnel were trained and certified to take blood pressure measurements in the dominant arm with a Tyco aneroid sphygmomanometer using American Heart Association standards and to perform the aortic PWV measurements (13). Three electrocardiographic (ECG) leads were attached: one to the right arm, one to the left arm and one to the left lower abdomen or leg providing a standard limb lead II ECG tracing. The head was usually turned to 45 – 60° away from the examiner and the right carotid pulse was gently palpated. The distance from the sternal notch to the point of the palpable carotid pulse was measured in mm and entered into the computer program. The right femoral pulse was palpated and the distance to the umbilicus from the sternal notch, and then from the umbilicus to the point of femoral palpation was measured in mm and also entered into the program. Next a Millar tonometer attached to an electronic module interface was placed perpendicular to the carotid pulse and repositioned in small increments until a stable wave form was observed (14). The operator captured 10 seconds of stable waveform and repeated the sequence using the femoral artery. After second waveform is capture the computer generates an estimate of aortic PWV with a standard deviation. If the standard deviation was more than 15% of the PWV value the study was repeated.
Laboratory measures
Hemoglobin and hematocrit values were measured directly at the laboratories of each center. Other standard laboratory testing (e.g. creatinine, glucose, etc.) was performed at a central lab in the University of Pennsylvania, as were lipid profiles and urine protein determinations. The eGFR was determined according to the abbreviated MDRD formula using creatinine values calibrated to the Cleveland Clinic Laboratory (15). Certain laboratory values are only available from the baseline visit [see Table 1].
Table 1.
Demographic and biochemical profile of PWV cohort
| ALL ELIGIBLE N = 3272 | WITHOUT PWV N = 716 | WITH PWV N = 2564 | P | |
|---|---|---|---|---|
| Male | 55.1% | 48.5% | 57.1% | <.0001 |
| Race | ||||
| Black | 42% | 48.9% | 40% | <.0001 |
| Other | 9.1% | 6% | 9.9% | . |
| White | 49.1% | 45.1% | 50.1% | . |
| Diabetes at baseline (%=Yes) | 46.8% | 53.9% | 44.9% | <.0001 |
| AGE (years) | 60.64 (10.81) | 60.5 (10.37) | 60.69 (10.99) | 0.6932 |
| eGFR (mL/min/1.73m2) | 39.74 (16.02) | 37.42 (16.02) | 40.68 (15.92) | <.0001 |
| Height (cm) | 168.75 (9.49) | 167.19 (9.68) | 169.36 (9.35) | <.0001 |
| Weight (kg) | 91.71 (23.52) | 99.45 (27.71) | 88.64 (20.87) | <.0001 |
| Body Mass Index (kg/m2) | 32.14 (7.91) | 35.51 (9.73) | 30.83 (6.64) | <.0001 |
| Systolic BP (mmHg) | 127.77 (22.04) | 129.1 (22.4) | 127.22 (21.87) | 0.0613 |
| Diastolic BP (mmHg) | 69.86 (12.8) | 69.82 (13.11) | 69.88 (12.67) | 0.9205 |
| Pulse Pressure (mm Hg) | 57.89 (19.67) | 59.23 (20.09) | 57.34 (19.48) | 0.0358 |
| Mean Arterial Pressure (mm Hg) | 89.13 (13.59) | 89.43 (13.79) | 89 (13.5) | 0.4795 |
| Heart Rate (beats/minute) | 67.98 (11.17) | 68.56 (10.93) | 67.73 (11.27) | 0.1045 |
| Hemoglobin (g/dL) | 12.67 (1.78) | 12.43 (1.81) | 12.77 (1.76) | <.0001 |
| Glucose (mg/dL) | 113.97 (48.47) | 118.2 (51.63) | 112.24 (47.03) | 0.0075 |
| Creatinine (mg/dL) | 2.24 (1.54) | 2.38 (1.61) | 2.18 (1.51) | 0.0055 |
| Triglycerides (mg/dL) | 148.74 (105.98) | 152.37 (90.44) | 147.3 (111.56) | 0.3167 |
| Low-density Lipoprotein (mg/dL) | 96.97 (33.89) | 93.09 (36.1) | 98.51 (32.86) | 0.0008 |
| High-density Lipoprotein (mg/dL) | 47.51 (15.99) | 46.02 (16.04) | 48.11 (15.94) | 0.0063 |
| Calcium (mg/dL) | 9.33 (0.52) | 9.29 (0.57) | 9.34 (0.5) | 0.0253 |
| *Phosphate (mg/dL) | 3.7 (0.66) | 3.81 (0.66) | 3.67 (0.65) | <.0001 |
| *Calcium Phosphate Product (Ca*Phos) | 33.95 (6.24) | 35.05 (6.16) | 33.64 (6.23) | <.0001 |
| *Parathyroid Hormone (pg/mL) | 73.16 (67.64) | 81.43 (75.89) | 70.81 (64.94) | 0.0003 |
| *Urine Protein (g/day) | 1 (2.19) | 1.21 (2.67) | 0.94 (2.03) | 0.0038 |
| *Urine Albumin g/day | 0.64 (1.54) | 0.77 (1.75) | 0.6 (1.47) | 0.0114 |
| *Hemoglobin A1C (%) | 6.61 (1.54) | 6.77 (1.64) | 6.56 (1.51) | 0.0020 |
| *Uric Acid (mg/dL) | 7.37 (1.9) | 7.74 (1.92) | 7.27 (1.89) | <.0001 |
Race
Race was queried for each participant at screening by asking if they consider their racial background as American Indian/Alaskan Native, Asian/Asian American, Black/African American, Native Hawaiian/Other Pacific Islander or White/Caucasian (participants could check more than one box). If a participant checked the box “White” and no other box they were considered as White/Caucasian. If they checked the box for Black/African American either alone or in combination with any other box they were considered Black/African American. If they did not check the White or Black box, or if they preferred not to answer this question they were considered “Other”.
Aortic PWV Data
Enrollment into the PWV ancillary study began in 2005. The data reported here represent aortic PWV measures obtained on subjects whose second annual follow up visit occurred on or before March 31, 2009, representing 79% of the entire eligible CRIC cohort.
Statistical Analyses
Continuous data are presented as mean ± standard deviation (S.D.) or [95% CI]. Categorical variables are expressed as proportions. Certain variables were pre-specified for analyses because other studies showed a relationship to aortic PWV (such as age, systolic blood pressure) or because they are felt to be important in kidney disease (such as calcium, hemoglobin). Univariable regression models for aortic PWV were used to assess the relationship between PWV and the selected variables. To summarize the association between aortic PWV and variables of interest, a multivariable linear regression model was developed. All parameters significant at a p=0.25 level in univariable regression were entered into both forward and backward selection algorithms. Where variables were known to be strongly correlated with each other (e.g. serum creatinine and eGFR) the one with the stronger association only was entered. Only those significant at the p=0.05 level in the multivariable model were retained in the final model. All multivariable models were adjusted for clinical site. Analyses were executed in SAS 9.1 (SAS Institute, Cary, NC).
We also conducted analyses which used inverse probability of sampling weights to adjust for nonrandom missingness of aortic PWV measurements explainable in terms of other measured covariates (16). These analyses had only trivial effects on the results, which are presented without the inverse probability weighting adjustment applied. Because PWV required a distance measurement from sternal notch to umbilicus and umbilicus to palpable femoral pulsation site, there may be an artifactual increase in the distance in participants with large waist circumferences. When we compared the femoral distance measurement (within gender) controlling for participant height we found that this distance measurement increased with progressive waist circumference increase. To address this we developed a regression-based method to adjust the femoral distance measurement; the details of this adjustment method are presented in the appendix. Once the adjusted femoral distance measure was obtained, the originally measured sternal-notch to carotid distance was subtracted from it (as per the Sphygmocor method) to determine the adjusted distance aspect of the PWV value.
Results
Demographic characteristics of all participants eligible for aortic PWV measurement at the year 2 follow up visit of the CRIC cohort are shown in Table 1. We anticipated data loss on approximately 20% of participants and we were unable to obtain or use measurements on 716 of 3280 eligible participants (21.8%). Separate columns of data in Table 1 show summary statistics of those within the CRIC cohort who did not have aortic PWV measurements (“WITHOUT PWV”) compared with those who did (“WITH PWV”). In those without aortic PWV measures either poor quality of the data (approximately 30%), or unsuccessful data capture from the femoral site related to obesity or arrhythmia (e.g. atrial fibrillation) (approximately 70%) were the principal reasons. Table 1 reflects the higher weight and larger BMI of those participants in whom we could not successfully perform aortic PWV measurements. In a few cases death or interval ESRD requiring dialysis or a transplant occurred before the second year follow-up visit.
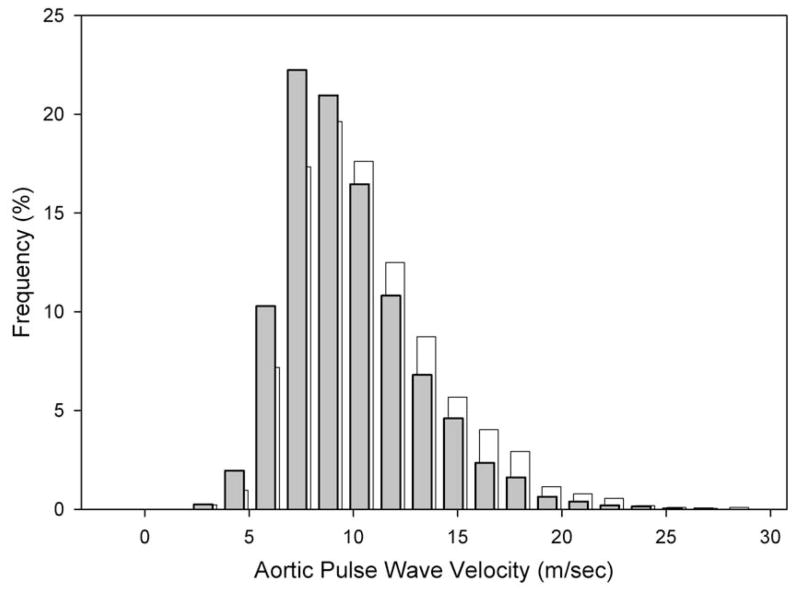
Figure 1 shows the frequency distribution of the waist circumference-adjusted aortic PWV determinations. The tertiles of the adjusted aortic PWV were < 7.7 m/sec, 7.7–10.2 m/sec and > 10.2 m/sec with an overall mean (± S.D.) value of 9.48 ± 3.03 m/sec [95% CI = 9.35–9.61 m/sec].
FIGURE 1.
Histogram depicting frequency of adjusted (for waist circumference) aortic PWV (PWV) in shaded bars (foreground) grouped in units of 1.5 meters/second in the CKD population of the CRIC study. The distribution of the unadjusted aortic PWV is shown in open bars (background) for reference.
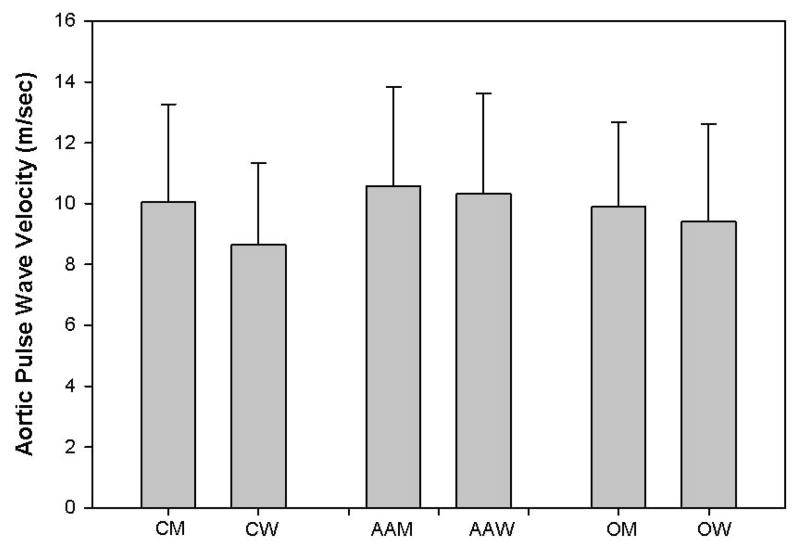
Figure 2 presents mean aortic PWV values for the cohort by gender and race. In multivariable analyses an interaction was noted between gender and race in that the association of aortic PWV with gender of Whites (men had higher aortic PWV) was significantly different than in Blacks (little difference between men and women).
FIGURE 2.
Mean ± S.D. of aortic PWV by race (C=Caucasian; AA=African American/Black; O=Other) and gender (M=Men; W=Women).
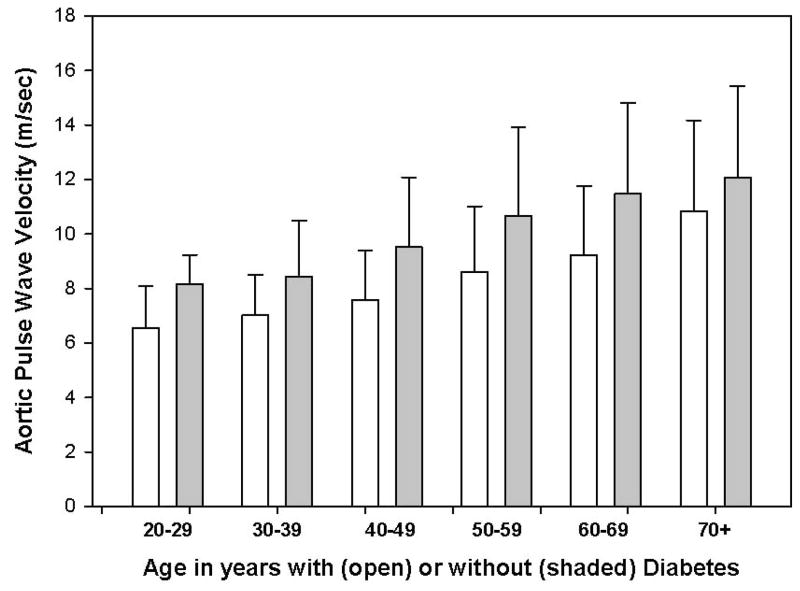
There was a positive relationship between age and aortic PWV as shown in Figure 3 (p<0.0001), which divided each decade into participants without diabetes (open bar) compared to participants with diabetes (solid bars). The mean waist circumference-adjusted aortic PWV in the cohort of those with diabetes (10.6 ± 3.2 m/sec) was significantly greater than those without diabetes (8.6 ± 2.6 m/sec; p<0.0001).
FIGURE 3.
Mean ± S.D. aortic PWV plotted against age in 10 year increments separated within each 10 year interval by the absence (open bar) or presence (shaded bar) of diabetes.
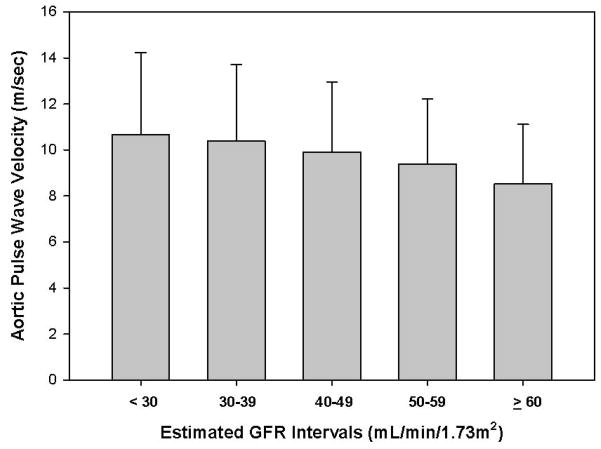
The inverse relationship between the estimated glomerular filtration rate (eGFR) and aortic PWV is depicted in Figure 4 (p<0.001). This relationship of eGFR to aortic PWV was similar in sub-cohort of subjects who underwent GFR measurement with 125I-iothalamate (1 of 3 participants had this performed; data not shown). Each unadjusted 10 mL/min/1.73m2 decrement in eGFR was associated with an average increase in aortic PWV of 0.4 m/sec.
FIGURE 4.
Mean ± S.D. aortic PWV plotted against estimated glomerular filtration rate (eGFR) in 10 mL/min/1.73m2 increments.
Table 2 displays the results of univariable regression of demographic, hemodynamic and laboratory data of our participants on aortic PWV shown three ways. The first group of columns regresses the unadjusted PWV on each independent variable individually. The second group of columns regresses the waist circumference adjusted PWV on the independent variables. The data for the third group of columns was derived by first performing a regression of the adjusted PWV on the mean arterial pressure and computing the residuals from that regression. Then those residuals were then regressed on each of the other independent variables. The data show the expected relationships of age, blood pressure and diabetes to PWV. In addition, the more novel findings of higher PWV in those of Black race, and the increase in PWV with declining kidney function (and factors associated with reduced kidney function such as increasing phosphorous level and lower hemoglobin level) are also noted.
Table 2.
Univariable linear regression
| Variable | Unadjusted PWV | PWV adjusted for waist circumference | Residual PWVadjusted for MAP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Reg coeff(SE) | R-sq | p-value | Reg coeff(SE) | R-sq | p-value | Reg coeff(SE) | R-sq | p-value | |
| Female (Yes) | −0.320 (0.14) | 0.00 | 0.0259 | −0.526 (0.13) | 0.01 | <.0001 | −0.402 (0.12) | 0.00 | <.01 |
| Black Race | 1.105 (0.15) | 0.02 | <.0001 | 0.856 (0.13) | 0.02 | <.0001 | 0.569 (0.13) | 0.01 | <.01 |
| Other Race | 0.327 (0.24) | 0.02 | 0.1805 | 0.238 (0.22) | 0.02 | 0.2714 | 0.141 (0.21) | 0.01 | 0.51 |
| Diabetes (Yes) | 2.645 (0.13) | 0.13 | <.0001 | 2.045 (0.12) | 0.11 | <.0001 | 2.026 (0.12) | 0.11 | <.01 |
| Age (10 year interval) | 1.238 (0.06) | 0.14 | <.0001 | 1.008 (0.05) | 0.13 | <.0001 | 1.052 (0.05) | 0.14 | <.01 |
| eGFR (10mL/min/1.73m2) | −0.463 (0.04) | 0.04 | <.0001 | −0.384 (0.04) | 0.04 | <.0001 | −0.380 (0.04) | 0.04 | <.01 |
| Height (cm) | −0.011 (0.01) | 0.00 | 0.1353 | 0.010 (0.01) | 0.00 | 0.1441 | 0.005 (0.01) | 0.00 | 0.42 |
| Weight (kg) | 0.028 (0.00) | 0.03 | <.0001 | 0.007 (0.00) | 0.00 | 0.0174 | 0.005 (0.00) | 0.00 | 0.08 |
| BMI (kg/m2) | 0.111 (0.01) | 0.04 | <.0001 | 0.019 (0.01) | 0.00 | 0.0438 | 0.015 (0.01) | 0.00 | 0.09 |
| Waist circumference (cm) | 0.058 (0.00) | 0.07 | <.0001 | 0.014 (0.00) | 0.01 | 0.0002 | 0.014 (0.00) | 0.01 | <.01 |
| SBP (10 mm Hg) | 0.596 (0.03) | 0.14 | <.0001 | 0.521 (0.03) | 0.14 | <.0001 | X | X | X |
| DBP (mm Hg) | −0.016 (0.01) | 0.00 | 0.0047 | −0.008 (0.00) | 0.00 | 0.0959 | X | X | X |
| MAP (mm Hg) | X | X | X | 0.041 (0.00) | 0.03 | <.01 | −0.000 (0.00) | 0.00 | 1.00 |
| Pulse Pressure (mm Hg) | 0.084 (0.00) | 0.21 | <.0001 | 0.071 (0.00) | 0.20 | <.0001 | 0.060 (0.00) | 0.15 | <.01 |
| Heart Rate (beats/minute) | 0.024 (0.01) | 0.01 | 0.0001 | 0.014 (0.01) | 0.00 | 0.0104 | 0.010 (0.01) | 0.00 | 0.08 |
| Number of BP medications | 0.786 (0.05) | 0.08 | <.0001 | 0.614 (0.05) | 0.07 | <.0001 | 0.559 (0.05) | 0.06 | <.0001 |
| Hemoglobin (g/dL) | −0.345 (0.04) | 0.03 | <.0001 | −0.247 (0.04) | 0.02 | <.0001 | −0.254 (0.04) | 0.02 | <.01 |
| Glucose (10 mg/dl) | 0.162 (0.01) | 0.05 | <.0001 | 0.130 (0.01) | 0.05 | <.0001 | 0.121 (0.01) | 0.04 | <.01 |
| Creatinine (mg/dL) | 0.733 (0.11) | 0.02 | <.0001 | 0.686 (0.10) | 0.02 | <.0001 | 0.578 (0.10) | 0.01 | <.01 |
| Triglycerides (10 mg/dl) | 0.017 (0.01) | 0.00 | 0.0069 | 0.011 (0.01) | 0.00 | 0.0588 | 0.010 (0.01) | 0.00 | 0.06 |
| LDL (10 mg/dL) | −0.062 (0.02) | 0.00 | 0.0044 | −0.049 (0.02) | 0.00 | 0.0106 | −0.068 (0.02) | 0.01 | <.01 |
| HDL (mg/dL) | −0.022 (0.00) | 0.01 | <.0001 | −0.014 (0.00) | 0.01 | 0.0005 | −0.013 (0.00) | 0.00 | <.01 |
| Calcium (mg/dL) | −0.298 (0.14) | 0.00 | 0.0356 | −0.259 (0.12) | 0.00 | 0.0374 | −0.181 (0.12) | 0.00 | 0.14 |
| *Phosphate (mg/dL) | 0.603 (0.11) | 0.01 | <.0001 | 0.402 (0.10) | 0.01 | <.0001 | 0.416 (0.10) | 0.01 | <.01 |
| *Calcium*Phosphate Product | 0.052 (0.01) | 0.01 | <.0001 | 0.033 (0.01) | 0.00 | 0.0013 | 0.036 (0.01) | 0.01 | <.01 |
| *PTH (pg/mL) | 0.008 (0.00) | 0.02 | <.0001 | 0.007 (0.00) | 0.02 | <.0001 | 0.006 (0.00) | 0.01 | <.01 |
| *Urine Protein (g/day) | 0.178 (0.04) | 0.01 | <.0001 | 0.142 (0.03) | 0.01 | <.0001 | 0.099 (0.03) | 0.00 | <.01 |
| *Urine Albumin (g/day) | 0.240 (0.05) | 0.01 | <.0001 | 0.189 (0.04) | 0.01 | <.0001 | 0.124 (0.04) | 0.00 | <.01 |
| *Hemoglobin A1C (%) | 0.668 (0.05) | 0.08 | <.0001 | 0.530 (0.04) | 0.07 | <.0001 | 0.502 (0.04) | 0.06 | <.01 |
| *Uric Acid (mg/dL) | 0.254 (0.04) | 0.02 | <.0001 | 0.169 (0.03) | 0.01 | <.0001 | 0.148 (0.03) | 0.01 | <.01 |
Table 3 shows the results of the multivariable linear regression. Age, kidney function decrement, serum glucose concentration, Black race, waist circumference, mean arterial pressure, male gender and diabetes had independent and significant associations with aortic PWV. Whereas no distinction based on gender was present prior to waist circumference adjustment, the post-adjustment results produced the significant association with male gender.
Table 3.
Multivariable linear regressions for selected variables and aortic PWV*
| Regression 1 | Regression 2 | |||
|---|---|---|---|---|
| VARIABLE | As-measured PWV Estimate (SE) | p-value | Adjusted PWV Estimate (SE) | p-value |
| Age (per 10 year interval) | 1.09 (0.06) | <.0001 | 0.95 (0.05) | <.0001 |
| eGFR (per 10mL/min/1.73m2) | −0.27 (0.04) | <.0001 | −0.23 (0.04) | <.0001 |
| Glucose (per 10 mg/dl) | 0.04 (0.01) | 0.0020 | 0.04 (0.01) | 0.0017 |
| Race Black vs. White | 0.41 (0.14) | 0.0026 | 0.39 (0.12) | 0.0016 |
| Race Other vs. White | 0.32 (0.25) | 0.2040 | 0.16 (0.23) | 0.4749 |
| MAP (per 1 mm Hg) | 0.05 (0.00) | <.0001 | 0.04 (0.00) | <.0001 |
| Waist Circumference (per 1 cm) | 0.03 (0.00) | <.0001 | −0.01 (0.00) | 0.0007 |
| Diabetes (Yes vs. No) | 1.67 (0.14) | <.0001 | 1.51 (0.13) | <.0001 |
| Female (vs. Male) | −0.04 (0.12) | 0.7534 | −0.31 (0.11) | 0.0054 |
Discussion
We performed aortic PWV measurements on a population of 2564 participants recruited specifically with impaired kidney function but not receiving chronic dialysis, of whom approximately one-half were diabetic. Consistent with studies in dialysis patients and other populations, we found that aortic PWV was higher with age, blood pressure and the presence of diabetes. Our investigation notes that in addition to these factors the participant’s blood glucose level (irrespective of diabetes presence), estimated kidney function, male gender and African-American ethnicity are independently associated with the aortic PWV value. Our study adds to the literature because it examined a large unique cohort specifically recruited because they have CKD, a patient population hitherto infrequently studied. The large sample size lends robustness to the findings. In addition the reliability of the measurements when done by different operators has been validated in this CKD population (17) supporting the validity of our findings. Other population studies of aortic PWV reported age-associated values similar to those we observed (1;18).
Our concern over waist circumference and its effects on the distance measurement are shared by others. In one study the authors used the carotid to femoral transit time statistically adjusted for height to estimate aortic PWV (10). The increasing prevalence of metabolic syndrome in CKD and the emerging role of obesity in the loss of kidney function indicate that the effect of waist circumference on carotid-femoral distance measurement techniques will need to adjust for this. Despite adjustment, waist circumference remained an independent and statistically significant predictor of PWV, in CKD (19).
The CRIC study was undertaken to enroll a longitudinal CKD cohort that would be ethnically diverse, spanning the ages of 21–74 with approximately 50% of its participants with diabetes to be generalizable to the clinical populations in practice settings (12). The recognition that reduced eGFR is related strongly to CVD morbidity and mortality has stimulated an ongoing search for biologic markers beyond traditional Framingham risk factors since these explain some but not all of the extra CVD burden in CKD (20;21) Thus, the pursuit of non-traditional factors like the determination of aortic PWV may help inform a portion of this challenge. The experience in ESRD suggests strongly that the aortic PWV is an important determinant of survival (7). Aortic PWV determinations have contributed to the epidemiology of CVD outcomes in otherwise healthy elderly people (4), diabetics (5) and those with hypertension (6). However, the influence of PWV on kidney as well as cardiovascular outcomes, and the determinants of aortic PWV in CKD patients not receiving dialysis, is less well studied.
Mourad and colleagues evaluated 1290 subjects, a mixed group composed of those with normal and impaired kidney function, and observed that those with the lowest tertile of kidney function showed an inverse relationship between creatinine clearance and aortic PWV (11). The work of Wang and colleagues in 102 subjects with an eGFR ranging across all 5 stages of CKD also suggested increasing aortic PWV as renal function declined (22). However, Hermans and colleagues in the setting of the Hoorn study examined the relationship between aortic PWV and the degree of kidney function and did not find that reduced renal function independently predicted central artery stiffness (10). Their study was weighted by 755 subjects with stages 2–3 CKD (mean eGFR of 60.6 mL/min/1.73 m2). They did note an association of PWV with urine albumin to creatinine ratio even after adjustment for age, gender and mean arterial pressure. Our study examined a larger, more ethnically diverse cohort with greater impairment renal function (mean eGFR of 40.7 mL/min/1.73m2). Our degree of proteinuria was numerically greater, and though univariable regression showed a relationship to PWV this disappeared in the multivariable regression analysis. Our results are in agreement with a study in Japan that followed 71 patients with CKD onto dialysis noting little change in aortic PWV as subjects transitioned onto dialysis (23).
Our data confirm the findings of Chaturvedi who reported higher aortic PWV in those of African descent without CKD and extends these findings to participants with CKD (24). Our data showing the relationship between glucose level and PWV also support the findings of the population-based Hoorn study (25).
Several reasons can be advanced for the progressive stiffness of the aorta as eGFR declines. Accumulation of advanced glycation end-products affecting vessel wall matrix and increase stiffness occurs in both diabetes and in kidney failure and may be related to the glucose findings we report (26;27). Vascular calcification promotes arterial stiffness and occurs in CKD as a result of disordered calcium, phosphorous and vitamin D metabolism, secondary hyperparathyroidism and changes in factors such as fibroblast growth factor 23 and fetuin A (28). Our findings with respect to divalent ion metabolism support this, but failed to show an independent effect since the changes in these variables are known to be strongly related to kidney function. Patients with decreased renal function also demonstrate signs of inflammation, with elevated levels of pro-inflammatory cytokines (29). Several studies show a relation between chronic inflammation, loss of vascular compliance and subclinical atherosclerosis in CKD (30;31). Cytokines affect endothelial function (32) and vascular remodeling (33). TGF-β, a pro-fibrotic cytokine stimulates the synthesis of the vasoconstrictor agent endothelin-1 (34), inhibits the production of nitric oxide (35) and increases renin secretion (36). These inflammatory cytokines are the subject of other CRIC ancillary studies and data regarding their levels in CRIC are still forthcoming.
Important limitations to our study include the distance measurement technique, the loss of data in which subject habitus (obesity) and other factors were not conducive to obtaining successful tracings and the absence of a normal control population. Those in whom we could not obtain suitable PWV readings were different [Table 1] from those with successful measurements which limits the generalizability of our data in CKD patients. The majority of participants in the CRIC study are on multiple antihypertensive medications, and the number of classes of BP medications prescribed was associated with higher mean PWV [Table 2]. The principal effects of antihypertensive drugs on PWV may be mediated through reduction in MAP rather than to non-blood pressure mediated alterations in large artery stiffness (37) and as shown in Table 3 the association between number of BP meds and PWV was not significant in multivariable regression [p=0.213]. Due to the cross sectional nature of our data we cannot infer directionality in the relationship of eGFR and PWV. It may be that reductions in eGFR lead to increases in PWV, or it may be vice versa, in that increases in PWV may contribute to reductions in eGFR. The longitudinal nature of CRIC, and the use of techniques (e.g. marginal structural models) as longitudinal data accumulates, may inform further the relationship between these variables.
In summary our findings in this large, diverse and unique CKD population show independent associations of age, kidney function, blood glucose concentrations, Black race, blood pressure, male gender and diabetes with aortic PWV. These are important associations in a group with such exceptional CVD burden and we anticipate that they will contribute to the development and pursuit of intervention strategies aimed at reducing both the cardiovascular burden and further kidney function loss in the CKD population which may be mediated substantially through increased aortic stiffness. We anticipate longitudinal data from our and other studies to pursue further the import of these findings.
Acknowledgments
Marshall Joffe, MD, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Sources of Support: These studies were supported by NIH/NIDDK grants including R01-DK-067390, U01-DK-060984, and NIH/NCRR grants UL1-RR024134, UL1 RR-025005, M01 RR-16500, UL1 RR-024989, M01 RR-000042, UL1 RR-024986, UL1RR029879, M01 RR-05096, UL1 RR-024131.
APPENDIX
Adjustment for waist circumference
An adjustment to account for possible waist-induced error in the sternal notch to femoral artery distance (sternal notch-femoral) was developed by focusing on adjusting the sternal notch-femoral distance and then using this distance to adjust the aortic PWV. A linear regression was run with the distance parameter as the outcome on height, waist, and the height*waist interaction. For each subject, the residual from the estimated regression of aortic PWV on these variables was calculated. This regression was run separately for men and women. Next, for each individual a reference waist was calculated based on the measured height and a reference value of 0.46 for the waist to height ratio (1). This reference waist value was combined with the regression coefficients to generate a predicted value for the distal distance, which is added to the residual to get an adjusted value:
The gender-specific beta coefficients and the residual are come from the regression estimates. The regression coefficients for men were 1.903 for β1, 3.413 for β2 and −0.005 for β3. For women the regression coefficients were 4.945 for β1, 8.932 for β2 and −0.039 for β3.
The adjusted distance variable (DTADJ) in turn is used to calculate an adjusted pulse wave velocity (PWV_ADJ):
(Performed using SAS v 9.1, SAS Institute, Cary, NC).
Examples: A man 174.4 cm tall with a waist circumference of 120.5 cm had a sternal notch-femoral distance of 645 mm and an unadjusted aortic PWV of 14.1 m/sec. After applying the regression his sternal notch-femoral distance was reduced to 533 mm and his adjusted aortic PWV was 11.7 m/sec. A man 175.2 cm tall with a waist circumference of 100.5 cm and a sternal notch-femoral distance of 600 mm had an unadjusted aortic PWV of 5.6 m/sec. Following regression his sternal-notch distance was adjusted to 545 mm and his adjusted aortic PWV was 5.1 m/sec.
Appendix Reference
- 1.Swiderski SJ. Thesis (M.S.) Air Force Institute of Technology; 2005. [accessed March 6th 2009]. Fit-To-Fight: Waist vs Waist/Height Measurements to Determine an Individual’s Fitness Level - A Study in Statistical Regression and Analysis. Available at: http://www.ntis.gov/search/index.aspx. [Google Scholar]
Chronic Renal Insufficiency Cohort (CRIC) Study
University of Pennsylvania
Scientific & Data Coordinating Center
Harold I. Feldman, MD, MSCE (PI)
J. Richard Landis, PhD
Shawn Ballard, MS
Zhen Chen, PhD
Denise Cifelli
Robert M. Curley, MS
James Dattilo
Marie Durborow
Stephen Durborow
Susan Eachus, PhD
Melanie Glenn, MPH
Asaf Hanish, MPH
Christopher Helker, MSPH
Maximilan Herlim
Amanda Hyre, PhD, MPH
Marshall Joffe, MD, PhD, MPH
Hopiy Kim
Stephen E. Kimmel, MD
Shiriki Kumanyika, PhD, MPH
Lisa Nessel, MSS, MLSP
Emile R. Mohler, III, MD
Gargi Parikh, MS
Amy Praestgard, MS
Nancy Robinson, MS
Leigh Rosen, MUEP
J. Sanford Schwartz, MD
Sandra Smith
Joan Stahl, MS
Valerie L. Teal, MS
Dawei Xie PhD
Peter Yang, PhD
University of Pennsylvania Medical Center
Raymond R. Townsend, MD (PI)
*Thomas Capolla, MD, ScM
Debbie Cohen, MD
Magdalena Cuevas
Mark J. Duckworth
Virginia Ford, MSN, CRNP
Colin M. Gorman
*Juan Grunwald, MD
Lucy Kibe, MS
*Mary B. Leonard, MD, MSCE
*Maureen Maguire, PhD
Stephanie McDowell
John Murphy, MD
*Muredach Reilly, MB
*Sylvia E. Rosas, MD
Wanda M. Seamon
Angie Sheridan, MPH
Karen Teff, MD
The Johns Hopkins University
Lawrence J. Appel, MD, MPH (PI)
Cheryl Anderson, PhD, MPH
Brad Astor, PhD, MPH
Jeanne Charleston, RN
Bernard Jaar, MD, MPH
Edgar “Pete” Miller, MD
Patience Ngoh
Hemalatha Venkatesh
J. Hunter Young, MD, MHS
University of Maryland
Jeffrey Fink, MD, MS (Co-PI)
Wanda Fink, RN, BSN
Afshin Parsa, MD, MPH
Beth Scism
Stephen Seliger, MD, MS
Matthew Weir, MD
University Hospitals of Cleveland Case Medical Center
Mahboob Rahman, MD (PI)
Renita Brooks
Valori Corrigan RN
Genya Kisin MA
Radhika Kanthety
Louise Strauss, RN
Jackson T. Wright, Jr., MD, PhD
MetroHealth Medical Center
Patricia Kao Co-PI
Kim Bauchens, RN
Jacqui Bjaloncik
Jeffrey Schelling, MD
John R. Sedor, MD
Mary Ann Shella, RN,BSN
Jacqueline Theurer
J. Daryl Thornton, MD, MPH
Cleveland Clinical Foundation
Martin J. Schreiber, MD (Co-PI)
Martha Coleman, RN
Richard Fatica, MD
David Frey
Darla Greenwald
Sandra Halliburton, PhD
Carol Horner, BSN, RN
Teresa Markle, BS
Sandra Pangonis, RN
Carmen Paradis, MD
MohammedA. Rafey, MD, MS
Annette Russo
Stephanie Slattery, RN
Jackie Soos
Rita Spirko, RN, MSN
Kay Stelmach, RN
Velma Stephens, LPN
University of Michigan at Ann Arbor
Akinlolu Ojo, MD, PhD (PI)
Anil Attili, MD
Jeff Briesmiester
Tonya L. Corbin, MD
Denise Cornish-Zirker, BSN
Crystal Gadegbeku, MD
Nancy Hill
Kenneth Jamerson, MD
Bruce Robinson, MD
Rajiv Saran, MD
Bonnie Welliver, BSN, CNN
Jillian Wilson
Eric Young, MD, MS
St. John’s Health System
Susan P. Steigerwalt, MD, FACP (Co-PI)
Keith Bellovich, DO
Jennifer DeLuca
Sherry Gasko, BSRN
Gail Makos, RN, MSN
Chantal Parmelee
Shahan Smith
Kathleen Walls
Wayne State University
John M. Flack, MD, MPH (Co-PI)
Andrew Johnson III, RN
Mary Maysura
Barbara Lloyd, MA, RD
Stephen Migdal, MD
M. Jena Mohanty, MD
Yanni Zhuang, BSN
University of Illinois at Chicago
James P. Lash, MD (PI)
Jose Arruda, MD
Carolyn Brecklin, MD
Janet Cohan, MSN
Michael Fischer, MD, MSPH
Leon Fogelfeld
Anne Frydrych, MS, RD
*Claudia Lora, MD
Onesema Martinez
Patricia Meslar, MSN
Kalyani Perumal, MD
Thomas Stamos, MD
Gregg Vachon
Paul Vaitkus, MD
*Eve Van Cauter, PhD
*Julio C. Vijil, MD
Tulane University Health Science Center
Jiang He, MD, PhD (PI)
Brent Alper, MD
Vecihi Batuman, M.D
Lydia A. Bazzano, MD, PhD
Bernadette Borja
Adriana Burridge
Jing Chen, MD, MSc
Catherine Cooke
Patrice Delafontaine, MD
Karen B. DeSalvo, MD, MPH, MSc
Vivian A. Fonseca, MD
Lee Hamm, MD
Michelle R. Hurly, RN, BSN
Eva Lustigova, MPH
*Paul Muntner, PhD
Lindsey Powers
Paul Whelton, MD, MSc
Kaiser Permanente of Northern California
Alan S. Go, MD (PI)
Lynn M. Ackerson, PhD
Pete Dorin, MPA
Daniel Fernandez
Rosy Fox
Nancy G. Jensvold, MPH
Joan C. Lo, MD
Juan D. Ordonez, MD, MPH
Rachel Perloff
Thida Tan, MPH
Daphne Thompson
Gina M. Valladares
Annette Wiggins, RN
Diana B. Wong, RN, MPH
Jingrong (Michelle) Yang, MA
University of California, San Francisco
Chi-yuan Hsu, MD, MSc (Co-PI)
Glenn M. Chertow, MD, MPH
Nisha Bansal, MD
Irina Gorodetskaya
*Vanessa Grubbs MD
*Manju Kurella, MD, MPH
Lowell Lo, MD
*Michael G. Shlipak, MD, MPH
*Kristine Yaffe, MD
NIDDK
John W. Kusek, PhD
Andrew S. Narva, MD
Scientific Advisory Committee
Kathy Faber-Langendoen, MD
Bryce A. Kiberd, MD
Elisa T. Lee, PhD
Julia Lewis, MD
William McClellan, MD, MPH
Timothy Meyer, MD
David Nathan, MD
John B. Stokes, MD
Herman Taylor, MD
Peter W. Wilson, MD
University of New Mexico
*Dominic Raj MD, DM
*Vallabh Shah, PhD
Consultants- Harvard School of Med
Paul M. Ridker, MD
Central Lab-University of Pennsylvania
Daniel J. Rader, MD
Anna DiFlorio
Ted Mifflin
Linda Morrell
Megan L. Wolfe
GFR Lab-Cleveland Clinic
Phillip Hall, MD
Sue Saunders
EBT Reading Center- UCLA
Mathew Budoff, MD
Chris Dailing
ECG Reading Center- Wake Forest
Ronald Prineas, MD, PhD
Elsayed Soliman, MD
Zhu-Ming Zhang, MD
Echo Reading Center- University of Pennsylvania
Martin St. John Sutton, MBBS
Martin G. Keane, MD, FACC, FAHA
University of Pennsylvania CTRC CTSA
UL1 RR-024134
The Johns Hopkins University
UL1 RR-025005
University of Maryland GRCR
M01 RR-16500
Case Western Reserve University Clinical and Translational Science Collaborative
(University Hospitals of Cleveland, Cleveland Clinic Foundation, and MetroHealth)
UL1 RR-024989
University of Michigan
GCRC grant number M01 RR-000042
CTSA grant number UL1 RR-024986
University of Illinois at Chicago
Clinical Research Center
M01 RR-013987-06
Tulane/LSU/Charity Hospital General Clinical
ResearchCenter
RR-05096
Kaiser NIH/NCRR UCSF-CTSI
UL1 RR-024131
*Ancillary Investigator
Footnotes
Conflict of Interest Statement: The Corresponding author (RRT) received funding from the NIH/NIDDK. There is no other COI to declare.
Reference List
- 1.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, et al. Prognostic value of aortic PWV as index of arterial stiffness in the general population. Circulation. 2006;113(5):664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 2.Bramwell JC, Hill AV. The velocity of the pulse wave in man. Proc Soc Lond (Biol ) 1922;93:298–306. [Google Scholar]
- 3.Bramwell JC, Hill A. Velocity of transmission of the pulse wave and elasticity of arteries. Lancet. 1922;i:891–892. [Google Scholar]
- 4.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, et al. Elevated aortic PWV, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111(25):3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 5.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106(16):2085–2090. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 6.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37(5):1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 7.Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation. 2001;103(7):987–992. doi: 10.1161/01.cir.103.7.987. [DOI] [PubMed] [Google Scholar]
- 8.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99(18):2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 9.O’Rourke MF, Mancia G. Arterial stiffness. J Hypertens. 1999;17(1):1–4. doi: 10.1097/00004872-199917010-00001. [DOI] [PubMed] [Google Scholar]
- 10.Hermans MM, Henry R, Dekker JM, Kooman JP, Kostense PJ, Nijpels G, et al. Estimated glomerular filtration rate and urinary albumin excretion are independently associated with greater arterial stiffness: the Hoorn Study. J Am Soc Nephrol. 2007;18(6):1942–1952. doi: 10.1681/ASN.2006111217. [DOI] [PubMed] [Google Scholar]
- 11.Mourad JJ, Pannier B, Blacher J, Rudnichi A, Benetos A, London GM, et al. Creatinine clearance, PWV, carotid compliance and essential hypertension. Kidney Int. 2001;59(5):1834–1841. doi: 10.1046/j.1523-1755.2001.0590051834.x. [DOI] [PubMed] [Google Scholar]
- 12.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14(7 Supple 2):S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 13.Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, et al. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460–2470. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- 14.Deloach SS, Townsend RR. Vascular stiffness: Its measurement and significance for epidemiologic and outcome studies. CJASN. 2008;3(1):184–192. doi: 10.2215/CJN.03340807. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 16.Robins JM, Rotnitzky A, Zhao L-P. Analysis of semiparametric regression models for repeated outcomes in the presence of missing data. Journal of the American Statistical Association. 1995;90:106–121. [Google Scholar]
- 17.Wimmer NJ, Townsend RR, Joffee MM, Lash JP, Go AS CRIC Study Investigators. Correlation between PWV and other measures of arterial stiffness in chronic kidney disease. Clinical Nephrology. 2007;68(3):133–143. [PubMed] [Google Scholar]
- 18.Shokawa T, Imazu M, Yamamoto H, Toyofuku M, Tasaki N, Okimoto T, et al. PWV predicts cardiovascular mortality: findings from the Hawaii-Los Angeles-Hiroshima study. Circ J. 2005;69(3):259–264. doi: 10.1253/circj.69.259. [DOI] [PubMed] [Google Scholar]
- 19.Safar ME, Czernichow S, Blacher J. Obesity, arterial stiffness, and cardiovascular risk. J Am Soc Nephrol. 2006;17(4 Suppl 2):S109–S111. doi: 10.1681/ASN.2005121321. [DOI] [PubMed] [Google Scholar]
- 20.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 21.Collins AJ, Li S, Gilbertson DT, Liu J, Chen SC, Herzog CA. Chronic kidney disease and cardiovascular disease in the Medicare population. Kidney Int Suppl. 2003;(87):S24–S31. doi: 10.1046/j.1523-1755.64.s87.5.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang MC, Tsai WC, Chen JY, Huang JJ. Stepwise increase in arterial stiffness corresponding with the stages of chronic kidney disease. Am J Kidney Dis. 2005;45(3):494–501. doi: 10.1053/j.ajkd.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Shinohara K, Shoji T, Tsujimoto Y, Kimoto E, Tahara H, Koyama H, et al. Arterial stiffness in predialysis patients with uremia. Kidney Int. 2004;65(3):936–943. doi: 10.1111/j.1523-1755.2004.00468.x. [DOI] [PubMed] [Google Scholar]
- 24.Chaturvedi N, Bulpitt CJ, Leggetter S, Schiff R, Nihoyannopoulos P, Strain WD, et al. Ethnic differences in vascular stiffness and relations to hypertensive target organ damage. J Hypertens. 2004;22(9):1731–1737. doi: 10.1097/00004872-200409000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Henry RM, Kostense PJ, Spijkerman AM, Dekker JM, Nijpels G, Heine RJ, et al. Arterial stiffness increases with deteriorating glucose tolerance status: the Hoorn Study. Circulation. 2003;107(16):2089–2095. doi: 10.1161/01.CIR.0000065222.34933.FC. [DOI] [PubMed] [Google Scholar]
- 26.Ueno H, Koyama H, Tanaka S, Fukumoto S, Shinohara K, Shoji T, et al. Skin autofluorescence, a marker for advanced glycation end product accumulation, is associated with arterial stiffness in patients with end-stage renal disease. Metabolism. 2008;57(10):1452–1457. doi: 10.1016/j.metabol.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Zieman SJ, Kass DA. Advanced glycation endproduct crosslinking in the cardiovascular system: potential therapeutic target for cardiovascular disease. Drugs. 2004;64(5):459–470. doi: 10.2165/00003495-200464050-00001. [DOI] [PubMed] [Google Scholar]
- 28.Townsend RR. Vascular compliance and arterial calcification: impact on blood pressure reduction. Curr Opin Nephrol Hypertens. 2008;17(1):93–98. doi: 10.1097/MNH.0b013e3282f331d7. [DOI] [PubMed] [Google Scholar]
- 29.Bolton CH, Downs LG, Victory JG, Dwight JF, Tomson CR, Mackness MI, et al. Endothelial dysfunction in chronic renal failure: roles of lipoprotein oxidation and pro-inflammatory cytokines. Nephrol Dial Transplant. 2001;16(6):1189–1197. doi: 10.1093/ndt/16.6.1189. [DOI] [PubMed] [Google Scholar]
- 30.London GM, Marchais SJ, Guerin AP, Metivier F, Adda H, Pannier B. Inflammation, arteriosclerosis, and cardiovascular therapy in hemodialysis patients. Kidney Int Suppl. 2003;84:S88–S93. doi: 10.1046/j.1523-1755.63.s84.25.x. [DOI] [PubMed] [Google Scholar]
- 31.Porazko T, Kuzniar J, Kusztal M, Kuzniar TJ, Weyde W, Kuriata-Kordek M, et al. IL-18 is involved in vascular injury in end-stage renal disease patients. Nephrol Dial Transplant. 2008 doi: 10.1093/ndt/gfn486. [DOI] [PubMed] [Google Scholar]
- 32.Nawroth PP, Stern DM. Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med. 1986;163(3):740–745. doi: 10.1084/jem.163.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leibovich SJ, Polverini PJ, Shepard HM, Wiseman DM, Shively V, Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature. 1987;329(6140):630–632. doi: 10.1038/329630a0. [DOI] [PubMed] [Google Scholar]
- 34.Kurihara H, Yoshizumi M, Sugiyama T, Takaku F, Yanagisawa M, Masaki T, et al. Transforming growth factor-beta stimulates the expression of endothelin mRNA by vascular endothelial cells. Biochem Biophys Res Commun. 1989;159(3):1435–1440. doi: 10.1016/0006-291x(89)92270-5. [DOI] [PubMed] [Google Scholar]
- 35.Roberts AB, Vodovotz Y, Roche NS, Sporn MB, Nathan CF. Role of nitric oxide in antagonistic effects of transforming growth factor-beta and interleukin-1 beta on the beating rate of cultured cardiac myocytes. Mol Endocrinol. 1992;6(11):1921–1930. doi: 10.1210/mend.6.11.1282674. [DOI] [PubMed] [Google Scholar]
- 36.Antonipillai I, Le TH, Soceneantu L, Horton R. Transforming growth factor-beta is a renin secretagogue at picomolar concentrations. Am J Physiol. 1993;265(4 Pt 2):F537–F541. doi: 10.1152/ajprenal.1993.265.4.F537. [DOI] [PubMed] [Google Scholar]
- 37.O’Rourke MF, Hashimoto J. Arterial stiffness: a modifiable cardiovascular risk factor? J Cardiopulm Rehabil Prev. 2008;28(4):225–237. doi: 10.1097/01.HCR.0000327179.21498.38. [DOI] [PubMed] [Google Scholar]