Abstract
Bone morphogenetic protein-15 (BMP-15) and growth and differentiation factor-9 (GDF-9) are oocyte-secreted factors that play essential roles in human folliculogenesis and ovulation. Their bioactivity is tightly regulated through phosphorylation, likely to occur within the Golgi apparatus of the secretory pathway. Here we show that Golgi apparatus casein kinase (G-CK) catalyzes the phosphorylation of rhBMP-15 and rhGDF-9. rhBMP-15, in particular, is an excellent substrate for G-CK. In each protein a single residue is phosphorylated by G-CK, corresponding to the serine residue at the sixth position of the mature region of both rhBMP-15 and rhGDF-9, whose phosphorylation is required for biological activity.
Keywords: oocyte-secreted factors, phosphorylation, Golgi-Casein Kinase
INTRODUCTION
Oocyte-secreted factors, such as bone morphogenetic protein-15 (BMP-15) and growth and differentiation factor-9 (GDF-9) play critical roles in regulating folliculogenesis and ovulation in mammals [1]. BMP-15 and GDF-9 are synthesized as precursors composed of a signal peptide, a proregion, and a biologically active mature protein [2-4]. Recently, we have shown that both BMP-15 and GDF-9 are phosphorylated and that phosphorylation is essential for bioactivity of the proteins [5-7]. Moreover, de-phosphorylated rhBMP-15 and rhGDF-9 exhibit antagonistic activity toward not only their phosphorylated counterparts but also toward each other, as well as rhBMP-7, a member of the TGF-ß superfamily that shares the same receptors. Thus, phosphorylation appears to be a means of regulating the bioactivity of rhBMP-15 and rhGDF-9, as well as other members of the TGF-ß superfamily. BMP-15 and GDF-9 are secreted proteins, indicating that phosphorylation is likely to occur in the Golgi apparatus of the secretory pathway. Based on the probable location of phosphorylation in the secretory pathway and the known phosphorylation site of rhBMP-15 at Ser6, we postulated that Golgi apparatus casein kinase (G-CK) could be the kinase implicated [8-10]. The consensus sequence targeted by G-CK, (Ser)-(Xaa)-(Glu or previously phosphorylated Ser) [8-10], is present in the hBMP-15 mature region coinciding with the phosphorylated residue at Ser6. Moreover, hGDF-9 contains a similar consensus sequence: (Ser6-Ser7-Glu8). Thus, both rhBMP-15 and rhGDF-9 are potential substrates for G-CK.
Identification of the kinase responsible for phosphorylating rhBMP-15 and rhGDF-9 is essential for further understanding the role of phosphorylation in regulation of bioactivity of the proteins. In the present study, we show that G-CK is able to phosphorylate rhBMP-15 and rhGDF-9. Additionally, we identified the residues phosphorylated by G-CK using mass spectrometry, and we show that they are those required for bioactivity.
MATERIALS AND METHODS
Production and purification of rhBMP-15 and rhGDF-9
rhBMP-15 and rhGDF-9, both tagged with a Flag epitope at the C terminus were prepared as described earlier [5,7].
Protein Kinase
G-CK was purified from the Golgi fraction of lactating mammary gland according to [11-13] and as described in the Supplementary Data section.
Phosphorylation assays
rhBMP-15 and rhGDF-9 phosphorylations were performed as described in [10-13] and in the Supplementary Data section.
Trypsin Digestion
After in vitro phosphorylation by G-CK, the proteins rhBMP-15 and rhGDF-9 were purified by SDS-PAGE and the bands corresponding to the different forms (precursor and mature proteins) were excised and subjected to in-gel reduction/alkylation followed by overnight digestion with trypsin (Promega) according to [14].
Phosphopeptides Enrichment and Mass Spectrometry
Phosphopeptides were enriched according to [15] using in-house made microcolumns of TiO2 and analyzed using a LCQ XP mass spectrometer (Thermo Scientific) coupled to a nano-HPLC system 1100 (Agilent Technologies) for the rhGDF-9 samples, whereas the rhBMP-15 samples were analyzed with a 4800 Plus MALDI TOF/TOF (Applied Biosystems). MS/MS data were then searched using Mascot search engine (Matix Science). For further details see the Supplementary Data section.
RESULTS
rhBMP-15 and rhGDF-9 are phosphorylated by G-CK with good kinetics parameters
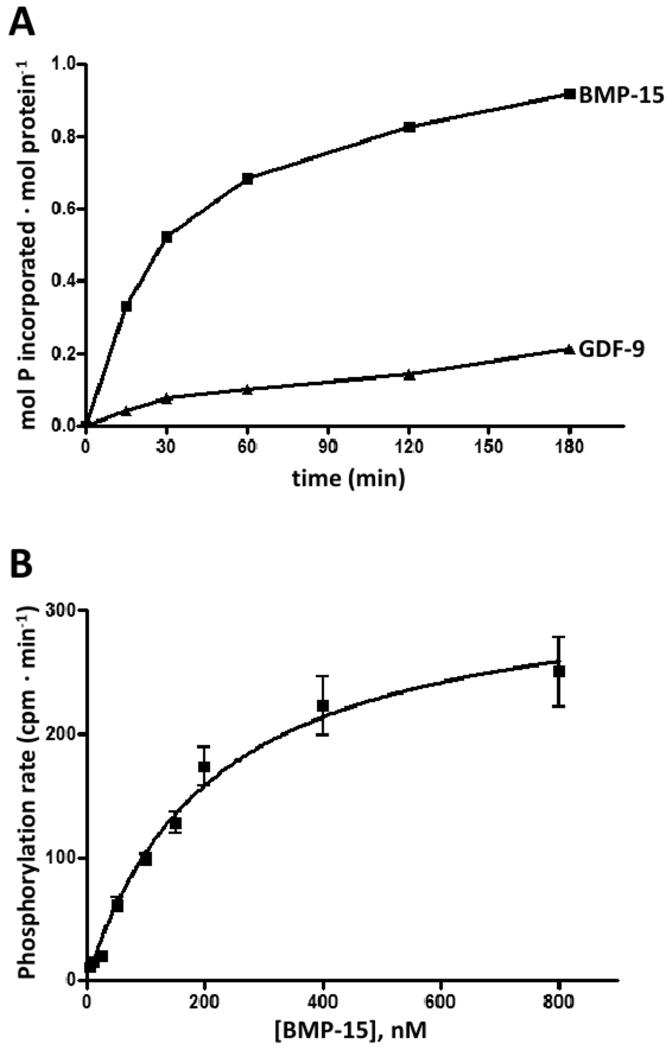
In order to evaluate whether rhBMP-15 and rhGDF-9 are susceptible to phosphorylation by G-CK, the proteins, each at 200 nM, were subjected to phosphorylation assays with highly purified G-CK from rat mammary gland [11]. The time course of phosphorylation (Fig. 1A) shows that rhBMP-15 was readily phosphorylated by the kinase, while rhGDF-9 was phosphorylated to a lesser extent. In particular, after 3 hours of incubation, up to 0.92 pmol and 0.13 pmol of phosphate/mol protein was incorporated into rhBMP-15 and rhGDF-9, respectively. Kinetic analysis allowed us to estimate an apparent Km value of 0.196 μM for rhBMP-15 phosphorylation (Fig. 1B). In the case of rhGDF-9 initial phosphorylation rates were too low and the kinetic constants could not be calculated.
Figure 1.
(A) Time course of phosphorylation of rhBMP-15 and rhGDF-9 (200 nM) by G-CK. Phosphorylation was performed as described in the Supplementary Data section. At different times reactions were stopped and phosphate incorporated was evaluated by SDS/PAGE and autoradiography. Its amount is expressed as mol P· mol prot−1. (B) Representative kinetic curve obtained by incubating G-CK with 50 μM [γ-32P]ATP and increasing concentrations of rhBMP-15 under conditions detailed in the Supplementary Data section. Values, expressed as cpm· min−1, are means of at least three determinations, with SEM. The Km value calculated by this experiment was 0.196 μM.
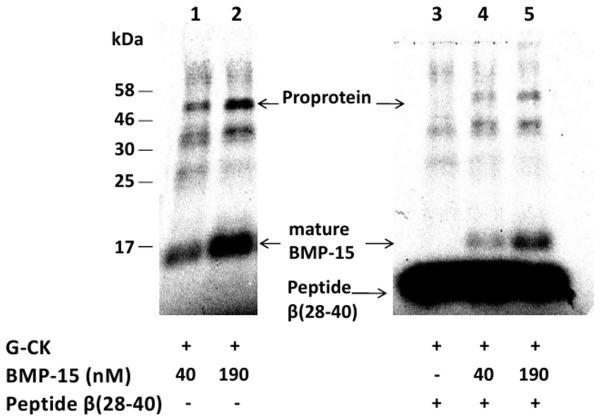
To rule out the possibility that protein kinase(s) other than G-CK are involved in the phosphorylation of BMP-15, we took advantage of the unique insensitivity of G-CK to staurosporine [11], a potent broad specificity inhibitor of protein kinases in general. Furthermore the β-casein 28-40 peptide that is phosphorylated only by G-CK was used as a competitor for BMP-15 phosphorylation by G-CK [10-11]. We found that 100 μM staurosporine, a concentration suppressing the activities of any other known protein kinase, has no effect on BMP-15 phosphorylation by G-CK. All our experiments were performed in the presence of 100 μM staurosporine unless differently indicated. Furthermore, as shown in Fig. 2, the intensity of rhBMP-15 phosphoradiolabeling was decreased by adding in the reaction the specific peptide substrate (500 μM) indicating that it competes with rhBMP-15 for phosphorylation by G-CK. Taken together, these results support the view that the kinase responsible for rhBMP-15 and rhGDF-9 phosphorylation is indeed G-CK.
Figure 2.
Effect of staurosporine and of the specific β-casein-derived peptide substrate β (28-40) on BMP-15 phosphorylation. BMP-15 either 40 nM (lanes 1 and 4) or 190 nM (lanes 2 and 5) was phosphorylated in the absence (lanes 1 and 2) or presence of 0.5 mM specific β-casein-derived peptide substrate β (28-40) (lanes 4 and 5) and in the presence of staurosporine 100 μM. After ten minutes incubation reactions were stopped, samples resolved by SDS-PAGE and phosphorylated bands were identified by direct scanning of the gel on a Cyclone Storage Phosphor System (Perkin-Elmer), as described in the Supplementary Data section. Controls without staurosporine displayed identical radiolabeling intensity of the phosphorylated bands (not shown).
Identification of the Phosphorylation Sites by Mass Spectrometry
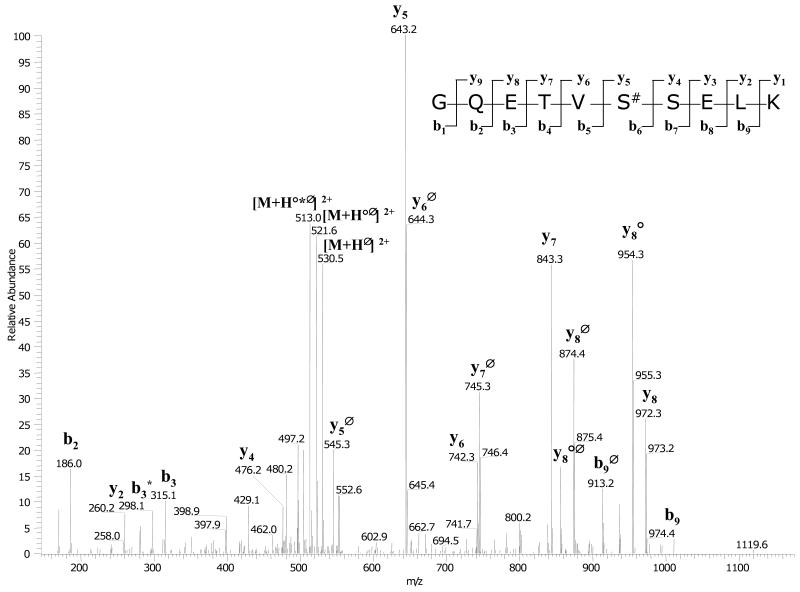
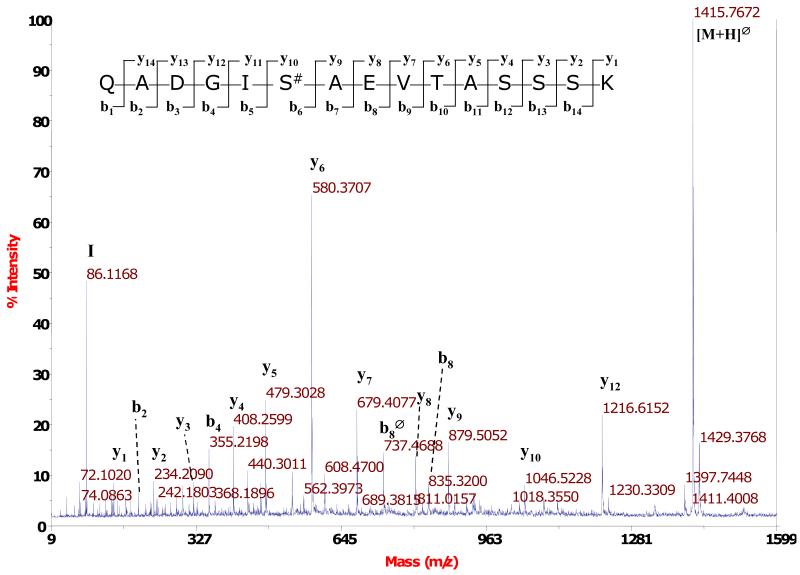
To identify the residues phosphorylated by G-CK, rhBMP-15 and rhGDF-9 were exhaustively phosphorylated by G-CK, and the phosphorylated residues were identified by trypsin digestion followed by LC-MS/MS analysis as detailed in the Experimental Procedures. Mass spectrometry analysis allowed us to detect several phosphopeptides belonging to rhBMP-15 and rhGDF-9, but only one phosphorylation site was identified for each of the proteins. All the MS2 spectra featuring loss of 98, 49 or 32.6 Da from the parent mass (strongly indicating that the peptide contains a phosphate group) and the relative MS3 spectra were manually inspected and interpreted to identify the peptide sequence and the phosphorylation sites. The high number of phosphopeptides identified was due to partial tryptic cleavages around the same phosphoresidue and to chemical modifications of the peptides As shown in Fig. 3, for the mature form of rhGDF-9 we could clearly identify Ser6 as phosphorylated. The same phosphorylated residue was also identified in the mature form of rhBMP-15 (Fig. 4). Both proteins are therefore phosphorylated at a Ser residue that matches the consensus sequence for G-CK [8-10]. The same analysis was also performed on the full length proteins (i.e. the proteins that have not been cleaved to give the mature form), obtaining similar results, since the same phosphopeptides were identified. Finally we searched for possible phosphorylated residues present in the proregion of the proproteins but we could not identify any phosphopeptides in these regions of either rhBMP-15 or rhGDF-9. Altogether, our data indicate that rhBMP-15 and rhGDF-9 are phosphorylated by G-CK at the same main residue (Ser6 of the mature forms) and this seems to be the only target of the kinase. Moreover, G-CK appears to be equally able to phosphorylate the proteins, at least in vitro, regardless of whether they are full-length or in their mature form.
Figure 3.
MS/MS spectrum of the phosphopeptide GQSTVS#SELK from GDF-9. After isolation of the phosphopeptides by TiO2 affinity chromatography, the analysis was carried out with an Ion trap mass spectrometer coupled to a nano-HPLC. In the spectrum are evident the intense losses of phosphate from the precursor ion and from the daughter ions (peaks marked with Δ). The y and b ion series give a clear indication of the peptide sequence and of the site of phosphorylation.
Figure 4.
MS/MS spectrum of the phosphopeptide QADGIS#AEVTASSSK from rhBMP-15. After isolation of the phosphopeptides by TiO2 affinity chromatography, the analysis was carried out with a MALDI TOF/TOF mass spectrometer. The base peak of the spectrum is due to the loss of phosphate (Δ) from the precursor ion. The y and b ion series give a clear indication of the peptide sequence and of the site of phosphorylation. The total mass of the peptide (1,513.76 Da) and the fragmentation pattern indicate that the peptide was subjected to the loss of ammonia from the N-terminus. This loss was probably due to an in-source fragmentation process.
DISCUSSION
Previously, we have shown that rhBMP-15 and rhGDF-9 are phosphorylated [6], and that phosphorylation is essential for bioactivity of the proteins [7]. In the present study, we identified G-CK as the kinase responsible for phosphorylating rhBMP-15 and rhGDF-9. These proteins are secreted proteins; thus, phosphorylation is likely to occur in the secretory pathway, specifically, within the Golgi apparatus. The 16 kDa mature region of rhBMP-15 was previously shown to be phosphorylated at a single site, Ser6 [5]. Based on these phosphorylation conditions, G-CK emerged as a candidate kinase [8-10]. G-CK has been detected in the Golgi apparatus of a variety of tissues [12], and has been shown to phosphorylate substrates such as progastrin [16-18], ACTH [19,20], IGF binding protein-1 [21] and osteopontin [22,23], in addition to β-casein [12,24]. G-CK targets proteins with a consensus sequence of (Ser)-(Xaa)-(Glu or previously phosphorylated Ser) [8-10]. The amino acids surrounding the rhBMP-15 phosphorylation site form the consensus sequence (Ser6-Ala7-Glu8), while the mature region of rhGDF-9 contains a similar consensus sequence: (Ser6-Ser7-Glu8). Thus, both rhBMP-15 and rhGDF-9 conform to the G-CK consensus.
Here, we show that rhBMP-15 is an excellent substrate for G-CK. A low apparent Km value of 0.196 μM was estimated from the phosphorylation reaction of rhBMP-15 by G-CK, indicating that the phosphorylation is physiologically relevant. While rhGDF-9 is also a substrate for G-CK, it was phosphorylated much more slowly, so kinetic analysis was not possible. We next used mass spectrometry to identify the residues phosphorylated by G-CK and found that Ser6, i.e. the residue found phosphorylated in BMP-15 [6] is the one readily phosphorylated by G-CK in the mature form of BMP-15. Also the mature form of rhGDF-9 was phosphorylated at Ser6. Both their residues conform to the consensus sequence of G-CK (S-X-E/Ps) as they display the sequences Ser6-Ala-Glu and Ser6-Ser-Glu in rhBMP-15 and rhGDF-9, respectively. Phosphorylation of the full-length rhBMP-15 and rhGDF-9 proteins, which have not been cleaved to yield the mature forms, with G-CK also revealed phosphorylation albeit weaker at the Ser6 residue of both proteins. No phosphorylation sites were detected in the proregion of either rhBMP-15 or rhGDF-9 proprotein. Thus, both rhBMP-15 and rhGDF-9 contain a single phosphorylation site located at Ser6 of the mature region of the proteins.
Our data add a new facet to the multifarious repertoire of functions of one of the most enigmatic protein kinases, G-CK. Believed for a long time to be a highly dedicated enzyme committed only to the phosphorylation of casein within lactating mammary gland, G-CK was later shown to be a pleiotropic protein kinase present in the Golgi apparatus of many tissues where it is presumably implicated in the phosphorylation of a wide variety of phosphoacceptor substrates displaying the motif pS-X-E/pS [12]. As unambiguously shown many years ago this motif is similar to but definitely distinct from the one recognized by another highly pleiotropic protein kinase, CK2 (S/T-X-X-E/D) [9]. This has made possible the development of an extremely selective peptide substrate unaffected by any other kinase [10], whose phosphorylation still represents the strongest criterion for G-CK identification, since the gene(s) expressing G-CK are still unknown, despite many efforts in different labs.
Recently G-CK activity has been also linked to the autism spectrum disorders as revealed by defective phosphorylation of salivary proteins at sites having the stigmata for being G-CK targets [25]. It appears therefore more and more urgent to fill this gap and disclose the gene encoding for this as yet orphan enzyme, responsible for the phosphorylation among many others substrates, of casein which was the first phosphoprotein to be described as early as in 1883 [26].
In conclusion, we have identified G-CK as the kinase responsible for phosphorylating rhBMP-15 and rhGDF-9. Interestingly, both rhBMP-15 and rhGDF-9 are phosphorylated at the same serine residue in the mature form of the proteins, Ser6. rhBMP-15 and rhGDF-9 each contain only a single phosphorylation site, further underscoring the potential importance of this posttranslational modification in regulation of bioactivity. These findings provide a new understanding of BMP-15 and GDF-9 regulation and may result in the identification of novel causes of human ovarian dysfunction.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by grants from AIRC and the European Commission (PRO-KINASERESEARCH 503467) to L.A. Pinna and from NIH RO1 HD41494 and NIH U54 HD012303 to S. Shimasaki.
Abbreviations
- BMP-15
bone morphogenetic protein-15
- GDF-9
growth and differentiation factor-9
- G-CK
Golgi apparatus casein kinase
- MALDI TOF/TOF
matrix-assisted laser-desorption/ionization time-of-flight/time-of-flight
- MS
mass spectrometry
- MS/MS
tandem mass spectrometry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr. Rev. 2004;25:72–101. doi: 10.1210/er.2003-0007. [DOI] [PubMed] [Google Scholar]
- [2].Massague J. The transforming growth factor-beta family. Ann. Rev. Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- [3].Chang H, Brown CW, Matzuk MM. Genetic analysis of the mammalian transforming growth factor-beta superfamily. Endocr. Rev. 2002;23:787–823. doi: 10.1210/er.2002-0003. [DOI] [PubMed] [Google Scholar]
- [4].Juengel JL, McNatty KP. The role of proteins of the transforming growth factor-beta superfamily in the intraovarian regulation of follicular development. Hum. Reprod. Update. 2005;11:143–160. doi: 10.1093/humupd/dmh061. [DOI] [PubMed] [Google Scholar]
- [5].Otsuka F, Yao Z, Lee TH, Yamamoto S, Erickson GF, Shimasaki S. Bone morphogenetic protein-15. Identification of target cells and biological functions. J. Biol. Chem. 2000;275:39523–39528. doi: 10.1074/jbc.M007428200. [DOI] [PubMed] [Google Scholar]
- [6].Saito S, Yano K, Sharma S, McMahon HE, Shimasaki S. Characterization of the post-translational modification of recombinant human BMP-15 mature protein. Protein Sci. 2008;17:362–370. doi: 10.1110/ps.073232608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McMahon HE, Sharma S, Shimasaki S. Phosphorylation of bone morphogenetic protein-15 and growth and differentiation factor-9 plays a critical role in determining agonistic or antagonistic functions. Endocrinology. 2008;149:812–817. doi: 10.1210/en.2007-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Meggio F, Boulton AP, Marchiori F, Borin G, Lennon DP, Calderan A, Pinna LA. Substrate-specificity determinants for a membrane-bound casein kinase of lactating mammary gland. A study with synthetic peptides. Eur. J. Biochem. 1988;177:281–284. doi: 10.1111/j.1432-1033.1988.tb14374.x. [DOI] [PubMed] [Google Scholar]
- [9].Meggio F, Perich JW, Meyer HE, Hoffmann-Posorske E, Lennon DP, Johns RB, Pinna LA. Synthetic fragments of beta-casein as model substrates for liver and mammary gland casein kinases. Eur. J. Biochem. 1989;186:459–464. doi: 10.1111/j.1432-1033.1989.tb15229.x. [DOI] [PubMed] [Google Scholar]
- [10].Lasa-Benito M, Marin O, Meggio F, Pinna LA. Golgi apparatus mammary gland casein kinase: monitoring by a specific peptide substrate and definition of specificity determinants. FEBS Lett. 1996;382:149–152. doi: 10.1016/0014-5793(96)00136-6. [DOI] [PubMed] [Google Scholar]
- [11].Tibaldi E, Arrigoni G, Brunati AM, James P, Pinna LA. Analysis of a sub-proteome which co-purifies with and is phosphorylated by the Golgi casein kinase. Cell. Mol. Life Sci. 2006;63:378–389. doi: 10.1007/s00018-005-5506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lasa M, Marin O, Pinna LA. Rat liver Golgi apparatus contains a protein kinase similar to the casein kinase of lactating mammary gland. Eur. J. Biochem. 1997;243:719–725. doi: 10.1111/j.1432-1033.1997.00719.x. [DOI] [PubMed] [Google Scholar]
- [13].Brunati AM, Contri A, Muenchbach M, James P, Marin O, Pinna LA. GRP94 (endoplasmin) co-purifies with and is phosphorylated by Golgi apparatus casein kinase. FEBS Lett. 2000;471:151–155. doi: 10.1016/s0014-5793(00)01378-8. [DOI] [PubMed] [Google Scholar]
- [14].Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. protoc. 2006;1:2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- [15].Thingholm TE, Jorgensen TJ, Jensen ON, Larsen MR. Highly selective enrichment of phosphorylated peptides using titanium dioxide. Nature protocols. 2006;1:1929–1935. doi: 10.1038/nprot.2006.185. [DOI] [PubMed] [Google Scholar]
- [16].Dockray GJ, Varro A, Desmond H, Young J, Gregory H, Gregory RA. Post-translational processing of the porcine gastrin precursor by phosphorylation of the COOH-terminal fragment. J. Biol. Chem. 1987;262:8643–8647. [PubMed] [Google Scholar]
- [17].Varro A, Desmond H, Pauwels S, Gregory H, Young J, Dockray GJ. The human gastrin precursor. Characterization of phosphorylated forms and fragments. Biochem. J. 1988;256:951–957. doi: 10.1042/bj2560951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Desmond H, Varro A, Young J, Gregory H, Nemeth J, Dockray GJ. The constitution and properties of phosphorylated and unphosphorylated C-terminal fragments of progastrin from dog and ferret antrum. Regul. Pept. 1989;25:223–233. doi: 10.1016/0167-0115(89)90264-4. [DOI] [PubMed] [Google Scholar]
- [19].Eipper BA, Mains RE. Phosphorylation of pro-adrenocorticotropin/endorphin-derived peptides. J. Biol. Chem. 1982;257:4907–4915. [PubMed] [Google Scholar]
- [20].Bennett HP, Brubaker PL, Seger MA, Solomon S. Human phosphoserine 31 corticotropin1-39. Isolation and characterization. J. Biol. Chem. 1983;258:8108–8112. [PubMed] [Google Scholar]
- [21].Jones JI, Busby WH, Jr., Wright G, Smith CE, Kimack NM, Clemmons DR. Identification of the sites of phosphorylation in insulin-like growth factor binding protein-1. Regulation of its affinity by phosphorylation of serine 101. J. Biol. Chem. 1993;268:1125–1131. [PubMed] [Google Scholar]
- [22].Sorensen ES, Hojrup P, Petersen TE. Posttranslational modifications of bovine osteopontin: identification of twenty-eight phosphorylation and three O-glycosylation sites. Protein Sci. 1995;4:2040–2049. doi: 10.1002/pro.5560041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lasa M, Chang PL, Prince CW, Pinna LA. Phosphorylation of osteopontin by Golgi apparatus casein kinase. Biochem. Biophys. Res. Commun. 1997;240:602–605. doi: 10.1006/bbrc.1997.7702. [DOI] [PubMed] [Google Scholar]
- [24].Price PA, Rice JS, Williamson MK. Conserved phosphorylation of serines in the Ser-X-Glu/Ser(P) sequences of the vitamin K-dependent matrix Gla protein from shark, lamb, rat, cow, and human. Protein Sci. 1994;3:822–830. doi: 10.1002/pro.5560030511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Castagnola M, Messana I, Inzitari R, Fanali C, Cabras T, Morelli A, Pecoraro AM, Neri G, Torrioli MG, Gurrieri F. Hypo-phosphorylation of salivary peptidome as a clue to the molecular pathogenesis of autism spectrum disorders. J. Proteome Res. 2008;7:5327–5332. doi: 10.1021/pr8004088. [DOI] [PubMed] [Google Scholar]
- [26].Hammarsten O. Zur Frage ob Caseín ein einheitlicher Stoff sei. Hoppe-Seyler’s Zeitschrift für Physiologische Chemie. 1883:227–273. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.