Abstract
Aging is accompanied by a general deterioration of fluid cognitive processes and a reduction in resting cerebral blood flow (CBF). While the two phenomena have been observed independently, it is uncertain whether individual differences in cerebral blood flow are reliably associated with cognitive functioning in older adults. Furthermore, previous studies have concentrated primarily on gross measures of cognition and global gray matter CBF, leaving open the possibility that perfusion of specific brain regions may relate differentially to distinct cognitive domains. The present study sought to provide a more focused treatment of CBF and cognitive function in the context of aging by investigating the relationships among aging, spatial memory and resting hippocampal blood flow, both between and within younger and older adult groups. Blood flow was quantified using a novel Flow-Enhanced Signal Intensity (FENSI) technique which provides a localized, functionally-relevant measure of volumetric flow across a given unit area. As expected, we found that aging was associated with poorer spatial memory and reduced resting CBF. Moreover, hippocampal blood flow was positively correlated with spatial memory performance in the older adult group, suggesting that increased blood flow to the hippocampus is associated with superior memory performance in older adults. These results demonstrate a region-specific CBF—cognition relationship and thereby offer new insight into the complex connection between the aging brain and behavior.
Keywords: hippocampus, cerebral blood flow, brain perfusion, cognition, memory, aging
1. Introduction
Aging is associated with a general decline in cognitive performance and a pattern of diffuse neurophysiological changes. Included among the changes in physiology is a significant decrease in resting gray matter cerebral blood flow (CBF) in older adults (Bentourkia et al., 2000; Leenders et al., 1990; Parkes et al., 2004; Slosman et al., 2001). Given the age-related deterioration of cognitive function and physiological integrity, it is only natural to cerebral blood flow associated with cognitive performance in aging? More precisely, is CBFinquire after the relationship between these two reliable phenomena. That is, is resting differentially associated with certain cognitive domains in aging? Even more specifically, is local CBF differentially associated with particular cognitive domains in aging?
In response to the first question, a handful of studies have interrogated the relationship between cognitive function and global gray matter perfusion in elderly adults. The results are conflicting. While some studies have reported positive correlations between cognition and resting CBF (Rabbitt et al., 2006), others have found negative associations (Bertsch et al., 2009) and still others have failed to find any relationship at all (Meyer et al., 1988; Poels et al., 2008).
With regard to the second question, Rabbitt et al. (2006) investigated the predictive power of carotid and basilar artery blood flow on performance on ten different cognitive tasks assessing fluid intelligence (gf), information-processing speed, memory and executive function in older adults. They found that CBF negatively predicted scores on eight of the tests and accounted for up to 36% of the age-associated variance in the speed scores. Therefore, it was concluded that CBF is a sensitive marker for neurophysiological changes
In light of the results of the aforementioned studies, particularly that of Rabbitt et al., one may ask whether a physiological index can be identified for distinct aspects of cognition. While global CBF has its largest influence on information processing speed, is it possible that local measures of blood flow could serve as a more powerful surrogate for other cognitive functions? To our knowledge, no study to date has explored associations between local blood flow and domain-specific cognitive performance within the context of nonpathological aging. Therefore, one cannot exclude the possibility that blood flow to a particular brain structure may have differential effects on cognitive processes involving the structure.
Our study was designed precisely to address this gap in the literature. More pointedly, our fundamental question was: is resting hippocampal blood flow correlated with spatial memory performance in aging? Our objectives were threefold: 1) to confirm age-related differences in spatial memory, 2) to examine age-related differences in resting hippocampal blood flow, and 3) to explore the relationship between individual differences in resting hippocampal blood flow and spatial memory as a consequence of aging. Numerous studies have established the association between spatial memory and the hippocampus, both in structure and in function (Erickson et al., 2009; Glikmann-Johnston et al., 2008; Ross and Slotnick, 2008). We chose a spatial short-term memory paradigm with a parametric manipulation in which performance has been shown to vary with age and hippocampal integrity (Erickson et al., 2009; Greenwood et al., 2005).
In order to demonstrate the specificity of spatial memory as opposed to general cognitive slowing, we also incorporated a measure of information processing speed in our study, i.e. a choice reaction time task. Previous studies have revealed that age-related slowing of processing speed functionally determines declines in several cognitive abilities including memory (Rabbitt et al., 2007; Salthouse, 1996). Therefore, we included a choice reaction time paradigm without a memory component to which we could compare our spatial memory measures and also use as a covariate in our statistical analyses.
With regard to blood flow, we targeted the hippocampus as our region of interest and selected the brainstem as a control area. Measurement of flow through the brainstem would be instrumental in establishing a selective association between hippocampal blood flow and spatial memory. We chose the brainstem as a control region because its involvement in cognitive processes does not overlap with that of the hippocampus, thereby providing an unbiased measurement of blood flow. Furthermore, the brainstem was clearly visible on the imaging slice and comparable in size to that of the hippocampus (see Figure 3), thus avoiding uncertainty and error associated with the localization of smaller brain structures.
Fig 3.
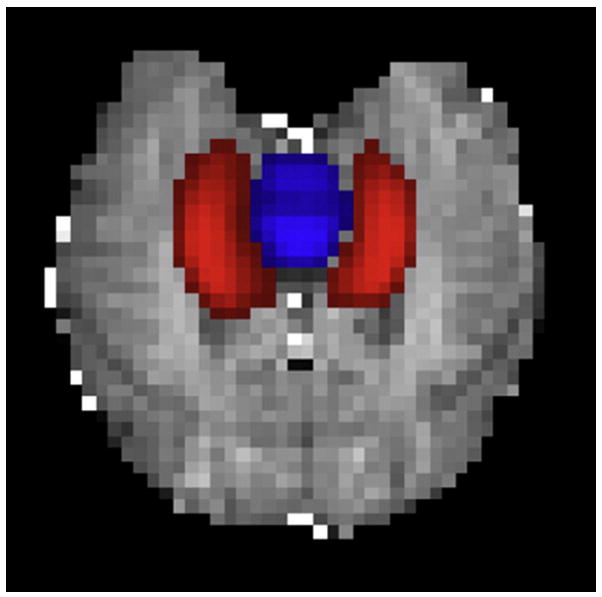
An example of one participant’s FENSI slice overlaid with her subject-specific hippocampal and brainstem masks.
To calculate blood flow through the regions of interest, we utilized a novel measurement technique: the Flow-Enhanced Signal Intensity (FENSI) method (Sutton et al., 2007). The FENSI technique computes volumetric flux (Q), i.e. the rate of volume flow across a unit area, in units of mL/min/cm2. An imaging slice is oriented in the brain to maximize the field of view of the area of interest, and movement of blood perpendicular to the slice is tracked over time to yield a value that represents highly-localized blood flow. The FENSI method was particularly advantageous to our study as it is non-invasive, is able to assess flow through a full axial cross-section of the hippocampus, and makes no assumptions about the transit time or path of blood; it is thus well-suited for aging studies (see D’Esposito et al., 2003 for a list of cerebrovascular alterations accompanying aging).
In view of the preexisting literature, we predicted significant age-related differences in spatial memory performance between the younger and older adult groups, with older adults performing more poorly on the task. We also expected a negative correlation between age and our measure of memory in elderly adults. Regarding aging and hippocampal blood flow, we hypothesized that elderly adults would exhibit reduced blood flow through the hippocampus and brainstem compared to younger adults and that within both age groups, age and blood flow would be negatively correlated through the two brain regions, thus confirming a global decrease in CBF (Parkes et al., 2004; Slosman et al., 2001). Finally, we predicted that within the older adult group, hippocampal blood flow, but not flow through the brainstem, would be positively associated with spatial memory performance. Such a finding would establish specificity in the relationship between local blood flow and a selective aspect of cognition in nonpathological aging.
2. Results
2.1. Cognitive performance and aging
2.1.1. Age-group differences in choice reaction time
The mean reaction times (RTs) and accuracy percentages for the younger and older adult groups in the choice reaction time task are displayed in Table 1. An independent-samples t test revealed a main effect of age group on reaction time, t(46) = −5.64, p < 0.001, with older adults responding more slowly than younger adults.
Table 1.
Mean Reaction Time and Accuracy ± One Standard Deviation for the Choice Reaction Time Task and Each Condition of the Spatial Memory Task for the Younger and Older Adult Groups
| Measures | Younger adults | Older adults |
|---|---|---|
| CRT reaction time | 470.5 ± 67.3 | 595.6 ± 85.2 |
| CRT accuracy | 0.97 ± .03 | 0.97 ± .04 |
| SM reaction time | ||
| 1 item | 614.6 ± 141.0 | 796.2 ± 168.4 |
| 2 items | 691.7 ± 156.3 | 917.5 ± 168.4 |
| 3 items | 754.8 ± 164.1 | 1005.0 ± 172.4 |
| Average | 687.0 ± 147.9 | 906.2 ± 163.9 |
| SM accuracy | ||
| 1 item | 0.94 ± 0.07 | 0.87 ± 0.13 |
| 2 items | 0.89 ± 0.07 | 0.81 ± 0.13 |
| 3 items | 0.87 ± 0.08 | 0.73 ± 0.14 |
| Average | 0.90 ± 0.07 | 0.80 ± 0.12 |
Note. Reaction time was measured in milliseconds and accuracy in percent correct. CRT, choice reaction time; SM, spatial memory.
2.1.2. Age-group differences in spatial memory
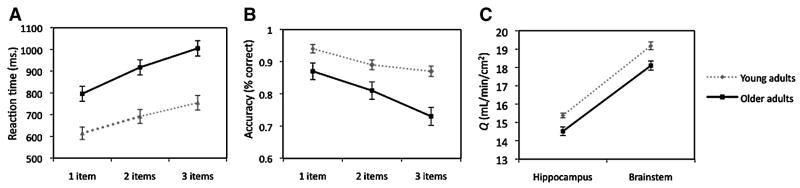
The mean reaction times and accuracy scores for the 1-, 2-, and 3-item conditions of the spatial memory task are presented in Table 1. Overall, the younger adults responded more quickly and more accurately on all conditions of the task (see Figure 1A). A repeated measures analysis of variance (ANOVA) confirmed the main effect of age group on reaction time, F(1, 46) = 23.65, p < 0.001, with faster RTs in the younger adult group. There was also a main effect of task condition, F(1.67, 76.71) = 128.19, p < 0.001 (after applying Greenhouse-Geisser correction, ε=0.83, due to violation of sphericity), such that participants responded more slowly with each increase in item number. Lastly, an age × condition interaction was observed, F(1.67, 76.71) = 5.07, p < 0.05 (after applying Greenhouse-Geisser correction), with older adults exhibiting a greater cost than younger adults with the parametric increase in load.
Fig. 1.
Graphs depicting age-group differences in spatial memory task performance and local cerebral blood flow. A. Mean reaction time ± one standard error for each memory task condition and age group, B. Mean accuracy ± one standard error for each memory task condition and age group, C. Mean local blood flow ± one standard error for each brain region and age group.
Regarding accuracy, there was a main effect of age group, F(1, 46) = 12.68, p < 0.001, with younger adults performing more accurately than older adults (see Figure 1B and Table 1). There was also a main effect of task condition, F(2, 92) = 37.79, p < 0.001, such that accuracy decreased with each increase in item load. Furthermore, there was a significant interaction between age group and task condition, F(2, 92) = 3.96, p < 0.05; that is, accuracy decreased more for the older than younger adults with increasing load.
2.1.3. Within-age-group partial correlations between age and spatial memory
Within the younger adult group, there were no significant correlations between age and spatial memory performance as indexed by reaction time either before or after removing the variance associated with choice reaction time (before correction: r(24) = −0.09, p = 0.67; after correction: r(21) = −0.08, p = 0.73 (see Table 3)). Within older adults however, age was positively correlated with reaction time after controlling for choice RT (r(21) = 0.48, p < 0.05 (see Table 4)) and marginally correlated before correction (r(24) = 0.36, p = 0.08). In other words, after accounting for the variance related with processing speed (i.e. choice RT), age was associated with slower reaction times on the spatial memory task within the older adult group. There were no significant correlations involving accuracy in either age group.
Table 3.
Partial Correlation Coefficients for Age, Cerebral Blood Flow and Spatial Memory Performance in the Younger Adult Group
| Age | CRT | SM RTa | SM Acc | |
|---|---|---|---|---|
| Age | 1.00** | −0.05 | −0.08 | −0.003 |
| Hipp Qb | 0.45* | 0.21 | −0.04 | 0.23 |
| BrStem Qb | 0.59** | 0.13 | −0.003 | 0.31 |
Note. CRT, choice reaction time; SM, spatial memory; RT, reaction time; Acc, accuracy; Hipp, hippocampus; BrStem, brainstem; Q, blood flow.
After removing the variance associated with choice reaction time
After removing the variance associated with sex
p < 0.05,
p < 0.01
Table 4.
Partial Correlation Coefficients for Age, Cerebral Blood Flow and Spatial Memory Performance in the Older Adult Group
| Age | CRT | SM RTa | SM Acc | |
|---|---|---|---|---|
| Age | 1.00** | 0.41* | 0.48* | 0.17 |
| Hipp Qb | −0.48* | −0.02 | −0.51* | 0.19 |
| BrStem Qb | −0.41* | −0.22 | 0.06 | −0.16 |
Note. CRT, choice reaction time; SM, spatial memory; RT, reaction time; Acc, accuracy; Hipp, hippocampus; BrStem, brainstem; Q, blood flow.
After removing the variance associated with choice reaction time
After removing the variance associated with sex
p < 0.05,
p < 0.01
2.2. Resting cerebral blood flow and aging
2.2.1. Age-group differences in resting cerebral blood flow
Mean values for resting blood flow through the hippocampus and the brainstem by age group are listed in Table 2. For both regions of the brain, younger adults exhibited greater blood flow than older adults (see Figure 1C). A repeated measures ANOVA confirmed the main effect of age on local CBF, F(1, 46) = 16.34, p < 0.001. There was also a main effect of region, F(1, 46) = 584.16, p < 0.001, with greater blood flow through the brainstem compared to the hippocampus.
Table 2.
Mean Regional Cerebral Blood Flow ± One Standard Deviation for the Younger and Adult Groups
| Brain Region | Younger adults | Older adults |
|---|---|---|
| Hippocampus | 15.20 ± 0.69 | 14.27 ± 1.12 |
| Brainstem | 19.18 ± 1.05 | 18.10 ± 1.44 |
Note. Blood flow was measured in mL/min/cm2
2.2.2. Within-age-group partial correlations between age and resting cerebral blood flow
Within younger adults, there was a positive correlation between age and blood flow through the hippocampus and brainstem after removing the variance associated with sex (hippocampus: r(21) = 0.45, p < 0.05; brainstem: r(21) = 0.59, p < 0.01 (see Table 3)). Within older adults however, there was a negative correlation between age and blood flow through both brain regions (hippocampus: r(21) = −0.48, p < 0.05; brainstem: r(21) = −0.41, p < 0.05 (see Table 4)). This suggests that increasing age is associated with greater resting CBF in younger adults and reduced resting CBF in older adults.
2.3. Resting cerebral blood flow and cognitive performance
2.3.1. Within-age-group partial correlations between resting cerebral blood flow and choice reaction time
There were no significant correlations between resting cerebral blood flow and choice reaction time in either younger or older adults (see Tables 3 and 4).
2.3.2. Within-age-group partial correlations between resting cerebral blood flow and spatial memory
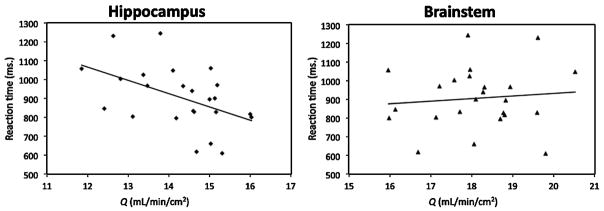
Partial correlations in the younger adult group did not reveal any reliable associations between CBF and spatial memory reaction time either before or after removing the variance associated with choice reaction time (before correction: hippocampus: r(21) = 0.10, p = 0.64; after correction: hippocampus: r(20) = −0.04, p = 0.88 (see Table 3)). Within the older adult group however, blood flow through the hippocampus was negatively correlated with reaction time on the spatial memory task (before correction: r(21) = −0.50, p < 0.05;after correction: r(20) = −0.51, p < 0.05 (see Table 4 and Figure 2)). In contrast, blood flow through the brainstem was not related to spatial memory performance either before or after controlling for processing speed (before correction: r(21) = 0.09, p = 0.69;after correction: r(20) = 0.06, p = 0.80 (see Table 4 and Figure 2)). Regarding accuracy, local blood flow through the hippocampus and brainstem were not correlated with spatial memory accuracy in either age group (see Tables 3 and 4).
Fig 2.
Scatterplots revealing associations between spatial memory performance and increased blood flow through the hippocampus, but not the brainstem, in older adults.
2.3.3. Age-group differences in resting hippocampal blood flow and spatial memory
The application of Fisher’s z transformation to compare the correlation coefficients between hippocampal blood flow and spatial memory reaction time between the younger and older adult groups yielded a z-score of 1.71, p < 0.05. To be exact, hippocampal blood flow was more predictive of spatial memory reaction time in older than younger adults.
3. Discussion
The specific aims of the present study were to investigate (1) age-related differences in spatial memory, (2) age-related differences in resting hippocampal blood flow, and (3) the relationship between individual differences in resting hippocampal blood flow and spatial memory as a result of aging. Aging differences in cognitive function and cerebral blood flow were examined both between younger and older adult groups as well as within the respective groups. The association between resting hippocampal blood flow and spatial memory first was considered separately for younger and older adults, and then with both age groups together in one statistical model.
3.1. Age-related differences in spatial memory
There was a significant difference between the two age groups with respect to both reaction time and accuracy on the spatial memory task. Overall, younger adults were faster and more accurate in performing the task. We also observed a significant slowing in choice reaction time in older adults. These findings are consistent with previous studies that have documented a steady decline in fluid cognitive functions with increased age (Park, 2000; Schaie, 2005) and specifically in processing speed (Salthouse, 1996) and short-term memory (Verhaeghen et al., 1993). We also found that older adults experienced a larger cost than younger adults on the more challenging conditions of the memory task. This is in line with studies that have shown a differential detriment to older adults with increased task difficulty (Erickson et al, 2007; Prakash et al., in press; Smith et al., 2001).
Within the healthy elderly group, aging was associated with longer reaction times on the spatial memory task both before and after removing the variance associated with processing speed. Therefore, the slower responses of older adults could be attributed to specific processes underlying the memory task, namely remembering the locations of objects. We did not find a relationship between age and spatial memory accuracy in our paradigm which may be due to the smaller variability in accuracy scores compared to reaction time measurements (see Table 1). In sum, aging was associated with slowing on the spatial memory task both between age groups as well as within the group of older adults, and reduced accuracy comparing younger and older adults.
3.2. Age-related differences in resting hippocampal blood flow
There was a clear difference between the two age groups in resting cerebral blood flow in the measured brain structures (hippocampus and brainstem). This global decrease is consistent with several experiments that have reported a relationship between aging and an overall reduction in gray-matter CBF (Bertsch et al., 2009; Leenders et al., 1990; Parkes et al., 2004; Slosman et al., 2001). The effect can be attributed, at least partially, to cerebrovascular alterations accompanying aging, including but not limited to ultrastructural changes in blood vessels, reduced vascular reactivity and tortuous cerebral vessels (D’Esposito et al., 2003).
As hypothesized, within the older adult group, there appeared significant negative correlations between age and blood flow through the hippocampus and brainstem. Within the younger adult group, however, a positive association emerged between age and resting blood flow through both regions. This latter finding was somewhat surprising given that a number of studies have reported a gradual decline in resting cerebral blood flow over the entire adult lifespan (Parkes et al., 2004; Slosman et al., 2001, Zhao et al., 2007). It should be noted though, that our study targeted two discrete age groups as opposed to a continuous distribution across the adult life, thus restricting comparability of the results. It is also possible that a quadratic function in the form of an inverted U may best capture the relationship between age and cerebral blood flow as opposed to a strictly linear one. Future studies encompassing a wider age range may be able to speak to the association of age and CBF from childhood into old-old adulthood.
3.3. Resting hippocampal blood flow and spatial memory
Younger and older adults first were analyzed separately to examine the relationship between individual differences in resting hippocampal blood flow and cognitive functioning. We did not find significant correlations between blood flow and information processing speed (i.e. performance on the choice reaction time task) in either age group which was unexpected in our older adult group (see Rabbitt et al., 2006). The discrepancy may be due to the disparate measures of blood flow in the two studies, i.e. global CBF in Rabbitt et al. vs. local CBF in the present study.
Turning our attention to correlations between local blood flow and spatial memory, we found that blood flow measurements through the hippocampus were negatively correlated with reaction time on the spatial memory task in older adults. The results were significant both before and after removing the variance from choice reaction time. In contrast, blood flow through the brainstem was not associated with memory performance. This dissociation suggests that in healthy older adults, spatial memory is coupled specifically with blood flow through the hippocampus, a structure that is implicated in memory for object locations (Postma et al., 2008; Smith and Milner, 1981). Moreover, the observed effects cannot be attributed merely to aging as evidenced by the widespread decrease in blood flow associated with increasing age, but local relationship between hippocampal blood flow and spatial memory.
Our pattern of results argues for a specific relationship between blood flow through the hippocampus and spatial memory in older adults. To summarize, hippocampal blood flow was correlated with spatial memory performance, but not with measures of processing speed, and brainstem blood flow was not correlated with spatial memory (see Table 4). We recognize that the lack of associations in the latter cases does not prove the local CBF—cognition link, but it does provide an interesting contrast and impetus for future research.
The identical analysis conducted in the group of younger adults did not yield any significant correlation between hippocampal blood flow and spatial memory performance. One possible reason for the null outcome in the younger adults is the smaller variances in blood flow and cognitive performance values in the younger group (see Tables 1 and 2). The limited individual differences in resting blood flow and memory performance may not be sufficient to yield statistically significant associations with our spatial memory indicators. It is also conceivable that decrements in cognitive performance may only manifest themselves behaviorally when CBF falls below a certain threshold, a threshold that is well below the normal range of values exhibited by healthy young adults.
A comparison of the correlation between hippocampal blood flow and spatial memory between younger and older adults revealed that hippocampal blood flow was more predictive of spatial memory reaction time in older compared to younger adults. This finding indicates that hippocampal blood flow may be a particularly sensitive marker in older adults to track declines in spatial memory performance.
Our discovery of a specific relationship between resting hippocampal blood flow and spatial memory in aging makes a novel contribution to an area that heretofore has focused primarily on global CBF values and gross measures of cognition. We intentionally targeted a particular structure of the brain, i.e. the hippocampus, and explored the association between resting blood flow through the structure and performance on a task utilizing the structure, i.e. spatial memory. Our results revealing a positive correlation between hippocampal blood flow and spatial memory in older adults are consistent with the aging literature that has shown a positive relationship between resting gray-matter CBF and cognitive functioning. In addition, our findings suggest that local blood flow may be a more precise marker of domain-specific cognitive decline as opposed to general CBF which exerts its greatest predictive power on processing speed (Rabbitt et al, 2006). In this way, the results reported in our study extend the preexisting body of knowledge while making a unique contribution to the inquiry of the implementation of cognitive processes in the human brain with respect to cerebral blood flow and aging.
3.4. Limitations
In the evaluation of our results and conclusions, it is prudent to acknowledge the limitations of our study. Firstly, we focused on two dichotomous age groups within the adult population, therefore restricting the interpretation and generalizability of our results. Future studies should incorporate a continuous distribution of adults across the lifespan to look at general trends and correlations in the broader context of adult aging. Secondly, our particular memory task precluded the acquisition of pure accuracy measures due to the restricted response window. A paradigm with a more generous response period would be more appropriate and even better still, several independent tests from which a memory construct could be created (Rabbitt et al., 2007). A third point of caution concerns the FENSI technique itself. While the experiment was well-controlled, the flow signal could have been adversely affected by magnetization transfer effects and subject motion. Progress is currently being made to address these issues. Finally, while we incorporated a control region, i.e. the brainstem, in our study to demonstrate a dissociation between spatial memory and blood flow through different areas of the brain, we did not have measurements of global CBF to which we could compare the differential effects of aging. It would be fruitful to quantify basilar and carotid arterial flow in parallel with local perfusion in future experiments to determine if local changes mirror global changes or if they are disproportionately greater than gross CBF reductions.
Despite these potential limitations however, we believe that our data are consistent with the extant literature on blood flow and aging and furthermore, that they augment this literature by revealing a specific relationship among aging, hippocampal blood flow and performance on a spatial memory task.
3.5. Conclusion
The present study demonstrated that aging is associated with inferior spatial memory and reduced resting cerebral blood flow. Moreover, we showed that resting hippocampal blood flow is positively correlated with spatial memory performance in elderly adults, suggesting a specific relationship between local blood flow and a particular aspect of cognition in the context of aging. Future studies should be designed to quantify blood flow through different brain structures and their associated cognitive abilities in comparison with gross CBF changes to strengthen the local versus global distinction.
4. Experimental Procedure
4.1. Participants
Twenty-four younger adults (16 female, mean age=22.96 years, SD=3.45, range=18–30 years) and twenty-four older adults (14 female, mean age=70.83, SD=5.99, range=61–86 years) participated in the study. All participants were pre-screened for suitability in the magnetic resonance imaging environment, e.g. no metallic implants that could interfere with the magnetic field or cause injury, no claustrophobia, no history of head trauma, and no neurological or neuropsychiatric illness. Exclusionary criteria included a score of less than 24 on the Mini-Mental State Exam (high score of 30), a score of greater than 18 on the Beck Depression Inventory (indicating moderate to severe depression), left- or equal-handedness as assessed by the Edinburgh Handedness Inventory, regular ingestion of any medication with neurological or psychological effects, a history of cerebrovascular disease or diabetes, as well as a resting blood pressure that exceeded 140/90 mmHg. Adults taking anti-hypertensive medication were not excluded from the experiment. The study was approved by the Institutional Review Board of the University of Illinois and all participants gave written consent to take part in the study.
4.2. Experimental design and procedure
Each participant underwent two testing sessions: a neuropsychological assessment followed by a FENSI scan in a 3 Tesla MR system. The sessions were separated by no more than two weeks (mean days elapsed=4.63, SD=2.97).
4.3. Cognitive measures
4.3.1. Spatial memory task
In this task, participants were presented with one, two, or three black dots that appeared at randomly-selected locations on the screen for 500 ms. Following the dot display, a fixation cross appeared for 3 s. At the end of the delay, a single red test dot appeared on the screen, either at the same location as one of the previous black dots (match), or at a novel location (nonmatch). Subjects had 2 s to decide whether the red test dot was a match or nonmatch to the spatial location of one of the initially presented dots and were asked to indicate their response by pressing the designated key on a computer keyboard (‘x’=nonmatch, ‘m’=match). Participants were instructed to answer as quickly and accurately as possible. The entire task consisted of 120 trials (40 trials for each set size divided into 20 match and 20 nonmatch conditions) and was preceded by several practice trials to acquaint the participants with the protocol. Reaction time and accuracy were recorded.
4.3.2. Choice reaction time task
Each trial began with the presentation of a centrally-located fixation cross on a computer screen which was displayed for 150, 350, 550 or 750 ms. Following the randomly selected interval, the letter “A” or “B” appeared either to the left or to the right of the cross. Subjects were instructed to respond as quickly as possible to the appearance of the letter stimulus by pressing the appropriate key (‘z’=‘A’, ‘m’=‘B’) while keeping their gaze focused on the fixation cross. The trial terminated with the subject’s response or the passage of 4 seconds, whichever occurred first. The entire task contained 8 practice trials and 48 experimental trials. Reaction time and accuracy were recorded.
4.4. Imaging data acquisition
The FENSI pulse sequence consisted of a series of 90° radiofrequency (RF) pulses with spoiling gradients followed by a spin-echo echo planar imaging (EPI) readout (Sutton et al., 2007). Briefly, two images were obtained in succession, one containing a “saturation module” of 30 RF pulses with spoiling gradients, and the other with only one RF pulse and spoiling gradient. The images were then subtracted to yield a 2D image representing blood velocity across a unit area of brain tissue (measured in mL/min/cm2).
Prior to the FENSI acquisition, a 10 mm thick slice of the brain was selected (heretofore referred to as the imaging slice) from which blood flow (Q) would be calculated. In the center of the imaging slice was situated a thinner slice (0.4 mm thick) which would serve as the target of the saturation module. During the saturation period, spins within the thin slice were saturated using RF pulses and allowed to flow or diffuse freely into the surrounding imaging slice. The molecules were quickly replaced by new molecules, which, in turn, were saturated. At the end of the module, the imaging slice was read, the resulting image revealing the quantity of spins that had been at some point during the saturation period within the thin volume. In this way, the FENSI technique was able to track slow-flowing blood in the smaller vessels.
The FENSI scan was a spin-echo sequence with a repetition time (TR) of 2000 ms, an echo time (TE) of 44 ms, a flip angle of 90°, and a total acquisition of 80 volumes. The imaging slice was centered in the hippocampal axial plane to maximize the view of the hippocampal formation (Beaurain et al., 1994). In order to locate each individual’s hippocampal axial plane, a high-resolution T2-weighted image was acquired (TR=2300 ms, TE=98 ms, flip angle=150°, slice thickness=2 mm) with 11 contiguous axial slices collected parallel to the long axis of the hippocampus.
To assist with registration and anatomical identification, two additional scans of the brain were taken: a T2*-weighted image with Blood Oxygenation Level Dependent (BOLD) contrast, and a high-resolution T1-weighted image. The T2*-weighted image was acquired using a fast echo-planar imaging (EPI) sequence (TR=2000 ms, TE=25 ms, flip angle=80°, slice thickness=3 mm, 10% gap) and contained 37 slices collected in an ascending fashion in the hippocampal axial plane. The image was oriented such that the center slice (slice number 19) corresponded to the FENSI slice. Two functional volumes were collected. The high-resolution T1-weighted brain image was acquired using a 3D MPRAGE (Magnetization Prepared Rapid Gradient Echo) protocol (TR=1800 ms, TE=3.87 ms, field of view=256 mm, flip angle=8°, slice thickness=1.3 mm) and consisted of 144 axial slices, parallel to the AC-PC axis. All images were collected on a Siemens Allegra 3T head-only scanner (Erlangen, Germany).
4.5. Imaging data processing
4.5.1. FENSI processing
FENSI images were analyzed offline using an in-house MATLAB program. Adjacent pairs of images were subtracted (control – tag) resulting in 40 volumes with an effective TR of 4 s. One 2D slice per individual (Q map) was generated by averaging together the 40 images on a voxel-by-voxel basis, thus representing mean blood flow for each voxel over the course of the scan.
4.5.2. Hippocampal localization procedure
For each subject, the left and right hippocampi were isolated using FMRIB’s automated subcortical segmentation program (FIRST v1.2; http://www.fmrib.ox.ac.uk/fsl) as applied to the anatomical T1-weighted image. Boundary correction was performed to exclude boundary voxels that were classified as not belonging to the hippocampus according to FMRIB’s FAST-based tissue classification system.
To locate each individual’s hippocampi on the FENSI slice, first the T2*-weighted image was registered to the subject’s MPRAGE image using FMRIB’s linear image registration tool (FLIRT v.5.5). Next, the reverse transformation was applied to the individual’s hippocampi to move the hippocampi from structural- to functional-image space. Finally, subject-specific hippocampal masks were created according to the intersection of the hippocampi and the center slice of the T2*-weighted image which corresponded to the FENSI slice. The identical procedure was applied to the brainstem to obtain subject-specific brainstem masks (see Figure 3).
To calculate an average value of blood flow for each brain structure on an individual-by-individual basis, we multiplied the appropriate subject-specific mask by the subject’s Q map and averaged over the non-zero voxels. In this way, mean values representing blood flow through the hippocampus and brainstem were computed for each participant.
4.6. Statistical analyses
4.6.1. Spatial memory and aging
The mean reaction times and accuracy percentages of all correct trials for the three set sizes of the spatial memory task were calculated separately. To investigate between-group age differences in spatial memory, we performed repeated measures analyses of variance (ANOVA) with RT and then accuracy at the three levels as the within-subjects factor and age group as the between-subjects factor. To examine within-group age differences in reaction time for the younger and older adult groups, we conducted partial correlations in each group, removing the variance associated with choice reaction time. We also performed bivariate correlations between age and reaction time without controlling for processing speed and we report the uncorrected values in section 2.1.3. To test for within-group age differences in accuracy, we carried out nonparametric bivariate correlations separately for each group. In all of our within-group analyses involving spatial memory performance, we used average values for reaction time since our main hypotheses are concerned with spatial memory broadly and its relationship with hippocampal blood flow. We have no reason to believe that the association is load-dependent given that all three conditions of the task involve spatial memory.
4.6.2. Resting hippocampal blood flow and aging
We performed repeated measures ANOVA with brain region as the within-subjects factor and age group as the between-subjects factor to assess differences in resting cerebral blood flow between younger and older adults. For within-group analyses, we conducted partial correlations among age, hippocampal blood flow and brainstem blood flow, removing the variance associated with sex since previous studies have demonstrated disparate CBF values in males and females (Parkes et al., 2004; Van Laere et al., 2001; Zhao et al., 2007). We chose to use an average value of hippocampal blood flow for each participant since our hypotheses do not involve any predictions about hemispheric differences.
4.6.3 Resting hippocampal blood flow and spatial memory
As the first step in our analysis, we carried out partial correlations separately in the younger and older adult groups. The variables that we analyzed were blood flow through each brain region of interest (hippocampus and brainstem), spatial memory reaction time, and spatial memory accuracy. The covariates that were entered were sex and choice reaction time (note: CRT was added only in the analyses involving spatial memory reaction time). We also report correlations prior to correcting for information processing speed in section 2.3.2. We did not control for age since age is closely related to both resting blood flow and spatial memory, and doing so would inappropriately dilute our variables of interest (see Miller and Chapman, 2001). To further make the case for a specific hippocampal blood flow—spatial memory relationship, we performed bivariate correlations between local blood flow and choice reaction time within each age group.
To examine whether the correlation coefficients between hippocampal blood flow and spatial memory reaction time were significantly different from each other in the younger and older adult groups, we performed Fisher’s z transformation (Fisher, 1921). From this calculation, we were able to compute a z-score that represents the difference between the two r values and thereby test the null hypothesis that the correlation coefficient is the same in the two age groups.
Acknowledgments
The authors would like to thank the following people for their assistance with data collection: Nancy Dodge. Holly Tracy, Matt VanPatter, Brittany Furler and Sandra Pahnke. This research was supported by grants from the National Institute on Aging (RO1 AG25667 and RO1 AG25032).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beaurain J, Dormont D, Semah F, Hasboun D, Baulac M. Hippocampal formations imaging with axial sections parallel to their longitudinal axis. Magn Reson Imaging. 1994;12:139–148. doi: 10.1016/0730-725x(94)92361-2. [DOI] [PubMed] [Google Scholar]
- Bentourkia M, Bol A, Ovanoiu A, Labar D, Sibomana M, Coppens A, et al. Comparison of regional cerebral blood flow and glucose metabolism in the normal brain: Effect of aging. J Neurol Sci. 2000;181:19–28. doi: 10.1016/s0022-510x(00)00396-8. [DOI] [PubMed] [Google Scholar]
- Bertsch K, Hagemann D, Hermes M, Walter C, Khan F, Naumann E. Resting cerebral blood flow, attention, and aging. Brain Res. 2009;1267:77–88. doi: 10.1016/j.brainres.2009.02.053. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: A challenge for neuroimaging. Nat Rev Neurosci. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Wadhwa R, Bherer L, Peterson M, Scalf P, et al. Training-induced plasticity in older adults: Effects of training on hemispheric asymmetry. Neurobiol Aging. 2007;28:272–283. doi: 10.1016/j.neurobiolaging.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Fisher RA. On the “probable error” of a coefficient of correlation deduced from a small sample. Metron. 1921;1:3–32. [Google Scholar]
- Glikmann-Johnston Y, Saling MM, Chen J, Cooper KA, Beare RJ, Reutens DC. Structural and functional correlates of unilateral mesial temporal lobe spatial memory impairment. Brain. 2008;131:3006–3018. doi: 10.1093/brain/awn213. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Lambert C, Sunderland T, Parasuraman R. Effects of Apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: Results from the National Institute of Mental Health’s BIOCARD study. Neuropsychology. 2005;19:199–211. doi: 10.1037/0894-4105.19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenders KL, Perani D, Lammertsma AA, Heather JD, Buckingham P, Healy M, et al. Cerebral blood flow, blood volume and oxygen utilization: Normal values and effect of age. Brain. 1990;113:27–47. doi: 10.1093/brain/113.1.27. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Rogers RL, Judd BW, Mortel KF, Sims P. Cognition and cerebral blood flow fluctuate together in multi-infarct dementia. Stroke. 1988;19:163–169. doi: 10.1161/01.str.19.2.163. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Park DC. The basic mechanisms accounting for age-related decline in cognitive function. In: Park D, Schwartz N, editors. Cognitive Aging: A Primer. Psychology Press; Philadelphia: 2000. pp. 3–21. [Google Scholar]
- Parkes LM, Rashid W, Chard DT, Tofts PS. Normal cerebral perfusion measurements using arterial spin labeling: Reproducibility, stability, and age and gender effects. Magn Reson Med. 2004;51:736–743. doi: 10.1002/mrm.20023. [DOI] [PubMed] [Google Scholar]
- Poels MM, Ikram MA, Vernooij MW, Krestin GP, Hofman A, Niessen WJ, et al. Total cerebral blood flow in relation to cognitive function: The Rotterdam scan study. J Cereb Blood Flow Metab. 2008;28:1652–1655. doi: 10.1038/jcbfm.2008.62. [DOI] [PubMed] [Google Scholar]
- Postma A, Kessels RP, van Asselen M. How the brain remembers and forgets where things are: The neurocognition of object-location memory. Neurosci Biobehav Rev. 2008;32:1339–1345. doi: 10.1016/j.neubiorev.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Prakash RS, Erickson KI, Colcombe SJ, Kramer AF. Age-related differences in the involvement of the prefrontal cortex in attentional control. Brain Cogn. doi: 10.1016/j.bandc.2009.07.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt P, Mogapi O, Scott M, Thacker N, Lowe C, Horan M, et al. Effects of global atrophy, white matter lesions, and cerebral blood flow on age-related changes in speed, memory, intelligence, vocabulary, and frontal function. Neuropsychology. 2007;21:684–695. doi: 10.1037/0894-4105.21.6.684. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Scott M, Thacker N, Lowe C, Jackson A, Horan M, et al. Losses in gross brain volume and cerebral blood flow account for age-related differences in speed but not in fluid intelligence. Neuropsychology. 2006;20:549–557. doi: 10.1037/0894-4105.20.5.549. [DOI] [PubMed] [Google Scholar]
- Ross RS, Slotnick SD. The hippocampus is preferentially associated with memory for spatial context. J Cogn Neurosci. 2008;203:432–446. doi: 10.1162/jocn.2008.20035. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Schaie KW. What can we learn from longitudinal studies of adult development? Res Hum Dev. 2005;23:133–158. doi: 10.1207/s15427617rhd0203_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slosman DO, Chicherio C, Ludwig C, Genton L, de Ribaupierre S, Hans D, et al. 133Xe SPECT cerebral blood flow study in a healthy population: Determination of t-scores. J Nucl Med. 2001;42:864–870. [PubMed] [Google Scholar]
- Smith EE, Geva A, Jonides J, Miller A, Reuter-Lorenz P, Koeppe RA. The neural basis of task-switching in working memory: Effects of performance and aging. Proc Natl Acad Sci USA. 2001;98:2095–2100. doi: 10.1073/pnas.98.4.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Milner B. The role of the right hippocampus in the recall of spatial location. Neuropsychologia. 1981;19:781–793. doi: 10.1016/0028-3932(81)90090-7. [DOI] [PubMed] [Google Scholar]
- Sutton BP, Ouyang C, Ching BL, Ciobanu L. Functional imaging with FENSI: Flow-enhanced signal intensity. Magn Reson Med. 2007;58:396–401. doi: 10.1002/mrm.21325. [DOI] [PubMed] [Google Scholar]
- Van Laere K, Versijpt J, Audenaert K, Koole M, Goethals I, Achten E, et al. 99mTc-EVD brain perfusion SPET: Variability, asymmetry and effects of age and gender in healthy adults. Eur J Nucl Med. 2001;28:8680–8685. doi: 10.1007/s002590100549. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Marcoen A, Goossens L. Facts and fiction about memory aging: A quantitative integration of research findings. J Gerontol. 1993;48:157–171. doi: 10.1093/geronj/48.4.p157. [DOI] [PubMed] [Google Scholar]
- Zhao M, Amin-Hanjani S, Ruland S, Curcio AP, Ostergren L, Charbel FT. Regional cerebral blood flow using quantitative MR angiography. AJNR Am J Neuroradiol. 2007;28:1470–1473. doi: 10.3174/ajnr.A0582. [DOI] [PMC free article] [PubMed] [Google Scholar]