Abstract
The goal of this study was to determine whether prevention of K+ loss can protect human corneal-limbal epithelial (HCLE) cells from UV-B induced apoptosis. Immunostaining for activated caspase-3 of HCLE cells exposed to 150 – 200 mJ/cm2 UV-B demonstrated induction of apoptosis 6 hrs after exposure. The number of apoptotic cells was decreased by incubation in medium with 25 or 100 mM K+. If this protection is due to a reduction of UV induced K+ loss then K+ channel blockers should also protect HCLE cells from UV-B. Caspase-8 activity induced by exposure to UV-B at 150 mJ/cm2 was significantly reduced when the cells were incubated in 0.3 µM BDS-I or 0.05–1 mM quinidine. Caspase-3 was also activated by UV-B and a reduction in activity was observed after incubation in 0.1–0.3 µM BDS-I and 0.1–1mM quinidine. Induction of DNA fragmentation, as measured by the TUNEL assay, was decreased by treatment with 0.3 µM BDS-I and 0.01–0.05 mM quinidine. Patch-clamp recording showed activation of K+ channels after exposure to UV-B and a decrease in outward K+ current was observed following application of BDS-I. Quinidine did not block K+ currents in HCLE cells, suggesting that the protective effect of quinidine occurs by a mechanism other than via K+ channels. The effect of the K+ channel blocker BDS-1 on HCLE cells exposed to UV-B confirms that preventing K+ efflux protects corneal epithelial cells from apoptosis. This suggests the elevated [K+] in tears may protect the corneal epithelium from effects of ambient UV-B.
1. Introduction
Lacrimal gland fluid and tears have a K+ concentration ([K+]) of 20–25 mM, which is markedly higher than the [K+] in extracellular fluid and fluids secreted by other exocrine glands (Botelho and Martinez, 1973; Atta et al., 1975; Rismondo et al., 1989). The overall hypothesis of this study is that the relatively high level of K+ in tears contributes to the protection of the corneal epithelium from the adverse effects of ambient ultraviolet-B (UV-B) exposure. Apoptosis caused by chemical agents and UV radiation involves activation of K+ channels causing loss of intracellular K+, which in turn leads to activation of apoptotic pathways (Bortner et al., 1997; Hughes et al., 1997; Wang et al., 2003). It has also been reported that apoptosis of lymphocytes and neurons can be inhibited by elevated extracellular [K+] (Hughes et al., 1997; Yu et al., 1997). The high [K+] in tears may therefore inhibit the loss of intracellular K+, reducing the activation of apoptotic mechanisms when ocular surface cells are exposed to UV-B radiation.
We recently reported that treatment with UV-B at levels relevant to ambient UV-B exposure (50–200 mJ/cm2) activates K+ channels of human corneal limbal epithelial (HCLE) cells (Singleton et al. 2009). This UV-B exposure also causes apoptosis of the HCLE cells by activating initiator caspase-8 and effector caspase-3, leading to DNA fragmentation. When cells exposed to UV-B were bathed in elevated extracellular K+ ([K+]o = 25–100 mM) the UV-B activated K+ currents were reduced. Incubation of the HCLE cells in isosmotic culture medium with high [K+]o at concentrations similar to that in tears also inhibited UV-B induced apoptosis and activation of caspases-3 and -8. These data indicate that decreasing the K+ gradient between intracellular and extracellular fluid reduces UV-B induced loss of K+ from cells, thereby inhibiting activation of apoptotic pathways.
If reducing the loss of intracellular K+ protects cells from apoptosis, then treatment of HCLE cells with K+ channel blockers should protect cells from adverse effects of UV-B in the same way as elevated [K+]o. This hypothesis was tested by exposing HCLE cells to UV-B followed by measurement of caspase-3 and -8 activation, DNA fragmentation and K+ currents, during incubation with the K+ channel modulators BDS-1 (Wang et al., 2004) and quinidine (Rae et al. 1992), both of which are reported to block K+ currents in corneal epithelial cells.
2. Materials and methods
2.1 Cell culture, UV-B exposure and enzyme assays
HCLE cells were maintained as monolayers in Keratinocyte-Serum Free medium (KSF-M, Invitrogen, Carlsbad, CA) as described by Gipson et al. (2003) and Singleton et al. (2009). Media with increased [K+] were prepared by mixing standard medium ([K+] = 5.5 mM) with a custom medium (Invitrogen) containing 100 mM K+ and reduced Na+ to maintain osmolarity.
Cells were exposed to 302 nm UV radiation (UV-B) at 80 to 200 mJ/cm2 using an Ultraviolet Products Model UVM-57 lamp (UVP, Upland, CA), as previously described (Singleton et al., 2009). UV-B intensity was measured with a Solarmeter Model 6.2 (Solartech, Inc., Harrison Twp., MI).
Activation of caspase-3 and caspase-8 was measured using fluorometric protease assay kits (Invitrogen) as previously described (Singleton et al., 2009). The enzyme activity was expressed as relative fluorescence units/mg protein, as measured with the Bio-Rad, Protein Assay (Bio-Rad, Hercules, CA). An APO-BrdU TUNEL assay kit, (Phoenix Flow Systems, San Diego, CA) was used according to manufacturer’s instructions to detect DNA fragmentation and the percentage of apoptotic cells was measured by flow cytometry (Singleton et al., 2009).
2.2 Caspase-3 immunofluorscence assay
HCLE cells were grown to confluence in four chamber Lab-Tek slides (Nalge Nunc International, Rochester, NY) and exposed to UV-B at 100, 150 or 200 mJ/cm2. After a 6 hour incubation in isosmotic KSF-M with K+ concentrations of 5.5, 25 or 100 mM (Singleton et al., 2009) the cells were washed in PBS and fixed in 100% ice cold methanol. Activated caspase-3 was detected using Cleaved Caspase-3 (Asp175) Antibody, Alexa Fluor 488 Conjugate (Cell Signaling Technology, Beverly, MA) according to manufacturer’s instructions. Cleaved Caspase-3 (Asp175) Blocking Peptide (Cell Signaling Technology) was used to ensure specificity of the cleaved caspase-3 antibody. The slides were cover slipped using Prolong Gold Antifade Reagent (Invitrogen) which contains DAPI to stain the nuclei. Cells were imaged using an Axiovert 200 imaging system with Axiovision software (Carl Zeiss Meditec, Inc., Thornwood, NJ). Large-scale mosaic images of Alexa Fluor 488 and DAPI stained cells were captured allowing analysis of all cells in each chamber on the slide. The cells in each channel were counted using ImageJ software (National Institutes of Health, Bethesda, MD). The number of activated caspase-3 positive cells was divided by the total number of cells to determine the fraction of apoptotic cells in each experimental condition.
2.3 Effects of K+ channel modulators on apoptotic pathways
Confluent HCLE cells were exposed to 150 mJ/cm2 UV-B and incubated at 37° C for 6 hours. The UV dosage, incubation time period and analysis methods were based on results of our previous study (Singleton et al., 2009). During the incubation period BDS-1 (0.01 – 0.3 µM) or quinidine (0.05 – 1 mM) was present in the culture medium. At the end of the incubation period attached cells and cells that had been shed into the medium were collected and pooled. Activation of apoptotic pathways and effects of the K+ channel modulators were then evaluated by measurement of activation of caspases-3 and -8 and the TUNEL assay.
2.4 Patch-clamp recording
HCLE cells were plated in a recording chamber at low density in bath solution containing (in mM) 140 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 10 HEPES and 10 glucose at pH 7.4. Whole-cell voltage-clamp current recordings were made using standard amphotericin-B perforated patch techniques. Patch pipettes (resistance 2–5 MΩ) were filled with a recording solution containing (in mM) 145 K-methanesulfonate, 2.5 MgCl2, 2.5 CaCl2, 5 HEPES and 0.25 mg/ml amphotericin-B (Sigma, St. Louis, MO)at pH 7.3. Recordings were made after the access resistance dropped below 20 MΩ. The holding potential was −80 mV and the recording protocol consisted of 250 msec duration voltage steps from −80 mV to +160mV in 10 mV increments. Current was low-pass filtered at 2 kHz, recorded at 10 kHz by an Axon Instruments Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA,), and analyzed by accompanying software (Clampex/Clampfit 9.2). Access resistance, capacitance, and leak resistance were compensated by the amplifier circuitry and by P/5 subtraction.
Control K+ currents were recorded, followed by exposure of cells to UV-B at 80 or 150 mJ/cm2. UV-B activated currents were then recorded and an AutoMate Scientific (Berkeley, CA) perfusion pencil used to apply the Kv3.4 channel blocker BDS-1 (Alamone Labs, Jerusalem, Israel) to cells at 1 µM (Wang et al., 2004) or quinidine (Sigma, St. Louis, MO) at 0.6 mM (Rae et al., 1992). Effects of the blockers on K+ currents were recorded, followed by washout of the blocker and recording of the UV activated currents in normal bath medium.
Results
3.1 Effect of elevated [K+]o on UV-B induced caspase-3 activation
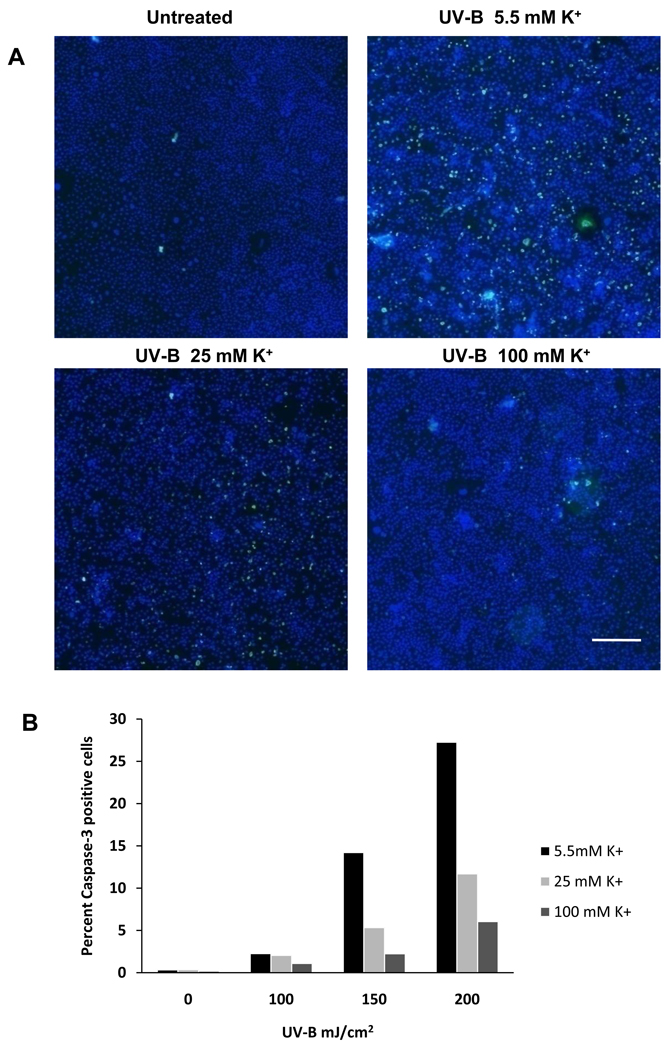
We previously measured effects of increased [K+]o on UV-B induced apoptosis using enzymatic assays (Singleton et al., 2009). The purpose of this experiment was to confirm those data by immunostaining of HCLE cells. The data presented are from one experiment that is representative of the results of 5 experiments. Calculations are based on counting 47,000 ± 2500 (mean ± SE) cells per chamber. UV-B at 100–200 mJ/cm2 caused a dose dependent increase in the percentage activated caspase-3 positive cells 6 hours after UV-B exposure. The number of activated caspase-3 positive cells was decreased by incubation of the cells in medium with 25 or 100 mM K+ (Fig. 1).
Fig. 1.
A. Immunostaining of HCLE cells exposed to UV-B at 150 mJ/cm2 and incubated for 6 hours in isosmotic medium with 5.5, 25 or 100 mM K+. Control cells were not exposed to UV-B and were incubated in medium with 5.5 mM K+. Apoptotic cells were stained with Cleaved Caspase-3 (Asp175) Antibody, Alexa Fluor 488 Conjugate antibody and all cells were counterstained with DAPI. Bar = 500 µM B. Effect of 100 – 200 mJ/cm2 UV-B and incubation in elevated [K+]o on caspase-3 activation in HCLE cells. 150 mJ/cm2 data are from cells shown in panel A. This experiment was repeated 5 times with similar results.
3.2 Effect of K+ channel modulators on UV-B induced activation of apoptotic pathways
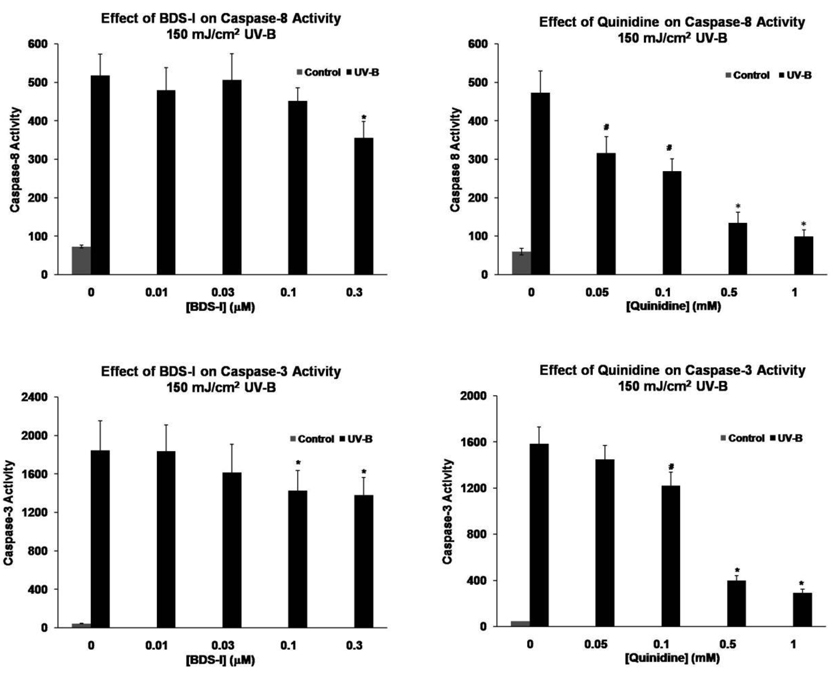
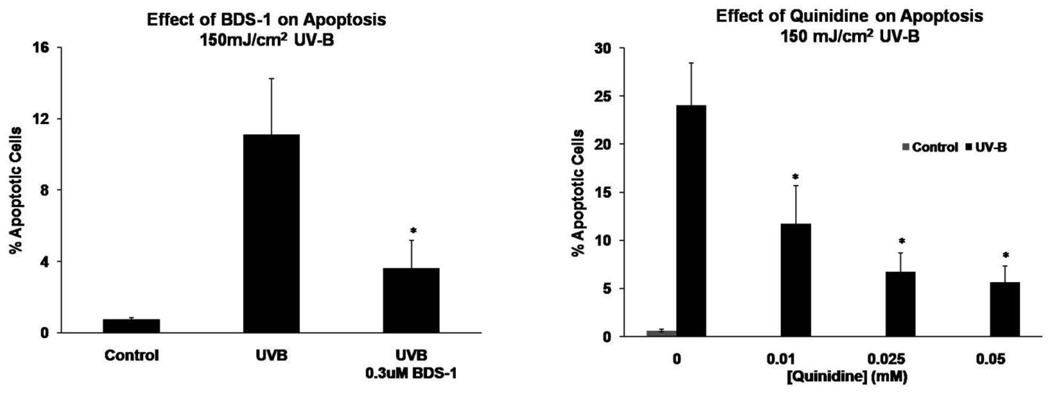
Exposure of HCLE cells to 150 mJ/cm2 UV-B resulted in marked activation of caspase-8 and caspase-3 and an increase in the number of TUNEL positive cells 6 hr after treatment (Fig. 2 and Fig. 3). When the cells were exposed to BDS-1 at 0.01–0.3 µM during the 6 hr incubation period a significant decrease in UV-B induced caspase-8 activation occurred at the 0.3 µM dose. UV-B activation of caspase-3 was significantly reduced by BDS-1 at 0.1 and 0.3 µM (Fig. 2). Based on these results UV-B induced DNA fragmentation was measured in the presence of 0.3 µM BDS-1 which resulted in a significant decrease in the number of TUNEL positive cells after UV-B treatment (Fig. 3.)
Fig. 2.
BDS-1 and quinidine inhibit activation of caspase-8 and caspase-3 in HCLE cells exposed to UV-B. Cells were exposed to UV-B and then incubated in culture medium with K+ blockers for 6 hours before measurement of caspase activity. Statistical analysis by 2-way ANOVA and Student-Newman-Kuels test, p ≤ 0.05, mean ± SE. BDS-1: caspase-8, * different than all other values, n = 6; caspase-3, *different from unmarked values, n = 6. Quinidine: caspase-8, #different from all other values. *different from all other values except control (n = 12); caspase-3, #different from all other values. *different from all other values but not from each other, n = 8.
Fig. 3.
BDS-1 and quinidine reduce the percentage of apoptotic HCLE cells in cultures exposed to UV-B. Cells were exposed to UV-B and then incubated in culture medium with K+ modulators for 6 hours before measurement of DNA fragmentation by the TUNEL assay and flow cytometry. Statistical analysis by ANOVA and Student-Newman-Kuels test, p ≤ 0.05, mean ± SE. BDS-1: *different from unmarked values, n = 6. Quinidine: *different from unmarked values but not from each other, n = 8.
When HCLE cells exposed to UV-B were incubated with 0.05 – 1 mM quinidine a dose-dependent decrease in UV induced caspase-8 activation occurred over the entire range while caspase-3 activation was inhibited by 0.1 – 1 mM quindine (Fig. 2). Based on the strong inhibition of caspase-8 and -3 activities at relatively low doses of quinidine, UV-B induced DNA fragmentation was measured in the presence of 0.01 – 0.05 mM quinidine. The percentage of TUNEL positive cells was significantly reduced over this entire range (Fig. 3).
3.3 Effect of K+ channel modulators on UV-B activated whole cell K+ current
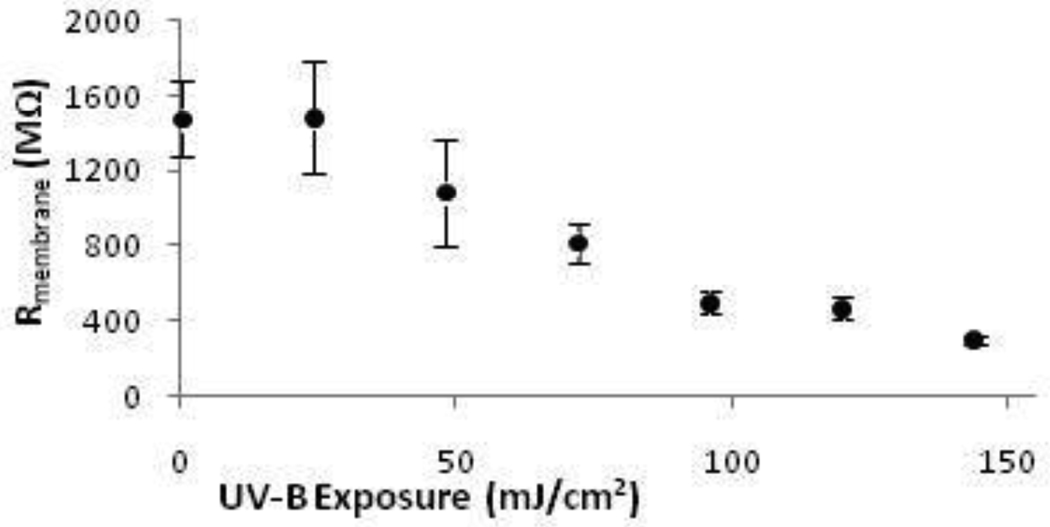
Whole-cell K+ currents measured in HCLE cells before UV-B exposure typically were very small, and in most cells barely rose above noise. With the recording pipette attached, cells were exposed to UV-B and membrane resistance was monitored throughout the exposure at a holding potential of −80 mV, which is below the resting potential of the cells. HCLE cells showed a dose-dependent decrease in membrane resistance at UV-B exposures up to 150 mJ/cm2 (Fig. 4). This is consistent with K+ channels being activated by UV-B even at membrane voltages at or below resting potential.
Fig. 4.
UV-B exposure decreased HCLE cell membrane resistance at voltages at or below resting potential. Membrane voltage was held at −80 mV and Rmembrane monitored during the UV-B exposure. Average Rmembrane decreased in a dose-dependent fashion from 1500 ± 200 MΩ prior to UV exposure to 304 ± 21 MΩ after 150 mJ/cm2 (mean ± SE, n = 20).
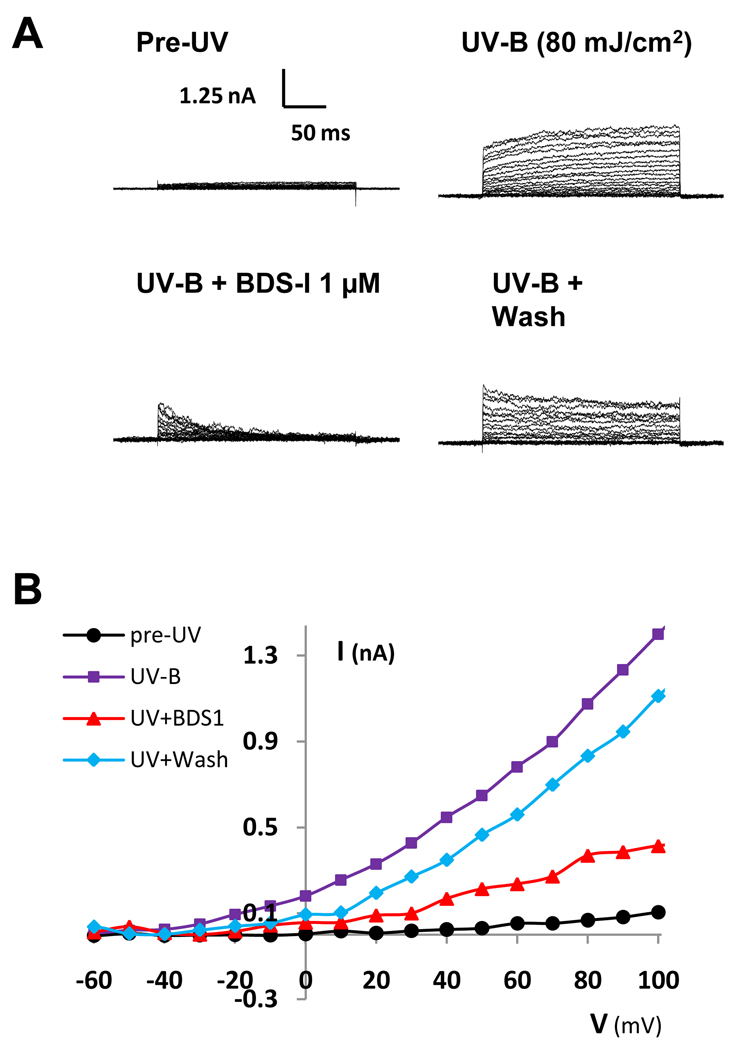
Whole-cell K+ currents measured within a minute after either 80 or 150 mJ/cm2 UV-B exposure showed a dramatic increase over control currents and often varied significantly in magnitude for 5–10 minutes after UV-B exposure before settling at elevated levels. When the Kv3.4 channel blocker BDS-1 (1 µM) bathed the cells, more than half the UV-activated K+ current was blocked. Washout of BDS-1 reversed the current block. An example of this response in one cell is shown in Figure 5. Summary data, including current-voltage curves of the K+ current response to UV-B exposure and BDS-1 block of K+ currents, has been published previously (Singleton et al. 2009).
Fig. 5.
BDS-1 (1 µM) reversibly inhibited UV-activated K+ currents in HCLE cells exposed to 80 mJ/cm2 UV-B. From a holding potential of −80mV, voltage steps of 250 ms duration were given in +10 mV intervals. UV-activated K+ currents were substantially larger than control currents within a minute after UV-B exposure. A. Current traces of a representative cell before UV-B exposure, 5 minutes after UV-B exposure before BDS-1 application, during BDS-1 application and after BDS-1 washout. B. Current-voltage (I-V) relationship of the same cell showing average currents before UV-B exposure (black), after UV-B exposure prior to drug application (violet), during BDS-1 (red), and during washout (blue).
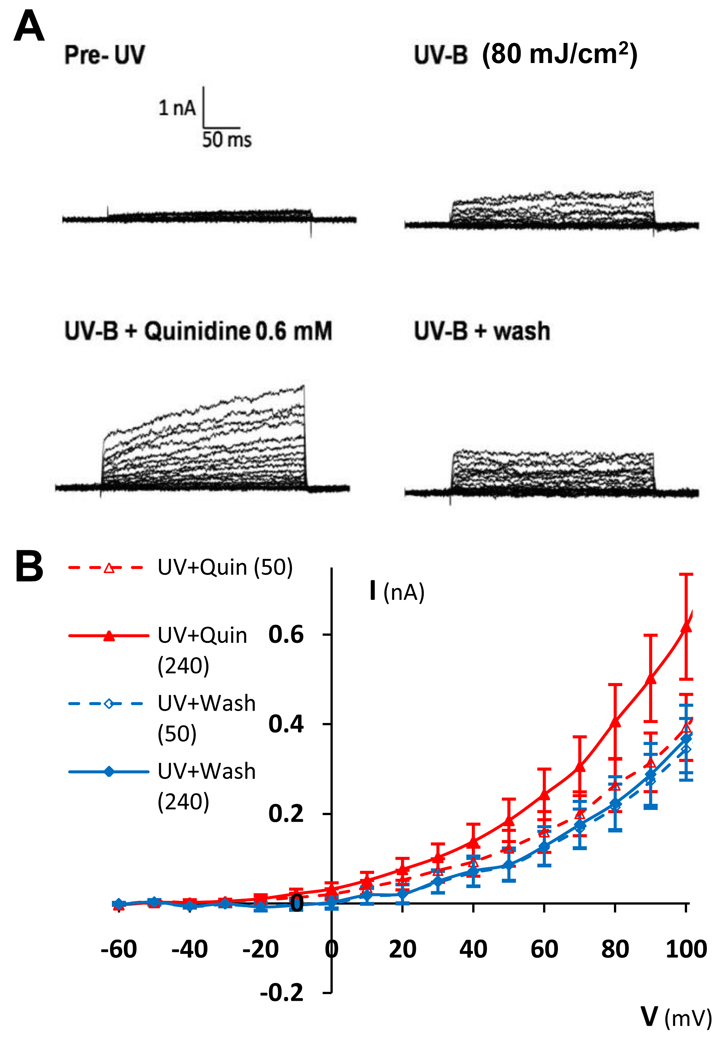
Quinidine (0.6 mM) had two effects on the K+ currents in HCLE cells exposed to 80 mJ/cm2 UV-B. First, in slightly less than two thirds of the cells (n = 13 out of 21) quinidine increased the slow-activating component of the K+ current. In Figure 6B this is shown as the separation between the solid red (quinidine) and solid blue (washout) curves. Second, in two thirds of the cells (n = 14 out of 21) quinidine slowed the activation of K+ currents in response to voltage steps. In Figure 6B this slowing of current activation is shown as the separation between the red dotted curve (quinidine, first 50 ms after voltage step) and red solid curve (quinidine, full 240 ms after voltage step). For command voltages in the range of +10 to +100 mV, during quinidine application the average K+ current during the first 50 ms after voltage steps was only 61% ± 3% of the average current during the subsequent 190 ms (mean ± SD). By comparison, during quinidine washout (blue curves) activation of K+ currents in response to voltage steps was faster. During washout the average K+ current during the first 50 ms after the voltage step was 94% ± 5% of the average current during the subsequent 190 ms.
Fig. 6.
Quinidine (0.6 mM) slowed the activation of K+ currents in response to voltage steps and increased the slow-activating portion of the current at high voltages in HCLE cells exposed to 80 mJ/cm2 UV-B. A. Current traces of a representative cell before UV exposure, 10 minutes after UV-B exposure before quinidine application, during quinidine application, and after quinidine washout. B. Average (n = 14) current-voltage (I-V) relationship of cells after UV-B exposure during quinidine application (red) and after quinidine washout (blue). Between +10 and +100 mV, the dashed red curve (quinidine, first 50 ms after voltage step) is 66% ± 3% (mean ± stdev) below the solid red curve (quinidine, full 240 ms), indicating a slowing of current activation. By comparison, the dashed blue curve (washout, first 50 ms) is 95% ± 5% (mean ± SD) below the solid blue curve (washout, full 240 ms).
It is not obvious from these data what effect quinidine has on the open-channel probability of K+ channels of HCLE cells at resting potential. Therefore, the effect of quinidine (0.6 mM) on the resting potential of HCLE cells exposed to 80 mJ/cm2 UV-B was monitored. Effects of quinidine were slight, with an average change in resting potential of −2.7 ± 1.2 mV relative to washout (n = 10, mean ± SD), which was not statistically significant. This result is not consistent with the hypothesis that quinidine reduced the open channel probability of K+ channels at resting potential.
To determine whether the effects of quinidine on K+ currents depended on UV-B dose, cells exposed to 150 mJ/cm2 were also tested. The K+ current response to quinidine in these cells was similar to cells exposed to 80 mJ/cm2 UV-B (data not shown).
4. Discussion
The results of this study generally support the hypothesis that reducing K+ efflux from corneal epithelial cells after exposure to UV-B reduces the activation of apoptotic pathways. Immunostaining of activated caspase-3 positive cells (Fig. 1) demonstrated the effect of UV-B on intact HCLE cells and the protection provided by elevated [K+]o. This confirms our previous biochemical assays (Singleton et al, 2009) and places the present study of K+ channel modulators in context. Caspase-3 was chosen for this experiment because it lies in the apoptotic pathway from early UV-B induced activation of caspase-8 to DNA fragmentation. Caspase-3, as an effector caspase that promotes the final steps in apoptosis, lies relatively early in the apoptosis cascade compared to later stages such as DNA fragmentation. For this reason the activated caspase-3 positive cells remained attached to the slide; however, TUNEL positive cells could not be detected by this method because they tend to slough into the cell culture medium (Singleton et al, 2009). Having shown that elevated [K+]o in the range found in tears, 25 mM, partially protects HCLE cells from UV-B induced apoptosis, we proceed to a discussion of effects of K+ channel modulators on UV-B induction of apoptosis.
BDS-1 is a blocker of the Kv3.4 channel, which has been reported to be expressed in rabbit corneal epithelial cells (Wang et. al., 2004). Adding BDS-1 to the bathing medium reduced the UV-B induction of the apoptotic pathway, as shown by a reduction in the UV-induced caspase-8 and caspase-3 activation and UV-induced DNA fragmentation (Fig. 2 and Fig. 3). The inhibition of apoptosis by BDS-1 was only seen at the highest doses used in this study and was not as strong as that of elevated [K+]o in our previous study. This is not surprising because there are other types of K+ channels in corneal epithelial cells in addition to KV3.4, for which BDS-1 is specific. Elevated [K+]o would reduce UV-B induced K+ efflux through all channel types, thereby maintaining a higher [K+]i than would be maintained by BDS-1 alone.
Quinidine also had the expected effect on the UV-B induced apoptotic pathway if it assumed that quinidine blocks K+ channels (Fig. 2 and Fig. 3). The strong, dose-dependent reduction in caspase activity and DNA damage in the presence of quinidine is consistent with a significant reduction in K+ efflux; however, as discussed below the electrophysiology data complicate this interpretation.
UV-B at levels relevant to ambient exposure caused a significant dose-dependent reduction in HCLE cell membrane resistance at voltages at or below resting potential (Fig. 4). UV-B exposure in this range also caused significant increases in whole-cell voltage-activated K+ currents at voltages above resting potential. This is consistent with UV-B directly or indirectly increasing the open probability of K+ channels in HCLE cells. As previously reported (Singleton et. al. 2009), increasing [K+] in the bathing solution decreases the efflux of K+ from HCLE cells through voltage-activated K+ channels. If the overall hypothesis that reducing the loss of K+ from cells inhibits apoptosis is correct, it should be possible to mimic this protective effect of high [K+]o with K+ channel modulators that decrease the open channel probability of UV-activated K+ channels. Therefore, having shown that BDS-1 and quinidine have the expected effect on apoptosis, their effect on UV-activated channel activity was investigated.
The Kv3.4 channel inhibitor BDS-1 (1 µM) reversibly blocked a large portion of the UV-activated K+ currents in every HCLE cell tested (Fig. 5). Since BDS-1 also reduced UV-induced apoptosis of HCLE cells, the simplest interpretation is that BDS-1 reduces UV-induced K+ efflux by blocking K+ channels.
Quinidine, an antiarrhythmia drug thought to function through its effects on K+ and Na+ channels (Pugsley, 2005; Schumacher et al., 2009), did not simply block K+ currents in HCLE cells. We observed slowing of channel-opening kinetics, increases in the slow-activating component of voltage-activated K+ currents and lack of effect on the resting potential when quinidine was applied to HCLE cells during whole cell recording. This shows that the effect of quinidine on intact cells is more complex than the reduction of open probability of large-conductance K+ channels from rabbit corneal epithelial cells that Rae et al.(1990, 1992) reported based on single channel recording. An alternative mechanism for the protective effect of quinidine is suggested by Schumacher et. al. (2009), who reported that quinidine caused HL-1 immortalized mouse atrial myocyte cells to internalize Kv1.5 channels. If this effect of quinidine is operative in HCLE cells, this process would reduce the number of K+ channels in the cell membrane and thereby reduce K+ efflux over longer periods of time. In our electrophysiology experiments quinidine was only applied for two or three minutes before being washed out, whereas in the caspase and TUNEL assay experiments cells were exposed to quinidine for several hours after UV-B exposure. Thus, it might be the case that quinidine reduces K+ efflux from UV-exposed HCLE cells over several hours not by directly decreasing the open probability of K+ channels at resting potentials, but rather by reducing the number of channels via which K+ can leave the cell. Schumacher’s observation provides an interesting basis for further studies of effects of quinidine on UV-induced apoptosis in corneal epithelial cells.
In summary, the data presented in this paper provide evidence that reducing K+ loss from corneal epithelial cells after exposure to UV-B inhibits apoptosis, although quinidine may protect cells from apoptosis by a mechanism unrelated to its effect on ion channels. The data lend support to our previous report that elevated [K+]o has a similar, albeit stronger and more consistent, effect on UV-B induced activation of apoptotic pathways. Taken together these observations suggest that the high K+ in the tears may contribute to protection of the cornea from ambient UV by modulating the loss of K+ from cells during UV exposure.
Acknowledgements
This work was supported by National Institutes of Health Grant R01 EY018100, National Science Foundation grant DBI-0520840 (JLU and LDH) and research fellowship support (LDH, JRL) from Calvin College. We thank Dr. Ilene Gipson, Schepens Eye Research Institute, Harvard Medical School for providing the HCLE cells and Dr. Mitchell Watsky, University of Tennessee College of Medicine, for consultation on patch-clamp recording.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atta AG, Maringoni RL, Borsio Netto JB, Silveira Filho B. Potassium and sodium levels in the initial saliva of the dog’s submandibular and parotid glands. Rev. Bras. Pesqui. Med. Bio. 1975;3:37–43. [PubMed] [Google Scholar]
- Bortner CD, Hughes FM, Cidlowski JA. A primary role for K+ and Na+ efflux in the activation of apoptosis. J. Biol. Chem. 1997;272:32436–32542. doi: 10.1074/jbc.272.51.32436. [DOI] [PubMed] [Google Scholar]
- Botelho SY, Martinez EV. Electrolytes in lacrimal gland fluid and in tears at various flow rates in the rabbit. Am. J. Physiol. 1973;225:606–609. doi: 10.1152/ajplegacy.1973.225.3.606. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Spurr-Michaud S, Argüeso P, Tisdale A, Ng TF, Russo CL. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest. Ophthalmol .Vis. Sci. 2003;44:2496–2506. doi: 10.1167/iovs.02-0851. [DOI] [PubMed] [Google Scholar]
- Hughes FM, Bortner CD, Purdy GD, Cidlowski JA. Intracellular K+ suppresses the activation of apoptosis in lymphocytes. J. Biol. Chem. 1997;272:30567–30576. doi: 10.1074/jbc.272.48.30567. [DOI] [PubMed] [Google Scholar]
- Pugsley MK, Walker MJ, Saint DA. Block of Na+ and K+ currents in rat ventricular myocytes by quinacainol and quinidine. Clin. Exp. Pharmacol. Physiol. 2005;32:60–65. doi: 10.1111/j.1440-1681.2005.04149.x. [DOI] [PubMed] [Google Scholar]
- Rae JL, Dewey J, Rae JS, Nesler M, Cooper K. Single Potassium Channels in Corneal Epithelium. Invest Ophthalmol Vis Sci. 1990;31:1799–1809. [PubMed] [Google Scholar]
- Rae JL, Dewey J, Rae JS. The large-conductance potassium ion channel of rabbit corneal epithelium is blocked by quinidine. Invest Ophthalmol Vis Sci. 1992;33:286–290. [PubMed] [Google Scholar]
- Rismondo V, Osgood TB, Leering P, Hattenhauer MG, Ubels JL, Edelhauser HF. Electrolyte composition of lacrimal gland fluid and tears of normal and vitamin A-deficient rabbits. CLAO J. 1989;15:222–229. [PubMed] [Google Scholar]
- Schumacher SM, McEwen DP, Zhang L, Arendt KL, Van Genderen KM, Martens JR. Antiarrhythmic drug-induced internalization of the atrial-specific K+ channel Kv1.5. Circ Res. 2009;19:1390–1398. doi: 10.1161/CIRCRESAHA.108.192773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton KR, Will DS, Schotanus MP, Haarsma LD, Koetje LR, Bardolph SL, Ubels JL. Elevated Extracellular Potassium Inhibits Apoptosis of Corneal Epithelial Cells Exposed to UV-B Radiation. Exp. Eye Res. 2009;89:140–151. doi: 10.1016/j.exer.2009.02.023. [DOI] [PubMed] [Google Scholar]
- Wang L, Fyffe RE, Lu L. Identification of a Kv3.4 channel in corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 2004;45:1796–1803. doi: 10.1167/iovs.03-1056. [DOI] [PubMed] [Google Scholar]
- Yu SP, Yeh CH, Sensi SL, Gwag BJ, Canzoniero LM, Farhangrazi ZS, Ying HS, Tian M, Dugan LL, Choi DW. Mediation of neuronal apoptosis by enhancement of outward potassium current. Science. 1997;278:114–117. doi: 10.1126/science.278.5335.114. [DOI] [PubMed] [Google Scholar]